Abstract
Background
Interleukin (IL)-33 is implicated in the pathophysiology of asthma and allergic diseases. However, our knowledge is limited regarding how IL-33 release is controlled. The transcription factor nuclear factor-erythroid-2-related factor 2 (Nrf2) plays a key role in antioxidant response regulation.
Objective
The goal of this project was to investigate the role of cellular oxidative stress in controlling IL-33 release in airway epithelium.
Methods
Complementary approaches were used that included human bronchial epithelial cells and mouse models of airway type-2 immunity that were exposed to fungus Alternaria extract. The clinically available Nrf2 activator 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid methyl ester (CDDO-Me) was used to evaluate the role of Nrf2-induced antioxidant molecules.
Results
Human bronchial epithelial cells produced reactive oxygen species (ROS) when they were exposed to Alternaria extract. ROS scavengers, such as glutathione (GSH) and N-acetyl cysteine, prevented extracellular secretion of ATP and increases in intracellular calcium concentrations that precede IL-33 release. Administration of CDDO-Me to mice enhanced expression of a number of antioxidant molecules in the lungs and elevated lung levels of endogenous GSH. Importantly, CDDO-Me treatment reduced allergen-induced ATP secretion and IL-33 release by airway epithelial cells in vitro and protected mice from IL-33 release and asthma-like pathological changes in the lungs.
Conclusions
The balance between oxidative stress and antioxidant responses plays a key role in controlling IL-33 release in airway epithelium. The therapeutic potential of Nrf2 activators needs to be considered for asthma and allergic airway diseases.
Keywords: Airway epithelial cells, IL-33, reactive oxygen species, Nrf2, asthma, lung
INTRODUCTION
The incidence of asthma and allergic diseases has increased over the past 50 years (1). However, the mainstay of asthma therapy is the use of adrenergic bronchodilators and corticosteroids, which has not changed for more than 40 years (2). Furthermore, severe corticosteroid-refractory patients with asthma represent a difficult current medical problem (3). The cost, tolerability, and side effects of asthma medications, including newly-developed biologics, also need to be considered (4). Therefore, there is a clear need for development of new drugs to treat asthma that complement or overcome the shortcoming of those that are currently available.
Cytokines produced by epithelial cells and other cell types at the barrier surface, including thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, play an important role in regulating type 2 immunity (5). Among these cytokines, IL-33 is unique in that it is constitutively expressed and stored in the nucleus of tissue cells (6). Nuclear localization is likely critical for IL-33 regulation because a lack of the nuclear localization domain causes extracellular leakage of the cytokine and subsequent lethal eosinophilic and neutrophilic inflammation of various organs (7). Abundant in vitro and in vivo evidence now supports roles for IL-33 in innate and adaptive type-2 immune responses (8). Evidence suggesting roles for IL-33 in human asthma and allergic diseases has been forthcoming (9, 10). Despite these advances in the field, the mechanisms responsible for production and release of IL-33 are still poorly understood.
Oxidative stress is involved in various biological and pathologic processes (11). The potential roles for oxidative stress in asthma and allergies have been studied (12). However, the results from therapeutic studies of these diseases with exogenous antioxidant agents have been inconclusive (13). A detailed mechanistic understanding of how oxidative stress is involved in the development and/or exacerbation of allergic airway inflammation is still lacking.
Recent studies show that a key element in the regulation of oxidative stress is the transcription factor nuclear factor-erythroid-2-related factor 2 (Nrf2) (14, 15). Under physiologic conditions, Nrf2 is localized and suppressed in the cytoplasm by binding to Kelch-like ECH-associated protein 1 (Keap1). Upon exposure to oxidative stress, Nrf2 translocates to the nucleus and initiates transcription of more than 200 antioxidant and cytoprotective genes (16). Because the Nrf2 pathway would provide broad control over oxidative stress responses by activating a number of antioxidant molecules, it could be a therapeutic target for a variety of diseases such as neurodegenerative disorders, diabetes complications, and cardiovascular diseases (17, 18). The Nrf2 pathway may be particularly relevant to the lungs because the organ is exposed to a variety of oxidative insults from our atmospheric environment, including tobacco smoke, air pollution, and allergens. Indeed, asthma is generally characterized by a loss of antioxidant activities (12). Thus, the goals of this project were to investigate the role of oxidative stress in controlling IL-33 release in airway epithelium and to examine the efficacy of Nrf2-activators for protecting hosts from type-2 immune responses and airway inflammation.
MATERIALS AND METHODS
Mice and reagents
BALB/cJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Il1rl1−/− (ST2−/−) mice on the BALB/c background (19) were a generous gift from Dr. Andrew N. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom) and were bred in the animal facility at the Mayo Clinic (Rochester, MN). Seven- to nine-week-old female mice were used in this study. The procedures and handling of the mice were reviewed and approved by the Mayo Clinic Institutional Animal Care and Use Committee. Extracts of Alternaria (Alternaria alternata), Aspergillus (Aspergillus fumigatus), German cockroach, and house dust mite (HDM) were purchased from Greer Laboratories (Lenoir, NC). The Nrf2 activator 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid methyl ester (CDDO-Me) was purchased from Cayman Chemical (Ann Arbor, MI). Reduced L-glutathione (GSH), N-acetylcysteine (NAC), resveratrol, uric acid (UA), and diphenyleneiodonium chloride (DPI) were purchased from Sigma-Aldrich (St. Louis, MO). IL-33 was purchased from eBioscience (San Diego, CA) and contained <0.1 ng/ml endotoxin.
Detailed Materials and Methods are fully described in the supporting information of this article.
