Summary
Lenalidomide (LEN) acts directly on multiple myeloma (MM) cells by inducing cereblon-mediated degradation of interferon regulatory factor 4, Ikaros (IKZF)1 and IKZF3, transcription factors that are essential for MM cell survival. The mucin 1 (MUC1) C-terminal transmembrane subunit (MUC1-C) oncoprotein is aberrantly expressed by MM cells and protects against reactive oxygen species (ROS)-mediated MM cell death. The present studies demonstrate that targeting MUC1-C with GO-203, a cell-penetrating peptide inhibitor of MUC1-C homodimerization, is more than additive with LEN in downregulating the WNT/β-catenin pathway, suppressing MYC, and inducing late apoptosis/necrosis. We show that the GO-203/LEN combination acts by synergistically increasing ROS and, in turn, suppressing β-catenin. LEN resistance has been linked to activation of the WNT/β-catenin→CD44 pathway. In this regard, our results further demonstrate that targeting MUC1-C is effective against LEN-resistant MM cells. Moreover, GO-203 resensitized LEN-resistant MM cells to LEN treatment in association with suppression of β-catenin and CD44. Targeting MUC1-C also resulted in downregulation of CD44 on the surface of primary MM cells. These findings, and the demonstration that expression of MUC1 and CD44 significantly correlate in microarrays from primary MM cells, provide support for combining GO-203 with LEN in the treatment of MM and in LEN-resistance.
Keywords: multiple myeloma, lenalidomide, MUC1, MYC, CD44
Introduction
Lenalidomide (LEN) is a highly effective immunomodulatory drug (IMiD) for the treatment of patients with newly diagnosed and relapsed multiple myeloma (MM) (Moreau, et al 2015, Nooka, et al 2015). However, refractoriness to LEN therapy has emerged as a significant clinical problem, prompting studies on the associated mechanisms of resistance. LEN acts directly on the MM cell, modulates the tumour microenvironment and activates the host immune response (Semeraro, et al 2013, Weisel and Kanz 2014). In regard to direct effects, LEN has been shown to inhibit MM cell proliferation by upregulating cyclin-dependent kinase (CDK) inhibitors, including p21 (also termed CDKN1A) (Escoubet-Lozach, et al 2009, Hideshima, et al 2000, Verhelle, et al 2007). LEN also inhibits nuclear factor-κB-induced pro-survival signals and promotes MM cell apoptosis (Chauhan, et al 2010, Mitsiades, et al 2002, Tai, et al 2005). Anti-MM cell activities of LEN are dependent on the expression of cereblon (CRBN), a component of the E3 ubiquitin ligase complex that also includes DDB1 and CUL4 (Lopez-Girona, et al 2012, Zhu, et al 2011). Targeting CRBN activity with LEN results in upregulation of p21 and cell cycle arrest (Lopez-Girona, et al 2012, Zhu, et al 2011). Other studies have demonstrated that sensitivity of MM cells to LEN is suppressed by activation of the WNT/β-catenin pathway (Bjorklund, et al 2011). In addition, LEN resistance has been associated with expression of the CD44 surface receptor, a target of WNT/β-catenin-mediated transcription (Bjorklund, et al 2014), which integrates the tumour microenvironment with properties of cell stemness (Yan, et al 2015).
Mucin 1 (MUC1) is a heterodimeric glycoprotein that is aberrantly expressed by MM cells and promotes their growth and survival (Baldus, et al 2007, Cloosen, et al 2006, Kawano, et al 2008). The MUC1 C-terminal transmembrane subunit (MUC1-C) functions as an oncoprotein by interacting with diverse signalling pathways that are associated with transformation (Kufe 2009a, Li, et al 2003). In this way, MUC1-C includes a 72 amino acid cytoplasmic domain that is intrinsically disordered and thereby has the capacity to serve as a substrate for multiple kinases and as a binding partner for diverse effectors of gene transcription (Kufe 2009a, Raina, et al 2015). For instance, the MUC1-C cytoplasmic domain is phosphorylated by GSK3β and binds directly to β-catenin (Yamamoto, et al 1997), linking MUC1-C to the WNT pathway. Binding of the MUC1-C cytoplasmic domain to β-catenin is also regulated by receptor tyrosine kinases (RTKs), SRC and protein kinase C (Li, et al 2001a, Li, et al 2001b, Ren, et al 2002). The functional significance of the MUC1-C/β-catenin interaction is supported by the demonstration that MUC1-C stabilizes β-catenin by blocking GSK3β-mediated phosphorylation of β-catenin and thereby its proteosomal degradation (Huang, et al 2005). Moreover, MUC1-C localizes with β-catenin on the promoters of WNT target genes, such as CCND1 and MYC, by forming complexes with TCF4/TCF7L2 and, in turn, promoting gene transcription (Bouillez, et al 2016, Rajabi, et al 2012, Tagde, et al 2016a). The MUC1-C cytoplasmic domain contains a CQC motif that is necessary and sufficient for MUC1-C (i) homodimerization (Leng, et al 2007, Raina, et al 2015, Raina, et al 2012), (ii) nuclear localization and (iii) its oncogenic function (Kufe 2009b, Leng, et al 2007, Raina, et al 2015, Raina, et al 2012). Interestingly, expression of MUC1-C with a CQC→AQA mutation in cancer cells is associated with decreases in anchorage-independent growth and tumourigenicity, consistent with a dominant-negative effect for transformation (Kufe 2009b, Leng, et al 2007). Based on these findings, we developed cell-penetrating peptides that target the MUC1-C CQC motif and block MUC1-C function (Kufe 2009b, Kufe 2013, Raina, et al 2015, Raina, et al 2012). In this way, we found that treatment of MM cells with the MUC1-C inhibitor GO-203 is associated with loss of survival in vitro and in tumour xenograft models (Yin, et al 2010, Yin, et al 2012).
The present studies demonstrate that targeting MUC1-C in combination with LEN is associated with downregulation of the WNT/β-catenin pathway. The results also show that the GO-203/LEN combination is effective in (i) suppressing expression of the WNT target genes, MYC and CD44, and (ii) inducing cell death. Moreover, we report that targeting MUC1-C attenuates LEN resistance in MM cells.
Materials and Methods
Cell culture
Human MM.1S, RPMI8226, H929 and U266 MM cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI2640 medium (Cellgro, Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (GEMINI, West Sacramento, CA, USA), 100 u/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine. Cells were treated with the MUC1-C inhibitor GO-203 ([R]9-CQCRRKN), the inactive control peptide CP-2 ([R]9-AQARRKN) (AnaSpec, Fremont, CA, USA), LEN (SelleckChem, Houston, TX, USA) or glutathione (GSH) (Sigma-Aldrich, St. Louis, MO, USA). Cells were selected for LEN resistance by exposure to increasing drug concentrations. To establish LEN-resistant cell lines, drug-naïve MM.1S and H929 cells were treated with 1 μM LEN for 5 days, at which time the cells were incubated with fresh 1 μM LEN-supplemented medium for an additional 5 days. Using this approach, exposure to LEN was progressively increased by 1 μM approximately every 10 days until reaching a concentration of 10 μM LEN.
