SUMMARY
The endogenous, free-running circadian period (τ) determines the phase relationship that an organism assumes when entrained to the 24-h day. We found a shorter circadian period in African-Americans compared to non-Hispanic European-Americans (24.07 vs 24.33 h). We speculate that a short circadian period, closer to 24 h, was advantageous to humans living around the equator, but when humans migrated north out of Africa, where the photoperiod changes with seasons, natural selection favored people with longer circadian periods. Recently, in evolutionary terms, immigrants came from Europe and Africa to America (“the new world”). The Europeans were descendents of people who had lived in Europe for thousands of years with changing photoperiods (and presumably longer periods), whereas Africans had ancestors who had always lived around the equator (with shorter periods). It may have been advantageous to have a longer circadian period while living in Europe early in the evolution of humans. In our modern world, however, it is better to have a shorter period, because it helps make our circadian rhythms earlier, which is adaptive in our early-bird dominated society. European-American women had a shorter circadian period than men (24.24 vs 24.41), but there was no sex difference in African-Americans (24.07 for both men and women). We speculate that selection pressures in Europe made men develop a slightly longer period than women to help them track dawn which could be useful for hunters, but less important for women as gatherers.
Keywords: race, ethnicity, Blacks, tau, chronotype, slavery
INTRODUCITON
The free-running circadian period (τ) is an important determinant of the phase relationship that an organism displays when entrained to the 24-h day. Shorter τs result in earlier circadian rhythms, whereas longer τs result in later rhythms. This relationship is predicted by oscillator (entrainment) theory (Aschoff, 1965), and has been shown in unicellular organisms, plants, insects and vertebrates (Aschoff and Pohl, 1978) including humans (Wright et al., 2005, Gronfier et al., 2007, Hasan et al., 2012, Eastman et al., 2015, Eastman et al., 2016).
We performed two studies comparing circadian rhythms in African-Americans to non-Hispanic European-Americans (Eastman et al., 2015, Eastman et al., 2016). Here we combine the τ data from both studies to explore sex and ancestry differences.
METHODS
The studies were approved by the Rush University Medical Center Institutional Review Board, and we obtained written informed consent.
There were 36 subjects in first study and 45 in the second. Eighteen subjects participated in both, and their data are averaged here yielding 63 unique individuals (Table 1).
Table 1.
Subject demographics and the free-running circadian period (τ). Mean ± SD.
| N | Age | Circadian Period (h) | |
|---|---|---|---|
| African-American | 32 | 31.9 ± 6.9 | 24.07 ± .16** |
| Men | 16 | 32.1 ± 8.0 | 24.07 ± .19 |
| Women | 16 | 31.6 ± 5.9 | 24.07 ± .13 |
| European-American | 31 | 28.5 ± 6.1 | 24.33 ± .23 |
| Men | 15 | 28.9 ± 6.3 | 24.41 ± .20* |
| Women | 16 | 28.3 ± 6.1 | 24.24 ± .23 |
| All | 63 | 30.2 ± 6.7 | 24.20 ± .23 |
p <.05 vs women
p <.0001 vs European-Americans
Subjects completed our Family/Ancestor Questionnaire and we accepted those who checked either Black/African-American or White for both biological parents, but not Hispanic or Latino. Buccal swabs were used to collect DNA samples, and were processed by AncestrybyDNA, DNA Diagnostics Center, Fairfield, OH. Results consisted of percents for each subject in 4 categories (European, Sub-Saharan African, East Asian, Indigenous Indian), and were used to confirm ancestry (largest % either European or African).
Subjects were not taking any prescription medications except for a few women on oral contraceptives. Exclusion criteria included body mass index>35 kg/m2 and night shift work in the preceding month.