RESULTS
Allergen exposure induces oxidative stress in bronchial epithelial cells
A strong association has been established between exposure to the fungus Alternaria alternata and allergic airway diseases, in particular severe asthma and life-threatening exacerbation (20, 21). Therefore, we investigated whether airway epithelial cells exhibit an oxidative stress response to Alternaria extract by using cell-permeable reactive oxygen species (ROS)-sensing fluorogenic probes. Human bronchial epithelial (hBE) cells were exposed to 100 μg/ml Alternaria extract for 15 minutes, and production of intracellular ROS was examined by a cytoplasm-localizing ROS probe (CellROX® Orange). As compared to hBE cells exposed to medium alone, significant increases in cytoplasmic fluorescence was detected in Alternaria-exposed hBE cells, consistent with an increase in ROS production within this compartment (Figure 1A,B). Similar findings were observed with nuclear-localizing probes (CellROX® Green) (Figure 1A,B). Kinetic studies showed that an increase in cytoplasmic ROS was observed as early as 5 minutes after cellular exposure to Alternaria extract, and that the level continued to increase at least for 20 minutes (Figure S1). Pretreatment with the ROS scavenger GSH (5 mM) abolished the increase in Alternaria-induced ROS production (Figure 1A,B). Altogether, these findings suggest that GSH-sensitive ROS are quickly produced within the cytoplasm and nucleus of hBE cells when they are exposed to Alternaria extract.
Figure 1.
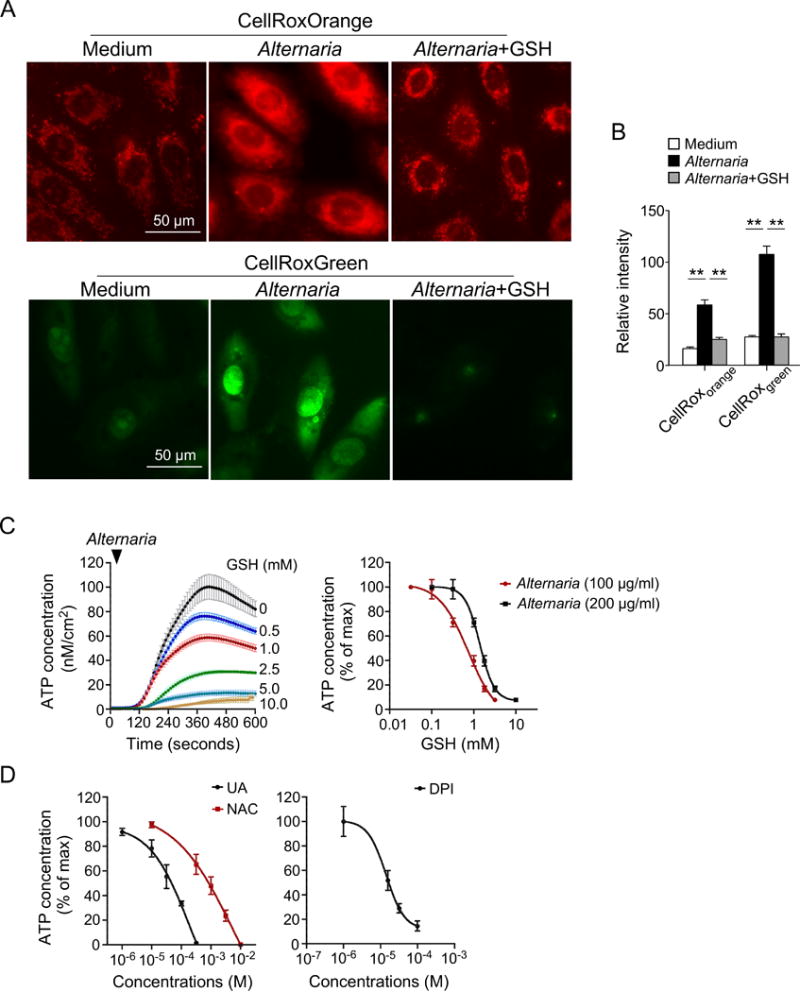
Oxidative stress is involved in ATP secretion by normal human bronchial epithelial (hBE) cells exposed to Alternaria extract. A, Fluorescence images of hBE cells labeled with CellROX® Orange (top panels) and CellROX® Green (lower panels). hBE cells were pretreated with GSH or medium alone, exposed to 100 μg/ml Alternaria extract for 15 minutes, and incubated with CellROX® Orange or CellROX® Green dye. B, Quantitative analysis of CellROX® Orange and CellROX® Green probe fluorescence intensities (n=25 for each condition). ** p<0.01 between groups indicated by horizontal lines. C, hBE cells were preincubated with GSH and exposed to Alternaria extract. Real-time release of ATP into extracellular medium was quantitated by a bioluminescent probe. Left panel shows concentration-dependent effects of GSH on Alternaria extract-induced ATP release kinetics (n=6). Right panel shows a concentration-response relationship with two different concentrations of Alternaria extract (n=6). D, Concentration-response relationship showing the effect of uric acid (UA), N-acetyl-cysteine (NAC), and diphenylene iodonium chloride (DPI) on Alternaria extract-induced ATP release (n=6).
Oxidative stress is involved in ATP secretion and increased [Ca2+]i
While the molecular mechanisms involved in IL-33 release are not fully understood, several studies indicate that increased extracellular ATP levels and intracellular calcium concentrations ([Ca2+]i) play a critical role in initiating IL-33 release (22–24). To investigate the relationship between Alternaria-evoked ROS production and ATP secretion, we pretreated hBE cells with varying concentrations of GSH. As shown previously (22, 25), exposure to Alternaria induced a rapid increase in extracellular ATP concentrations that was inhibited by GSH (Figure 1C); the IC50 for 100 μg/ml Alternaria extract was 0.73±0.04 mM GSH. Furthermore, greater concentrations of GSH were required to inhibit ATP release induced by greater concentrations of Alternaria extract (i.e. 200 μg/ml). Alternaria-evoked ATP release was also inhibited by other common ROS scavengers, including UA and NAC (Figure 1D), which had IC50 values of 0.24±0.03 mM and 0.71±0.04 mM, respectively (n=6). In addition, pretreatment of hBE cells with DPI, which inhibits mitochondrial ROS production (26), also inhibited ATP release with an IC50 of 14.1±1.2 μM (n=6).
