Abstract
Female mice were immunized intravaginally with gonococcal outer membrane vesicles (OMV) plus microencapsulated IL-12, and challenged using an established model of genital infection with Neisseria gonorrhoeae. Whereas sham-immunized and control animals cleared the infection in 10–13 days, those immunized with OMV plus IL-12 cleared infection with homologous gonococcal strains in 6–9 days. Significant protection was also seen after challenge with antigenically distinct strains of N. gonorrhoeae, and protective anamnestic immunity persisted for at least 6 months after immunization. Serum and vaginal IgG and IgA antibodies were generated against antigens expressed by homologous and heterologous strains. Iliac lymph node CD4+ T cells secreted IFNγ, but not IL-4, in response to immunization, and produced IL-17 in response to challenge regardless of immunization. Antigens recognized by immunized mouse serum included several shared between gonococcal strains, including two identified by immunoproteomics approaches as EF-Tu and PotF3. Experiments with immunodeficient mice showed that protective immunity depended upon IFNγ and B cells, presumably to generate antibodies. The results demonstrated that immunity to gonococcal infection can be induced by immunization with a non-living gonococcal antigen, and suggest that efforts to develop a human vaccine should focus on strategies to generate Th1-driven immune responses in the genital tract.
Keywords: Neisseria gonorrhoeae, vaccine, immune response, antibody, interferon-γ, murine model
INTRODUCTION
Neisseria gonorrhoeae is a well adapted and exclusively human pathogen that causes the sexually transmitted infection, gonorrhea. The most recent estimates based on data collected in 2012 indicate an annual incidence of 78 million new cases worldwide 1. The reported incidence in the United States is >350,000 cases per year 2, although the real incidence is believed to exceed 800,000 cases per year 3. In the absence of a vaccine, control depends upon effective screening and diagnosis followed by prompt antibiotic treatment, which are not available in all parts of the world. However, N. gonorrhoeae has rapidly developed resistance to all classes of antibiotics that have been deployed against it, including most recently fluoroquinolones and extended-spectrum cephalosporins, giving rise to fears that gonorrhea might become untreatable 4, 5. Infection usually presents as a mucopurulent discharge, cervicitis in women and urethritis in men, but >50% of infections in women may be clinically inapparent 6. Men typically become aware of infection within a few days, but it is increasingly recognized that asymptomatic infection can also occur in men. Women bear the greater burden of morbidity, since if left untreated gonorrhea can ascend to the upper reproductive tract and cause salpingitis, leading to tubal scarring, infertility, pelvic inflammatory disease, and increased risk for ectopic pregnancy which can be life-threatening. In men untreated infection can progress to prostatitis and epididymitis. Newborns delivered through an infected birth canal can acquire eye infections that lead to blindness. In both sexes, N. gonorrhoeae can invade systemically, giving rise to disseminated gonococcal infection with septic arthritis and dermatitis being the most common manifestations. In addition, untreated gonorrhea enhances the transmission and acquisition of HIV by up to 5-fold 7.
The emergence of multiple-drug-resistant strains of N. gonorrhoeae has led the WHO and the US Centers for Disease Control and Prevention to call for new approaches to treatment and renewed efforts at vaccine development 8. Previous attempts to develop a vaccine have come to nothing 9. A small-scale trial of a killed whole cell vaccine in Alaska in the 1970s was unsuccessful 10. A major effort to develop a vaccine based on gonococcal pilus succeeded in inducing protective antibody responses against strains bearing antigenically similar pili, but the extensive variability of the pilin protein among naturally occurring N. gonorrhoeae strains rendered this vaccine totally ineffective in a field trial 11. A more recent effort was made to develop a vaccine based on gonococcal porin, the major outer membrane protein 12, but plans for a clinical trial were apparently abandoned.
Vaccine efforts are complicated by the extensive antigenic variability of N. gonorrhoeae, in which most major surface antigens, including lipooligosaccharide (LOS), porin, pilin, and the opacity proteins (Opa) are subject to phase-variable expression (LOS, Opa, pilus), allelic variation (porin, Opa), or recombinatorial expression (pilin) (reviewed in 9, 13). In addition, it is widely recognized that gonorrhea can be acquired repeatedly with little or no evidence of effective immunity being developed as a result of therapeutic recovery from infection. Anecdotal evidence going back to the early 20th century indicates that gonorrhea may ultimately be self-limiting, suggesting that an infected person might eventually develop a sufficient immune response to eliminate the pathogen, but ethical considerations prohibit prospective investigation of this. Furthermore, with the possible exception of chimpanzees, there are no animal models that closely mimic human infection and disease to permit vaccine studies that can be readily translated into clinical trials 14.
However, a small animal model of genital tract gonococcal infection has been established, using estradiol-treated female mice 15, 16, and at present this remains the only model in which the response of an intact mammalian immune system to genital gonococcal infection can be prospectively studied and manipulated. In this model, infection is maintained for approximately 10–20 days (depending on various experimental factors) but is eventually eliminated probably because N. gonorrhoeae is not adapted to colonize mice. Nevertheless, this period of infection provides an opportunity to evaluate host immune responses and to test strategies of immunization that inhibit infection and lead to accelerated clearance. Notably, infection does not result in specific serum or local genital antibody responses 16, 17. Moreover, although some strains of mice (especially BALB/c, but not C57BL/6) develop a neutrophil infiltrate into the vagina within a few days, there is no evidence for the induction of adaptive type 1 or type 2 T helper (Th1, Th2) cell responses 16–19. In contrast, a Th17 response occurs with the production of IL-17 and IL-22 19, which upregulate the secretion of innate antimicrobial proteins by epithelial cells and the recruitment of neutrophils. Abrogation of IL-17-mediated responses with neutralizing antibody or in IL-17 receptor-knockout mice results in diminished neutrophil influx and prolonged infection, suggesting that innate defense mechanisms contribute to clearance 19. Furthermore, if mice are allowed to recover from infection and then reinfected, the course of infection is exactly the same as in age-matched control mice, and there is no evidence for recall of any anamnestic immune response, either elevated antibodies or enhanced Th1, Th2, or Th17 cellular responses 16, 17. These findings are reminiscent of the human immune response to uncomplicated gonococcal infection, which likewise is minimal with respect to both antibody and T cell-mediated responses, regardless of history of prior infection 20, 21. IL-17 is reported to be elevated in humans infected with N. gonorrhoeae 22, 23.
Further study of the responses to genital gonococcal infection in mice demonstrated that N. gonorrhoeae upregulates the production of the immunosuppressive cytokines, TGFβ and IL-10 24, 25. Counteracting these cytokines with neutralizing antibodies allows the emergence of Th1-driven responses including anti-gonococcal IgG and IgA antibodies in serum and vaginal secretions, establishment of immune memory, and accelerated clearance of inection 24, 25. Subsequent re-infection of such mice without further anti-TGFβ or anti-IL-10 treatment resulted in resistance to re-infection, and the recall of antibody responses to higher levels as well as Th1 (and Th17) cellular responses. These findings imply that N. gonorrhoeae suppresses adaptive immune responses, and that reversal of the induced immunosuppression enables the development of protective immunity. We have further developed this approach to counter-manipulating the ability of N. gonorrhoeae to suppress adaptive immune responses that would eliminate it, by treating gonococcus-infected mice intravaginally (i.vag.) with IL-12 encapsulated in microspheres (IL-12/ms) 26. This too permits the development of protective immunity that not only accelerates clearance of the existing infection, but also generates resistance to repeated infection. Because this treatment in effect converts the infection into a live vaccine, we hypothesized that microencapsulated IL-12 would serve as an adjuvant for a locally administered non-living gonococcal vaccine. To test this hypothesis, we have immunized mice i.vag. with a gonococcal outer membrane vesicle (OMV) preparation administered with or without IL-12/ms. OMV were selected as a vaccine immunogen because they contain most of the surface antigens in native conformation, not denatured by heat or chemical inactivation. Moreover, meningococcal vaccines have been successfully developed based on OMV preparations from Neisseria meningitidis 27. The results demonstrate the generation of a Th1-driven, antibody-dependent, protective immune response that persists for at least several months and is effective against antigenically diverse strains of N. gonorrhoeae.
