Abstract
Objectives
To determine the associations between local (pericardial) fat and incident CV disease (CVD) events and cardiac remodeling independent of markers of overall adiposity.
Background
The impact of pericardial fat—a local fat depot encasing the heart—on myocardial function and long-term cardiovascular (CV) prognosis independent of systemic consequences of adiposity or hepatic fat is an area of active debate.
Methods
We studied 4,234 participants enrolled in the Multi-Ethnic Study of Atherosclerosis with concomitant cardiac magnetic resonance (CMR) imaging and computed tomographic (CT) measurements for pericardial fat volume and hepatic attenuation (a measure of liver fat). Poisson and Cox regression were used to estimate the annualized risk of incident hard atherosclerotic CVD (ASCVD), all-cause death, heart failure, all-cause CVD, hard CHD and stroke as a function of pericardial and hepatic fat. Generalized additive models were used to assess the association between CMR indices of left ventricular (LV) structure and function and pericardial fat. Models were adjusted for relevant clinical, demographic, and cardiometabolic covariates.
Results
MESA participants with higher pericardial and hepatic fat were more likely to be older, more frequently male, and had a higher prevalence of cardiometabolic risk factors (including dysglycemia, dyslipdemia, hypertension), as well as adiposity-associated inflammation. Over a median 12.2-year follow-up (IQR 11.6–12.8 years), pericardial fat was associated with a higher rate of incident hard ASCVD (standardized hazard ratio [SHR] 1.22, 95% confidence interval [CI] 1.10–1.35, P=0.0001; hepatic fat by CT was not significantly associated with hard ASCVD (SHR 0.96, 95% CI 0.86–1.08, P=0.52). Higher pericardial fat was associated with greater indexed LV mass (37.8 vs. 33.9 g/m2.7, highest vs. lowest quartile, P<0.01), LV mass-to-volume ratio (1.2 vs. 1.1, highest vs. lowest quartile, P<0.01). In adjusted models, a higher pericardial fat volume was associated with greater LV mass (P<0.0001) and concentricity (P<0.0001).
Conclusion
Pericardial fat is associated with poorer CVD prognosis and LV remodeling, independent of insulin resistance, inflammation and CT measures of hepatic fat.
Keywords: Hepatic fat, Pericardial fat, Obesity, Cardiac magnetic resonance imaging, Remodeling
INTRODUCTION
Obesity is associated with subclinical abnormalities in cardiovascular (CV) structure and function that predispose to heart failure (HF)1–5. The adverse CV effects of obesity are postulated to originate in part from pro-inflammatory adipose tissue depots that exert toxic effects on the heart either locally (e.g., pericardial fat) or remotely via inflammation or insulin resistance. Although several recent investigations have suggested that visceral and hepatic fat may play a primary role in CV disease (CVD) risk and myocardial remodeling6,7, an emerging body of evidence supports the idea that pathologic expansion of pericardial fat—generally felt to be a cache of metabolic fuel for the heart—may function as a “visceral-like” fat depot, promoting atrial fibrillation, coronary artery calcification, and vascular disease7–9. Indeed, a case-control study conducted early during the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that pericardial fat, but not body mass index (BMI) or waist circumference (markers of overall and regional adiposity), was associated with coronary heart disease (CHD)10. Given a potential metabolic and pro-inflammatory role for pericardial fat, evaluating its impact on CV risk and structure is warranted.
To address this important issue, we investigated the association between pericardial fat and CVD outcomes after accounting for systemic markers of adiposity (insulin resistance and inflammation) and measures of hepatic fat in MESA. We further sought to understand the relationship between pericardial fat and CV structure using comprehensive cardiac magnetic resonance (CMR) assessment of LV structure and function. We hypothesized that greater pericardial fat would be associated with poorer CVD outcomes and prevalent abnormalities in LV structure and function by CMR.
METHODS
Study population
The overall design of the MESA study has been described previously11. Briefly, the MESA is a longitudinal cohort study consisting of 6,814 men and women aged 45 to 84 years, free of clinical CV disease at study enrollment. Participants were enrolled from July 2000 through August 2002 in Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN12. At the baseline visit (Exam 1), standardized questionnaires were used to collect information on demographics, income and education, medical history, medication use, smoking status, alcohol use, and physical activity. Physical activity was calculated based on duration and intensity of total intentional exercise (MET-min/week). Resting blood pressure, fasting blood glucose and insulin, BMI, and dysglycemia status were also assessed at Exam 1, as described13. The homeostatic model assessment was used to quantify insulin resistance (HOMA-IR; fasting insulin × fasting glucose/40513). C-reactive protein was measured as described14.
From the initial MESA cohort studied at Exam 1, we excluded patients with missing liver attenuation (or liver attenuation values > 200 Hounsfield units, N=201), missing pericardial fat volume (N=25), those not included in CMR assessment of LV structure and function (N=1,804), cirrhosis (N=9), significant alcohol use (>7 drinks per week in women and >14 drinks per week in men; N=232), and self-reported cancer (N=345) or kidney disease (N=99), yielding 4,234 MESA participants for the final analytic sample. Protocols were approved by the Institutional Review Board at each participating institution, and all MESA participants provided written informed consent.
CV and adiposity imaging
CMR imaging was performed at 1.5 T at the baseline examination, as previously described5, 15, 16. MASS software (version 4.2, Medis, The Netherlands) was used to perform analyses at a single reading center in a blinded fashion. Computed tomographic (CT) imaging was used to determine hepatic attenuation17 (an index of liver fat content, higher values indicating less liver fat content) and pericardial fat volume10 (indexed to height), as described. Briefly, liver attenuation was measured by placement of regions of interest (>100 mm2) in the right hepatic lobe17. Pericardial fat included both epicardial and paracardial fat, as previously published10. Briefly, the boundaries of pericardial fat volume were circumscribed by slices 15 mm above and 30 mm below the left main coronary artery (superior/inferior) and chest wall and aorta or bronchus (anterior/posterior). Fat was defined by attenuation (between −190 and −30 Hounsfield units), with the volume defined as sum of fat-containing voxels.
We chose LV mass (indexed to height2.7 18) and LV mass-to-volume ratio as our primary cardiac geometry endpoints in this study. Secondary endpoints included LVEF, LV end-diastolic volume (indexed to height2.7). Rate of incident hard CV disease events was our primary clinical endpoint with rates for all CV disease and incident HF as secondary endpoints.