ROS scavengers were also effective in preventing the prolonged increase in [Ca2+]i after Alternaria exposure. As shown in Figure 2A,B, Alternaria initiated an increase in [Ca2+]i within 7 minutes that continued for at least 15 minutes; pretreatment with 5 mM GSH completely inhibited this response. Similarly, pretreatment with 100 μM DPI prior to stimulation with Alternaria extract inhibited the increase in [Ca2+]i (Figure 2B). Together, these findings suggest that oxidative stress is involved in ATP release and sustained elevation of [Ca2+]i in airway epithelial cells exposed to Alternaria.
Figure 2.
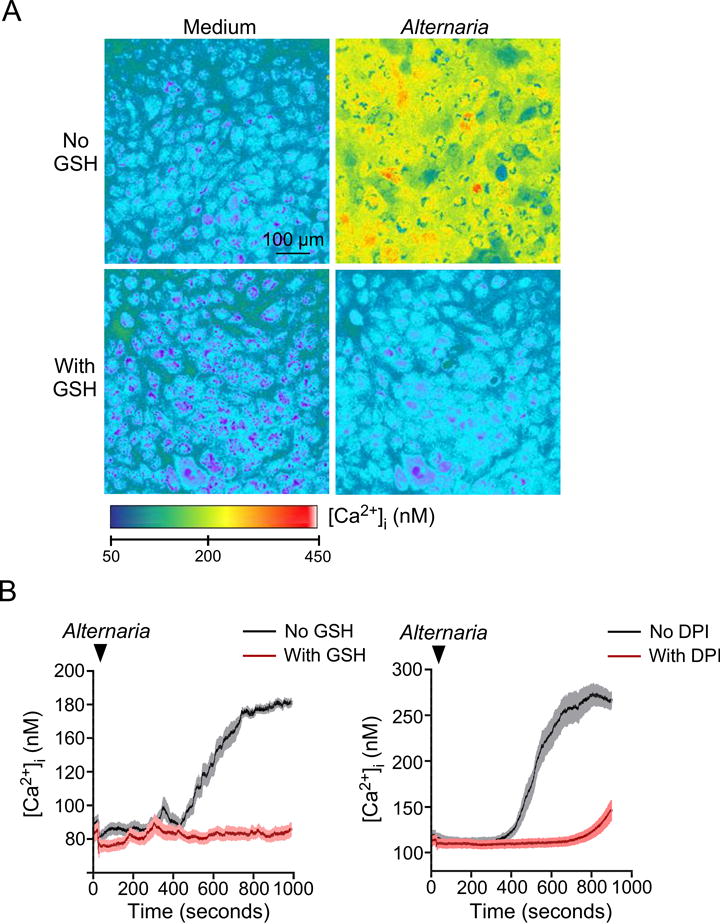
GSH and DPI block increases in [Ca2+]i in human bronchial epithelial (hBE) cells exposed to Alternaria extract. hBE cells were cultured in chamber slides, loaded with Fura-2 AM, and mounted on the stage of an inverted microscope equipped for fluorescence imaging. The cells were pretreated with 5 mM GSH, 100 μM diphenylene iodonium chloride (DPI) or medium alone and exposed to 100 μg/ml Alternaria extract. A, Representative images 15 minutes after exposure to Alternaria extract. B, Kinetics of [Ca2+]i in hBE cells (n=6 experiments).
Oxidative stress is involved in allergen-induced IL-33 release by hBE cells
Because oxidative stress is involved in acute cellular responses to Alternaria exposure, we examined whether oxidative stress is involved in IL-33 secretion. IL-33 is abundantly expressed by basal airway epithelial cells, but expression is lost when these cells differentiate into ciliated epithelial cells (27). Human primary airway epithelial cells derived from nasal scrapings or bronchial brushings and primary hBE cells from commercial sources (e.g. Lonza and Lifeline Cell Technology) contain small and varying amounts of IL-33 protein (22). Therefore, we created a new human airway epithelial cell model that allows us to evaluate the effects of various pharmacologic agents on IL-33 release. We introduced the full-length human IL-33 gene and a transposase gene into immortalized hBE cells to achieve stable expression of full-length IL-33 (hBE33 cells; Figure 3A) (see the Materials and Methods section in the supporting information for more details). hBE33 cells have a long lifespan and stably express IL-33 even after a number of passages (1082±142 pg IL-33/105 cells, n=10). IL-33 protein was localized to the nuclei of hBE33 cells (Figure 3B) as in native hBE cells (6, 22).
Figure 3.
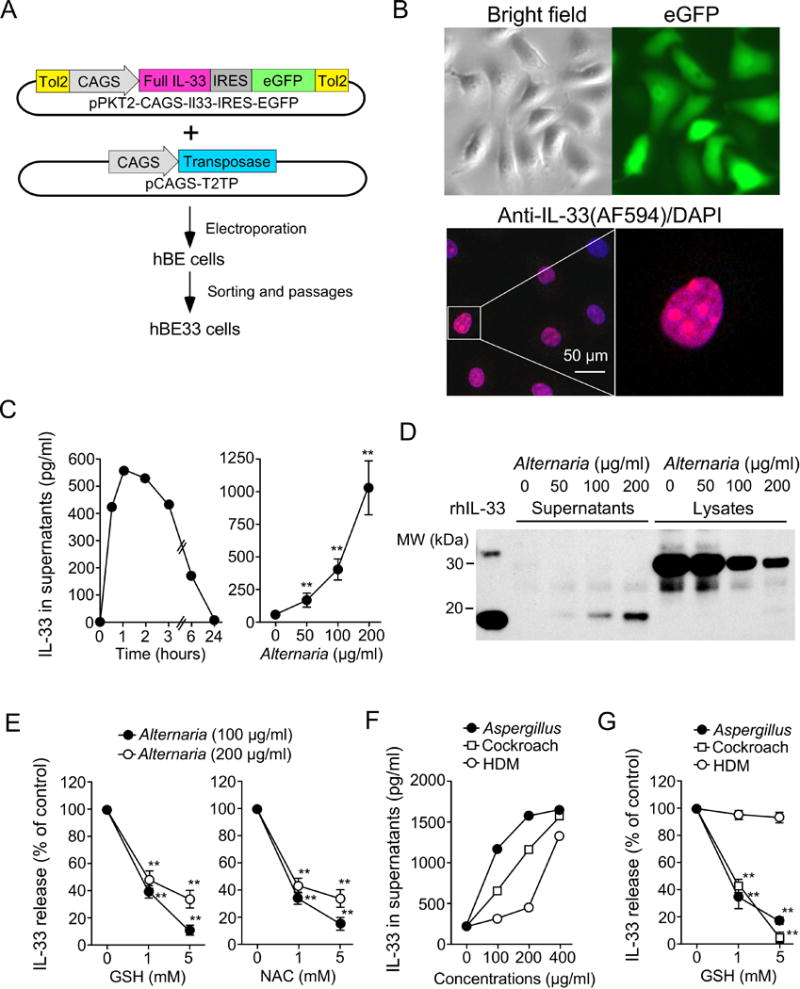
ROS scavengers inhibit IL-33 secretion by human bronchial epithelial (hBE) cells exposed to allergen extracts. A, Schematic diagram showing generation of hBE cells that constitutively express IL-33 (hBE33 cells). B, Confocal microscopic images of hBE33 cells. Upper panels show bright field and eGFP images. Lower panels show hBE33 cells stained with rabbit anti-human IL-33 and DAPI. C, IL-33 release by hBE33 cells exposed to Alternaria extract. Left panel shows kinetics of IL-33 release following exposure to 100 μg/ml Alternaria extract (representative of two experiments). Right panel shows a concentration-response relationship at the 1-hour time point (n=12, ** p<0.01 compared to no Alternaria). D, hBE33 cells were exposed to the indicated concentrations of Alternaria extract for 1 hour. Cell-free supernatants and cellular lysates were analyzed by Western blot. Data shown are representative of three experiments. E, hBE33 cells were preincubated with GSH (left panel) or NAC (right panel) and exposed to the indicated concentrations of Alternaria extract for 1 hour. IL-33 concentrations in supernatants are normalized to the values without GSH or NAC as 100% and presented as mean±SEM (n=4 experiments) ** p<0.01 compared to no GSH or NAC. F, hBE33 cells were exposed to allergen extracts for 1 hour. Data are representative of three experiments. G, hBE33 cells were preincubated with GSH and exposed to allergen extracts for 1 hour. IL-33 concentrations in supernatants were normalized to the values without GSH as 100% and presented as mean±SEM (n=4 experiments) ** p<0.01 compared to no GSH.
