Abstract
G protein-coupled receptor kinases (GRKs) are classically known for their role in regulating the activity of the largest known class of membrane receptors, which influence diverse biological processes in every cell type in the human body. As researchers have tried to uncover how this family of kinases, containing only 7 members, achieves selective and coordinated control of receptors, they have uncovered a growing number of non-canonical activities for these kinases. These activities include phosphorylation of non-receptor targets and kinase-independent molecular interactions. In particular, GRK2, GRK3, and GRK5 are the predominant members expressed in the heart. Their canonical and non-canonical actions within cardiac and other tissues have significant implications for cardiovascular function in healthy animals and for the development and progression of disease. This review summarizes what is currently known regarding the activity of these kinases, and particularly the role of GRK2 and GRK5 in the molecular alterations that occur during heart failure. This review further highlights areas of GRK regulation that remain poorly understood and how they may represent novel targets for therapeutic development.
Keywords: GPCR, GRK, cardiovascular
Introduction to GPCR Signaling and GRK Regulation
G protein-coupled receptors (GPCRs) comprise a large family of diverse membrane receptors that translate the binding of extracellular stimuli such as photons, neurohormones, chemokines, or ions into complex intracellular signaling cascades that regulate the function of all eukaryotic cells (1, 2). This occurs through ligand binding to the receptor, inducing a conformational change that stimulates the activation and dissociation of the associated heterotrimeric G protein subunits, Gα and Gβγ, which then bind effector proteins to alter cellular activity (3–5). Given the importance of GPCR signaling in determining cellular homeostasis and function, it is not surprising that receptor signaling is tightly controlled, and that this control is through the phosphorylation of ligand-bound receptors via GPCR kinases (GRKs) (6). Phosphorylation of receptors by GRKs triggers desensitization via binding of β-arrestin, sterically blocking further G protein activation and attenuating downstream effector signaling (3–6). β-arrestins not only induce receptor desensitization and internalization, but downstream of GRK actions they can initiate intracellular signaling through kinase scaffolding functions, independent of G protein activity (7). In fact, endogenous GPCR ligands stimulate both G protein and arrestin signaling that can be biased by the GRK involved, while synthetic ligands may be designed to show bias for a particular pathway (8, 9). Thus, β-arrestin 1 and 2 in the heart may undergo GRK-arrestin signaling and play an important role in regulating normal and compromised cardiomyocyte function. This exemplifies the historically classical function of GRKs in regulating GPCR activity.
GRKs represent a subgroup of the AGC protein kinase family of serine/threonine kinases, which includes PKA, PKG, and PKC, due to the sequence similarity within their kinase domains (10). A thorough review of the known literature regarding GRK structure, localization, GPCR activity, cardiac function, and regulation has recently been published (11). The GRK family is composed of 7 known members that have been classified into 3 subfamilies based on their sequence and structural similarities. One subfamily is the rhodopsin kinases including GRK1 and GRK7, that are solely expressed in the retina and regulate opsin signaling (12). Additionally, there is the β-adrenergic receptor kinase (βARK) subfamily with GRK2 and GRK3, and the GRK4-like subfamily that includes GRK4, GRK5, and GRK6 (6). While GRK4 is predominantly expressed in the testes (13), GRK2, 3, 5, and 6 are ubiquitously expressed with GRK2 and GRK5 being the predominant GRKs in the heart (14). GRK2, GRK3, GRK5, GRK6, and particularly GRK4 have also been shown to play important roles in regulating blood pressure and hypertension through activities in the heart, vasculature and kidneys. These important contributions to overall cardiovascular function are outside the scope of this article, but have recently been reviewed in detail (15). All GRKs have a tri-domain structure with a central catalytic domain flanked by amino (N) and carboxyl (C) terminal domains containing elements involved in GRK regulation and key binding elements for effectors and protein-protein interactions (3). Importantly, the N-terminal 30 amino acids that are critical for recognition of the activated receptor are highly conserved, while the C-terminus that is necessary for membrane localization is rather divergent between GRK subfamilies (16, 17).