Immunoblot analysis
Cell lysates were prepared as described (Hasegawa, et al 2016, Yin, et al 2010). Soluble proteins were analysed by immunoblotting with anti-MUC1-C (NeoMarkers, Fremont, CA, USA) anti-β-catenin (Calbiochem, San Diego, CA, USA), anti-MYC (Abcam, Cambridge, MA, USA), anti-TCF4, anti-PKCδ, anti-MCL-1 (Santa Cruz, Dallas, TX, USA), anti-β-actin (Sigma-Aldrich, St. Louis, MO, USA), anti-TIGAR, anti-CRBN (Abcam, Cambridge, MA, USA), anti-CD44, anti-poly ADP ribose polymerase (PARP), and anti-BCL-XL (BCL2L1) (Cell Signaling, Danvers, MA, USA). Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescense (GE Healthcare, Piscataway, NJ, USA).
Cell viability and apoptosis assays
Cell viability was assessed by staining with Alamar blue (Invitrogen, Carlsbad, CA, USA). For assessment of cell death, cells were incubated with propidium iodide (PI)/annexin V-fluoresecin isothiocyanate (BD Biosciences, San Jose, CA, USA) for 15 min at room temperature and then analysed by flow cytometry.
Measurement of reactive oxygen species (ROS)levels
Cells were incubated with 5 μM carboxy-2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes, Eugene, OR, USA) for 30 min to assess H2O2-mediated oxidation to 2′7′-dichlorodihydrofluorescein (DCF) as measured at an excitation wavelength of 480 nm and an emission wavelength of 590 nm (Yin, et al 2012).
Determination of NADPH and GSH levels
Intracellular NADPH concentrations were measured using the Enzychrom NADP/NADPH Assay Kit (BioAssay Systems, Hayward, CA, USA). Intracellular GSH concentrations were assayed using the Bioxytech GSH-400 kit (OXIS International, Burlingame, CA, USA).
Determination of GO-203/LEN interactions
The combined effects of GO-203 and LEN were determined by isobologram analysis using the CalcuSyn software program (Version 2.0) (Biosoft, Cambridge, UK). Using this approach, a combination index (CI) of <1.0 reflects synergism and a CI >1.0 indicates antagonism (Tagde, et al 2016b, Tagde, et al 2014).
Purification of primary MM cells
Bone marrow aspirates were obtained from MM patients under approval of the Institutional Review Board of the Dana-Farber Cancer Institute. Mononuclear cells were isolated by density gradient centrifugation through Ficoll-Paque (GE Healthcare) and plasma cells were purified (>95% CD138+) by positive selection with anti-CD138 magnetic activated cell separation microbeads (Miltenyi Biotec, Auburn, CA, USA) (Mimura, et al 2014).
Analysis of MUC1 and CD44 cell surface expression
For analysis of MUC1 expression, cells were incubated with anti-MUC1-N antibody (Kufe, et al 1984) for 1 h on ice, washed and incubated with phycoerythrin (PE)-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA), washed and then analysed by flow cytometry. For analysis of CD44 expression, cells were incubated with anti-CD44-PE-cyanin 7 (eBioscience, San Diego, CA, USA) for 1 h on ice, washed and then analysed by flow cytometry.
Data analysis
Statistical significance was determined using the Student’s t-test (GraphPad Software, La Jolla, CA, USA), where * denotes p<0.01; ** denotes p<0.05 and # denotes not significant.
Bioinformatics analysis
Microarray data obtained from MM patient samples were downloaded from the Gene Expression Omnibus (GEO, Bethesda, MD, USA) under accession number GSE2658 (n=559) (Hanamura, et al 2006, Zhan, et al 2007). Raw signal intensities were RMA normalized across patients. Expression of MUC1 and CD44 was assessed using the Spearman correlation coefficient (Li, et al 2011, Tagde, et al 2016a, Tagde, et al 2016b, Takahashi, et al 2015).
Results
Targeting MUC1-C is effective in combination with LEN
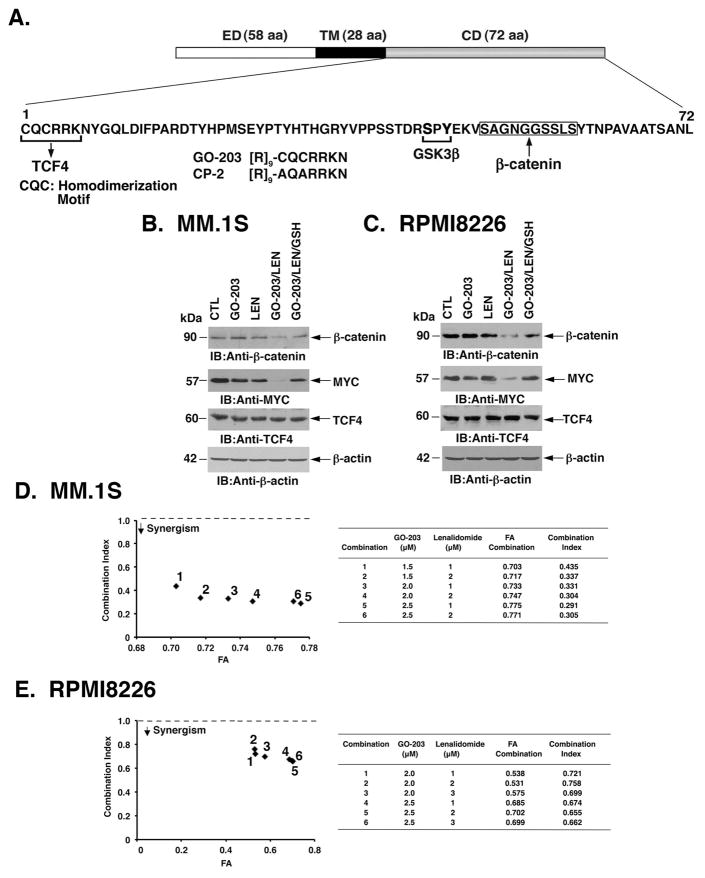
The WNT/β-catenin pathway is of importance to LEN sensitivity of MM cells (Bjorklund, et al 2011). In this context, MUC1-C has been shown to stabilize β-catenin (Huang, et al 2005, Yamamoto, et al 1997), invoking the possibility that targeting MUC1-C could be effective in combination with LEN. Accordingly, we exposed MM cells to the MUC1-C inhibitor GO-203, a cell-penetrating peptide that blocks MUC1-C homodimerization and function (Raina, et al 2015, Raina, et al 2012, Raina, et al 2011, Rajabi, et al 2016) (Fig. 1A). Treatment of MM.1S cells with 2.5 μM GO-203 or 2 μM LEN had no apparent effect on β-catenin levels (Fig. 1B). Significantly, however, treatment with GO-203 and LEN resulted in downregulation of β-catenin (Fig. 1B). The WNT/β-catenin pathway has been linked to MYC expression in MM cells (Sukhdeo, et al 2007). Moreover, we have shown that MUC1-C drives MYC activation in MM cells by a β-catenin-mediated mechanism (Tagde, et al 2016a). In those studies, treatment of MM cells with 5 μM GO-203 markedly downregulated β-catenin and MYC (Tagde, et al 2016a). Accordingly, we used a lower concentration of 2.5 μM GO-203 in the present work to assess the effects of combining GO-203 with LEN. In this way, we found that the GO-203/LEN combination markedly suppresses MYC, and as a control, not the TCF4 transcription factor (Fig. 1B). As such, we