When hBE33 cells were exposed to Alternaria extract, IL-33 was released into cell-free supernatant as early as 30 minutes after exposure (Figure 3C). Western blot analysis showed that processed 19-kDa IL-33 was released into the extracellular media, while full-length 31-kDa IL-33 was retained within the cells (Figure 3D). Importantly, ROS scavengers, including GSH and NAC, significantly inhibited Alternaria-evoked IL-33 secretion (Figure 3E, p<0.01). IL-33 release was also observed when hBE33 cells were stimulated with extracts of other common allergens, including Aspergillus, cockroach, and HDM (Figure 3F). GSH and NAC inhibited IL-33 release induced by cockroach or Aspergillus extract, but not IL-33 release induced by HDM extract (Figure 3G). GSH did not affect production of RANTES by hBE cells that were exposed to 100 μg/ml Alternaria extract (Figure S2), suggesting that it is unlikely that GSH causes non-specific inhibitory effects on hBE cells.
Oxidative stress is involved in Alternaria-induced IL-33 release in vivo
To investigate the role of oxidative stress in IL-33 release in vivo, we used a mouse model. When naïve mice were exposed to Alternaria extract, BAL IL-33 levels increased within 1 hour and declined thereafter, while lung levels of IL-33 reached a peak 6 hours after exposure (Figure 4A). After 1 hour, processed 19-kDa IL-33 was detected in BAL fluids, while the full-length 31-kDa IL-33 remained in the lung tissues (Figure 4B), which was similar to the observations from the hBE33 in vitro model (Figure 3D). Importantly, administration of the ROS scavengers GSH and NAC to the airways abolished Alternaria-induced IL-33 secretion and type-2 cytokine production (Figure 4C,D). However, GSH did not affect IL-33-driven type-2 cytokine production (Figure 4D). Thus, oxidative stress plays a key role in allergen-induced IL-33 secretion in airway epithelium both in vitro and in vivo.
Figure 4.
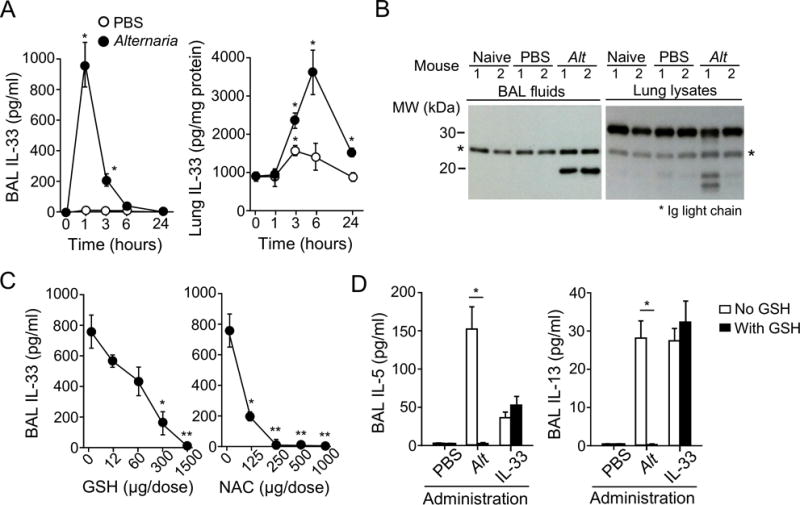
ROS scavengers inhibit IL-33 release and type-2 immune responses in mice exposed to Alternaria extract. A, Naïve BALB/c mice were administered i.n. 50 μg Alternaria extract or PBS. IL-33 levels in BAL fluid supernatants (left panel) and lung lysates (right panel) were analyzed by ELISA. Data are presented as mean±SEM of five mice and are representative of two experiments. * p<0.05 compared to PBS. B, BAL fluid supernatants and lung lysates from naïve mice or those administered i.n. PBS or Alternaria extract after 1 hour were analyzed by Western blot. The results are representative of three experiments with two mice/group. C, Alternaria extract was administered i.n. with various doses of GSH or NAC to naïve BALB/c mice. IL-33 levels in BAL supernatants were analyzed 1 hour later. Data are mean±SEM and are representative of two experiments with three mice/group. *p<0.05 and **p<0.01 compared to no GSH or NAC. D, Naïve BALB/c mice were administered i.n. Alternaria extract or IL-33 with or without 1 mg GSH. BAL fluids were collected 6 hours later. Data are mean±SEM and are representative of two experiments with four mice/group. *p<0.05 between the groups indicated by horizontal lines.