RESULTS
Intravaginal immunization of mice with gonococcal OMV plus IL-12/ms accelerates clearance of challenge infection with N. gonorrhoeae
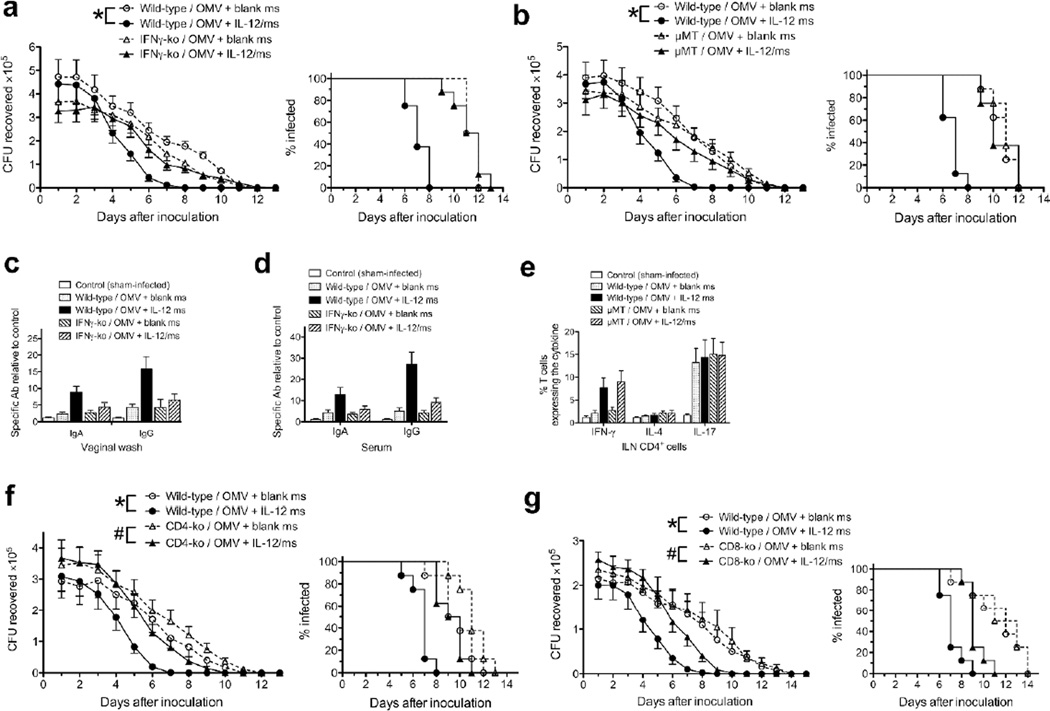
Groups of 8 female BALB/c mice were immunized i.vag. with gonococcal OMV (strain FA1090; 40µg protein) plus IL-12/ms (1µg IL-12), or with OMV plus control (blank) ms; two additional control groups were sham-immunized with IL-12/ms or with blank ms alone. Immunizations were repeated 1 week and 2 weeks later, and all mice were challenged after a further 2 weeks by i.vag. instillation of N. gonorrhoeae FA1090 (5 × 106 CFU). Control (sham-immunized) mice, or mice immunized with OMV plus blank ms cleared the infection commencing at day 7 post-challenge and were all cleared by day 15; median days of clearance were 10–13. There was no significant difference in the clearance rates between these three control groups (Fig. 1a). However, mice immunized with OMV plus IL-12/ms cleared the infection beginning at day 6 and were all cleared by day 9; median clearance =7.5 days compared to 12 days in mice immunized with OMV plus blank ms (P <0.01, Kaplan-Meier; Suppl. Table 1 and Fig. 1a). This experiment was repeated twice more with similar results (see Suppl. Table 1 and Suppl. Fig. 1). Further examples of replication of this finding can be seen in subsequent experiments reported below, e.g., Figs. 1d, 4, 5a and c (and Figs. 5b, d, and e for other gonococcal strains), and Figs. 7a, b, f, and g (with C57BL/6 mice)
Fig. 1.
I.vag immunization with gonococcal OMV plus IL-12/ms induced resistance to genital infection with N. gonorrhoeae, and generated an immune response. a: Mice were immunized 3 times at 7-day intervals with OMV (40µg protein) from strain FA1090 plus control (blank) ms or IL-12/ms (1µg IL-12); control mice were sham-immunized with either blank ms, or with IL-12/ms alone. Two weeks after the last immunization, all mice were challenged by i.vag. inoculation with N. gonorrhoeae strain FA1090 (5 × 106 CFU), and infection was monitored by vaginal swabbing and plating. Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), * P <0.01 (ANOVA); right panel: % of animals remaining infected at each time point, P <0.01 (Kaplan-Meier analysis, log-rank test, OMV plus IL-12/ms vs. OMV plus blank ms). b: Vaginal wash (left) and serum (right) antibodies against strain FA1090 in samples collected after termination (day 15), shown as mean ±SEM, N=5 samples; # P <0.05, * P <0.01, Student’s t. c: Intracellular cytokine staining in CD4+ cells recovered from ILN at termination (day 15), shown as mean ±SEM, N=3 samples, % of CD4+ staining for each cytokine; * P <0.01 Student’s t. d: Mice were immunized twice at a 14-day interval with gonococcal (Ngo) OMV (40µg protein) plus blank ms or IL-12/ms (1µg IL-12); control mice were sham-immunized with blank ms alone or with NTHI OMV (40µg protein) plus IL-12/ms (1µg IL-12). Two weeks later, all mice were challenged with N. gonorrhoeae FA1090 (5 × 106 CFU). Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), * P <0.01 (ANOVA, gonococcal OMV plus IL-12/ms vs. gonococcal OMV plus blank ms); right panel: % of animals remaining infected at each time point, P <0.0001 (Kaplan-Meier analysis, log-rank test, gonococcal OMV plus IL-12/ms vs. gonococcal OMV plus blank ms).
Fig. 4.
Resistance to gonococcal (FA1090) challenge persisted for at least 6 months after immunization with two doses of gonococcal (FA1090) OMV plus IL-12/ms. Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), * P <0.01 (ANOVA, gonococcal OMV plus IL-12/ms vs. gonococcal OMV plus blank ms); right panel: % of animals remaining infected at each time point, P <0.001 (Kaplan-Meier analysis, log-rank test, gonococcal OMV plus IL-12/ms vs. gonococcal OMV plus blank ms).
Fig. 5.
Resistance to heterologous gonococcal challenge. a: One month after immunization with FA1090 OMV plus IL-12/ms or blank ms, mice were challenged with N. gonorrhoeae strain FA1090 (homologous challenge) or strain MS11 (heterologous challenge). Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), * P <0.001 (ANOVA, for comparisons shown); right panel: % of animals remaining infected at each time point, P <0.02 for FA1090 challenge, IL-12/ms vs. blank ms; P <0.001 for MS11 challenge, IL-12/ms vs. blank ms (Kaplan-Meier analysis, log-rank test). b: Mice immunized with MS11 OMV were resistant to challenge with N. gonorrhoeae FA1090. Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), P <0.01 (ANOVA); right panel: % of animals remaining infected at each time point), P <0.01 (Kaplan-Meier analysis, log-rank test). c: Mice immunized with FA1090 OMV were resistant to challenge with N. gonorrhoeae FA19. Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), * P <0.01 (ANOVA, for comparisons shown); right panel: % of animals remaining infected at each time point, P <0.01, IL-12/ms vs. blank ms for FA1090 challenge; P <0.0001, IL-12/ms vs. blank ms for FA19 challenge (Kaplan-Meier analysis, log-rank test), N=8 mice. d: Mice immunized with FA19 OMV were resistant to challenge with N. gonorrhoeae FA1090. Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), P <0.01 (ANOVA); right panel: % of animals remaining infected at each time point), P <0.01 (Kaplan-Meier analysis, log-rank test). e: Mice immunized with FA1090 OMV were resistant to challenge with clinical isolate GC68. Left panel: recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), P <0.01 (ANOVA); right panel: % of animals remaining infected at each time point), P <0.01 (Kaplan-Meier analysis, log-rank test).
Fig. 7.
Resistance to challenge induced by immunization with gonococcal OMV plus IL-12/ms depended on IFNγ and B cells. a: Course of infection (FA1090) in IFNγ-ko vs wild-type mice immunized with FA1090 OMV; left panel, recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), • P <0.01 (ANOVA); right panel, % of animals remaining infected at each time point, P <0.0001 for wild-type mice, IL-12/ms vs. blank ms (Kaplan-Meier analysis, log-rank test). b: Course of infection (FA1090) in µMT vs. wild-type mice immunized with FA1090 OMV; left panel, recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), • P <0.01 (ANOVA); right panel, % of animals remaining infected at each time point, P <0.0001 for wild-type mice, IL-12/ms vs. blank ms (Kaplan-Meier analysis, log-rank test). Vaginal wash (c) and serum (d) antibody responses in IFNγ-ko vs wild-type (mean ±SEM, N=5 samples) assayed at termination (day 13). IgA and IgG responses in vaginal wash and serum were significant (P <0.05, Student’s t, OMV plus blank ms vs. OMV plus IL-12/ms) for wild-type mice, but not for IFNγ-ko mice. e: T cell cytokine responses in µMT vs. wild-type mice (mean ±SEM, N=3 samples) assayed at termination (day 13). IFNγ response to immunization with OMV plus blank ms vs. OMV plus IL-12/ms was significant (P <0.01) for both wild-type and µMT mice (ANOVA). f: Course of infection (FA1090) in CD4-ko vs wild-type mice immunized with FA1090 OMV; left panel, recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), # P <0.05, • P <0.01 (ANOVA) for comparisons shown; right panel, % of animals remaining infected at each time point, P <0.001 for wild-type mice IL-12/ms vs. blank ms, P <0.01 for CD4-ko mice IL-12/ms vs. blank ms (Kaplan-Meier analysis, log-rank test). g: Course of infection (FA1090) in CD8-ko vs wild-type mice immunized with FA1090 OMV; left panel, recovery (CFU) of N. gonorrhoeae (mean ±SEM, N=8 mice), # P <0.05, • P <0.01 (ANOVA) for comparisons shown; right panel, % of animals remaining infected at each time point, P <0.001 for wild-type mice IL-12/ms vs. blank ms, P <0.02 for CD8-ko mice IL-12/ms vs. blank ms (Kaplan-Meier analysis, log-rank test).
Serum and vaginal wash samples collected after clearance (at termination, day 15 post-inoculation) were assayed for antibodies against intact gonococci (FA1090) by ELISA. This showed that mice immunized with OMV plus IL-12/ms had developed the highest levels of vaginal and serum IgG and IgA antibodies, whereas those mice immunized with OMV plus blank ms developed much lower levels of these antibodies (Fig. 1b). Mice that were unimmunized and sham-infected showed no antibodies detectable above assay background at the starting dilutions, and mice immunized with blank ms alone and infected also did not develop detectable antibodies (Fig. 1b). Iliac lymph node (ILN) mononuclear cells collected at the same time were stained for surface CD4 expression and for intracellular cytokines, and analyzed by flow cytometry. This revealed that only mice immunized with OMV plus IL-12/ms generated CD4+/IFNγ+ (and CD8+/IFNγ+) T cells, whereas no mice developed significant numbers of CD4+/IL-4+ T cells (Fig. 1c; see also Suppl. Fig. 2). However, all mice that were infected with N. gonorrhoeae regardless of immunization developed CD4+/IL-17+ T cells (Fig. 1c and Suppl. Fig. 2), as observed previously 19, 26. These findings are all consistent with the results of our previous experiments, in which mice that were first infected with N. gonorrhoeae and then treated i.vag. with IL-12/ms cleared the infection significantly faster than infected mice that were untreated or treated with blank ms, and that IL-12/ms-treated mice developed serum and vaginal IgG and IgA anti-gonococcal antibodies, as well as IFNγ-secreting Th1 cells in the draining ILN 26.