focused our studies of combining GO-203 with LEN on the WNT/β-catenin pathway. Treatment of MM cells with GO-203 results in the disruption of redox balance (Yin, et al 2010, Yin, et al 2012). Addition of exogenous GSH or the GSH precursor N-acetylcysteine (NAC) has been used to re-establish GO-203-induced decreases GSH levels and block ROS-induced MM cell death (Yin, et al 2012, Yin, et al 2014). We therefore added GSH to the GO-203/LEN-treated MM.1S cells and found reversal of β-catenin and MYC downregulation (Fig. 1B). Similar results were obtained when RPMI8226 cells were treated with GO-203 and LEN; that is (i) more than additive effects in suppressing β-catenin and MYC, and (ii) attenuation of this response with GSH (Fig. 1C). Based on these findings and the dependence of MM cells on MYC for survival (Delmore, et al 2011, Holien, et al 2012, Mertz, et al 2011), we examined whether the combination of GO-203 and LEN at different concentrations of these agents induces synergistic anti-MM activity. MM.1S cells were treated with GO-203 at 1.5, 2 and 2.5 μM and with LEN at 1 and 2 μM based on the effects of each agent alone (Fig. 1D, left). Isobologram analysis of cell viability demonstrated that the combination of these agents is more than additive, with CI values <1 (Fig. 1D, right). Analysis of RPMI8226 cells treated with GO-203 and LEN confirmed the more than additive anti-MM activity of these agents (Fig. 1E). GO-203 and LEN also induced synergistic killing of U266 MM cells (Fig. S1A), supporting the notion that these agents are effective when used in combination. Of note, a narrow range of GO-203 concentrations was used in the combination studies based on the steep dose-response curves for this agent. The fraction affected (FA) values are reflective of using this approach and the CI values obtained are consistently more than additive for MM.1S, RPMI8226, U266 and H929 (Figs. 1D and 1E; Figs. S1A and S1B) cells.
Figure 1. Combining GO-203 and LEN suppresses β-catenin, MYC and survival.
A. Schema of the MUC1-C subunit with amino acid sequence of the intrinsically disordered cytoplasmic domain. Highlighted are the sites that interact with GSK3β and β-catenin, linking MUC1-C to the WNT signalling pathway. The MUC1-C CQC motif is necessary for MUC1-C homodimerization, nuclear import and oncogenic function. The MUC1-C CQC motif is the target of the cell-penetrating GO-203 peptide. CP-2 is a control peptide with a CQC→AQA alteration that does not interact with the cytoplasmic domain. CD, cytoplasmic domain; ED, extracellular domain; TM transmembrane domain. B and C. MM.1S (B) and RPMI8226 (C) cells left untreated (control; CTL) and treated with (i) 2.5 μM GO-203 alone each day for 72 h, (ii) 2 μM lenalidomide (LEN) alone for 72 h, or (iii) GO-203 combined with LEN for 72 h. GO-203/LEN-treated cells were also incubated in the presence of 5 mM GSH for 72 h. Lysates were immunoblotted with the indicated antibodies. D and E. MM.1S (D) and RPMI8226 (E) cells were treated with (i) the indicated concentrations of GO-203 alone each day for 72 h, (ii) the indicated concentrations of LEN alone for 72 h, and (iii) GO-203 combined with LEN for 72 h. Mean cell survival was assessed in triplicate by Alamar blue assays. Numbers 1–6 in the graphs (left) represent combinations listed in the tables (right). FA, fraction affected. The results are representative of 3 independent experiments.
Combining GO-203 and LEN disrupts redox balance
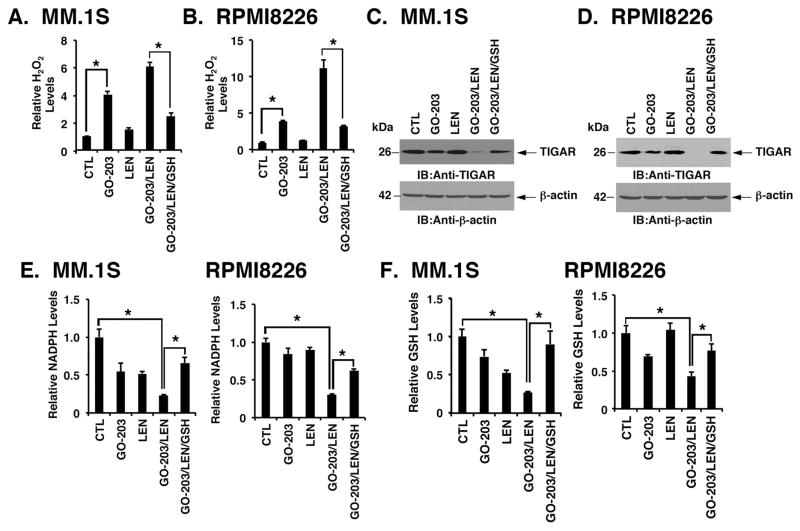
The observation that GSH reverses the combined effects of GO-203/LEN on suppression of β-catenin and MYC prompted studies of these agents on ROS levels. Notably, the GO-203/LEN combination was associated with greater increases in H2O2 than that obtained with either agent alone in MM.1S (Fig. 2A) and RPMI8226 (Fig. 2B) cells. Targeting MUC1-C in MM cells increases ROS, partly by decreasing expression of the fructose-2,6-bisphosphatase p53-inducible regulator of glycolysis and apoptosis (TIGAR) and thereby shunting of the glycolytic intermediate glucose-6-phosphate (G6P) into the pentose phosphate pathway (PPP) (Yin, et al 2010, Yin, et al 2012). Interestingly, the GO-203/LEN combination was more effective in downregulating TIGAR than either agent alone (Figs. 2C and 2D). These results invoked the possibility that the combination could affect shunting into the PPP and, in turn, the production of NADPH and GSH (Patra and Hay 2014). Indeed, GO-203/LEN treatment of MM.1S and RPMI8226 cells was associated with suppression of NADPH (Fig. 2E) and GSH (Fig. 2F) levels that were greater than that found with either agent alone. Moreover, these MM cells responded to the GO-203/LEN combination with decreases in TIGAR (Figs. 2C and 2D), NADPH (Figs. 2E) and GSH (Figs. 2F) that were reversed in part with GSH. Similar results were obtained with U266 cells (Figs. S2A–S2D), indicating that the interaction between GO-203 and LEN is mediated by a ROS-mediated mechanism.
Figure 2. Combining GO-203 with LEN induces oxidative stress.