Activation of the antioxidant transcription factor Nrf2 protects epithelial cells from oxidative stress and IL-33 release
Oxidative stress is a result of an imbalance between ROS generation and its removal by various antioxidant molecules (28). Recent studies show that a key element in the regulation of antioxidant molecules is the master transcription factor Nrf2 (14–16). In human lung epithelial cells, treatment with the stilbenoid resveratrol induces Nrf2 activation that subsequently inhibits cigarette smoke-induced oxidative stress (29). Similarly, the synthetic oleanane triterpenoid CDDO-Me, which is an Nrf2 activator, is being tested in clinical trials for several diseases (17, 18, 30). Therefore, we examined the effects of resveratrol and CDDO-Me on Alternaria-evoked oxidative stress in hBE cells.
Pretreatment of hBE cells with 50 nM CDDO-Me or 25 μM resveratrol for 24 hours blocked the increase in CellROX® Orange fluorescence induced by 100 μg/ml Alternaria extract (Figure 5A,B, Figure S3A). Similarly, the ATP secretion evoked by Alternaria exposure was abolished in hBE cells pretreated with CDDO-Me or resveratrol (Figure 5C, Figure S3B). Pretreatment with CDDO-Me or resveratrol also inhibited Alternaria-induced increases in [Ca2+]i (Figure 5D,E, Figure S3C), suggesting that pharmacologic Nrf2 activators inhibit allergen-induced ROS production in epithelial cells and protect them from subsequent ATP release and increases in [Ca2+]i.
Figure 5.
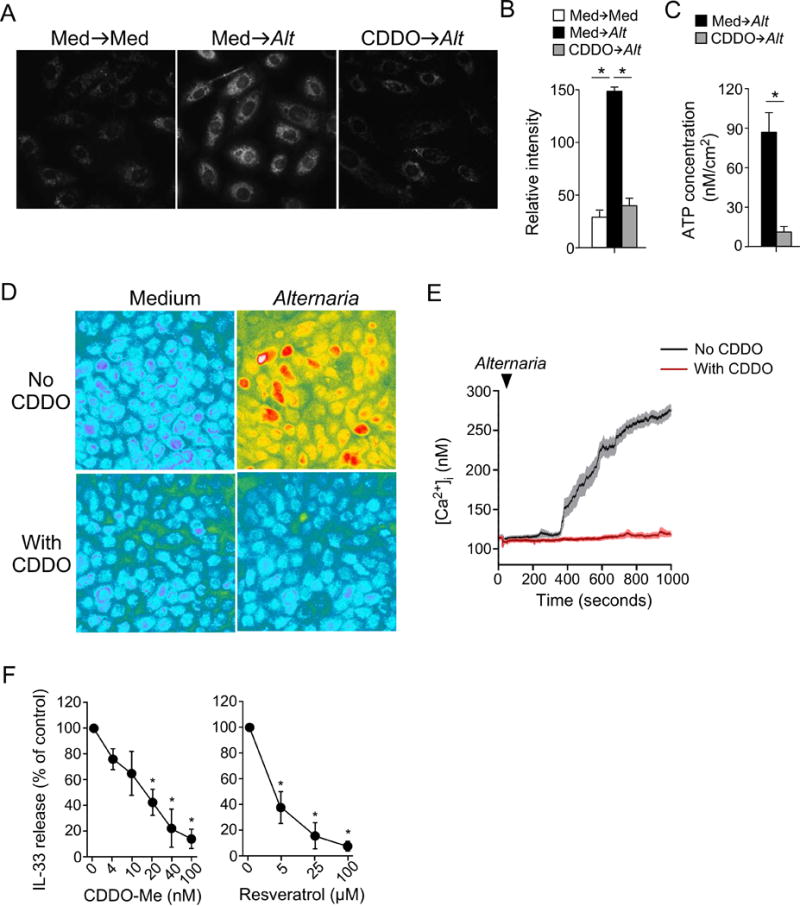
Treatment with Nrf2 activators inhibits oxidative stress and IL-33 release in human bronchial epithelial (hBE) cells. A, hBE cells were preincubated with medium alone or 50 nM CDDO-Me for 24 hours and exposed to Alternaria extract, followed by CellROX® Orange dye. Representative images are shown. B, Quantitative analysis of CellROX® Orange probe fluorescence intensities (mean±SEM, n=25). * p<0.05 between groups indicated by horizontal lines. C, hBE cells were treated similar to those in A. ATP release into extracellular medium was quantitated (mean±SEM, n=4). * p<0.05 between groups indicated by horizontal lines. D,E, hBE cells were preincubated with medium alone or CDDO-Me for 24 hours, and exposed to Alternaria extract. Representative images are shown in Panel D and kinetics changes in [Ca2+]i are shown in Panel E. Data are representative of four experiments. F, hBE33 cells were preincubated with the indicated concentrations of CDDO-Me or resveratrol for 24 hours and exposed to Alternaria extract. IL-33 concentrations in cell-free supernatants were normalized to the values without drugs as 100%. Data are presented as mean±SEM from three experiments. * p<0.05 compared to no drugs.