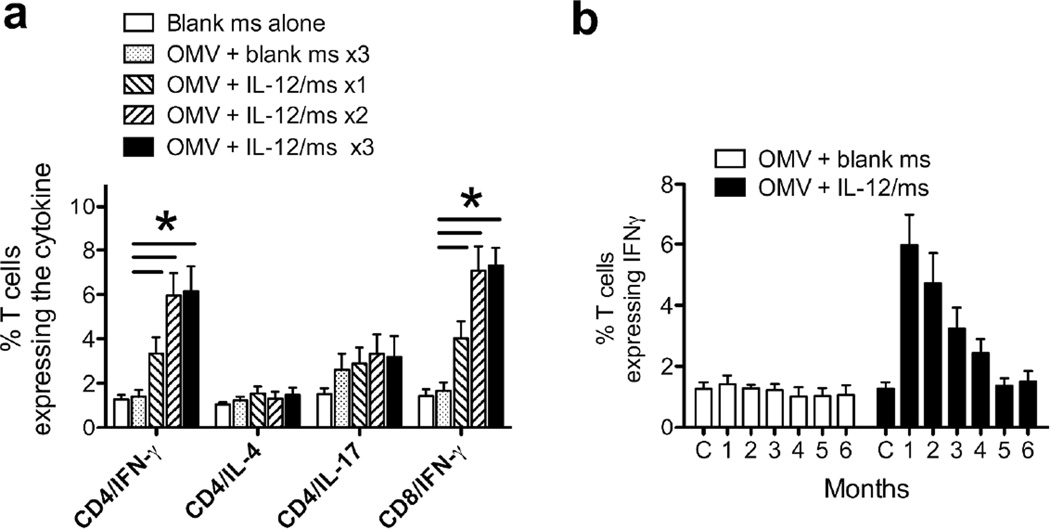
Further experiments were performed to determine the minimum number of immunizations required to induce immune resistance to infection. A single dose of OMV plus IL-12/ms given i.vag. did not consistently generate resistance to challenge, but two doses of OMV (40µg protein) plus IL-12/ms (1µg IL-12) given at an interval of 2 weeks were sufficient to induce similar resistance to infection; median clearance =8 days (Fig. 1d; Suppl. Table 1). In addition, control immunization with OMV prepared from non-typable Haemophilus influenzae (NTHI) plus IL-12/ms failed to induce resistance to N. gonorrhoeae; median clearance =13 days (Fig. 1d; Suppl. Table 1). This induced antibodies to NTHI but not to N. gonorrhoeae (Suppl. Fig. 3a,b) and generated IFNγ-producing CD4+ and CD8+ cells in the ILN (Suppl. Fig. 3c).
Intravaginal immunization with gonococcal OMV plus IL-12/ms induces persistent gonococcus-specific antibody responses and Th1 cellular responses
To characterize the local and systemic immune responses after immunization with 1, 2, or 3 doses of gonococcal OMV plus IL-12/ms and before challenge, serum, vaginal washes, and ILN were collected from immunized and control mice 2 weeks after the last immunization. Serum anti-gonococcal IgM antibodies were at low levels with little difference between experimental groups. IgA and IgG antibodies were not detectable above background in vaginal wash or serum samples of mice given blank ms alone. Intravaginal immunization with 3 doses of gonococcal OMV plus blank ms elevated vaginal and serum anti-gonococcal IgA and IgG antibodies but to a lesser degree than immunization with OMV plus IL-12/ms (Fig. 2a). In contrast, immunization with one dose of OMV plus IL-12/ms induced low levels of anti-gonococcal antibodies in both serum and vaginal wash; a second dose elevated antibody production, but no further elevation was seen after 3 doses (Fig. 2a). Antibodies appeared to be specific for N. gonorrhoeae, as they were not detected above control levels against E. coli or NTHI. Assay of IgG subclass antibodies in both vaginal wash and serum showed a predominance of IgG2a, with lesser amounts of IgG1 and IgG2b and low levels of IgG3 (Suppl. Fig. 4). The production of anti-gonococcal IgA and IgG antibodies in vaginal wash and serum peaked at 3 months after immunization with 2 doses of OMV plus IL-12/ms, and were still detectable at 6 months after immunization (Figs. 2b,c).
Fig. 2.
Antibody responses generated by immunization with gonococcal OMV plus IL-12/ms, prior to gonococcal challenge. a: Vaginal wash (left panel) and serum (right panel) antibodies assayed by ELISA 2 weeks after the last immunization with 1, 2, or 3 doses of gonococcal OMV (40µg protein) plus IL-12/ms (1µg IL-12). Control samples were obtained from mice sham-immunized with blank ms (3 doses); additional mice were immunized 3x with gonococcal OMV plus blank ms. Data shown as mean ±SEM, N=5 samples, # P <0.05, * P <0.01 relative to control samples (ANOVA). Duration of vaginal wash (b) and serum (c) antibodies in mice immunized with 2 doses of FA1090 OMV plus blank ms or IL-12/ms; data shown as mean ±SEM, N=5 samples; C, control samples from unimmunized mice.
Flow cytometric analysis of ILN cells revealed increased numbers of IFNγ+/CD4+ and IFNγ+/CD8+ T cells from mice immunized with OMV plus IL-12/ms compared with those from control mice (Fig. 3a). As observed with the antibody responses, one immunization was sufficient to induce IFNγ production, and it was further elevated by 2 immunizations; 3 doses did not further increase it. In contrast, immunization with OMV plus IL-12/ms did not significantly increase the numbers of IL-4+/CD4+ and IL-17+/CD4+ T cells relative to controls (Fig. 3a). To determine whether the induced IFNγ+/CD4+ (and IFNγ+/CD8+) T cells were specific for gonococcal antigens, CD4+ cells isolated from ILN were preloaded with CFSE, cultured in vitro for 3 days in the presence of antigen-presenting cells with gonococcal OMV or without stimulation as controls, and their proliferation was assessed by flow cytometry. CD4+ cells from the ILN of immunized mice proliferated significantly more, and produced significantly more IFNγ, in response to stimulation in vitro, than the cells from control mice (Suppl. Fig. 5a). No production of IL-4 was seen, but IL-17 was generated by CD4+ T cells cultured with gonococcal OMV, regardless of immunization, similar to what was observed previously with murine cells stimulated with gonococcal antigen in vitro 24 (Suppl. Fig. 5a). IFNγ production by ILN CD4+ T cells remained elevated, albeit at slowly declining levels, for 4 months after immunization (Fig. 3b).
Fig. 3.
a: T cell cytokine responses in ILN cells induced by immunization with gonococcal OMV plus IL-12/ms 2 weeks after the last immunization with 1, 2, or 3 immunizations with gonococcal OMV (40µg protein) plus IL-12/ms (1µg IL-12). Control ILN were obtained from mice sham-immunized with blank ms (3 doses) and additional mice were immunized 3x with gonococcal OMV plus blank ms. Data shown as mean ±SEM, N=3 samples, % of CD4+ or CD8+ cells staining for each cytokine. * P < 0.01 (Student’s t test) comparing immunization with IL-12/ms vs. blank ms. b: Duration of IFNγ responses in CD4+ ILN cells 1–6 months after two immunizations with gonococcal OMV plus IL-12/ms or with OMV plus blank ms. Data shown as mean ±SEM, N=3 samples, % of CD4+ cells staining for IFNγ; C, control ILN from unimmunized mice.
In addition, vaginas were excised from euthanized mice 3 days after the last immunization and RNA was extracted from the whole tissue. RT-PCR analysis showed that, in comparison to controls, immunization with gonococcal OMV plus IL-12/ms significantly enhanced the expression of mRNA for IFNγ but not for IL-4 or IL-17 (Suppl. Fig. 5b). IFNγ mRNA expression in vaginal tissue, and production of IFNγ by ILN CD4+ cells remained elevated for up to 2 months following i.vag. immunization with gonococcal OMV plus IL-12/ms (Suppl. Fig. 5c). These findings support the cytokine expression results obtained with cells from the draining ILN.
Duration of vaccine-induced resistance to infection
To evaluate the duration of immune resistance, groups of 8 mice were immunized with gonococcal OMV plus IL-12/ms, and were challenged with the same strain (FA1090) of N. gonorrhoeae at 2, 4, or 6 months after immunization. Compared to age-matched controls that were either sham-immunized or immunized with OMV plus blank ms, mice immunized with OMV plus IL-12/ms were resistant to N. gonorrhoeae infection when challenged at 2 or 4 months after immunization; median clearance in controls =11–11.5 days vs. 7 days in immunized mice (Suppl. Table 1). Similar results were obtained in a replicate experiment when mice were challenged 6 months after immunization; median clearance in controls =9.5–10 days vs. 6.5 days in mice immunized with OMV plus IL-12/ms (Fig. 4; Suppl. Table 1). After termination anti-gonococcal antibodies were detected in serum and vaginal washes (Suppl. Figs. 6a,b). IFNγ-(but not IL-4-) secreting CD4+ T cells were present in ILN (Suppl. Fig. 6c). Notably, the antibody and IFNγ responses detected after challenge were higher than those before challenge (compare with Figs. 2b,c, and 3b) implying recall of memory. As observed previously, IL-17-secreting T cells were always found after challenge with N. gonorrhoeae, regardless of immunization (Suppl. Fig. 6c). Longer time periods were not evaluated because mice become increasingly resistant to gonococcal infection as they age.