Outcomes Ascertainment
CV events in MESA were defined as “hard” CHD (non-fatal myocardial infarction, resuscitated sudden cardiac arrest or CHD death), “hard” atherosclerotic CVD (ASCVD) (hard CHD, stroke [not TIA], stroke death), all-cause CVD (hard ASCVD, definite angina, probable angina followed by revascularization, other atherosclerotic death or other CV disease death) or HF (probable HF: symptoms [e.g. shortness of breath or edema], plus HF diagnosis by treating physician and treatment for HF; definite HF: symptoms, HF diagnosis by treating physician, treatment for HF and one of the following: pulmonary edema or congestion by chest x-ray, dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction)19. Incident hard ASCVD was our primary clinical endpoint with all-cause CVD, hard CHD, all cause mortality, stroke and incident HF as secondary endpoints.
Statistical analysis
Baseline clinical, demographic, biochemical, and CMR indices were compared across quartiles of pericardial and hepatic fat using one-way analysis of variance (ANOVA) or Wilcoxon testing for continuous covariates (depending on data normality) and chi-square test for categorical covariates. Event rate regression was performed with Poisson models to estimate the association between pericardial or hepatic fat and rates of incident CVD (as defined above) per year. Cox regression was performed to estimate adjusted hazard ratios. Given the potential for non-linear associations between imaging and clinical outcomes with pericardial and hepatic fat, we used generalized additive models (GAM) to delineate the form of the association between pericardial fat and each imaging outcome and developed final models using linear, quadratic and/or cubic terms as required based on visual inspection of the results of GAMs and confirmed by analysis of variance. Each model was adjusted for age, sex, race/ethnicity, pericardial fat-adjusted BMI and waist circumference residuals (as a measure of “excess” adiposity beyond that explained by pericardial fat), cigarette smoking status, total intentional exercise, HOMA-IR, C-reactive protein, diabetes status, systolic blood pressure, hypertension medication use, LDL and statin medication use. Survival models were also adjusted for coronary artery calcium score. Models where pericardial fat was the primary predictor of interest were also adjusted for hepatic fat and vice versa. Given potential collinearity among BMI, waist circumference and liver and pericardial fat, we utilized fat depot-adjusted BMI and waist circumference residuals in our regression models (as opposed to waist BMI and circumference alone); this approach has been taken in prior epidemiologic studies to limit multicollinearity effects20, 21. Covariates were comprehensively selected based on expert experience of the most relevant confounding variables for which reliable assessments were available. We assessed for the presence of effect modification by age, sex, race/ethnicity, diabetes and metabolic syndrome using multiplicative interaction terms. Finally, we evaluated incremental value of pericardial fat and liver steatosis in Cox models using net reclassification improvement (NRI), relative integrated discrimination index (IDI) and c-index. Confidence intervals for NRI and IDI were estimated using bootstrapping with 1000 resamples. SAS 9.4 (SAS Institute, Cary, NC) and R (R project, www.r-project.org) were used for analysis, and a two-tailed P-value <0.05 was considered significant.
RESULTS
Clinical and biochemical characteristics by pericardial and hepatic fat
Baseline demographic, clinical, and biochemical measures at Exam 1 stratified by pericardial fat quartiles are shown in Table 1. Compared to those in the lowest quartile (least pericardial fat), MESA participants in the highest quartile of pericardial fat were more likely to be older males with dysglycemia (by diabetes status, fasting glucose, and HOMA-IR), proatherogenic dyslipidemia (lower HDL, higher triglycerides), systemic inflammation (by CRP, PAI-1 and fibrinogen), greater weight, and more proteinuria. We observed similar results with hepatic fat (Supplementary Table 1)
Table 1.
Baseline characteristics for study participants, stratified by pericardial fat quartile. Values are median (interquartile range) or n (%). Nonparametric tests (continuous values) or chi-square testing (categorical values) were used to determine P values. Abbreviations: HU = Hounsfield unit; HDL = high-density lipoprotein; BP = blood pressure; BMI = body mass index; HOMA-IR = homeostatic model assessment of insulin resistance; LDL = low-density lipoprotein; PAI = plasminogen activator inhibitor.
| Quartile 1 (4.15 to 29.28 cm3/m) |
Quartile 2 (29.29 to 41.46 cm3/m) |
Quartile 3 (41.46 to 56.80 cm3/m) |
Quartile 4 (56.81 to 194.38 cm3/m) |
|||
|---|---|---|---|---|---|---|
| Variable | N=1058 | N=1059 | N=1059 | N=1058 | P-Value All Quartiles | P-Value Q1 vs. Q4 |
| Age, yrs | 55 (49–65) | 59 (52–68) | 63 (55–70) | 65 (57–72) | <0.01 | <0.01 |
|
| ||||||
| Male | 398 (20%) | 459 (23%) | 497 (25%) | 657 (33%) | <0.01 | <0.01 |
|
| ||||||