MM.1S and RPMI8226 cells left untreated (CTL) and treated with (i) 2.5 μM GO-203 alone each day for 72 h, (ii) 2 μM lenalidomide (LEN) alone for 72 h, or (iii) GO-203 combined with LEN for 72 h. GO-203/LEN-treated cells were also incubated in the presence of 5 mM GSH for 72 h. A and B. The indicated cells were analysed for relative hydrogen peroxide levels (mean±SD of 3 determinations) as compared with that obtained with control cells (assigned a value of 1). C and D. Lysates were immunoblotted (IB)with the indicated antibodies. E and F. The indicated cells were analysed for relative NADPH (E) and glutathione (GSH) (F) levels (mean±SD of 3 determinations) as compared with that obtained with control cells (assigned a value of 1). These results are representative of 3 independent experiments. * p<0.01.
GO-203/LEN combination induces ROS-mediated cell death
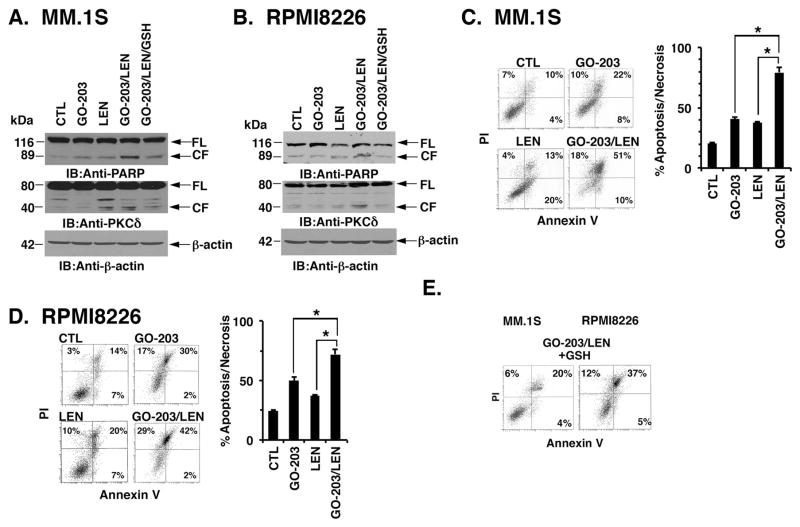
In concert with the effects of GO-203/LEN on disruption of redox balance, we found that the GO-203/LEN combination is effective in inducing cleavage of the caspase-3 substrates, PARP and PKCδ, and that these responses are attenuated by GSH (Figs. 3A and B). To extend this analysis, we assessed the effects of GO-203 and LEN on cell death as determined by PI/annexin V staining. Using this approach, the percentage of apoptotic/necrotic MM.1S cells was increased by the combination (79%) as compared to that obtained with GO-203 (40%) or LEN (37%) alone (Fig. 3C). Treatment of RPMI8226 cells with the GO-203/LEN combination was also associated with increased apoptotic/necrotic death (Fig. 3D). Additionally, GO-203/LEN-induced death of both MM.1S and RPMI8226 (Fig. 3E) cells was attenuated by GSH, consistent with a ROS-mediated mechanism.
Figure 3. The GO-203/LEN combination induces cell death by a ROS-dependent mechanism.
MM.1S and RPMI8226 cells were left untreated (CTL) and treated with (i) 2.5 μM GO-203 alone each day for 72 h, (ii) 2 μM lenalidomide (LEN) alone for 72 h, and (iii) GO-203 combined with LEN for 72 h. GO-203/LEN-treated cells were also incubated in the presence of 5 mM GSH for 72 h. A and B. Lysates were immunoblotted (IB) with the indicated antibodies. FL, full length. CF, cleaved fragment. C and D. The indicated cells were incubated with propidium iodide (PI)/annexin V and analysed by flow cytometry (left). The percentage of PI and/or annexin V positive cells is included in the panels (left). The results are expressed as the percentage (mean±SD of 3 determinations) of apoptotic/necrotic cells (right). E. The indicated cells were incubated with PI/annexin V and analysed by flow cytometry. The percentage of PI and/or annexin V positive cells is included in the panels. The results are representative of 3 independent experiments. * p<0.01.
Effects of targeting MUC1-C in LEN-resistant (LR) cells
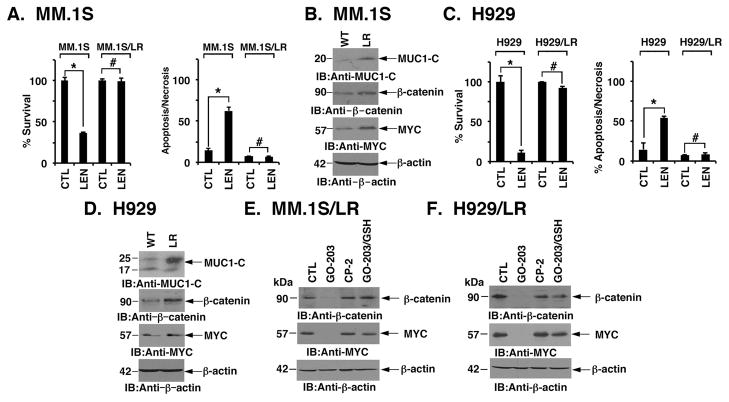
To assess the effects of targeting MUC1-C in a setting of LEN resistance, we grew MM.1S cells in the presence of increasing concentrations of LEN. In this way, we generated MM.1S/LR cells selected for survival in 10 μM LEN (Fig. 4A). Notably, LEN resistance was associated with upregulation of MUC1-C, β-catenin and MYC (Fig. 4B). Selection of H929 cells for LEN resistance (Fig. 4C) was also associated with selective increases in MUC1-C, β-catenin and MYC expression (Fig. 4D). In contrast to the consistent activation of the MUC1-C→β-catenin→MYC pathway, LEN resistance had varying effects on levels of the BCL2 family proteins, BCL-XL and MCL1 (Figs. S3A and S3B). In addition and in comparison to the drug naïve cells, LEN-induced increases in ROS were abrogated in LEN resistant cells (S Figs. S3C and S3D). As found for drug-naïve MM.1S cells, treatment of MM.1S/LR cells with GO-203 resulted in suppression of β-catenin and MYC (Fig. 4E). By contrast, treatment with the control inactive CP-2 peptide (Fig. 1A) had no apparent effect (Fig. 4E). Similar results were obtained with H929/LR cells (Fig. 4F), indicating that LEN resistance is associated with increases in WNT/β-catenin signalling, which are sensitive to targeting MUC1-C with GO-203.
Figure 4. LEN-resistant MM cells respond to GO-203 with downregulation of β-catenin and MYC.