Immunization induces resistance to heterologous strains of N. gonorrhoeae
An important consideration for any vaccine is that it should be effective against different strains of the pathogen, as well as those antigenically homologous to the immunizing strain. N. gonorrhoeae is well known for its antigenic variability involving most of its surface antigens through multiple molecular mechanisms. We therefore determined whether i.vag. immunization with one strain of gonococcal OMV would be effective against challenge with other strains to a similar extent as challenge with the same strain. At first, mice (8 per group) were immunized i.vag. with OMV prepared from strain FA1090 plus IL-12/ms or blank ms, and were challenged one month later with N. gonorrhoeae strains FA1090 or MS11 (5 × 106 CFU). Immunization with FA1090 OMV induced resistance to challenge with either FA1090 or MS11 to similar extents (Fig. 5a; Suppl. Table 2). After challenge and clearance antibodies were elevated to similar levels against MS11 and the Th1 responses indicated by IFNγ+/CD4+ T cells in ILN were similarly enhanced (Suppl. Fig. 7a,b,c). In a reciprocal manner, immunization with MS11 OMV (plus IL-12/ms) induced resistance to challenge with strain FA1090 (Fig. 5b; Suppl. Table 2).
Gonococcal strains FA1090 and MS11 both possess porin of the same major type (PorB.1B) although of different subtypes 28, 29. Therefore, to determine whether the major porin type is integral to immune resistance, further experiments were performed with strain FA19 (PorB.1A). Immunization with FA1090 OMV (plus IL-12/ms) induced resistance to challenge with FA19 (Fig. 5c; Suppl. Table 2). Antibody responses assayed at termination showed cross-reactivity against FA19, and IFNγ+/CD4+ T cells in ILN were elevated (Suppl. Fig. 7d,e,f). Reciprocally, immunization with FA19 OMV induced resistance to challenge with strain FA1090 (Fig. 5d; Suppl. Table 2). Other immunization and challenge combinations (i.e., MS11 against FA19, and vice versa) likewise showed similar cross-resistance (Suppl. Table 2).
N. gonorrhoeae strains FA1090, MS11, and FA19 have been widely used in many laboratories and extensively subcultured since their original isolation. As a result, it is possible that they have acquired mutations and become altered in some of their characteristics. Therefore we also challenged immunized mice with novel clinical strains that have been minimally passaged in vitro since their isolation 30. Mice immunized with FA1090 OMV plus IL-12/ms were also resistant to challenge with clinical isolates GC68 (a PorB.1B strain; Fig. 5e; Suppl. Table 2) and GC69 (PorB.1A; Suppl. Table 2).
Antigens targeted by immunization-induced antibodies
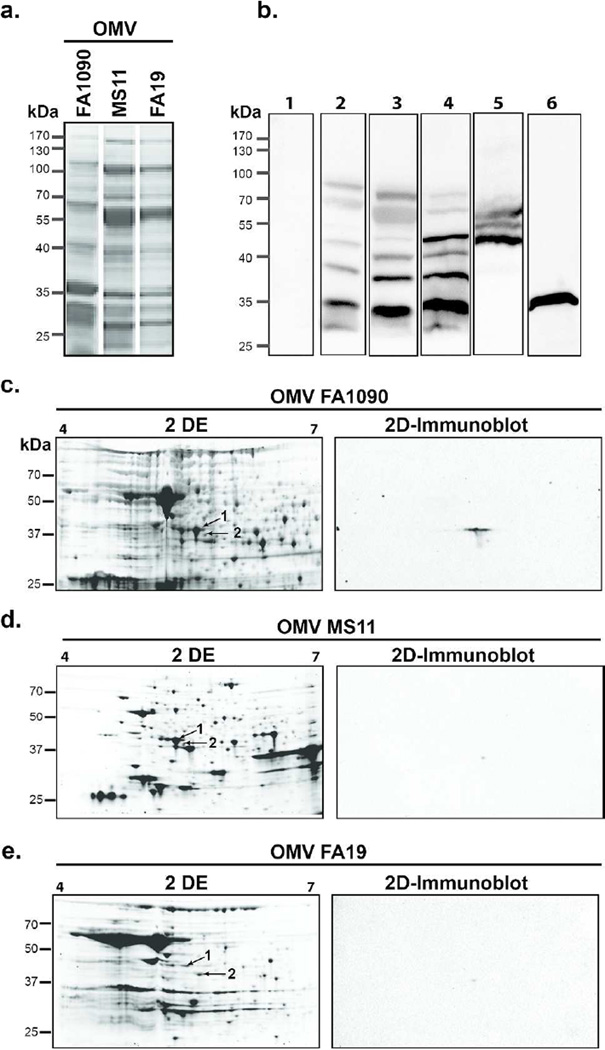
When examined by one-dimensional (1D) SDS-PAGE, the protein profiles of FA1090, MS11, and FA19 OMV were similar, but with apparent quantitative as well as qualitative variations (Fig. 6a). This was expected based on a previous study using comparative high-throughput proteomic analysis of OMV from these gonococcal strains 31. Western blot analyses of serum from one mouse (#1) immunized with FA1090 OMV plus IL-12/ms against FA1090, MS11 or FA19 OMV separated by SDS-PAGE revealed IgG antibodies reactive with bands migrating at approximately 35–80kDa, with reactivity against bands present in OMV from all three strains strains (Fig. 6b, lanes 2–4). One of these bands at approximately 35kDa may correspond to porin, as H5 antibody reacted with a band of similar mobility (Fig. 6b, lane 6). Another serum (#2) displayed strong reactivity against three bands migrating at approximately 45–65kDa (Fig. 6b, lane 5), possibly similar to antigen bands detected by antibodies in mice that had been intranasally immunized with OMV 32. In an effort to identify the ~45–65-kDa antigens, we used immunoproteomic approaches consisting of two-dimensional (2D) SDS-PAGE separation of OMV and parallel 2D SDS-PAGE followed by immunoblotting (2D–immunoblot) and mass spectrometry. The three 2D protein maps of OMV revealed by Flamingo staining showed numerous protein species and significant differences in the OMV proteome between FA1090, MS11, and FA19 strains (Fig. 6c,d,e). In contrast, the blotted protein maps showed two spots (Spot 1 and Spot 2) of masses corresponding to 45kDa and 43kDa, and pI 5.2 and 5.5, respectively (FA1090 OMV; Fig. 6c), or one spot (Spot 1) with an approximate mass 45kDa and pI 5.2 (MS11 and FA19 OMV; Fig. 6d,e). Mass spectrometry analysis of the tryptic peptides obtained from Spot 1 and Spot 2 (Fig. 6c,d,e) revealed as top hits translation elongation factor-Tu (EF-Tu) and a putative periplasmic polyamine-binding protein, PotF3, respectively (Suppl. Table 3). EF-Tu appeared as the most confident antigen as it was immunoreactive in all three 2D–immunoblots and was identified with the highest confidence (score ranging from 485.0 to 947.1) and coverage (64.2 to 90.6) in all OMV preparations (Suppl. Table 3).
Fig. 6.
Immunoproteomics of gonococal OMV. a: SDS-PAGE of OMV preparations from N. gonorrhoeae strains FA1090, MS11, and FA19, stained with Coomassie blue. b: Western blot analysis of mouse sera tested on gonococcal OMV preparations separated by SDS-PAGE. Lane 1, control serum from a mouse immunized with FA1090 OMV plus blank ms, tested against FA1090 OMV; lanes 2–4, serum #1 from a mouse immunized with FA1090 OMV plus IL-12/ms, tested against OMV from FA1090 (lane 2), MS11 (lane 3), or FA19 (lane 4); lane 5, serum #2 from a mouse immunized with FA1090 OMV plus IL-12/ms, tested against OMV from FA1090; lane 6, antibody H5 (anti-porin PIB3) tested against FA1090 OMV. c–e: proteome maps of gonococcal OMV derived from FA1090 (c), MS11 (d), and FA19 (e) revealed by 2D electrophoresis and Flamingo fluorescent staining (left panels) and their corresponding immunoblots (right panels) obtained by probing with mouse serum #2. Immunoreactive spots subjected to MS/MS analysis are labeled as spots 1 and 2 (arrows). Molecular mass marker (kDa) indicated on the left.
Immune resistance to N. gonorrhoeae depends on IFNγ and antibody
To determine whether the protective effect of immunization with OMV adjuvanted with IL-12/ms is dependent on IFNγ or antibody responses, or on immunity governed by CD4+ or CD8+ T cells, we performed immunization experiments using mutant C57BL/6 mice deficient in IFNγ (IFNγ-ko) B cells (µMT), CD4+ T cells (CD4-ko), or CD8+ T cells (CD8-ko). Groups of 8 C57BL/6 wild-type (control) and immumnodeficient mice were immunized with FA1090 OMV plus IL-12/ms or blank ms, and challenged with N. gonorrhoeae FA1090 (5 × 106 CFU) one month later. The course of vaginal gonococcal infection was not altered in unimmunized immunodeficient mice relative to wild-type controls. All wild-type and immunodeficient mice started to reduce the recoverable gonococcal load from day 7–11 and had cleared the infection by day12–14 (median 9–13 days), similar to BALB/c mice used in experiments described in the previous sections (Figs. 7a, b, f, and g; Suppl. Table 4).