The retinal GRK1 and GRK7 contain sequences that can be targeted for prenylation, and this hydrophobic moiety may act as a lipid anchor to facilitate their association with the plasma membrane (18, 19). Similarly, GRK4 and GRK6 contain palmitoylation sites, and these C-terminal cysteine modifications tether the GRKs to the membrane (20–22). GRK5 is unique in this subfamily in that is does not contain this palmitoylation site. Instead, GRK5 contains a positively charged amphipathic helix (N-terminal residues 25–31) that acts as a lipid-binding element, tethering it to phosphatidylinositol 4, 5 bisphosphate (PIP2) such that GRK5 is constitutively membrane bound (23, 24). Unlike the GRK4 subfamily, GRK2 and GRK3 are not independently capable of membrane association and rely upon binding to the heterotrimeric G-protein subunit Gβγ. G-protein γ subunits are isoprenylated through a covalent cysteine modification that mediates the membrane association of Gβγ subunits (25). Following receptor activation and dissociation of the heterotrimeric G-proteins, GRK2 and GRK3 associate with membrane bound Gβγ through their C-terminal pleckstrin homology domain, thereby facilitating their membrane translocation (26–28). The pleckstrin homology domain then further orients GRK2 at the site of GPCR action via association with membrane phospholipids such as PIP2 (29, 30). The critical interaction of GRK2 and GRK3 with Gβγ subunits is further affected by the expression and localization of different β and γ subunit isoforms (31–33) that may partially account for the receptor selectivity of these GRKs. No matter the method, membrane localization is critical for the classical function of GRKs, where close proximity to the receptors facilitates GRK activation and subsequent phosphorylation of the agonist bound receptors. The details regarding activation of GRKs and the mechanism by which they phosphorylate receptors is outside the scope of this article, but the current understanding of these processes and how they are being further investigated has been recently described (24, 34).
Of the many functions of GRKs, the best characterized is this classical phosphorylation of agonist-bound GPCRs to regulate their signaling. GRKs mediate homologous desensitization of GPCRs via phosphorylation of agonist-occupied receptors that in turn promotes the binding of β-arrestin, sterically blocking further G protein activation and attenuating downstream effector signaling (3–5). β-arrestin binding then serves as a scaffold for association of the internalization machinery, and following internalization receptors may be targeted to lysosomes for degradation or recycled through early endosomal compartments back to the plasma membrane (35). Receptor recycling plays a critical role in determining the active pool of receptors at the membrane, since receptor de-phosphorylation and dissociation of β-arrestin occurs within these endosomal compartments (5). Therefore, through this classical function, GRKs contribute significantly to controlling the duration and capacity for GPCR signaling in the cell. In addition to the classical activity of these enzymes, ongoing research has demonstrated a great diversity in the function of GRKs, including phosphorylation of non-GPCR substrates and numerous phosphorylation-independent regulatory binding partners. These studies suggest that such alternate or non-canonical activities of GRKs are more prevalent than expected. Further, that they embody significant, functional interactions that alter cardiac physiology and disease progression. These non-canonical activities of GRKs and the role they play specifically in cardiovascular function in healthy tissue and during disease will be the focus of this review article.
GRK2
GRK2 was the first GRK identified in the heart and was originally named β-adrenergic receptor (βAR) kinase 1 (βARK1) due to phosphorylation of activated βARs and other GPCRs (Figure 1-1) (36). Further, GRK2 has been shown to be intimately involved in heart failure (HF) progression. GRK2 is the most abundant GRK isoform in the heart and it’s mRNA, protein and activity is elevated (3–4 fold) in failing human hearts (37) and animal models of HF (38–41). Several mouse models support a critical and facilitative role for GRK2 in HF development. The role of GRK2 in the regulation of βAR signaling in the healthy and diseased myocardium and the pursuit for therapeutic inhibition of GRK2 has recently been reviewed in detail (42).
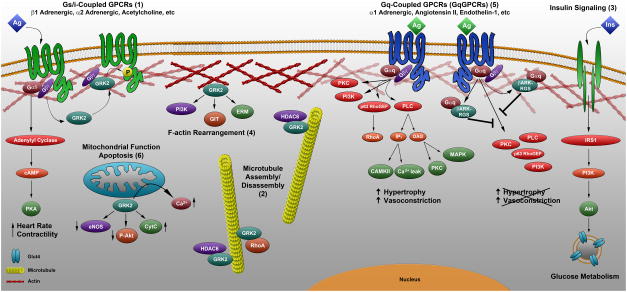
Figure 1.
Summary diagram of the predominant canonical and non-canonical activities of GRK2 that alter cardiac physiology and disease progression. (1) The historically classical function of GRK2 in regulating GPCR activity wherein phosphorylation of β1ARs triggers desensitization to attenuate downstream effector signaling. (2) Phosphorylation of non-GPCR targets HDAC6, tubulin, synucleins, and ezrin for GRK2-mediated regulation of cytoskeletal rearrangements and cell migration. (3) GRK2 as a negative regulator of insulin-dependent glucose uptake and insulin signaling by an unknown mechanism. (4) GRK2-mediated recruitment of PI3K to the membrane for enhanced internalization of agonist bound βARs and less well understood interactions for regulating endocytosis. (5) Selective interaction of the GRK2 N-terminal peptide βARKrgs, but not full-length GRK2, with Gαq in the heart, thereby inhibiting activation of Gαq-coupled GPCR effectors to reduce hypertrophic signaling. (6) GRK2 localization to the mitochondrial outer membrane, where phosphorylation of non-GPCR targets reduces the induction of vital cell survival pathways such as Akt and nitric oxide, thereby altering mitochondrial biogenesis and cell survival.