To examine the effects of these Nrf2 activators on IL-33 release, hBE33 cells were pretreated with various concentrations of CDDO-Me and resveratrol and then exposed to Alternaria extract. CDDO-Me and resveratrol inhibited IL-33 release with IC50s of approximately 15 nM and 3 μM, respectively (Figure 5F). CDDO-Me at 50 nM and resveratrol at 25 μM nearly abolished IL-33 release, consistent with their inhibitory effects on ATP secretion and [Ca2+]i increases. Altogether, these findings suggest that pharmacologic activators of the Nrf2 pathway are effective at preventing oxidative stress and subsequent IL-33 release in allergen-exposed airway epithelial cells.
CDDO-Me activates the expression of multiple antioxidant molecules in the lungs
To examine whether Nrf2 activators modulate the balance between oxidants and antioxidants in the lungs, we administered CDDO-Me to mice and used PCR arrays to screen for expression of antioxidant molecule genes in the lungs. Mice were given 50 μg CDDO-Me i.p. daily for 18 days, which is equivalent to roughly 12.5 mg CDDO-Me per dose in humans (31). As a reference, human non-cancer clinical trials have used a daily oral dose of 20 mg to 150 mg CDDO-Me (32). The genes with expression in CDDO-Me-treated mice that were more than doubled compared to DMSO-treated control mice included glutamate-cysteine ligase catalytic (Gclc), glutathione S-transferase pi 1 (Gstp1), NADPH:quionone oxidoreductase (Nqo1), thioredoxin 1 (Txnrd1), aldehyde oxidase 1 (Aox1), sulfiredoxin 1 (Srxn1), and heat shock protein family member 1A (Hspa1a) (Figure S4). These are all previously reported Nrf2 target genes (28).
To examine whether CDDO-Me modulates GSH levels in the lungs, we treated mice with 100 μg CDDO-Me i.p. daily for 3 days and then exposed them to 50 μg Alternaria extract (Figure S5A). As expected, GSH was constitutively detected in both BAL fluids and lung tissue in control mice treated with DMSO. CDDO-Me treatment further elevated the levels of GSH in BAL fluids and lungs by 98% and 40%, respectively (Figure S5B). When CDDO-Me-treated mice were exposed to Alternaria extract, significant reductions were observed in IL-33 released into airway lumens (p<0.05).
Nrf2 activation inhibits allergic airway inflammation
Given the inhibitory effects of CDDO-Me on IL-33 release, we investigated whether Nrf2 activation affects airway inflammation and pathology induced by allergen exposure in vivo. Naïve BALB/c mice were treated with CDDO-Me or DMSO (control) and exposed to Alternaria extract three times per week for 2 weeks (Figure 6A). In this sub-chronic exposure model, the control mice developed marked airway eosinophilia and increases in IL-5 and IL-13 protein expression in lungs (Figure 6B); total serum IgE levels also increased. Alternaria-induced airway eosinophilia and enhanced expression of type-2 cytokines in lungs were significantly decreased in mice deficient in the IL-33 receptor ST2 (Figure S6), suggesting that IL-33 plays a key role in this model. Importantly, these allergic immune responses were nearly abolished (airway eosinophilia and IgE expression) or significantly inhibited (IL-5 and IL-13 expression, p<0.05 and p<0.01, respectively) in mice treated with CDDO-Me (Figure 6B). Histologic examination of lung tissues showed peribronchial infiltration of inflammatory cells and mucus hyperplasia in Alternaria-exposed control mice; these responses were significantly reduced in mice treated with CDDO-Me (Figure 6C,D, p<0.05).
Figure 6.
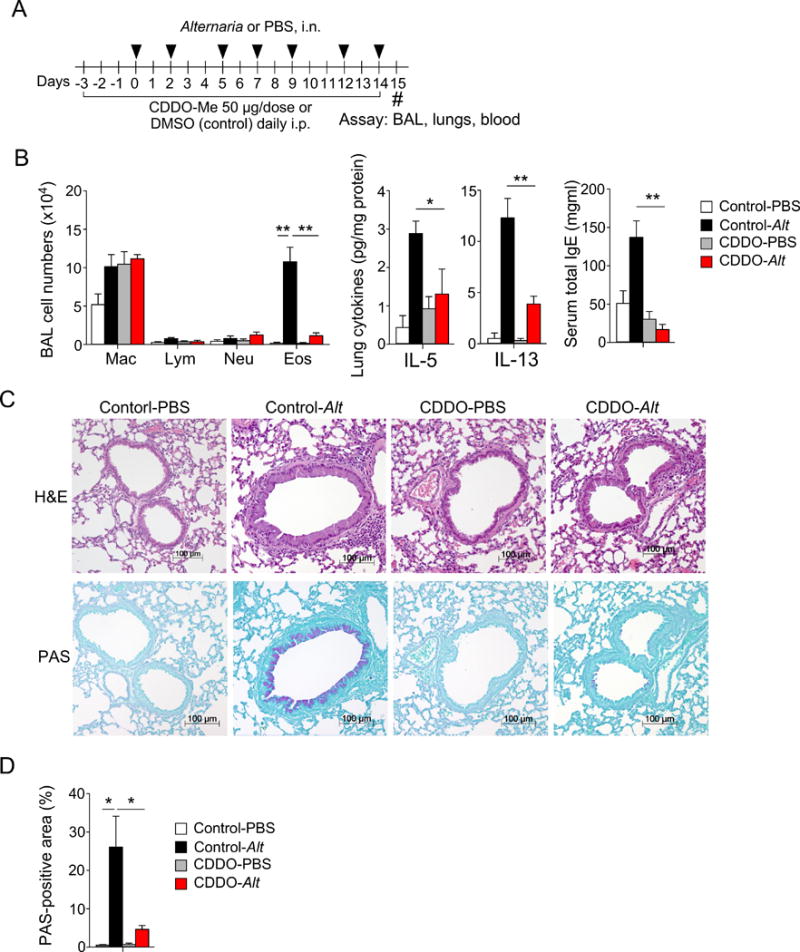
A Nrf2 activator inhibits airway inflammation and pathology in mice exposed repeatedly to Alternaria extract. A, Schematic diagram. Naïve BALB/c mice were pretreated daily with 50 μg CDDO-Me i.p. for 3 days and exposed i.n. to Alternaria extract (10 μg) 3 times/week for 2 weeks. Daily CDDO-Me treatment was continued throughout the experiment. Samples were collected 24 hours after the last Alternaria exposure. B, BAL fluids were analyzed by differential cell counts (left panel); cytokine levels in lung lysates (middle panel) and total IgE in sera (right panel) were analyzed by ELISA. Data are presented as mean±SEM from a pool of two experiments (n=7 or 10 mice in each group). *p<0.05 and **p<0.01 between groups indicated by horizontal lines. C, Lung specimens were analyzed by H&E and PAS staining. D, PAS-positive areas were quantitated by image analysis as described in the Materials and Methods. Data are presented as mean±SEM (n=3 mice in each group). *p<0.05 between groups indicated by horizontal lines.