In contrast to wild-type mice, clearance of gonococcal infection was not accelerated in IFNγ-ko or µMT mice immunized with OMV plus IL-12/ms compared to immunization with OMV plus blank ms (Fig. 7A,B; Suppl. Table 4; Suppl. Fig. 8a,b). Thus deficiency of either IFNγ or B cells abrogated the adjuvant effect of IL-12/ms in generating immune resistance to genital gonococcal infection. The production of gonococcus-specific vaginal and serum IgA and IgG antibodies induced by OMV plus IL-12/ms in wild-type mice was abrogated in IFNγ-ko mice (Fig. 7c,d), and as expected there was no generation of IFNγ by the ILN cells of immunized IFNγ-ko mice (not shown). Likewise, in µMT mice there was no detectable antibody response to immunization (not shown). In contrast, the numbers of IFNγ+/CD4+ T cells in ILNs of µMT mice immunized with gonococcal OMV plus IL-12/ms were not affected, and there was no IL-4 response, while IL-17 responses remained unaltered (Fig. 5e). These findings indicate that resistance induced by immunization with gonococcal OMV plus IL-12/ms depended on both IFNγ and B cells, the latter presumably to produce gonococcus-specific antibodies.
The protective effect of immunization with gonococcal OMV plus IL-12/ms was incompletely diminished in CD4-ko, and partially diminished also in CD8-ko mice, in comparison with wild-type controls (Fig. 7f,g; Suppl. Table 4; Suppl. Fig. 8c,d). These findings suggest that the requirement for CD4+ T cells to generate immune resistance could be partially compensated by other cells, including CD8+ or NK cells, which can also produce IFNγ. However, CD8+ cells appeared to be less critical for protective immunity.
DISCUSSION
We have demonstrated for the first time that a vaccine-induced state of immune resistance to genital gonococcal infection can be reliably generated by an intact mammalian immune system. This state of immunity appears to depend on antibody production by B cells, and on the generation of IFNγ mainly by CD4+ T cells. I.vag. vaccination of mice with gonococcal OMV plus IL-12/ms as an adjuvant induced serum and vaginal IgG and IgA antibodies against gonococcal antigens, and IFNγ-secreting CD4+ and CD8+ T cells in the draining ILN. Both Th1 cellular and antibody responses persisted for several months after immunization, and were capable of eliciting resistance to challenge with N. gonorrhoeae for at least 6 months, with the recall of immune memory. I.vag. immunization with gonococcal OMV alone, either without adjuvant or with control (blank) ms, induced only weak antibody responses with no detectable IFNγ production, and no significant resistance to challenge infection. Control immunization with OMV prepared from NTHI plus IL-12/ms did not generate immune resistance or antibodies cross-reactive with N. gonorrhoeae, although an IFNγ response was induced. Thus, while IFNγ appears to be necessary for resistance to N. gonorrhoeae, without specific antibodies it is not sufficient.
It should be noted that all mice eventually clear genital gonococcal infection, usually within 2–3 weeks regardless of immunization or other treatment. We previously reported that IL-17-dependent responses contribute to this since infection is prolonged in IL-17RA-deficient mice 19, and furthermore, i.vag. treatment with microencapsulated IL-17 shortens the course of infection, importantly however without enhancing resistance to re-infection 26. Nevertheless, the infection is eventually eliminated in untreated or in IL-17RA-deficient mice, probably because N. gonorrhoeae, as an exclusively human pathogen, is not adapted to survive for longer periods in mice. For example, N. gonorrhoeae cannot utilize murine transferrin as a source of iron 33, nor effectively bind murine factor H or C4B-binding protein to inhibit complement activation 34, nor exploit murine epithelial cell receptors as effectively as it exploits the human equivalents 35, 36. Nevertheless, we have repeatedly found that IL-12/ms, given i.vag. either as a therapeutic treatment of an existing infection 26, or as an adjuvant with gonococcal OMV vaccine (this study), enhances Th1-driven protective immunity revealed by a significantly shortened course of genital gonococcal infection. Notably, a similar dose of free, soluble IL-12 is ineffective 26, indicating that sustained release of IL-12 from the slowly hydrolyzing microspheres over several days is necessary to induce the protective immune responses. Possibly, however, the microspheres also facilitate uptake by the genital tissues, as reported for another microparticulate formulation 37.
What is perhaps most remarkable about the findings presented here, given the well-known and extensive antigenic variation shown by N. gonorrhoeae, is that resistance extended to heterologous strains as well as against the homologous strain from which the OMV vaccine was prepared. The full extent of this cross-protection is unknown at present, but our results show that immunization with OMV derived from strain FA1090 enhances resistance equally well against strains MS11 and FA19, and vice versa, and that resistance extends to clinical isolates of N. gonorrhoeae in addition to these “laboratory strains”. Among the major gonococcal surface antigens, we know that FA1090, MS11, and FA19 differ in their porin (PorB) molecules. FA1090 and MS11 possess different subtypes of PorB.1B, and FA19 has PorB.1A 28. Although not as well characterized, the Opa proteins encoded in their genomes differ 29, 38, and their LOS are different 39. Opa proteins and LOS glycan chains are also phase-variable, resulting in the expression of different antigenic epitopes 40. Recent quantitative proteomic studies revealed additional differences and commonalities in the composition of the extracellular proteome (cell envelopes and naturally secreted OMV) existing between these gonococcal strains 31.
Consistent with cross-protective immunity, ELISA analysis of antibodies induced by immunization revealed quantitatively similar levels of antibodies detectable against the different strains, with respect to both IgG and IgA in serum and vaginal washes. The antibodies appeared to be specific for N. gonorrhoeae as they were not detected against E. coli or NTHI, and they were not generated by immunization with OMV prepared from NTHI. Western blot analysis of serum IgG antibodies, however, revealed evidence of antigens shared between different strains of N. gonorrhoeae. Bands migrating at 45–65kDa resembled those observed in an earlier study on intranasal immunization with gonococcal OMV, which found modest partial protection against genital gonococcal infection 32. These bands migrated at higher molecular mass than major gonococcal outer membrane proteins such as porin and Opa which are in the range 30–40kDa. Although in these studies we did not observe putative antibodies against gonococcal Rmp (~25kDa), it will be important to determine whether anti-Rmp antibodies are induced, because of their counter-protective effect in blocking complement activation by antibodies against other antigens 41. Further studies on many more samples of serum and vaginal wash from immunized mice need to be undertaken to determine the range of gonococcal antigens detected by the induced antibodies.
Immunoproteomics technology has provided vaccine candidate antigens for many infectious diseases 42, 43, and our initial efforts have identified two novel gonococcal vaccine candidates, EF-Tu in FA1090, MS11, and FA19 OMV, and PotF3 also in FA1090. Corroborating these findings both proteins were also identified in quantitative proteomic profiling of cell envelopes and OMV derived from four common gonococcal isolates 31. EF-Tu is of particular interest as it has been identified in both spots and in all analyzed OMV. EF-Tu is commonly perceived as a cytosolic GTP-binding protein and an essential factor in protein synthesis, however only 20–30% of its total cellular pool is involved in protein synthesis at any given time 44. EF-Tu is encoded by at least two genes and has been identified previously in gonococcal periplasm as well as on the surface of bacterial cell envelopes and OMVs 45–47. In N. gonorrhoeae two separate open reading frames of identical sequences encode EF-Tu (NGO1842 and NGO1858; Suppl. Table 3). In support of our findings, EF-Tu has been shown to exhibit many functions associated with pathogenesis and to elicit antibody responses during infection by several bacteria, and by a meningococcal group B OMV vaccine 48–51. The role of PotF3 in gonococcal pathogenesis and immunity is unknown at this point; however, PotF2 appears to function as a polyamine-binding protein, and spermine inhibits gonococcal biofilm formation 52. Further immunoproteomic analysis of additional samples of serum and vaginal wash from immunized mice is likely to reveal many more, hitherto unsuspected gonococcal antigens that might contribute to protective immunity against N. gonorrhoeae.
Immune resistance induced by i.vag. immunization with gonococcal OMV plus IL-12/ms depended on IFNγ, the key cytokine in Th1-driven responses, as IFNγ-deficient mice did not generate protective immunity and antibody responses were substantially diminished. Likewise, immune resistance was abrogated in µMT mice which lack B cells and hence the ability to generate antibodies, although they retained IFNγ production. Consistent with Th1-driven immunity, serum and vaginal IgG antibodies were mainly of the IgG2a subclass, which in mice is particularly effective in mediating complement activation by the classical pathway, and in opsonization for phagocytosis by neutrophils and macrophages 53. However, it is unclear at this point which mechanisms of anti-gonococcal defense are most important for the observed protection in the murine genital tract. Murine vaginal secretions contain both IgG, probably derived largely from the circulation, and locally produced secretory IgA 54. Both isotypes of anti-gonococcal antibodies were induced in response to i.vag. immunization with OMV plus IL-12/ms, but their relative contribution to defense against genital gonococcal infection is unknown at this point. The role of IFNγ may have been to skew the IgG isotype response towards IgG2a 55, but it is also possible that it enhanced phagocytosis by upregulating the expression of high-affinity FcγR-I on neutrophils and macrophages, and by activating macrophages. Other mechanisms of IFNγ-driven T cell-mediated defense cannot be ruled out, although these might be considered less likely against a predominantly extracellular bacterial infection. In addition it is possible that IL-12 may have had direct effects on B cells, which express the IL-12 receptor 56, and can respond by differentiating and generating antibodies 57–60. This may also help to explain why CD4-deficiency did not totally abrogate the adjuvant effect of IL-12/ms on immunization with OMV for inducing protective responses. In addition, other cells including CD8+ T cells as well as possibly NK cells, can serve as an alternative source of IFNγ; deficiency of CD8 was not strongly detrimental to the adjuvant effect of IL-12/ms.