| Race/ethnicity | <0.01 | <0.01 | ||||
| White, Caucasian | 367 (24%) | 330 (22%) | 359 (24%) | 448 (30%) | ||
| Chinese American | 113 (19%) | 172 (30%) | 164 (28%) | 131 (23%) | ||
| Black, African-American | 429 (36%) | 326 (28%) | 271 (23%) | 152 (13%) | ||
| Hispanic | 149 (15%) | 231 (24%) | 265 (27%) | 327 (34%) | ||
|
| ||||||
| Smoking status | <0.01 | <0.01 | ||||
| Never | 598 (27%) | 575 (26%) | 586 (26%) | 494 (22%) | ||
| Former | 316 (22%) | 351 (24%) | 349 (24%) | 434 (30%) | ||
| Current | 140 (27%) | 131 (25%) | 122 (23%) | 127 (24%) | ||
|
| ||||||
| Glycemic control | <0.01 | <0.01 | ||||
| Normoglycemia | 914 (29%) | 846 (27%) | 757 (24%) | 638 (20%) | ||
| Impaired fasting glucose | 62 (11%) | 104 (19%) | 156 (28%) | 228 (41%) | ||
| Untreated diabetes | 11 (10%) | 22 (20%) | 38 (34%) | 41 (37%) | ||
| Treated diabetes | 66 (16%) | 85 (21%) | 106 (26%) | 148 (37%) | ||
|
| ||||||
| Metabolic syndrome component | ||||||
| Waist circumference, cm | 86.9 (80.0–94.5) | 93.7 (86.5–100.8) | 98.9 (91.7–106.5) | 105.0 (97.7–113.2) | <0.01 | <0.01 |
| Triglycerides, mg/dl | 86 (63–121) | 107 (78–152) | 119 (84–169) | 143 (100–197) | <0.01 | <0.01 |
| HDL, mg/dl | 55 (45–67) | 50 (42–59) | 47 (40–56) | 43 (37–51) | <0.01 | <0.01 |
| Systolic BP, mm Hg | 117 (105–133) | 122 (109–138) | 124 (113–139) | 129 (116–144) | <0.01 | <0.01 |
| Fasting glucose, mg/dl | 84 (79–91) | 88 (82–96) | 91 (84–100) | 95 (87–108) | <0.01 | <0.01 |
|
| ||||||
| Weight, kg | 69.2 (60.2–78.9) | 73.1 (63.6–83.7) | 78.2 (67.7–89.3) | 85.4 (74.5–96.8) | <0.01 | <0.01 |
|
| ||||||
| BMI, kg/m2 | 24.6 (22.5–27.4) | 26.4 (23.8–29.4) | 28.0 (25.5–31.2) | 30.1 (27.3–33.6) | <0.01 | <0.01 |
|
| ||||||
| Waist to hip ratio | 0.9 (0.8–0.9) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 1.0 (0.9–1.0) | <0.01 | <0.01 |
|
| ||||||
| Fasting insulin, mU/L | 6.3 (4.9–8.6) | 7.4 (5.8–10.3) | 8.8 (6.4–12.4) | 11.1 (8.0–15.5) | <0.01 | <0.01 |
|
| ||||||
| HOMA-IR | 1.3 (1.0–2.0) | 1.6 (1.2–2.4) | 2.0 (1.4–3.0) | 2.7 (1.9–3.9) | <0.01 | <0.01 |
|
| ||||||
| Cholesterol, mg/dl | 191 (170–214) | 194 (173–218) | 193 (170–217) | 191 (171–214) | 0.04 | 0.34 |
|
| ||||||
| LDL, mg/dl | 114 (94–134) | 119 (99–139) | 118 (98–139) | 115 (96–136) | <0.01 | 0.04 |
|
| ||||||
| Taking hypertension medication | 262 (18%) | 315 (21%) | 418 (28%) | 478 (32%) | <0.01 | <0.01 |
|
| ||||||
| Taking statins | 97 (16%) | 150 (24%) | 173 (28%) | 197 (32%) | <0.01 | <0.01 |
|
| ||||||
| Urine albumin-creatinine ratio, mg/g | 4.5 (3.0–7.8) | 5.0 (3.1–9.7) | 5.6 (3.6–10.9) | 6.2 (3.7–14.6) | <0.01 | <0.01 |
|
| ||||||
| Inflammatory biomarkers | ||||||
| C-reactive protein, mg/l | 1.1 (0.5–2.6) | 1.6 (0.7–3.9) | 2.2 (0.9–4.8) | 2.4 (1.1–4.7) | <0.01 | <0.01 |
| Fibrinogen antigen, mg/dl | 321 (286–374) | 331 (291–380) | 341 (297–391) | 351 (309–398) | <0.01 | <0.01 |
| Interleukin-6, pg/ml | 0.9 (0.6–1.5) | 1.0 (0.7–1.6) | 1.2 (0.8–1.8) | 1.4 (1.0–2.2) | <0.01 | <0.01 |
| Oxidized LDL, mg/dl (N=667) | 0.7 (0.5–1.0) | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.8 (0.6–1.3) | 0.01 | <0.01 |
| PAI-1, ng/ml (N=647) | 11.0 (6.0–19.0) | 17.0 (9.0–30.5) | 21.5 (10.0–37.0) | 30.0 (18.0–49.0) | <0.01 | <0.01 |
| Tumor necrosis factor-α, pg/ml (N=1867) | 1157 (1007–1353) | 1232 (1070–1429) | 1291 (1109–1525) | 1420 (1230–1684) | <0.01 | <0.01 |
|
| ||||||
| Liver attenuation (Hounsfield units) | 64.0 (60.0–68.5) | 63.0 (57.5–68.0) | 60.5 (54.0–66.5) | 58.5 (49.0–64.5) | <0.01 | <0.01 |
Pericardial fat but not liver steatosis is associated with incident CV disease, independent of systemic effects of adiposity
Over a median follow-up of 12.2 years (IQR 11.6–12.8 years), study participants experienced 337 hard ASCVD events, 481 all-cause CVD, 193 incident HF events, hard CHD events, 549 deaths from any cause, 219 hard CHD events and 142 strokes. The unadjusted annualized event rates for hard ASCVD, hard CHD, death, and HF are shown in Figure 1 and for all-cause CVD and stroke in Supplemental Figure 1. Pericardial, but not hepatic, fat was associated with a progressive rise in risk of all-cause CVD, hard ASCVD, and HF. In adjusted Cox regression, for each standard deviation increase in pericardial fat, there was a 22% increase in incident hard ASCVD events (HR 1.22 [95% CI 1.10–1.35], p=0.0001) (Table 3), with similar associations for other outcomes. Across quartiles, increased pericardial fat was consistently associated with worsening outcomes (Supplemental Figure 2). Conversely, there was no relationship between liver fat by CT and any of these end points in either event rate or Cox regression models (Table 3). There was no evidence of effect modification by sex, race, age, diabetes or metabolic syndrome.
Figure 1. Associations between pericardial and hepatic fat and cardiovascular events.
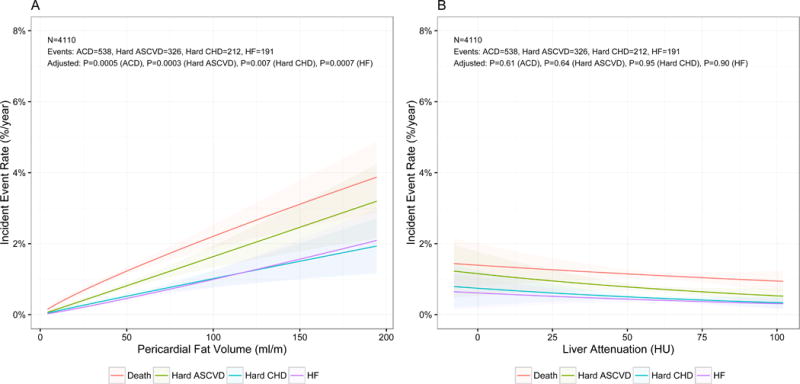
Event rate regression for association between pericardial (panel A) or hepatic (panel B) fat and annualized rates of all-cause death (red), hard cardiovascular (CVD) events (cyan), hard coronary heart disease (CHD) events (green) and heart failure (purple). Fully adjusted p-values are presented.