A. Drug-naïve MM.1S and lenalidomide (LEN)-resistant MM.1S/LR cells were left untreated (CTL) and treated with 10 μM LEN for 96 h. Percentage survival (mean±SD of 3 determinations) was assessed by Alamar blue assays (left). Cells were also incubated with PI/annexin V and analysed by flow cytometry (right). The results are expressed as the percentage (mean±SD of 3 determinations) of apoptotic/necrotic cells. B. Lysates from wild-type (WT) and LEN-resistant (LR) MM.1S cells were immunoblotted (IB) with indicated antibodies. C. Drug-naïve H929 and LEN-resistant H929/LR cells were left untreated (CTL) and treated with 10 μM LEN for 96 h. Percentage survival (mean±SD of 3 determinations) was assessed by Alamar blue assays (left). Cells were incubated with PI/annexin V and analysed by flow cytometry (right). The results are expressed as the percentage (mean±SD of 3 determinations) of apoptotic/necrotic cells. D. Lysates from wild-type (WT) and LEN-resistant (LR) H929 cells were immunoblotted with indicated antibodies. E and F. MM.1S/LR (E) and H929/LR (F) cells were left untreated (CTL) or treated with 4 μM GO-203 or CP-2 each day for 72 h. GO-203-treated cells were also incubated in the presence of 5 mM GSH for 72h. Lysates were immunoblotted with the indicated antibodies. These results are representative of 3 independent experiments. * p<0.01; # not significant.
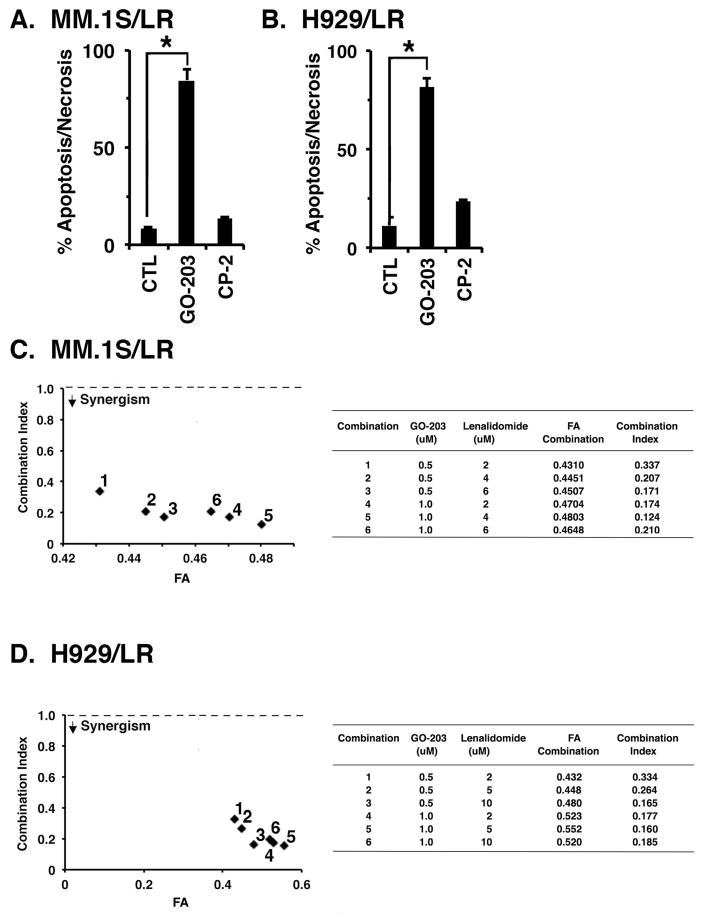
Targeting MUC1-C attenuates LEN resistance
MM cells are dependent on MYC for survival (Delmore, et al 2011, Holien, et al 2012, Mertz, et al 2011, Tagde, et al 2016a). In this context, the demonstration that targeting MUC1-C downregulates MYC in LEN-resistant cells invoked the potential for sensitivity to GO-203-induced killing. Indeed, MM.1S/LR cells were sensitive to treatment with GO-203, as determined by induction of apoptosis/necrosis (Fig. 5A). As a control, treatment with the inactive peptide CP-2 (Fig. 1A) had little, if any, effect (Fig. 5A). H929/LR cells were also sensitive to GO-203-, but not CP-2-induced apoptosis/necrosis (Fig. 5B), further supporting a lack of cross-resistance between GO-203 and LEN. To extend this line of investigation, we examined whether the GO-203/LEN combination is synergistic in the presence of LEN resistance. Isobologram analysis of MM.1S/LR cell viability at different concentrations of these agents demonstrated that the combination of GO-203 and LEN is more than additive, with a CI value <1 (Fig. 5C). Similar results were obtained when H929/LR cells were treated with the GO-203/LEN combination (Fig. 5D), indicating that GO-203 reverses LEN resistance.
Figure 5. Targeting MUC1-C reverses LEN resistance.
A. MM.1S/LR cells were left untreated (CTL) and treated with 4 μM GO-203 or CP-2 each day for 72 h. B. H929/LR cells were left untreated (CTL) and treated with 4 μM GO-203 or CP-2 each day for 72 h. Cells were incubated with propidium iodide/annexin V and analysed by flow cytometry. The results are expressed as the percentage (mean±SD of 3 determinations) of apoptotic/necrotic cells. C and D. MM.1S/LR (C) and H929/LR (D) cells were treated with (i) the indicated concentrations of GO-203 alone each day for 72 h, (ii) the indicated concentrations of LEN alone for 72 h, and (iii) GO-203 combined with LEN for 72 h. Mean cell survival was assessed in triplicate by Alamar blue assays. Numbers 1–6 in the graphs (left) represent combinations listed in the tables (right). FA, fraction affected. The results are representative of 3 independent experiments. * p<0.01.
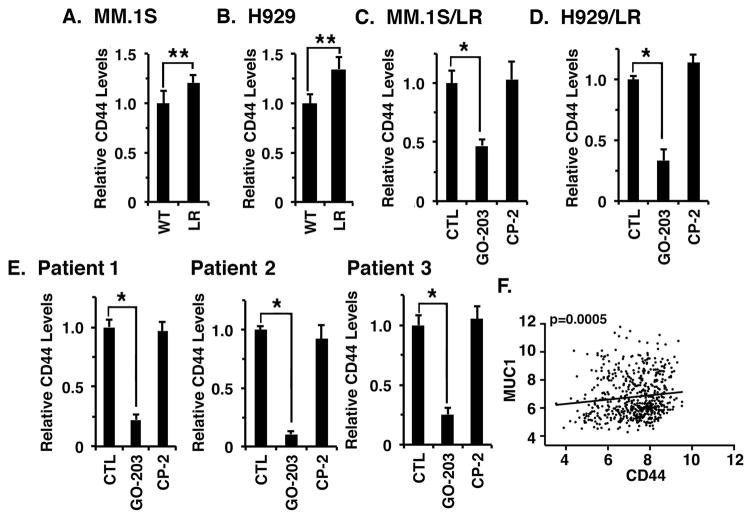
Effects of targeting MUC1-C on CD44 expression
Resistance of MM cells to LEN has been linked to involvement of WNT/β-catenin and CD44 (Bjorklund, et al 2014, Bjorklund, et al 2011). In this context, analysis of MM.1S (Fig. 6A) and H929 (Fig. 6B) cells for CD44 expression demonstrated significant increases in association with LEN resistance. CD44 expression is regulated by diverse signals, including the WNT/β-catenin pathway (Smith, et al 2014, Wong and Pignatelli 2002, Yan, et al 2015). Accordingly, we questioned if targeting MUC1-C affects CD44 levels in MM cells. Indeed, treatment of MM.1S/LR cells with GO-203, but not CP-2, was associated with marked suppression of CD44 (Fig. 6C). H929/LR cells also responded to GO-203 with downregulation of CD44 expression (Fig. 6D). To determine whether the results obtained with MM cell lines extend to primary MM cells, bone marrow samples from patients with active LEN-resistant MM were separated into CD138+ populations. Treatment of MUC1-positive CD138+ cells from LEN-sensitive Patient 1 with GO-203 was associated with suppression of CD44 expression as determined by flow cytometry (Fig. 6E, left; Fig. S4). Similar results were obtained with MUC1-expressing MM cells obtained from LEN-resistant Patient 2 (Fig. 6E, middle; Fig. S4) and LEN-resistant Patient 3 (Fig. 6E, right; Fig. S4), confirming that targeting MUC1-C is associated with downregulation of CD44 expression. To further investigate the relationship between MUC1 and CD44 in MM, we analysed the Gene Expression Omnibus (GEO) dataset GSE2658 obtained from primary MM cells (n=559). Notably, we found that MUC1 levels significantly correlated with CD44 expression (Fig. 6F), further supporting the notion that, as found for MYC (Tagde, et al 2016a), MUC1-C also contributes to CD44 expression.