Two major questions are the extent to which these findings relate to immunity to gonorrhea in humans, and whether this approach to vaccination can be developed for human use. The answers are not straightforward, in part because the absence of a demonstrable state of protective immunity to N. gonorrhoeae in humans means that the determinants or even correlates of protection are unknown 9. Previous studies in humans with uncomplicated genital gonococcal infection have revealed a paucity of antibody responses in serum and genital secretions 20, 21. In addition, cytokine responses were weak, although inflammatory cytokines such as IL-1 and IL-6 were elevated in some women who were co-infected with Chlamydia trachomatis or Trichomonas vaginalis in addition to N. gonorrhoeae 20. However, these studies performed nearly 20 years ago were limited by the technology for measuring cytokines available at the time, and by the existing knowledge of cytokines. For example, although IL-17 had been discovered in 1995 61, its significance for anti-microbial defense did not become widely appreciated until after 2005 when the Th17 subset of lymphocytes was defined 62, 63. There is therefore a need to update these studies on human immune responses in gonorrhea, using current multiplex technology for detecting multiple cytokines in limited amounts of samples, and including more recently discovered cytokines.
While the mouse model does not fully mimic the human infection or the consequent disease 64, nevertheless it remains the only currently available model for the prospective investigation of immune responses to N. gonorrhoeae. There are certain parallels between what is known about the immune response of mice and humans to N. gonorrhoeae. In the absence of intervention in either species, the immune response to uncomplicated lower tract infection in females is minimal: there is little or no specific antibody response, and no evidence for the generation of effective, recallable immune memory involving Th1 or Th2 cells. However, two reports indicate that humans infected with gonorrhea have elevated IL-17 22, 23, as do female mice infected with N. gonorrhoeae 19. Both mice and humans can be re-infected with N. gonorrhoeae with apparently no effective protection, whether measured in terms of probablity of acquisition, duration or intensity of infection, or disease, arising from prior exposure. Therefore, while it would be mistaken to assume that findings in the murine model are directly translatable to humans, our findings raise questions and specific hypotheses for evaluation in humans.
The prevailing paradigm for lack of effective immunity to gonorrhea holds that the extensive antigenic variation shown by N. gonorrhoeae, involving most of its major surface antigens, coupled with multiple mechanisms for resisting complement, enable it to evade whatever adaptive immune responses the host may develop. Our studies in mice, presaged by conclusions derived from the studies of Hedges et al. in humans 20, 21, reveal that, in addition, N. gonorrhoeae suppresses adaptive immune responses by inducing high levels of regulatory cytokines TGFβ and IL-10, and type 1 regulatory T cells 17, 24, 25. Counter-manipulating this suppression by means of neutralizing antibodies to TGFβ and IL-10 17, 25, or by the local administration of microencapsulated IL-12 26, allows Th1-governed responses to emerge, with the development of specific antibodies, establishment of memory, and accelerated clearance of infection.
The current study builds on these findings in the context of vaccination, by demonstrating that mice can be immunized against N. gonorrhoeae by the i.vag. administration of a non-living vaccine (OMV) with a Th1-driving adjuvant, IL-12/ms. Whether this approach is applicable to humans remains to be determined. Most likely, i.vag. immunization will not be acceptable, and is inapplicable for males; therefore another route of immunization will be needed. In addition, other Th1-driving adjuvants may be required. However, the findings imply that a vaccine against N. gonorrhoeae may indeed be feasible despite earlier setbacks and subsequent pessimism 64, and they serve to indicate the type of immune responses that will need to be induced to generate protective immunity.
METHODS
Mice
All mice, including wild-type BALB/c and C57BL/6 mice, B6.129S7-Ifngtm1Ts/J (IFNγ-deficient), B6.129S2-Ighmtm1Cgn/J (B cell-deficient; also known as µMT), B6.129S2-Cd4tm1Mak/J (CD4-deficient), and B6.129S2-Cd8atm1Mak/J (CD8-deficient) mice on a C57BL/6 background, were purchased from Jackson Laboratories (Bar Harbor, ME). BALB/c mice were used for the experiments unless otherwise specified. Mice were maintained in a BSL2 facility in the Laboratory Animal Facility at the University at Buffalo, which is fully accredited by AAALAC. All animal use protocols were approved by the Institutional Animal Care and Use Committee of the University at Buffalo.
Bacteria
N. gonorrhoeae strain FA1090 was provided by Dr Janne Cannon (University of North Carolina at Chapel Hill); strain MS11 was provided by Dr Daniel Stein (University of Maryland); strain FA19, and clinical isolates were obtained from the collection of clinical strains maintained at the University of North Carolina at Chapel Hill. The original clinical strains have been stored at –80°C since initial isolation in 1992 and have been minimally subcultured 65. For use in the murine infection model, N. gonorrhoeae strains 9087 and 0336 were transformed with the streptomycin-resistant rpsL gene from strain FA1090 to generate strains GC68 and GC69, respectively. E. coli K12 was provided by Dr Terry Connell (University at Buffalo). Non-typeable Haemophilus influenzae (NTHI) strain 6P24H1 was provided by Dr Timothy Murphy (University at Buffalo). N. gonorrhoeae was cultured on GC agar supplemented with hemoglobin and Isovitalex (BD Diagnostic Systems, Franklin Lakes, NJ) and the resultant growth was checked for colony morphology consistent with Opa protein and pilus expression. NTHI was cultured on GC agar supplemented with hemoglobin only. E. coli was cultured on BHI agar. Bacteria were harvested from plates and the cell density was determined as detailed previously 24.
IL-12 microspheres
Murine IL-12 (Wyeth, Philadelphia, PA) was encapsulated into poly-lactic acid microspheres using the Phase Inversion Nanoencapsulation technology as previously described except that bovine serum albumin was replaced by sucrose (0.1 %, w/w) 66. Blank microspheres were prepared in the same way but without IL-12.
Gonococcal outer membrane vesicles (OMV)
After 18–22 h culture on supplemented GC agar, N. gonorrhoeae was harvested from plates into ice-cold lithium acetate buffer (pH 5.8) and passed through a 25-gauge needle 10–12 times to sheer the outer membranes from the bacteria. The suspensions were spun in microfuge tubes at 13,000 RPM for 1 min. The supernatants were collected and ultracentrifuged at 107,000×g for 2 h. The pellet was washed with 50 mM Tris-HCl (pH 8.0) and resuspended in PBS. Protein was assayed with the Micro BCA protein kit (Thermo Scientific, Rockford, IL) or RC DC Protein Assay kit (Bio-Rad, Hercules, CA).
Immunization schedule and mouse vaginal infection model
Groups of 8 female mice between 7 and 9 weeks old were immunized i.vag. with gonococcal OMV (40µg protein) of various strains as described, plus IL-12/ms (1µg IL-12) or blank ms in a total volume of 40µl PBS; control groups were sham-immunized with IL-12/ms or with blank ms alone. Mice were immunized 1 to 3 times with a 7–14 day interval, as indicated. After a further 2 weeks to 6 months, immunized mice were infected with 5 × 106 CFU live N. gonorrhoeae as previously described,15, 26 with the modification that 0.5 mg Premarin (Pfizer, Philadelphia, PA) was used as estradiol administered s.c. on days –2, 0, and 2. Vaginal swabs collected daily were quantitatively cultured on GC agar supplemented with hemoglobin, Isovitalex, and selective antibiotics (vancomycin, streptomycin, nisin, colistin, and trimethoprim) to determine the bacterial colonization loads.15, 26 The limit of detection was 100 colony-forming units (CFU) recovered per mouse. Gonococcal recovery was counted by an individual who was “blinded” to the experimental treatments, and all experiments were repeated 2 or 3 times for verification.
Assay of serum and mucosal antibodies
Samples of vaginal wash and serum were collected from the mice at the indicated time points.17, 26 Gonococcus-specific IgA, IgG, IgM, and IgG subclass antibodies IgG1, IgG2a, IgG2b, and IgG3 in vaginal washes and sera were measured by ELISA on plates coated with whole gonococci, using undiluted vaginal wash and 10-fold diluted serum as starting dilutions. 17, 26 Total IgA, IgG, and IgM concentrations in secretions were assayed by ELISA on plates coated with anti-IgA, -IgG, or -IgM antibodies (Southern Biotech, Birmingham, AL). H5 mouse monoclonal antibody (specific for N. gonorrhoeae porin serovar PIB3) or affinity-purified mouse IgA, IgG, and IgM (Southern Biotech) were used to establish standard curves. Bound antibodies were detected by alkaline phosphatase-conjugated goat anti-mouse IgA, IgG, IgM, IgG1, IgG2a, IgG2b, or IgG3 antibody (Southern Biotech) and p-nitrophenylphosphate substrate (Southern Biotech). Plates were read in a VersaMax microplate reader with SoftMax software (Molecular Devices, Sunnyvale, CA) or an ELX800 Universal microplate reader with KC Junior software (Bio-Tek Instruments, Winooski, VT). Antibody data were expressed as relative (fold increase) to the antibody levels detected in control samples (from sham-immunized mice) assayed simultaneously.
Flow cytometry
Isolated cells were washed with staining buffer twice, then incubated with the indicated antibodies for 30 min on ice, washed, and analyzed on a FACSCalibur cytometer. For intracellular staining, cells were first fixed with Cytofix/Cytoperm (eBioscience). Antibodies to mouse CD4, CD8, IFNγ, IL-4, and IL-17A conjugated with FITC, PE, or allophycocyanin were purchased from eBioscience.
Lymphocyte isolation and culture
Mononuclear cells were isolated from aseptically harvested ILN using Histopaque 1083 (Sigma-Aldrich, St Louis, MO) density gradient centrifugation and pooled from 2 or 3 mice to provide sufficient numbers of cells for culture. CD4+ T cells were purified through negative selection using a Dynal CD4 cell isolation kit (Invitrogen, Carlsbad, CA). Cells were cultured in 24-well culture plates at a density of 2 × 106 cells/ml in the presence of equal numbers of mitomycin C-inactivated spleen cells to serve as APC, either with no stimulus or with 2 × 107 N. gonorrhoeae cells.