Table 3. Adjusted Effect Sizes.
Adjustments are as specified in text. Abbreviations: LVMI, LV mass index; LVEDVI, LV end-diastolic volume index; LVMtoV, LV mass-to-volume ratio; LVEF, LV ejection fraction.
| Outcome | Model | Adjusted Standardized HR (95% CI) | P-Value | Adj. Calibration χ2 | P-Value | C-Index (95% CI) | P-Value | Relative IDI (95% CI) | NRI (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Death | |||||||||
|
| |||||||||
| Base | N/A | N/A | 8.42 | 0.49 | 0.8 (0.78–0.82) | ref | N/A | N/A | |
|
| |||||||||
| + PFV | 1.18 (1.09–1.28) | <0.0001 | 14.35 | 0.11 | 0.8 (0.78–0.82) | 0.03 | 2.7% (0.8%– 5%) | 0.225 (0.137–0.317) | |
|
| |||||||||
| + Liver | 0.92 (0.84–1.01) | 0.06 | 7.55 | 0.58 | 0.8 (0.78–0.82) | 0.45 | 0.4% (−0.4%– 1.3%) | 0.177 (0.086–0.273) | |
|
| |||||||||
|
| |||||||||
| All CVD | |||||||||
|
| |||||||||
| Base | N/A | N/A | 15.95 | 0.07 | 0.79 (0.77–0.81) | ref | N/A | N/A | |
| + PFV | 1.17 (1.08–1.28) | 0.0002 | 21 | 0.01 | 0.79 (0.77–0.81) | 0.05 | 2.4% (0.2%–4.7%) | 0.169 (0.072–0.271) | |
|
| |||||||||
| + Liver | 0.96 (0.87–1.06) | 0.40 | 12.66 | 0.18 | 0.79 (0.77–0.81) | 0.62 | 0.1% (−0.4%–0.5%) | 0.055 (−0.043–0.16) | |
|
| |||||||||
|
| |||||||||
|
Hard ASCVD |
|||||||||
|
| |||||||||
| Base | N/A | N/A | 6.8 | 0.66 | 0.78 (0.76–0.81) | ref | N/A | N/A | |
|
| |||||||||
| + PFV | 1.22 (1.10–1.35) | 0.0001 | 5.65 | 0.77 | 0.79 (0.76–0.81) | 0.14 | 4.7% (1.3%–8.6%) | 0.257 (0.148–0.371) | |
|
| |||||||||
| + Liver | 0.96 (0.86–1.08) | 0.52 | 5.56 | 0.78 | 0.78 (0.76–0.81) | 0.75 | 0.4% (−0.2%–0.9%) | 0.02 (−0.09–0.129) | |
|
| |||||||||
|
| |||||||||
|
Hard CHD |
|||||||||
|
| |||||||||
| Base | N/A | N/A | 5.1 | 0.83 | 0.81 (0.78–0.84) | ref | N/A | N/A | |
|
| |||||||||
| + PFV | 1.24 (1.09–1.40) | 0.0009 | 5.99 | 0.74 | 0.81 (0.79–0.84) | 0.47 | 5.9% (1.5%–10.7%) | 0.299 (0.164–0.442) | |
|
| |||||||||
| + Liver | 0.94 (0.81–1.08) | 0.37 | 10.53 | 0.31 | 0.81 (0.78–0.84) | 0.61 | 1.1% (−0.1%–2.5%) | 0.029 (−0.103–0.171) | |
|
| |||||||||
|
| |||||||||
| CHF | |||||||||
|
| |||||||||
| Base | N/A | N/A | 7.24 | 0.61 | 0.85 (0.83–0.87) | ref | N/A | N/A | |
|
| |||||||||
| + PFV | 1.27 (1.12–1.45) | 0.0003 | 10.85 | 0.29 | 0.85 (0.83–0.88) | 0.28 | 5.9% (1.3%–11%) | 0.277 (0.131–0.427) | |
|
| |||||||||
| + Liver | 1.00 (0.86–1.16) | 1.00 | 7.41 | 0.59 | 0.85 (0.83–0.87) | 0.2 | 0% (−0.1%–0.1%) | 0.074 (−0.073–0.229) | |
|
| |||||||||
|
| |||||||||
| Stroke | |||||||||
|
| |||||||||
| Base | N/A | 13.05 | 0.16 | 0.78 (0.74–0.81) | ref | N/A | N/A | ||
|
| |||||||||
| + PFV | 1.20 (1.02–1.40) | 0.03 | 8.6 | 0.48 | 0.78 (0.75–0.82) | 0.4 | 4% (−0.2%–8.7%) | 0.199 (0.015–0.373) | |
|
| |||||||||
| + Liver | 1.00 (0.84–1.19) | 0.96 | 12.51 | 0.19 | 0.78 (0.74–0.81) | 0.83 | 0% (−0.1%–0.1%) | 0.07 (−0.096–0.233) | |
Adjusted for age, sex, race/ethnicity, excess BMI, excess waist circumference, cigarette smoking status, total intentional exercise, HOMA-IR, C-reactive protein, diabetes status, systolic blood pressure, hypertension medication use, LDL, statin medication use and coronary artery calcium score. Models where pericardial fat was the primary predictor of interest were also adjusted for hepatic fat and vice versa.
Addition of pericardial fat to clinical parameters and coronary calcium score resulted in favorable risk reclassification for all outcomes (NRI for hard ASCVD of 0.257 [95% CI 0.164–0.442]) and improved risk discrimination for all outcomes except stroke (relative IDI for hard ASCVD 5.9% [95% CI 1.5%–10.7%]). Addition of liver attenuation to clinical models and calcium score did not result in improved risk reclassification for any outcome except death from any cause (NRI 0.177 [95% CI 0.086–0.273]). Liver attenuation did not improve risk discrimination for any of the outcomes studied.
Pericardial fat and liver steatosis are associated with adverse LV remodeling, independent of systemic effects of adiposity and hepatic fat
Given the association between pericardial fat and outcomes, we investigated whether extent of pericardial fat was related to cardiac geometry and function. CMR indices of myocardial structure and function are shown in Table 2, stratified by pericardial fat. Relative to participants in the lowest quartile, those with the highest pericardial fat volume had greater LV mass and more concentric LV remodeling. There was no difference in LV ejection fraction, which was preserved, by pericardial fat.