DISCUSSION
The role of IL-33 in asthma and allergic diseases has been increasingly recognized (8–10). However, little information is available regarding the regulatory mechanisms of this cytokine. A novel concept derived from this study is that airway epithelial cells develop an oxidative stress response upon exposure to airborne allergens and that this response is critical for initiation of a series of biochemical events, such as ATP secretion and [Ca2+]i increases, that lead to extracellular release of processed IL-33. Inhibition of oxidative stress by exogenous ROS scavengers strongly suppressed these cellular events. Furthermore, pharmacologic activation of endogenous antioxidant molecules by Nrf2 activators reduced IL-33 release by airway epithelial cells and prevented development of asthma-like airway inflammation in mice. Altogether, these findings suggest that oxidative stress plays a pivotal role in regulating IL-33 release by airway epithelium.
ROS are well-conserved cellular response molecules, as are ATP and calcium. A complex network of these molecules likely takes place in response to cellular stresses (33). Furthermore, a recent study showed that allergen-induced ATP secretion activates the NADPH oxidase dual oxidase 1 (DUOX1), leading to redox-dependent activation of Src/EGFR, which mediates IL-33 processing and secretion (24). In this study, the intracellular levels of ROS increased roughly in parallel to ATP secretion (Figure 1, Figure S1), and the increase in ROS levels preceded the increase in [Ca2+]i (Figure 2). Furthermore, the inhibitory effects of ROS scavengers and DPI indicate that allergen-induced ROS initiates ATP secretion and the increase in [Ca2+]i (Figure 1). Thus, oxidative stress is likely involved both upstream and downstream of ATP release in allergen-exposed airway epithelial cells. The molecular mechanisms that explain how ROS initiate ATP release are an active area of research. For example, hBE cells express connexin 43 (Cx43), which serves as a hemichannel to mediate ATP release in the plasma membrane of various cell types (34). Exposure of hBE cells to oxidants, such as H2O2, induces PI3K signaling leading to the activation of AKT (35), which directly phosphorylates Cx43 on Ser-369 and Ser-373 residues, thus inducing Cx43 opening (36). Therefore, ROS induced by allergen exposure could activate AKT-dependent phosphorylation of Cx43 and trigger initial ATP secretion. Future studies are necessary to investigate this hypothesis.
Generally, the redox state of the healthy lung is reducing due to the abundance of antioxidant molecules available on the surface of, and within, airway epithelial cells (37). Potential roles for imbalance in this homeostasis in the pathophysiology of asthma have been recognized (12). In this regard, Nrf2 is likely the most important regulator of antioxidant molecules (14–16, 28). Significant effort has been taken recently to develop pharmacologic agents that selectively activate the Nrf2 pathway (32). Indeed, one of these agents, CDDO-Me, enhances expression of various endogenous antioxidant molecules in wild-type mice, but not in Nrf2−/− mice (38). In this study, treatment with CDDO-Me enhanced steady-state expression of glutamate-cysteine ligase (GCL), thyoredoxin reductase (TXNRD), NADPH quinone dehydrogenase (NQO1), and glutathione S-transferase pi 1 (GSTP1) in mouse lungs (Figure S4). GCL catalyzes GSH synthesis and TXNRD catalyzes reduction of cystine to cysteine, thus promoting GSH synthesis (28). NADPH is a reducing agent and is required for the regeneration of GSH and TXNRD. GSTP1 is a ROS-detoxifying enzyme. Therefore, these molecules likely work together to promote potent antioxidant responses in the lungs. Indeed, CDDO-Me treatment increased the steady-state BAL and lung levels of GSH by 98% and 40%, respectively (Figure S5B).
When used in an in vitro allergen exposure model, CDDO-Me at nanomolar concentrations prevented increases in intracellular ROS and extracellular IL-33 release (Figure 5). CDDO-Me also partially inhibited IL-33 release in vivo in mice (Figure S5B) and significantly inhibited airway inflammation when mice were exposed repeatedly to Alternaria extract (Figure 6). We acknowledge that potential off-target effects of CDDO-Me cannot be ruled out, as with any pharmacologic agent. Indeed, while we verified activation of a number of Nrf2-target genes (Figure S4), the efficacy of CDDO-Me may involve other molecules that may or may not be regulated by redox, such as peroxisome proliferation-activated receptor γ (PPARγ) and the nuclear factor-κB pathway (39). Nonetheless, epithelium-specific activation of Nrf2 through a conditional Keap1 knockout strategy demonstrated cytoprotective effects of Nrf2 in mouse airways (40). Furthermore, airway administration of exogenous GSH prevented IL-33 release and the ensuing innate type-2 immune response when mice were exposed to extracts of Alternaria (Figure 4). In contrast, GSH did not affect IL-33-induced production of type-2 cytokines, suggesting that it does not inhibit activation of group 2 innate lymphoid cells directly. Thus, activation of Nrf2 and production of antioxidant molecules, such as GSH, likely create a protective environment in allergen-exposed airway epithelium.