Proliferation assays
Cells were labeled with carboxymethyl fluorescein succinimide ester (CFSE; Sigma-Aldrich).67 CFSE-labeled cells were then washed twice in PBS, recounted, and stimulated as described above. Cultured cells were harvested and then stained with allophycocyanin-conjugated anti-mouse CD4 antibody. The data were acquired by gating on the CD4+ cell populations in a FACSCalibur cytometer. The attenuation of CFSE fluorescence was used to measure cell proliferation.
Cytokine ELISA
IFNγ, IL-4, and IL-17A levels were measured in triplicate using ELISA kits purchased from eBioscience.
Real-time RT-PCR
Total cellular RNA of whole vaginas harvested from the mice was isolated with RNeasy Mini Kits (Qiagen, Valencia, CA), and was transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time RT-PCR was performed on an iCycler iQ detection system (Bio-Rad) using Sybergreen (Bio-Rad) for real-time monitoring of the PCR. The primers used were as follows: IFNγ, 5’-TAC TGC CAC GGC ACA GTC ATT GAA-3’, 5’-GCA GCG ACT CCT TTT CCG CTT CCT-3’; IL-4, 5’-GAA GCC CTA CAG ACG AGC TCA-3’, 5’-ACA GGA GAA GGG ACG CCAT-3’; IL-17A, 5’-TCA GGG TCG AGA AGA TGC TG-3’, 5’-TTT TCA TTG TGG AGG GCA GA-3’; β-actin, 5’-CCT AAG GCC AAC CGT GAA AAG-3’, 5’-GAG GCA TAC AGG GAC AGC ACA-3’. Relative quantification of target genes was analyzed based on the threshold cycle (Ct) determined by Bio-Rad iQ5 optical system software.
Western Blot
N. gonorrhoeae OMV preparations were boiled for 5 min in sodium dodecyl sulfate (SDS) loading buffer containing 2-mercaptoethanol. Protein quantification was done with the RC DC Protein Assay kit. Ten micrograms of protein from each sample was separated on 10% polyacrylamide SDS electrophoresis gels. Protein bands were transferred onto nitrocellulose membranes using the electrophoresis transfer system (Bio-Rad, Hercules, CA, USA). The nitrocellulose membranes were blocked with PBS containing 3% skim milk overnight at 4°C before incubation for 2 h with serum samples diluted 1:200, or vaginal wash samples diluted 1:20 in PBS containing 3% skim milk. Specific antibodies bound to N. gonorrhoeae OMV preparations were detected with horseradish peroxidase-conjugated goat anti-mouse-IgG (Santa Cruz Biotechnology, Paso Robles, CA) at a dilution of 1:4000. The Pierce detection kit was used for chemiluminescent detection and images were collected with a ChemiDoc MP imaging system (Bio-Rad).
Immunoproteomics
Protein concentration in OMV was measured using DC Protein Assay Kit (Bio-Rad). Samples of OMV [300µg and 50µg of protein, for two dimensional (2D) SDS-PAGE-MS/MS analysis and immunoblotting, respectively] were precipitated overnight in 90% acetone, washed twice with 100% ice-cold acetone and air-dried. Protein pellets were reconstituted in 200µL of rehydration buffer (7M urea, 2M thiourea, 2% CHAPS, 2% ASB-14, 1% DTT, 2mM TBP, 2% 3–10 IPG buffer, trace of Orange G) and used to rehydrate pH 4–7 ReadyStrip IPG strips (Bio-Rad) overnight at 25°C. Isoelectric focusing was carried out using the PROTEAN® i12™ IEF System (Bio-Rad) for a total of 26,000Vh with the following settings: 50µA current limit, 8000V rapid ramp for 26,000Vh, 750V hold. The second dimension (2D) SDS-PAGE was performed using Criterion TGX Any kD gels (Bio-Rad). The proteins were stained overnight in Flamingo fluorescent stain (Bio-Rad) and the spots were visualized using the ChemiDoc Imaging System (Bio-Rad). For immunoblotting, separated proteins were transferred onto PVDF membranes using the TurboBlott transfer system (Bio-Rad). The membranes were blocked for 2h in 5% milk in PBS Tween, and probed by overnight incubation with sera from immunized mice, followed by incubation with anti-mouse HRP-conjugated antibodies (Bio-Rad). Spots were visualized using Clarity Western ECL Substrate and ChemiDoc MP Imaging System (Bio-Rad). Proteins on membranes were stained with Novex Reversible Membrane Protein Stain (InVitrogen) to overlay positions of selected “anchor” spots with the Flamingo-stained 2D gels. Matching spots were excised and the proteins were trypsin digested as described 68. Samples containing extracted peptides were desalted using ZipTip C18 (Millipore, Billerica, MA) and eluted with 70% acetonitrile/0.1% TFA, and dried in a speed vac. Desalted peptides were brought up in 2% acetonitrile in 0.1% formic acid (20µL) and analyzed (2µL) by LC/ESI MS/MS with a Thermo Scientific Easy-nLC II (Thermo Scientific, Waltham, MA) nano HPLC system coupled to a hybrid Orbitrap Elite ETD (Thermo Scientific) mass spectrometer using an instrument configuration as described 69. In-line de-salting was accomplished using a reversed-phase trap column (100µm × 20mm) packed with Magic C18AQ (5-µm 200Å resin; Michrom Bioresources, Auburn, CA) followed by peptide separations on a reversed-phase column (75µm × 250mm) packed with Magic C18AQ (5-µm 100Å resin; Michrom Bioresources) directly mounted on the electrospray ion source. A 30-minute gradient from 7% to 35% acetonitrile in 0.1% formic acid at a flow rate of 400nL/min was used for chromatographic separations. The heated capillary temperature was set to 300°C and a spray voltage of 2750V was applied to the electrospray tip. The Orbitrap Elite instrument was operated in the data-dependent mode, switching automatically between MS survey scans in the Orbitrap (AGC target value 1,000,000, resolution 240,000, and injection time 250 milliseconds) with MS/MS spectra acquisition in the linear ion trap (AGC target value of 10,000, and injection time 100msec). The 20 most intense ions from the Fourier-transform (FT) full scan were selected for fragmentation in the linear trap by collision-induced dissociation with normalized collision energy of 35%. Selected ions were dynamically excluded for 15sec with a list size of 500 and exclusion mass by mass width ±0.5. Data analysis was performed using Proteome Discoverer 1.4 (Thermo Scientific). All identified peptides were searched against a N. gonorrhoeae database (FA1090, FA19, and MS11) combined with cRAP.fasta, a database of common contaminants (http://www.thegpm.org/crap/); this creates a list of proteins commonly found in proteomics experiments that are present by accident or unavoidable contamination. Trypsin was set as the enzyme with maximum missed cleavages set to 2. The precursor ion tolerance was set to 10 ppm and the fragment ion tolerance was set to 0.8 Da. Variable modifications included oxidation on methionine (+15.995 Da) and carbamidomethyl on cysteine (+57.021 Da). Data were searched using Sequest HT. All search results were run through Percolator for scoring
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Data on the effect of immunization on recovery of N. gonorrhoeae after inoculation were analyzed using two-way ANOVA for repeated measures with Fisher’s protected least significant difference post-hoc tests. In addition, Kaplan-Meier analysis with log-rank tests was used to compare clearance of infection (defined as the first of 3 successive days of zero recovery) between treatment groups. For immune response data, unpaired two-tailed t tests were used to compare the mean values between two groups, or ANOVA with Bonferroni post-hoc tests was used for multiple comparisons. P <0.05 was considered statistically significant. Statistical analyses were performed using Microsoft Excel or Prism 5 (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank Tara Phillips and Julianny Perez (TherapyX, Inc.), and James E. Anderson (University of North Carolina) for excellent technical assistance, and Philip Gafken and Lisa Jones (Oregon State University) for data collection.
These studies were supported by US National Institutes of Health grants R43-AI104067 and R43-AI115877 to YL, P30-AI050410 (Virology, Immunology and Microbiology Core of the University of North Carolina Center for AIDS Research grant) to MMH, R01-AI117235 to AES, and P30-CA015704 (Proteomics Facility, Fred Hutchinson Cancer Research Center, Seattle, WA).
Footnotes
AUTHOR CONTRIBUTIONS
YL and MWR conceived and designed the animal experiments, interpreted and analyzed the data, and wrote the manuscript with input from MMH, AES, and AEJ; YL and LAH performed animal experiments; LAH and WL performed in vitro analyses; MMH collected, characterized, and transformed clinical neisserial isolates; RAZ and AES designed and performed proteomics analyses; AEJ conceived, developed, and refined the animal model and advised on its use and interpretation; NKE conceived and developed the cytokine microencapsulation process. All authors read and approved the final manuscript.
DISCLOSURES
NKE has ownership interest in TherapyX, Inc., which is developing sustained-release nanoparticulate adjuvants for inflammatory disease therapy. YL and LAH are salaried employees of TherapyX, Inc. MWR serves as a paid consultant for TherapyX, Inc. Other authors declare no conflicts of interest.