Table 2.
Left ventricular structure and function, stratified by quartiles of pericardial fat. Values are median (interquartile range). Kruskal-Wallis and Wilcoxon rank-sum testing were used to determine P values. Abbreviations: LVMI = left ventricular mass, indexed by height2.7; LVEF = left ventricular ejection fraction; LVEDVI= left ventricular end-diastolic volume, indexed by height2.7, LVMtoV = left ventricular mass to volume ratio.
| Pericardial fat volume
| ||||||
|---|---|---|---|---|---|---|
| CMR Remodeling Index | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value All Quartiles | P-value Q1 vs. Q4 |
| LVMI, g/m2.7 | 33.9 (29.1–38.8) | 34.9 (30.9–39.8) | 36.5 (32.0–42.1) | 37.8 (33.2–43.8) | <0.01 | <0.01 |
|
| ||||||
| LVEDVI, ml/m2.7 | 31.5 (27.7–35.3) | 31.4 (27.7–35.1) | 32.1 (27.8–36.2) | 31.8 (27.8–36.6) | 0.02 | 0.04 |
|
| ||||||
| LVMtoV, g/ml | 1.1 (0.9–1.2) | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) | 1.2 (1.1–1.4) | <0.01 | <0.01 |
|
| ||||||
| LVEF, % | 69.0 (63.6–73.5) | 70.1 (65.4–74.2) | 70.5 (65.4–74.7) | 69.2 (64.2–74.5) | <0.01 | 0.01 |
In generalized additive models (adjusted as described in Methods; Figure 2) to delineate the form of the relationship between fat depots and CMR parameters, greater pericardial fat was associated with increased LV mass index and LV concentricity, with increasing effects seen with increasing pericardial fat (P-values for quadratic relations of <0.0001 for both, Table 4). Pericardial fat appeared to exhibit a curvilinear relationship with LV ejection fraction, with a steeper decline in LV ejection fraction at the highest levels of pericardial fat (P=0.01 for both linear and quadratic terms). Liver attenuation was not associated with ejection fraction (P=0.31) but did have qualitatively similar relationships with LV mass index and LV concentricity as pericardial fat (Supplemental Figure 1). Interestingly, liver attenuation was associated with LV end diastolic volume with a complex relationship suggesting that increasing fatty infiltration was associated with greater dilation until a saturation point with wide confidence intervals for those with the highest degrees of fatty infiltration.
Figure 2. Relationships between pericardial fat and left ventricular geometry and function.
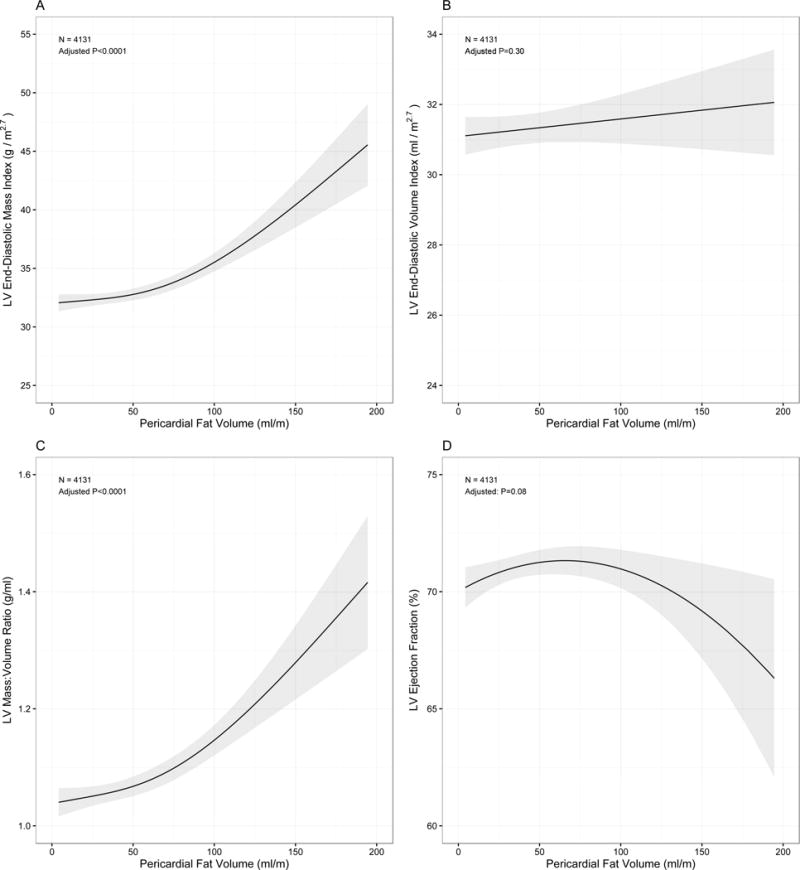
Associations for LV mass (panel A), LV mass-to-volume ratio (panel C) and ejection fraction were curvilinear (panel D), with significant quadratic terms (Table 4). There was no significant relationship noted between pericardial fat and LV volume (panel B). Graphs and p-values are derived from fully adjusted generalized additive spline models with grey bands represent 95% confidence intervals.
Table 4. Linear models for association with cardiac remodeling parameters.