Finally, recent discoveries of epithelium-derived cytokines that mediate type-2 immune responses, such as IL-25, IL-33, and TSLP (5), opened a new window for the treatment of patients with asthma and allergic diseases. These molecules and their receptors can be targeted directly with biologic agents such as antibodies (4, 41). Alternatively, the pathways leading to production and secretion of these molecules can be altered by pharmacologic agents. In this regard, our findings, together with those of others (24), suggest that oxidative stress serves as a key checkpoint by which airway epithelium initiates IL-33 release in response to allergen exposure. Furthermore, GSH levels in lung epithelial lining fluid decreases quickly after allergen exposure in humans (42), and a lack of Nrf2 favors Th2-type immune responses in airways (43). Therefore, activation of Nrf2 and its target antioxidant molecules may provide an excellent opportunity for regulating airway redox states and disease processes. Thus, the potential utility of Nrf2 activators for treatment of patients with asthma and related disorders must be considered as Nrf2 activators have already been examined in other medical fields (16–18, 28, 30). Furthermore, identification of biomarkers to evaluate airway oxidative stress in patients with asthma will be necessary to identify appropriate patient populations and to evaluate treatment outcomes.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HL117823) and the Mayo Foundation. MU is a recipient of 2012 Banyu Life Science Foundation International Fellowship. We thank Dr. Andrew N. McKenzie for providing the Il1rl1−/− mice, and LuRaye S. Eischens for secretarial help. This work was supported by grants from the National Institutes of Health (R01 AI71106, R01 HL117823) and the Mayo Foundation.
Footnotes
Additional Supporting Information is found in the online version of this article:
Appendix S1. Materials and Methods
Figure S1.. Kinetic analysis of oxidative stress.
Figure S2.. GSH does not affect Alternaria-induced RANTES production by human bronchial epithelial (hBE) cells.
Figure S3.. Treatment with the Nrf2 activator resveratrol inhibits oxidative stress, ATP secretion, and increased [Ca2+]i in hBE cells exposed to Alternaria extract.
Figure S4.. A Nrf2 activator increases expression of antioxidant molecules in lungs.
Figure S5.. A Nrf2 activator inhibits IL-33 release induced by acute Alternaria exposure.
Figure S6. Alternaria-induced airway inflammation is dependent on the IL-33/ST2 pathway.
AUTHOR CONTRIBUTIONS
M.U., E.LA, N.P., H.K. and S.M.O. designed the studies and experiments. M.U., E.L.A, D.L.S., N.P., P.J.M., and K.I. performed the experiments. M.U., E.L.A, N.P., H.K., and S.M.O. interpreted the data. M.U., E.L.A, H.K., and S.M.O. wrote the manuscript. All the authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest in relation to this manuscript.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011;128:495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 3.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126:2394–2403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ. 2014;349:g5517. doi: 10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 5.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PloS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, et al. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun. 2014;55:33–41. doi: 10.1016/j.jaut.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd CM, Saglani S. Epithelial cytokines and pulmonary allergic inflammation. Curr Opin Immunol. 2015;34:52–58. doi: 10.1016/j.coi.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 11.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Sign. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Macias H, Romieu I. Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J Allergy Clin Immunol. 2014;133:1237–1244. doi: 10.1016/j.jaci.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 15.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Sawaf O, Clarner T, Fragoulis A, Kan YW, Pufe T, Streetz K, et al. Nrf2 in health and disease: current and future clinical implications. Clin Sci. 2015;129:989–999. doi: 10.1042/CS20150436. [DOI] [PubMed] [Google Scholar]
- 17.Howden R. Nrf2 and cardiovascular defense. Oxid Med Cell Long. 2013;2013:104308. doi: 10.1155/2013/104308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan SM, de Haan JB. Combating oxidative stress in diabetic complications with Nrf2 activators: how much is too much? Commun Free Rad Res. 2014;19:107–117. doi: 10.1179/1351000214Y.0000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 22.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hristova M, Habibovic A, Veith C, Janssen-Heininger YM, Dixon AE, Geiszt M, et al. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J Allergy Clin Immunol. 2016;137:1545–1556 e1511. doi: 10.1016/j.jaci.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Grady SM, Patil N, Melkamu T, Maniak PJ, Lancto C, Kita H. ATP release and Ca2+ signalling by human bronchial epithelial cells following Alternaria aeroallergen exposure. J Physiol (Lond) 2013;591:4595–4609. doi: 10.1113/jphysiol.2013.254649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 27.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Disc. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 29.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 30.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Pharmacology and Toxicology. 2005 Jul; J:\!GUIDANC\5541fnlcln1.doc 07/06/05. [Google Scholar]
- 32.Wang YY, Yang YX, Zhe H, He ZX, Zhou SF. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug Design Develop Ther. 2014;8:2075–2088. doi: 10.2147/DDDT.S68872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Yang J, Zhou X, Xiao Q, Lu Y, Xia L. High glucose induces dysfunction of airway epithelial barrier through down-regulation of connexin 43. Exp Cell Res. 2016;342:11–19. doi: 10.1016/j.yexcr.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Krick S, Wang J, St-Pierre M, Gonzalez C, Dahl G, Salathe M. Dual Oxidase 2 (Duox2) Regulates Pannexin 1-mediated ATP Release in Primary Human Airway Epithelial Cells via Changes in Intracellular pH and Not H2O2 Production. J Biol Chem. 2016;291:6423–6432. doi: 10.1074/jbc.M115.664854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batra N, Riquelme MA, Burra S, Kar R, Gu S, Jiang JX. Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J Biol Chem. 2014;289:10582–10591. doi: 10.1074/jbc.M114.550608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax. 2005;60:693–700. doi: 10.1136/thx.2004.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh J, Jenkins RE, Wong M, Olayanju A, Powell H, Copple I, et al. Identification and quantification of the basal and inducible Nrf2-dependent proteomes in mouse liver: biochemical, pharmacological and toxicological implications. J Proteomics. 2014;108:171–187. doi: 10.1016/j.jprot.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64:972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sussan TE, Gajghate S, Chatterjee S, Mandke P, McCormick S, Sudini K, et al. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am J Physiol Lung Cell Mol Physiol. 2015;309:L27–36. doi: 10.1152/ajplung.00398.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyman O, Kaegi C, Akdis M, Bavbek S, Bossios A, Chatzipetrou A, et al. EAACI IG Biologicals task force paper on the use of biologic agents in allergic disorders. Allergy. 2015;70:727–754. doi: 10.1111/all.12616. [DOI] [PubMed] [Google Scholar]
- 42.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 43.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