REFERENCES
- 1.Newman L, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Sexually transmitted disease surveillance 2014. Atlanta, GA: Department of Health and Human Services; 2015. [Google Scholar]
- 3.NIAID. The Antibacterial Resistance Program of the National Institute of Allergy and Infectious Diseases: Current Status and Future Directions. US Department of Health and Human Services; 2014. [Google Scholar]
- 4.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 2012;366:485–487. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 5.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hook EW, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, et al., editors. Sexually Transmitted Diseases. 3rd. New York: McGraw-Hill; 1999. pp. 451–466. [Google Scholar]
- 7.WHO. Global strategy for the prevention and control of sexually transmitted infections : 2006 – 2015 : breaking the chain of transmission. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 8.Gottlieb SL, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine. 2016;34:2939–2947. doi: 10.1016/j.vaccine.2016.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerse AE, Bash MC, Russell MW. Vaccines against gonorrhea: current status and future challenges. Vaccine. 2014;32:1579–1587. doi: 10.1016/j.vaccine.2013.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg L, et al. Gonococcal vaccine studies in Inuvik. Can. J. Public Health. 1974;65:29–33. [PubMed] [Google Scholar]
- 11.Boslego JW, et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine. 1991;9:154–162. doi: 10.1016/0264-410x(91)90147-x. [DOI] [PubMed] [Google Scholar]
- 12.Wetzler LM, Blake MS, Gotschlich EC. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J. Exp. Med. 1988;168:1883–1897. doi: 10.1084/jem.168.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell MW, Hook EW, Gonorrhe . In: Vaccines for Biodefense and Emerging and Neglected Diseases. Stanberry LR, Barrett ADT, editors. Amsterdam/London: Elsevier; 2009. pp. 963–981. [Google Scholar]
- 14.Arko RJ. Animal models for pathogenic Neisseria species. Clin. Microbiol. Rev. 1989;2(Suppl):S56–S59. doi: 10.1128/cmr.2.suppl.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 1999;67:5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, et al. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17β-estradiol-treated mice. Vaccine. 2008;26:5741–5751. doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Russell MW. Diversion of the immune response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by treatment with anti-TGF-β antibody generates immunological memory and protective immunity. mBio. 2011;2 doi: 10.1128/mBio.00095-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packiam M, Veit SJ, Anderson DJ, Ingalls RR, Jerse AE. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 2010;78:433–440. doi: 10.1128/IAI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges SR, Sibley D, Mayo MS, Hook EW, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 21.Hedges SR, Mayo MS, Mestecky J, Hook EW, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagliardi MC, et al. Circulating levels of interleukin-17A and interleukin-23 are increased in patients with gonococcal infection. FEMS Immunol. Med. Microbiol. 2011;61:129–132. doi: 10.1111/j.1574-695X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 23.Masson L, et al. Relationship between female genital tract infections, mucosal interleukin-17 production and local T helper type 17 cells. Immunology. 2015;146:557–567. doi: 10.1111/imm.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Islam E, Jarvis GA, Gray-Owen S, Russell MW. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-β-dependent mechanisms. Mucosal Immunol. 2012;5:320–331. doi: 10.1038/mi.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Liu W, Russell MW. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 2014;7:165–176. doi: 10.1038/mi.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Egilmez NK, Russell MW. Enhancement of adaptive immunity to Neisseria gonorrhoeae by local intravaginal administration of microencapsulated IL-12. J. Infect. Dis. 2013;208:1821–1829. doi: 10.1093/infdis/jit354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holst J, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum. Vacc. Immunother. 2013;9:1241–1253. doi: 10.4161/hv.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkins C, Barkley KB, Carbonetti NH, Coimbre AJ, Sparling PF. Immunobiology of purified recombinant outer membrane porin protein I of Neisseria gonorrhoeae . Mol. Microbiol. 1994;14:1059–1075. doi: 10.1111/j.1365-2958.1994.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 29.Hobbs MM, et al. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front. Microbiol. 2011;2:123. doi: 10.3389/fmicb.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox KK, et al. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae . Am. J. Epidemiol. 1999;149:353–358. doi: 10.1093/oxfordjournals.aje.a009820. [DOI] [PubMed] [Google Scholar]
- 31.Zielke RA, Wierzbicki IH, Weber JV, Gafken PR, Sikora AE. Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Molec. Cell. Proteomics. 2014;13:1299–1317. doi: 10.1074/mcp.M113.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plante M, et al. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae . J. Infect. Dis. 2000;182:848–855. doi: 10.1086/315801. [DOI] [PubMed] [Google Scholar]
- 33.Schryvers AB, Stojiljkovic I. Iron acquisition systems in the pathogenic Neisseria . Mol. Microbiol. 1999;32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 34.Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11:87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 35.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sintsova A, et al. Selection for a CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract. Infect. Immun. 2015;83:1372–1383. doi: 10.1128/IAI.03123-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howe SE, Konjufca VH. Protein-coated nanoparticles are internalized by the epithelial cells of the female reproductive tract and induce systemic and mucosal immune responses. PLoS One. 2014;9:e114601. doi: 10.1371/journal.pone.0114601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole JG, Jerse AE. Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops. PLoS One. 2009;4:e8108. doi: 10.1371/journal.pone.0008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erwin AL, Haynes PA, Rice PA, Gotschlich EC. Conservation of the lipooligosaccharide synthesis locus lgt among strains of Neisseria gonorrhoeae: requirement for lgtE in synthesis of the 2C7 epitope and of the beta chain of strain 15253. J. Exp. Med. 1996;184:1233–1241. doi: 10.1084/jem.184.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apicella MA, et al. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect. Immun. 1987;55:1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice PA, Vayo HE, Tam MR, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulton KM, Twine SM. Immunoproteomics: current technology and applications. Methods Mol. Biol. 2013;1061:21–57. doi: 10.1007/978-1-62703-589-7_2. [DOI] [PubMed] [Google Scholar]
- 43.Klade CS. Proteomics approaches towards antigen discovery and vaccine development. Curr. Opin. Mol. Ther. 2002;4:216–223. [PubMed] [Google Scholar]
- 44.Jacobson GR, Rosenbusch JP. Abundance membrane association of elongation factor Tu in E. coli . Nature. 1976;261:23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- 45.Porcella SF, Belland RJ, Judd RC. Identification of an EF-Tu protein that is periplasm-associated and processed in Neisseria gonorrhoeae . Microbiology. 1996;142:2481–2489. doi: 10.1099/00221287-142-9-2481. [DOI] [PubMed] [Google Scholar]
- 46.Jaskunas SR, Lindahl L, Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli . Nature. 1975;257:458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- 47.Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrometry Rev. 2008;27:535–555. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- 48.Nieves W, et al. Immunospecific responses to bacterial elongation factor Tu during Burkholderia infection and immunization. PLoS One. 2010;5:e14361. doi: 10.1371/journal.pone.0014361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marques MA, Chitale S, Brennan PJ, Pessolani MC. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae . Infect. Immun. 1998;66:2625–2631. doi: 10.1128/iai.66.6.2625-2631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vipond C, et al. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics. 2006;6:3400–3413. doi: 10.1002/pmic.200500821. [DOI] [PubMed] [Google Scholar]
- 51.Carrasco SE, et al. Borrelia burgdorferi elongation factor EF-Tu is an immunogenic protein during Lyme borreliosis. Emerg. Microbes Infect. 2015;4:e54. doi: 10.1038/emi.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goytia M, et al. Characterization of a spermine/spermidine transport system reveals a novel DNA sequence duplication in Neisseria gonorrhoeae . FEMS Microbiol. Lett. 2015;362:fnv125. doi: 10.1093/femsle/fnv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 54.Wu H-Y, Abdu S, Stinson D, Russell MW. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect. Immun. 2000;68:5539–5545. doi: 10.1128/iai.68.10.5539-5545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snapper CM, Peschel C, Paul WE. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 56.Airoldi I, et al. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J. Immunol. 2000;165:6880–6888. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]
- 57.Jelinek DF, Braaten JK. Role of IL-12 in human B lymphocyte proliferation and differentiation. J. Immunol. 1995;154:1606–1613. [PubMed] [Google Scholar]
- 58.Thibodeaux DK, et al. Autocrine regulation of IL-12 receptor expression is independent of secondary IFN-γ secretion and not restricted to T and NK cells. J. Immunol. 1999;163:5257–5264. [PubMed] [Google Scholar]
- 59.Skok J, Poudrier J, Gray D. Dendritic cell-derived IL-12 promotes B cell induction of Th2 differentiation: a feedback regulation of Th1 development. J. Immunol. 1999;163:4284–4291. [PubMed] [Google Scholar]
- 60.Metzger DW. Interleukin-12 as an adjuvant for induction of protective antibody responses. Cytokine. 2010;52:102–107. doi: 10.1016/j.cyto.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Z, et al. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 62.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 63.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards JL, Jennings MP, Apicella MA, Seib KL. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine developmen.t. Crit. Revs. Microbiol. 2016:1105782. doi: 10.3109/1040841X.2015.1105782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hobbs MM, et al. Molecular typing of Neisseria gonorrhoeae causing repeated infections: Evolution of porin during passage within a community. J. Infect. Dis. 1999;179:371–381. doi: 10.1086/314608. [DOI] [PubMed] [Google Scholar]
- 66.Egilmez NK, Jong YS, Mathiowitz E, Bankert RB. Tumor vaccination with cytokine-encapsulated microspheres. Methods Mol. Med. 2003;75:687–696. doi: 10.1385/1-59259-324-0:687. [DOI] [PubMed] [Google Scholar]
- 67.Chen JC, Chang ML, Muench MO. A kinetic study of the murine mixed lymphocyte reaction by 5,6-carboxyfluorescein diacetate succinimidyl ester labeling. J. Immunol. Methods. 2003;279:123–133. doi: 10.1016/s0022-1759(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 68.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 69.Yi EC, Lee H, Aebersold R, Goodlett DR. A microcapillary trap cartridge-microcapillary high-performance liquid chromatography electrospray ionization emitter device capable of peptide tandem mass spectrometry at the attomole level on an ion trap mass spectrometer with automated routine operation. Rapid Commun. Mass Spectrom. 2003;17:2093–2098. doi: 10.1002/rcm.1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.