Figure 6. Targeting MUC1-C downregulates CD44 expression.
A and B. The indicated wild-type (WT) and lenalidomide (LEN) resistant (LR) MM.1S (A) and H929 (B) cells were analysed for CD44 expression by flow cytometry. The results are expressed as relative CD44 levels (mean±SD of 3 determinations) as compared to that obtained for WT cells (assigned a value of 1). C and D. MM.1S/LR (C) and H929/LR (D) cells were left untreated (CTL) and treated with 4 μM GO-203 or CP-2 for 72 h. The results are expressed as relative CD44 levels (mean±SD of 3 determinations) as compared to that obtained for CTL cells (assigned a value of 1). The results are representative of 3 independent experiments. E. Primary CD138+ MM cells from Patient 1 (LEN-sensitive), Patient 2 (LEN-resistant) and Patient 3 (LEN-resistant) were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 for 72 h. The results are expressed as relative CD44 levels (mean±SD of 3 determinations) as compared to that obtained for CTL cells (assigned a value of 1). F. Microarray gene expression data from GEO dataset GSE2658 (n=559) was RMA normalized and the correlation between MUC1 and CD44 expression in MM patients was assessed by Spearman correlation, where p<0.05 was considered as statistically significant. * p<0.01; ** p<0.05.
Discussion
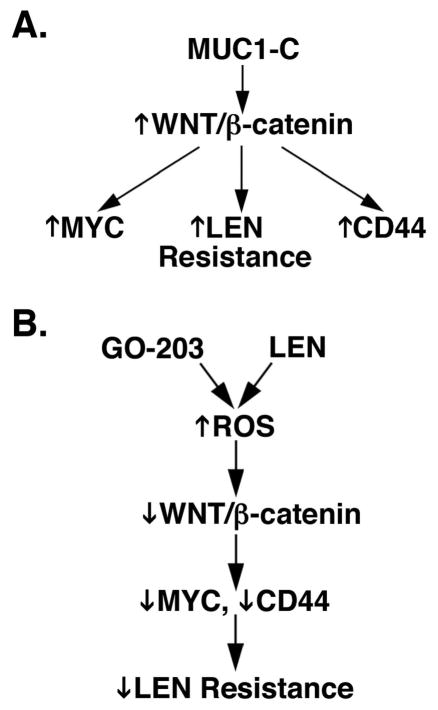
LEN is an effective agent in the treatment of MM; however, most patients invariably develop resistance to this agent (Moreau, et al 2015, Nooka, et al 2015). LEN resistance has been linked, at least in part, to activation of the WNT/β-catenin pathway (Bjorklund, et al 2014, Bjorklund, et al 2011). The findings that MUC1-C is aberrantly expressed in MM cells (Baldus, et al 2007, Cloosen, et al 2006, Kawano, et al 2008) and promotes activation of WNT/β-catenin signalling (Fig. 7A) (Huang, et al 2005, Yamamoto, et al 1997) therefore provided support for the notion that MUC1-C contributes to LEN resistance. Indeed, the present results demonstrate that, whereas LEN alone has little, if any effect, on β-catenin, treatment of MM cells with low concentrations of GO-203 in combination with LEN is associated with suppression of β-catenin levels. The GO-203/LEN combination was also effective in downregulating expression of the WNT target gene, MYC. Notably, these effects of the GO-203/LEN combination on β-catenin and MYC were reversed by the antioxidant GSH, providing evidence for a ROS-mediated mechanism (Fig. 7B). In this context, ROS negatively modulates the WNT pathway by downregulation of β-catenin (Finkel 2011, Shin, et al 2004). Targeting MUC1-C in MM cells has been linked to increases in ROS and, in turn, ROS-mediated decreases in TIGAR, NADPH and GSH (Yin, et al 2010, Yin, et al 2012). Interestingly, the GO-203/LEN combination resulted in significant increases in ROS compared to that with either agent alone. In concert with these results, GO-203/LEN was highly effective in downregulating TIGAR by a ROS-mediated mechanism and in suppressing NADPH and GSH levels. Moreover, the GO-203/LEN combination was synergistic in killing MM cells by ROS-induced apoptosis/necrosis. These findings thus provided support for a model in which targeting MUC1-C in combination with LEN disrupts redox balance and thereby suppresses the WNT/β-catenin pathway and survival (Fig. 7B).
Figure 7. Proposed models supporting the role of MUC1-C in LEN resistance.
A. Schema depicting involvement of MUC1-C in activating the WNT/β-catenin pathway and thereby the induction of MYC, lenalidomide (LEN) resistance and CD44. B. Targeting MUC1-C with GO-203 and treatment with LEN induces reactive oxygen species (ROS) levels, which in turn (i) suppress WNT/β-catenin signalling (ii) downregulate MYC and CD44 expression, and (iii) reverse LEN resistance.
Previous work demonstrated that targeting MUC1-C is synergistic with bortezomib (BTZ) in inducing ROS-mediated MM cell death (Yin, et al 2012). Moreover, GO-203 re-sensitized BTZ-resistant MM cells to BTZ treatment, as evidenced by the combination synergistically increasing ROS levels and cell death (Yin, et al 2012). The finding that targeting MUC1-C in MM cells is effective in combination with LEN thus prompted further studies to determine whether GO-203 has activity in the setting of LEN resistance. For this purpose, we generated LEN-resistant MM cells and found them somewhat more sensitive to GO-203 as compared to LEN-naïve MM cells. In addition, when LEN-resistant cells were treated with the GO-203/LEN combination, there was greater killing than that obtained with GO-203 or LEN alone. These findings supported the premise that GO-203 attenuates resistance to LEN. In further support, treatment of LEN-resistant MM cells with the GO-203/LEN combination also resulted in marked increases in ROS that were not observed with either agent alone. Treatment of LEN-resistant MM cells with GO-203/LEN was also associated with more than additive decreases in NADPH and GSH, consistent with the disruption of redox balance observed in drug-naïve MM cells. Additionally, LEN-resistant MM cells responded to the GO-203/LEN combination with more than additive loss of survival. Addiction to MYC has been reported for MM cells based on induction of death in response to a MYC inhibitor (Holien, et al 2012). In addition, targeting MYC transcription with the BET bromodomain inhibitor JQ1 inhibits MM cell survival and tumour growth (Delmore, et al 2011, Mertz, et al 2011). Thus, the effects of treating LEN-resistant MM cells with GO-203 could be a result of MYC downregulation. However, based on the finding that GSH reverses the effects of the GO-203/LEN combination on LEN-resistant cells, our results support a model in which these agents act more than additively by disruption of redox balance and thereby downregulation of WNT/β-catenin signalling (Fig. 7B). Thus, as found for BTZ-resistant MM cells (Yin, et al 2012), targeting MUC1-C with GO-203 could be effective in certain settings of LEN-resistance.