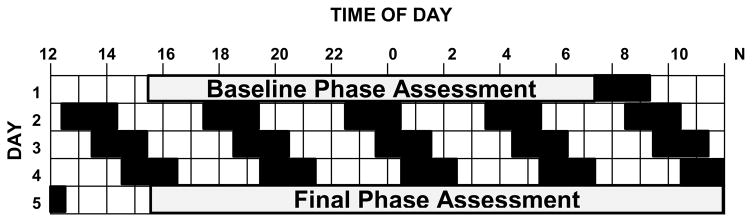
During the 5 days of the protocol (Fig 1) subjects were in temporal isolation. They were required to remain in bed during the dark periods even if they could not sleep. While awake they ate, watched pre-recorded movies and TV or engaged in other sedentary activities in dim light, 21±11 lux. The ultradian LD cycle was either a 5-h day (3 h of wake alternating with 2 h in bed in the dark) or a 4-h day (2.5 h wake and 1.5 h in bed).
Figure 1.
Protocol diagram for determining the free-running circadian period (τ). The ultradian LD cycle produces forced desynchrony (FD). Black shows times for sleep in the dark. Period was calculated from the change in the dim light melatonin onset (DLMO) from baseline to final phase assessment.
The DLMO, a measure of the phase of the circadian clock, was obtained during phase assessments. Saliva samples were collected every 30 min in very dim light (<5 lux) using Salivettes (Sarstedt, Newton, NC, USA). Saliva samples were centrifuged, frozen, and radioimmunoassayed for melatonin by SolidPhase, Inc. (Portland, Maine, USA). The sensitivity (limit of detection) of the assay was 0.9 pg/ml.
Data Analysis
Melatonin profiles were smoothed with a LOWESS curve (GraphPad Prism). The threshold for determining the DLMO was 25% of the distance from the fitted minimum value to the fitted maximum.
To calculate τ the difference between DLMOs on the baseline and final phase assessment was divided by 4 (because there were 4 days between these DLMOs) and then added to 24 (when the DLMO delayed), or subtracted from 24 (when the DLMO advanced). Results are presented as means ± SD unless otherwise indicated. Prism was used for data analysis.
For more detailed methods see Eastman et al (2015, 2016).
RESULTS
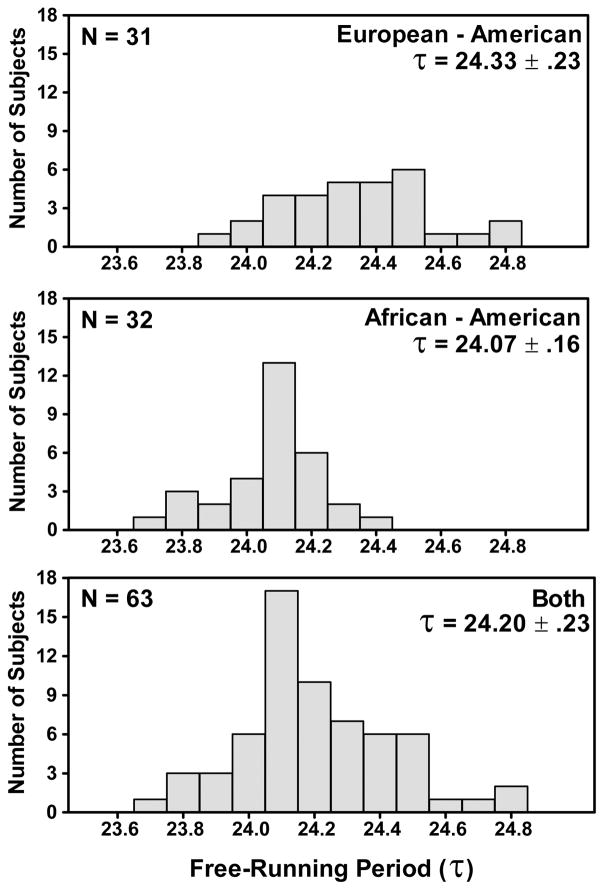
Figure 2 shows that the shapes of the distributions of τ were very different in the African-Americans compared to the European-Americans. For the African-Americans the histogram was shifted left (τ was shorter, Table 1, t=5.181, df=61, p<.0001). The τs of the African-Americans were closer to 24 h; the absolute difference from 24 h was .14 ± .10 h vs .34 ± .21 h for the European-Americans (t=4.855, df=61, p<.0001). More African-Americans than European-Americans had τs<24.0 h (6/32=19% vs 1/31=3%, Chi-Square p=.05).