Adjustments as specified in text. Abbreviations: LVMI, LV mass index; LVEDVI, LV end-diastolic volume index; LVMtoV, LV mass-to-volume ratio; LVEF, LV ejection fraction
| Predictor | Outcome | Estimated β Coefficient | Standardized β Coefficient | Adjusted P-Value |
|---|---|---|---|---|
| Pericardial Fat (ml/m) | LVMI (g/m2) | Quadratic: 0.0004 ± 0.00004 | Quadratic: 0.12 ± 0.01 | Quadratic: <0.0001 |
|
| ||||
| LVEDVI (ml/m2) | Linear: 0.005 ± 0.005 | Linear: 0.02 ± 0.02 | Linear: 0.30 | |
|
| ||||
| LVMtoV (g/ml) | Quadratic: 0.00001 ± 0.000001 | Quadratic: 0.12 ± 0.02 | Quadratic: <0.0001 | |
|
| ||||
| LVEF (%) | Linear: 0.04 ± 0.02 Quadratic: −0.0003 ± 0.0001 |
Linear: 0.12 ± 0.05 Quadratic: −12 ± 0.05 |
Linear: 0.01 Quadratic: 0.01 |
|
|
| ||||
|
| ||||
| Liver | LVMI (g/m2) | Linear: −0.07± 0.01 | Linear: −0.10 ± 0.01 | Linear: <0.0001 |
|
| ||||
| Attenuation (HU) | ||||
|
| ||||
| LVEDVI (ml/m2) | Linear: 0.12 ± 0.04 Quadratic: −0.001 ± 0.0004 |
Linear: 0.20 ± 0.07 Quadratic: −0.26 ± 0.07 |
Linear: 0.004 Quadratic: 0.0002 |
|
|
| ||||
| LVMtoV (g/ml) | Linear: −0.005 ± 0.002 Quadratic: 0.00004 ± 0.0000 |
Linear: −0.24 ± 0.07 Quadratic: 0.18 ± 0.07 |
Linear: 0.0007 Quadratic: 0.01 |
|
|
| ||||
| LVEF (%) | Linear: 0.01 ± 0.01 | Linear: 0.02 ± 0.02 | Linear: 0.27 | |
Adjusted for age, sex, race/ethnicity, excess BMI, excess waist circumference, cigarette smoking status, total intentional exercise, HOMA-IR, C-reactive protein, diabetes status, systolic blood pressure, hypertension medication use, LDL and statin medication use. Models where pericardial fat was the primary predictor of interest were also adjusted for hepatic fat and vice versa.
Given previous reports of sex- and race-specific heterogeneity in cardiac remodeling and risk, we used adjusted GAMs to explore the relationship between cardiac remodeling phenotypes and sex and race (Figure 4). Women exhibited linear increases in LV end-diastolic volume for each increment in pericardial fat volume (interaction P<0.0001) relative to men, but no significant heterogeneity in response of LV mass, ejection fraction or concentricity to pericardial fat by sex. By race, we observed heterogeneity in response of LV mass and LV volume with increasing pericardial fat. Interestingly, the impact of increasing pericardial fat appeared to be particularly harmful on LV mass and LV volume among diabetics compared to non-diabetics. Similar heterogeneity was not seen based on the presence of metabolic syndrome. Conversely, for liver steatosis, effect modification was only observed for the effects of gender and race on LV mass (Supplemental Figure 4).
DISCUSSION
In this multi-racial/multi-ethnic, community-based population, pericardial fat was significantly associated with CVD outcomes and modestly associated with cardiac structure and function, a relationship that persisted after adjustments for CVD and cardiometabolic risk factors, including insulin resistance, systemic inflammation, and imaging indices of hepatic fat. Pericardial fat was linearly associated with LV mass and concentricity, suggesting a concentric LV remodeling phenotype with increasing pericardial fat. LV function, on the other hand, exhibited a curvilinear association with pericardial fat, which after further inspection appeared to be driven by greater LV dysfunction at higher pericardial fat in African Americans. In exploratory analyses by sex, we observed a greater LV mass and volume in response to greater pericardial fat in women. Taken together, these results suggest that pericardial fat (a local fat depot) may have effects on LV structure and function and outcome, independent of the systemic impact of dysfunctional adiposity (e.g., insulin resistance, inflammation) and hepatic attenuation (a CT measure of hepatic fat).
Obesity is strongly associated with cardiovascular disease22, with obesity-related geometric changes in the ventricle related to prognosis23, 24. Under normal physiologic circumstances, pericardial (epicardial) fat is critical to normal myocardial homeostasis, serving as a local source of free-fatty acids as metabolic fuel and in a buffering capacity for excess substrates to prevent lipotoxicity25. However, expansion of the pericardial fat depot that accompanies obesity may be associated with similar biochemical consequences as visceral adipose tissue expansion or hepatic steatosis26, including elaboration of tumor necrosis factor-α, interleukin-6 and -11, and lower adiponectin, potentially independent of BMI27–29. In turn, this pro-inflammatory milieu has been linked to abnormalities in ventricular structure30, 31 and incident HF32. Accordingly, pericardial and other similar fat depots have been associated with prevalent abnormalities in cardiac structure6, including coronary artery disease, hypertrophy, vascular stiffness, coronary vasomotor dysfunction, and diastolic function8, 9, 18, 29, 33, 34.
Several seminal investigations have begun to define a role of pericardial fat relative to other fat depots in CVD risk. In a comprehensive study of nearly 1,000 participants from the Framingham Heart Study, clinical anthropometric indices (e.g., BMI and weight) and CT-determined visceral and pericardial fat were associated with greater CMR-determined LV mass and volume6. After adjustment for weight or visceral fat, associations with pericardial fat were insignificant (except for left atrial dimension in men), prompting the conclusion that overall obesity (or at least remote effects from visceral fat) may have a greater impact on myocardial structure relative to any local effect of pericardial fat. In a recent, smaller study of 75 individuals with non-alcoholic fatty liver disease, Graner et al. found that visceral adipose tissue and hepatic triglyceride content were closely related to CMR diastolic function7, suggesting that any associations between pericardial or intramyocardial fat with cardiac remodeling is likely a consequence of collinearity with visceral or hepatic fat.
Certainly, the physiologic similarities and collinearity between fat depots renders separating their independent contributions on cardiac risk in an observational study difficult35, 36. Most prior work has not involved biomarkers of inflammation and insulin resistance mechanistically central to both dysfunctional adiposity and cardiac remodeling in obesity. Furthermore, epicardial, hepatic, and visceral fat (in conditions of nutritional excess) exhibit a similar pro-inflammatory phenotype, suggesting that efforts to account for their independent influences in regression models may be difficult. Collectively, these reports suggest that visceral adiposity and hepatic fat—potentially functioning via remote endocrine effects (e.g., adiponectin, inflammation, insulin) or indirect influence on cardiometabolic disease (e.g., hypertension, diabetes)—may be the prime impetus for myocardial hypertrophy and dysfunction in obesity.