Increasing evidence has supported the importance of the WNT/β-catenin pathway and MYC in promoting MM cell growth and survival (Derksen, et al 2004, Tagde, et al 2016a). As a result, the development of agents that target the β-catenin/TCF complex in MM cells has been a focus of investigation (Sukhdeo, et al 2007). Targeting MUC1-C with GO-203 abrogates the interaction of MUC1-C with β-catenin and TCF4, and the occupancy of these complexes on the promoters of WNT target genes (Rajabi, et al 2012). In this context, MUC1-C drives MYC expression by β-catenin-mediated activation of the MYC promoter in MM cells (Tagde, et al 2016a). Thus, targeting MUC1-C with silencing or pharmacologically with GO-203 results in the downregulation of β-catenin and MYC (Tagde, et al 2016a). To extend the role of MUC1-C in conferring LEN resistance, we also investigated the effects of targeting MUC1-C on activation of the WNT/β-catenin target gene, CD44 (Wielenga, et al 1999). In this regard, CD44 is overexpressed in LEN-resistant MM cell lines and primary cells (Bjorklund, et al 2014). Moreover, silencing CD44 attenuates MM cell adhesion and LEN resistance (Bjorklund, et al 2014). In concert with the effects of targeting MUC1-C on suppression of WNT/β-catenin signalling, we found that GO-203 treatment of LEN-naïve and -resistant MM cell lines is associated with downregulation of CD44 levels (Fig. 7B). Similar results were obtained with primary CD138+ MM cells. Moreover, analysis of microarray data obtained from primary MM cells provided further support for a significant relationship between MUC1 and CD44, indicating that targeting MUC1-C could contribute to the treatment of LEN-sensitive and -resistant MM by suppressing CD44 expression (Fig. 7B). Based on these findings, we are currently analysing a larger cohort of primary MM samples from patients who are resistant to LEN treatment. Those studies are monitoring the effects of targeting MUC1-C on β-catenin, MYC and CD44 expression as potential markers of sensitivity to LEN exposure ex vivo.
Other studies demonstrating that histone deacetylase inhibitors (HDACi) are synergistic with LEN in inducing MM cell killing (Hideshima, et al 2015) lend support for further investigations that combine GO-203 with an HDACi and LEN for the treatment of LEN-resistant disease. With regard to translational relevance, a Phase I trial of GO-203 has been completed in patients with advanced solid tumours. Moreover, based on the marked synergy between GO-203 and decitabine in AML (Tagde, et al 2016b), a Phase IIb trial of this combination is underway for patients with relapsed/refractory acute myeloid leukaemia. Therefore, the present findings could provide the experimental basis for treatment of LEN-resistant MM with GO-203 alone or in combination with other targeted agents.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers P50CA100707 and PO1CA78378.
Footnotes
Author Contributions
LY, AT, KA, DA and DK conceived the studies.
LY, AT, Y-TT and TH performed the experiments.
AT and RG performed the bioinformatic analyses.
LY, AT, KA, DA and DK wrote the manuscript.
Conflict of Interest Disclosures
D.K. holds equity in Genus Oncology and is a consultant to the company. The other authors disclosed no potential conflicts of interest.
References
- Baldus SE, Palmen C, Thiele J. MUC1 (EMA) expressing plasma cells in bone marrow infiltrated by plasma cell myeloma. Histol Histopathol. 2007;22:889–893. doi: 10.14670/HH-22.889. [DOI] [PubMed] [Google Scholar]
- Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM, Wang M, Shah JJ, Orlowski RZ. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. J Biol Chem. 2011;286:11009–11020. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund CC, Baladandayuthapani V, Lin HY, Jones RJ, Kuiatse I, Wang H, Yang J, Shah JJ, Thomas SK, Wang M, Weber DM, Orlowski RZ. Evidence of a role for CD44 and cell adhesion in mediating resistance to lenalidomide in multiple myeloma: therapeutic implications. Leukemia. 2014;28:373–383. doi: 10.1038/leu.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillez A, Rajabi H, Pitroda S, Jin C, Alam M, Kharbanda A, Tagde A, Wong K, Kufe D. Inhibition of MUC1-C suppresses MYC expression and attenuates malignant growth in KRAS mutant lung adenocarcinomas. Cancer Res. 2016;76:1538–1548. doi: 10.1158/0008-5472.CAN-15-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Singh AV, Aujay M, Kirk CJ, Bandi M, Ciccarelli B, Raje N, Richardson P, Anderson KC. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood. 2010;116:4906–4915. doi: 10.1182/blood-2010-04-276626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloosen S, Gratama J, van Leeuwen EB, Senden-Gijsbers BL, Oving EB, von Mensdorff-Pouilly S, Tarp MA, Mandel U, Clausen H, Germeraad WT, Bos GM. Cancer specific Mucin-1 glycoforms are expressed on multiple myeloma. Br J Haematol. 2006;135:513–516. doi: 10.1111/j.1365-2141.2006.06331.x. [DOI] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, Brady HA, Gandhi AK, Schafer PH, Muller GW, Worland PJ, Chan KW, Verhelle D. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69:7347–7356. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamura I, Huang Y, Zhan F, Barlogie B, Shaughnessy J. Prognostic value of cyclin D2 mRNA expression in newly diagnosed multiple myeloma treated with high-dose chemotherapy and tandem autologous stem cell transplantations. Leukemia. 2006;20:1288–1290. doi: 10.1038/sj.leu.2404253. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Takahashi H, Rajabi H, Alam M, Suzuki Y, Yin L, Tagde A, Maeda T, Hiraki M, Sukhatme V, Kufe D. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7:11756–11769. doi: 10.18632/oncotarget.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- Hideshima T, Cottini F, Ohguchi H, Jakubikova J, Gorgun G, Mimura N, Tai YT, Munshi NC, Richardson PG, Anderson KC. Rational combination treatment with histone deacetylase inhibitors and immunomodulatory drugs in multiple myeloma. Blood Cancer J. 2015;5:e312. doi: 10.1038/bcj.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holien T, Vatsveen TK, Hella H, Waage A, Sundan A. Addiction to c-MYC in multiple myeloma. Blood. 2012;120:2450–2453. doi: 10.1182/blood-2011-08-371567. [DOI] [PubMed] [Google Scholar]
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- Kawano T, Ahmad R, Nogi H, Agata N, Anderson K, Kufe D. MUC1 oncoprotein promotes growth and survival of human multiple myeloma cells. Int J Oncology. 2008;33:153–159. [PMC free article] [PubMed] [Google Scholar]