Figure 2.
Frequency histograms of the endogenous free-running circadian periods. τ was longer in European-Americans (p<.0001).
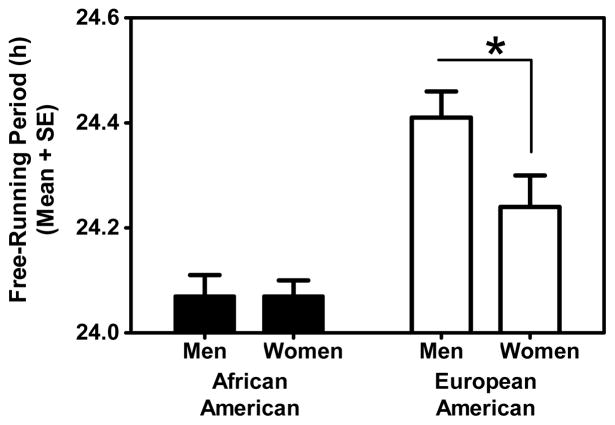
Figure 3 and Table 1 show that for European-Americans τ was shorter in women than men (t=2.187, df=29, p=.037, 2-tailed). In contrast, τ was identical in African-American women and men.
Figure 3.
For European-Americans the free-running period was shorter in women than men (p<.05), but African-American women and men had identical circadian periods.
DISCUSSION
We found that τ was shorter in African-Americans than European-Americans, confirming previous results (Eastman et al., 2012).
In the current study we found that τ was shorter in European-American women compared to European-American men, but there was no sex difference in African-Americans. Duffy et al (2011) pooled data from several FD studies (N=157), and found that τ for women was 6 min shorter than for men. In the current study, we also found a shorter τ in women than men, but only in European-Americans, and it was slightly larger (10 min). The difference in τ between ancestry groups (European vs African), however, was even larger (16 min). Duffy et al did not specify the race/ancestry of their subjects, but if they had a greater proportion of African-American men than women, that could decrease the difference between the sexes. An FD study from the UK did not find a sex difference in τ (Lazar et al., 2012). They attributed this to their smaller sample (N=31), but it could also be from not considering ancestry. Wever (1984) found a sex difference in τ when subjects free-ran in the underground bunker with synchronized rhythms, but not during spontaneous internal synchronization when the underlying period was revealed by the temperature rhythm (c.f.(Eastman, 1984).
The sex and ancestry differences in τ may seem trivial, but Duffy et al reasoned that the 6 min difference in τ would produce a 28 min difference in phase position when entrained to the 24-h day based on their previous data on the melatonin rhythm phase angle difference vs τ (Wright et al., 2005). If we used the same constant (28/6), then the sex difference we found in τ would produce an entrained sex difference of 47 min, the ancestry difference in τ would produce an entrained difference of 75 min, and the difference in τ between European-American and African-American men would produce an entrained difference of 93 min.
Among European-Americans, why would males have a longer τ than females? We can only speculate. If a longer τ does help animals to track dawn when living at higher latitudes where the time of dawn changes across seasons (Pittendrigh and Daan, 1976), then perhaps it was more important for men as hunters than for women as gatherers.
Differences in τ among species are well known (Refinetti, 2016), differences in τ among inbred strains of the species mouse are often found (e.g., (Possidente and Stephan, 1988) and several studies provide data on τ sex differences in animals besides humans. One study found a shorter τ in female Sprague-Dawley rats (Schull et al., 1989), small or no sex differences were found in the hamster, Mesocricetus auratus (Zucker et al., 1980, Davis et al., 1983), and no sex difference was found in mice (Kuljis et al., 2013, Possidente and Stephan, 1988) or a bird species, Parus major (Helm and Visser, 2010). Aschoff (1979) reviewed work showing a shorter period in male chaffinches, but no sex difference in the house sparrow or pocket mouse. In the diurnal rodent Octodon degus, males had a shorter τ than females (Hummer et al., 2007, Labyak and Lee, 1995, Lee and Labyak, 1997). Presumably latitude and other selection pressures contribute to differences in τ.