Nevertheless, there is an emerging literature implicating pericardial fat in the pathogenesis of a variety of CV diseases37–40. Here, we demonstrate pericardial fat is associated with incident CVD over long-term follow-up as well as cardiac structure, independent of selected measured influences of dysfunctional adiposity, defined by cardiometabolic risk factors, insulin resistance, inflammation, and imaging surrogates of hepatic fat content. In exploratory analyses, we found complex sex- and race-based heterogeneity in cardiac structural associations with pericardial fat, including greater hypertrophy and concentric LV remodeling in females, and a non-linear relationship between pericardial fat and LV function in African Americans. While these observations arise from secondary analyses and require confirmation in larger, emerging multi-ethnic cohorts worldwide, they are concordant with prior laboratory and clinical research: in animal models of obesity and diabetes, cardiac remodeling is observed early in the pathogenesis of obesity/diabetes41, and HF with preserved LV ejection fraction risk is higher specifically in older, obese women42. Furthermore, African-Americans harbor a disproportionate risk of subclinical LV dysfunction43 and higher CVD-related mortality44. Given meaningful improvements in risk discrimination and reclassification without additional radiation exposure, it would be reasonable to consider assessment of pericardial fat in patients undergoing coronary calcium scoring, though further efforts to define whether this approach improves outcomes merits investigation. Indeed, imaging-based metrics of adiposity (e.g., hepatic steatosis) may be helpful in identifying risk for metabolic diseases after statin initiation45. Overall, further investigation into the relevance of adiposity by sex and race on subclinical remodeling and HF risk is warranted.
The strength of our study is the combination of multi-modality imaging conducted at the same MESA study visit to quantify hepatic and pericardial fat and CV structure and function alongside inflammation and insulin resistance in a large, multi-ethnic/racial prospective observational cohort. Nevertheless, our results should be viewed in the context of several limitations. By confining our exposures to those measured at the baseline study visit in MESA, we were able to investigate subclinical markers of cardiac remodeling concomitant to the adjudication of liver and pericardial fat quantification in a large sample size. We did not include quantitative abdominal visceral fat (performed after the baseline MESA visit), which we previously found was associated with LV remodeling. Therefore, it is possible that associations between pericardial fat and cardiac structure may attenuate (or disappear) after adjustment for visceral fat, as in Framingham6. Nevertheless, adjustment by hepatic attenuation, insulin resistance, and inflammation (systemic effects of visceral adiposity) did not eliminate relationships with pericardial fat. In addition, direct MRI spectroscopic measures of hepatic lipid content used in prior, smaller studies of this question7 would be more specific than CT attenuation (and therefore stronger in prognostic/structural assessments), though these were not available to us. Further investigations in larger, multi-ethnic cohorts with detailed imaging phenotypes across all adiposity depots may further address these relevant issues.
In conclusion, in a large, multi-racial, multi-ethnic population of American adults, we demonstrated that pericardial fat is associated with adverse subclinical alterations in myocardial structure, systolic function, and CVD outcomes. Pericardial fat may influence cardiac structural and functional abnormalities independent of traditional cardiometabolic mechanisms of inflammation and insulin resistance. Further studies investigating mechanisms of action of pericardial fat on cardiac remodeling as well as interventions specifically targeting pericardial fat may mitigate future HF risk in the growing obese population.
Clinical Perspectives
Competency in Medical Knowledge
The quantity of pericardial fat, but not the degree of hepatic steatosis as measured by CT attenuation, was related to cardiovascular outcomes and improved risk discrimination and reclassification. Although both metrics were related to left ventricular geometry and function, the relationships were generally stronger for pericardial fat.
Translational Outlook
Quantification of pericardial fat among patients in whom a calcium score CT is performed may have clinical value. Additional studies are required to confirm and to evaluate the cost effectiveness of this added measure.
Supplementary Material
Figure 3. Modification of relationships between pericardial fat and left ventricular geometry and function by sex, race and diabetes.
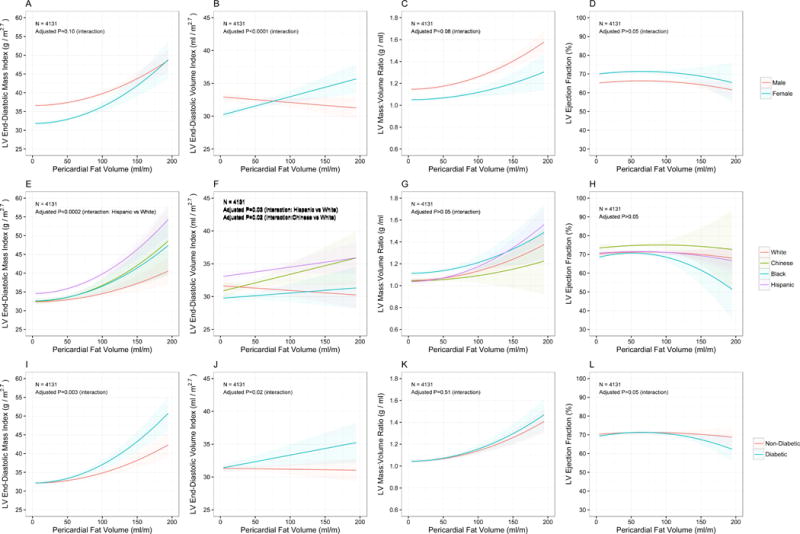
Plots in top row (A-D) evaluate effect modification by sex (male=red, female=cyan) while the middle row (E-H) evaluate effect modification by race (white=red, Chinese=green, black=blue, Hispanic=purple) and the lower row (I-L) evaluate effect modification by diabetes (non-diabetic=red, diabetic=cyan).
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Grant Funding: This research was supported by R01 HL071739 (to MB), R01 HL-085323 (to JZD) and contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. Dr. Shah is funded by the American Heart Association. This work forms part of the research areas contributing to the translational research portfolio of the Cardiovascular Biomedical Research Unit at Barts, which is supported and funded by the National Institute for Health Research.
ABBREVIATIONS
- BMI
body mass index
- CMR
cardiac magnetic resonance
- CRP
C-reactive protein
- CT
computed tomography
- CHD
coronary heart disease
- CV
cardiovascular
- CVD
cardiovascular disease
- HOMA-IR
homeostatic model assessment for insulin resistance
- IQR
interquartile range
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LVMI
left ventricular mass, indexed
- MESA
Multi-Ethnic Study of Atherosclerosis
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: Dr. Murthy has minor stockholdings in General Electric. Dr. Petersen is Consultant to Circle Cardiovascular Imaging (Calgary, Canada) and GlaxoSmithKline. Dr. Budoff receives grant funding from General Electric and the National Institutes of Health.