- Kufe D. Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer. 2009a;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther. 2009b;8:1201–1207. doi: 10.4161/cbt.8.13.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–1081. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, Kufe D. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- Li Q, Birkbak NJ, Gyorffy B, Szallasi Z, Eklund AC. Jetset: selecting the optimal microarray probe set to represent a gene. BMC Bioinformatics. 2011;12:474. doi: 10.1186/1471-2105-12-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β and β-catenin. J Biol Chem. 2001a;276:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- Li Y, Ren J, Yu W, Li G, Kuwahara H, Yin L, Carraway KL, Kufe D. The EGF receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and β-catenin. J Biol Chem. 2001b;276:35239–35242. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, Cathers B, Rychak E, Gaidarova S, Chen R, Schafer PH, Handa H, Daniel TO, Evans JF, Chopra R. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ., 3rd Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N, Hideshima T, Shimomura T, Suzuki R, Ohguchi H, Rizq O, Kikuchi S, Yoshida Y, Cottini F, Jakubikova J, Cirstea D, Gorgun G, Minami J, Tai YT, Richardson PG, Utsugi T, Iwama A, Anderson KC. Selective and potent Akt inhibition triggers anti-myeloma activities and enhances fatal endoplasmic reticulum stress induced by proteasome inhibition. Cancer Res. 2014;74:4458–4469. doi: 10.1158/0008-5472.CAN-13-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi N, Treon SP, Anderson KC. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: therapeutic applications. Blood. 2002;99:4079–4086. doi: 10.1182/blood.v99.11.4079. [DOI] [PubMed] [Google Scholar]
- Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–3084. doi: 10.1182/blood-2014-09-568915. [DOI] [PubMed] [Google Scholar]
- Nooka AK, Kastritis E, Dimopoulos MA, Lonial S. Treatment options for relapsed and refractory multiple myeloma. Blood. 2015;125:3085–3099. doi: 10.1182/blood-2014-11-568923. [DOI] [PubMed] [Google Scholar]
- Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, Shimamura T, Shapiro G, Supko J, Kharbanda S, Kufe D. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther. 2011;10:806–816. doi: 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Ahmad R, Rajabi H, Panchamoorthy G, Kharbanda S, Kufe D. Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. International journal of oncology. 2012;40:1643–1649. doi: 10.3892/ijo.2011.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Agarwal P, Lee J, Bharti A, McKnight C, Sharma P, Kharbanda S, Kufe D. Characterization of the MUC1-C cytoplasmic domain as a cancer target. PLoS One. 2015;10:e0135156. doi: 10.1371/journal.pone.0135156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi M, Kufe D. MUC1-C oncoprotein induces TCF7L2 transcription factor activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem. 2012;287:10703–10713. doi: 10.1074/jbc.M111.323311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi H, Tagde A, Alam M, Bouillez A, Pitroda S, Suzuki Y, Kufe D. DNA methylation by DNMT1 and DNMT3b methyltransferases is driven by the MUC1-C oncoprotein in human carcinoma cells. Oncogene. 2016;35:6439–6445. doi: 10.1038/onc.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Li Y, Kufe D. Protein kinase C β regulates function of the DF3/MUC1 carcinoma antigen in β-catenin signaling. J Biol Chem. 2002;277:17616–17622. doi: 10.1074/jbc.M200436200. [DOI] [PubMed] [Google Scholar]
- Semeraro M, Vacchelli E, Eggermont A, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Lenalidomide-based immunochemotherapy. Oncoimmunology. 2013;2:e26494. doi: 10.4161/onci.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Kim CG, Jho EH, Rho MS, Kim YS, Kim YH, Lee YH. Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett. 2004;212:225–231. doi: 10.1016/j.canlet.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Smith SM, Lyu YL, Cai L. NF-kappaB affects proliferation and invasiveness of breast cancer cells by regulating CD44 expression. PLoS One. 2014;9:e106966. doi: 10.1371/journal.pone.0106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, Mitsiades C, Anderson KC, Carrasco DR. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagde A, Singh H, Kang MH, Reynolds CP. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma. Blood Cancer J. 2014;4:e229. doi: 10.1038/bcj.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagde A, Rajabi H, Bouillez A, Alam M, Gali R, Bailey S, Tai YT, Hideshima T, Anderson K, Avigan D, Kufe D. MUC1-C drives MYC in multiple myeloma. Blood. 2016a;127:2587–2597. doi: 10.1182/blood-2015-07-659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagde A, Rajabi H, Stroopinsky D, Gali R, Alam M, Bouillez A, Kharbanda S, Stone R, Avigan D, Kufe D. MUC1-C induces DNA methyltransferase 1 and represses tumor suppressor genes in acute myeloid leukemia. Oncotarget. 2016b;7:38974–38987. doi: 10.18632/oncotarget.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, Song W, Podar K, Hideshima T, Chauhan D, Schlossman R, Richardson P, Treon SP, Grewal IS, Munshi NC, Anderson KC. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005;65:11712–11720. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R, Raina D, Hasegawa M, Suzuki Y, Tagde A, Bronson RT, Weichselbaum R, Kufe D. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene. 2015;34:5187–5197. doi: 10.1038/onc.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, Schafer PH, Chen R, Glezer E, Ferguson GD, Lopez-Girona A, Muller GW, Brady HA, Chan KW. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- Weisel K, Kanz L. Lenalidomide. Recent Results Cancer Res. 2014;201:347–357. doi: 10.1007/978-3-642-54490-3_21. [DOI] [PubMed] [Google Scholar]
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Path. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NA, Pignatelli M. Beta-catenin--a linchpin in colorectal carcinogenesis? Am J Path. 2002;160:389–401. doi: 10.1016/s0002-9440(10)64856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- Yan Y, Zuo X, Wei D. Concise Review: Emerging role of CD44 in cancer stem cells: A promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4:1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Ahmad R, Kosugi M, Kufe T, Vasir B, Avigan D, Kharbanda S, Kufe D. Survival of human multiple myeloma cells is dependent on MUC1 C-terminal transmembrane subunit oncoprotein function. Mol Pharm. 2010;78:166–174. doi: 10.1124/mol.110.065011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Kosugi M, Kufe D. Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by downregulating TIGAR expression and depleting NADPH. Blood. 2012;119:810–816. doi: 10.1182/blood-2011-07-369686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Kufe T, Avigan D, Kufe D. Targeting the MUC1-C oncoprotein is synergistic with bortezomib in downregulating TIGAR and inducing ROS-mediated multiple myeloma cell death. Blood. 2014;123:2997–3006. doi: 10.1182/blood-2013-11-539395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F, Barlogie B, Arzoumanian V, Huang Y, Williams DR, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Zangari M, Dhodapkar M, Shaughnessy JD., Jr Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109:1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.