Arguments have been made that having a τ slightly different from 24 h, but not greatly different, is adaptive (Daan and Beersma, 2002, Johnson et al., 2003, Hut and Beersma, 2011). The shorter τ of African-Americans has the advantage that a smaller daily correction is needed to achieve entrainment. Although a longer τ may have been advantageous early in the evolution of humans living north with changing photoperiods, this may no longer be true in today’s modern urban world with electric lights providing a constant photoperiod. Long τs could lead to extreme eveningness, social jet lag, and the delayed sleep phase disorder.
Acknowledgments
Support: NIH grant R01NR007677 to CIE.
Footnotes
CONFLICT OF INTEREST
No conflicts of interest.
AUTHOR CONTRIBUTIONS
C.I.E. conceived, designed and coordinated the studies and wrote the manuscript. V.A.T. performed data analysis and graphed figures. S.J.C. helped revise the paper.
References
- Aschoff J. The phase-angle difference in circadian periodicity. In: ASCHOFF J, editor. Circadian Clocks. North-Holland Publishing Co; Amsterdam: 1965. pp. 262–76. [Google Scholar]
- Aschoff J. Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Zeitschrift fur Tierpsychologie. 1979;49:225–49. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Pohl H. Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschaften. 1978;65:80–84. doi: 10.1007/BF00440545. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DGM. Circadian frequency and its variablity. In: KUMAR V, editor. Biological Rhythms. Narosa Publishing House; New Delhi, India: 2002. pp. 24–37. [Google Scholar]
- Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. American Journal of Physiology. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI. Are separate temperature and activity oscillators necessary to explain the phenomena of human circadian rhythms? In: MOORE-EDE MC, CZEISLER CA, editors. Mathematical Models of the Circadian Sleep-Wake Cycle. Raven Press; New York: 1984. pp. 81–103. [Google Scholar]
- Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans) Chronobiol Int. 2012;29:1072–77. doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Suh C, Tomaka VA, Crowley SJ. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep. 2015;5:8381. doi: 10.1038/srep08381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ. Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci Rep. 2016;6:36716. doi: 10.1038/srep36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A. 2007;104:9081–6. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, Santhi N, Lazar AS, et al. Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB J. 2012;26:2414–23. doi: 10.1096/fj.11-201699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B, Visser ME. Heritable circadian period length in a wild bird population. Proc Biol Sci. 2010;277:3335–42. doi: 10.1098/rspb.2010.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R586–97. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- Hut RA, Beersma DG. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philos Trans R Soc Lond B Biol Sci. 2011;366:2141–54. doi: 10.1098/rstb.2010.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiology International. 2003;20:741–74. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- Kuljis DA, Loh DH, Truong D, et al. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–12. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58:573–85. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- Lazar AS, Santhi N, Hasan S, et al. Circadian period and the timing of melatonin onset in men and women: predictors of sleep during the weekend and in the laboratory. J Sleep Res. 2012;22:155–9. doi: 10.1111/jsr.12001. [DOI] [PubMed] [Google Scholar]
- Lee TM, Labyak SE. Free-running rhythms and light- and dark-pulse phase response curves for diurnal Octodon degus (Rodentia) Am J Physiol. 1997;273:R278–86. doi: 10.1152/ajpregu.1997.273.1.R278. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- Possidente B, Stephan FK. Circadian period in mice: Analysis of genetic and maternal contributions to inbred strain differences. Behavior Genetics. 1988;18:109–17. doi: 10.1007/BF01067080. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Circadian Physiology. 3 CRC Press, Taylor & Francis Group; Boca Raton, FL: 2016. [Google Scholar]
- Schull J, Walker J, Fitzgerald K, et al. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989;46:341–6. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Wever RA. Sex differences in human circadian rhythms: Intrinsic periods and sleep fractions. Experientia. 1984;40:1226–34. doi: 10.1007/BF01946652. [DOI] [PubMed] [Google Scholar]
- Wright KP, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–77. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of the circadian system in the golden hamster. Am J Physiol. 1980;238:R97–101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]