References
- 1.Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98:116–20. doi: 10.1016/j.amjcard.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Ventura HO, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100:1460–4. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–92. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D’Agostino RB, Sr, O’Donnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–91. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graner M, Nyman K, Siren R, Pentikainen MO, Lundbom J, Hakkarainen A, Lauerma K, Lundbom N, Nieminen MS, Taskinen MR. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.001979. [DOI] [PubMed] [Google Scholar]
- 8.Atakan A, Macunluoglu B, Kaya Y, Ari E, Demir H, Asicioglu E, Kaspar C. Epicardial fat thickness is associated with impaired coronary flow reserve in hemodialysis patients. Hemodialysis international International Symposium on Home Hemodialysis. 2014;18:62–9. doi: 10.1111/hdi.12091. [DOI] [PubMed] [Google Scholar]
- 9.Tok D, Cagli K, Kadife I, Turak O, Ozcan F, Basar FN, Golbasi Z, Aydogdu S. Impaired coronary flow reserve is associated with increased echocardiographic epicardial fat thickness in metabolic syndrome patients. Coron Artery Dis. 2013;24:191–5. doi: 10.1097/MCA.0b013e32835d75d1. [DOI] [PubMed] [Google Scholar]
- 10.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 13.Shah RV, Abbasi S, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, Kwong R, Bluemke DA, Lima JA, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.01.053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–79. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnett DK, McClelland RL, Bank A, Bluemke DA, Cushman M, Szalai AJ, Jain N, Gomes AS, Heckbert SR, Hundley WG, Lima JA. Biomarkers of inflammation and hemostasis associated with left ventricular mass: The Multiethnic Study of Atherosclerosis (MESA) Int J Mol Epidemiol Genet. 2011;2:391–400. [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 17.Tota-Maharaj R, Blaha MJ, Zeb I, Katz R, Blankstein R, Blumenthal RS, Budoff MJ, Nasir K. Ethnic and sex differences in fatty liver on cardiac computed tomography: the multi-ethnic study of atherosclerosis. Mayo Clin Proc. 2014;89:493–503. doi: 10.1016/j.mayocp.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbasi SA, Hundley WG, Bluemke DA, Jerosch-Herold M, Blankstein R, Petersen SE, Rider OJ, Lima JA, Allison MA, Murthy VL, Shah RV. Visceral adiposity and left ventricular remodeling: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25:667–76. doi: 10.1016/j.numecd.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 21.Ngueta G, Laouan-Sidi EA, Lucas M. Does waist circumference uncorrelated with BMI add valuable information? Journal of epidemiology and community health. 2014;68:849–55. doi: 10.1136/jech-2014-204005. [DOI] [PubMed] [Google Scholar]
- 22.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56:369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Lavie CJ, Patel DA, Milani RV, Ventura HO, Shah S, Gilliland Y. Impact of echocardiographic left ventricular geometry on clinical prognosis. Progress in cardiovascular diseases. 2014;57:3–9. doi: 10.1016/j.pcad.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Progress in cardiovascular diseases. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue: far more than a fat depot. Cardiovascular diagnosis and therapy. 2014;4:416–29. doi: 10.3978/j.issn.2223-3652.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Parisi V, Rengo G, Pagano G, D’Esposito V, Passaretti F, Caruso A, Grimaldi MG, Lonobile T, Baldascino F, De Bellis A, Formisano P, Ferrara N, Leosco D. Epicardial adipose tissue has an increased thickness and is a source of inflammatory mediators in patients with calcific aortic stenosis. Int J Cardiol. 2015;186:167–9. doi: 10.1016/j.ijcard.2015.03.201. [DOI] [PubMed] [Google Scholar]
- 28.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 29.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–6. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 30.Arnett DK, McClelland RL, Bank A, Bluemke DA, Cushman M, Szalai AJ, Jain N, Gomes AS, Heckbert SR, Hundley WG, Lima JA. Biomarkers of inflammation and hemostasis associated with left ventricular mass: The Multiethnic Study of Atherosclerosis (MESA) International journal of molecular epidemiology and genetics. 2011;2:391–400. [PMC free article] [PubMed] [Google Scholar]
- 31.Harhay MO, Tracy RP, Bagiella E, Barr RG, Pinder D, Hundley WG, Bluemke DA, Kronmal RA, Lima JA, Kawut SM. Relationship of CRP, IL-6, and fibrinogen with right ventricular structure and function: the MESA-Right Ventricle Study. Int J Cardiol. 2013;168:3818–24. doi: 10.1016/j.ijcard.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–95. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 34.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, Grundy SM, de Lemos JA, Drazner MH. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–7. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarma S, Levine BD. Trimming the fat, do we know where to begin? Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002836. [DOI] [PubMed] [Google Scholar]
- 36.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 37.Al-Rawahi M, Proietti R, Thanassoulis G. Pericardial fat and atrial fibrillation: Epidemiology, mechanisms and interventions. Int J Cardiol. 2015;195:98–103. doi: 10.1016/j.ijcard.2015.05.129. [DOI] [PubMed] [Google Scholar]
- 38.Wu CK, Tsai HY, Su MY, Wu YF, Hwang JJ, Tseng WY, Lin JL, Lin LY. Pericardial fat is associated with ventricular tachyarrhythmia and mortality in patients with systolic heart failure. Atherosclerosis. 2015;241:607–14. doi: 10.1016/j.atherosclerosis.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, Rocha Goncalves F, Gama V, Leite-Moreira A. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol. 2014;114:1663–9. doi: 10.1016/j.amjcard.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Picard FA, Gueret P, Laissy JP, Champagne S, Leclercq F, Carrie D, Juliard JM, Henry P, Niarra R, Chatellier G, Steg PG. Epicardial adipose tissue thickness correlates with the presence and severity of angiographic coronary artery disease in stable patients with chest pain. PLoS One. 2014;9:e110005. doi: 10.1371/journal.pone.0110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden MA, Tesch GH, Julius TL, Rosli S, Love JE, Ritchie RH. Earlier onset of diabesity-Induced adverse cardiac remodeling in female compared to male mice. Obesity (Silver Spring) 2015;23:1166–77. doi: 10.1002/oby.21072. [DOI] [PubMed] [Google Scholar]
- 42.Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–35. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Race-ethnic differences in subclinical left ventricular systolic dysfunction by global longitudinal strain: A community-based cohort study. Am Heart J. 2015;169:721–6. doi: 10.1016/j.ahj.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 45.Shah RV, Allison MA, Lima JA, Bluemke DA, Abbasi SA, Ouyang P, Jerosch-Herold M, Ding J, Budoff MJ, Murthy VL. Liver fat, statin use, and incident diabetes: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;242:211–7. doi: 10.1016/j.atherosclerosis.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