In addition to the canonical function of this enzyme, ongoing research has demonstrated great diversity in the functional roles of GRK2 (7, 43). GRK2 is ubiquitously expressed and participates in diverse biological functions including cell migration, inflammation, insulin sensitivity and vascular biology (44–48); however, the mechanisms by which GRK2 participates in these cellular functions remain to be determined. An underlying mechanism for these diverse functions may be the phosphorylation-independent desensitization of diverse GPCRs, including endothelin, α and β adrenergic, muscarinic cholinergic, angiotensin, and thyrotropin releasing hormone (TRK) receptors, among others, in distinct cellular populations. Further, GRK2 has emerged as a complex regulator of cellular migration, acting through classical GPCR desensitization during chemotaxis in addition to numerous non-canonical substrates and protein interactions. For example, GRK2 dynamically binds to and phosphorylates histone deacetylase 6 (HDAC6), activating deacetylase activity at α-tubulin subunits within the leading edge of migrating cells to promote cell motility (Figure 1-2) (49). In addition, phosphorylation of non-receptor substrates including tubulin, synucleins, and ezrin may underlie GRK2 function in endocytosis and cytoskeletal rearrangements for cell migration (48). A recent review thoroughly outlined the role of GRK2 in chemotaxis and gradient sensing, cell polarization and migration, and cell adhesion and the implications of these diverse functional roles of GRK2 for cellular function and pathophysiology (50). In addition, GRK2 has been identified as an important negative regulator of insulin-dependent glucose uptake and insulin signaling (Figure 1-3). Heterozygous KO of GRK2 improves glucose transport, whole-body glucose and insulin tolerance, and insulin signaling pathways in relevant animal models of insulin resistance (51–54); however the underlying mechanism has not been elucidated. These studies indicate that the overall impact of GRK2 levels and activity on diverse cellular functions depends significantly on the particular stimuli and cell type in consideration. More importantly, these studies indicate that a complete understanding of the role of GRK2 in immune cell migration and infiltration will expand our knowledge of this enzyme in regulating scar formation and fibrosis in the heart following cardiac injury. Further, GRK2 regulation of insulin signaling and cardiac metabolism will provide a more encompassing view of the importance of this enzyme in cardiac metabolism and function during disease progression.
GRK2 has also been shown in vitro to interact with numerous cell signaling and trafficking proteins (55–58). For many of the GRK2 interacting partners, such as the Raf kinase inhibitor protein RKIP, GIT proteins, and mitogen activated protein kinases (MAPK), the functional consequences on GRK2 activity have been described, while the specific amino acid binding domain on GRK2 is not yet known (Figure 1-4) (59, 60). Similarly, phosphoinositide 3-kinase (PI3K) has been shown to interact with GRK2 through its 197 amino acid PIK domain, resulting in GRK2-mediated recruitment of PI3K to the membrane and enhanced internalization of agonist bound βARs (Figure 1-4) (61). These cellular proteins may interact with GRK2 directly or in complex, potentially as members of scaffolded nodal signaling systems. In contrast, the binding domain of proteins such as Akt have been defined, amino acids 492–689 in the C-terminus of GRK2; however, this interaction inhibits Akt by an unknown mechanism (59). Some GRK2 binding partners have presumed binding domains within the N-terminus and the C-terminal pleckstrin homology (PH) domain such as caveolin (residues 63–71 and 567–584) (62) and calmodulin (residues 18–37 and 593–689) (63). Caveolins are known scaffolding proteins that serve to inhibit or enhance signaling through compartmentalization of GPCRs and kinases within distinct membrane microdomains (64–68), and caveolin 1 or 3 binding to GRK2 has been shown to inhibit GRK2-mediated phosphorylation (62, 64, 69). Similarly, calmodulin binding directly inhibits GRK2 activity (63). Overall, though, little is known regarding what distinct signaling complexes GRK2 participates in at any given time, the localization of the complexes within the cell, and the full consequences of these regulatory binding interactions for cardiomyocyte or other cell type function in vivo.
A domain of interest for determining the mechanism of action of GRK2 is the Regulator of G protein signaling (RGS) - like domain identified within the N-terminus (amino acids 51–173) of GRK2. This domain has been shown to bind to and regulate Gαq activity in vitro (70–73), an interaction that could prove an interesting therapeutic target as Gαq represents the final common trigger of maladaptive cardiac hypertrophy (Figure 1-5). Transgenic (Tg) mice with cardiac-specific overexpression of Gαq have a phenotype of decompensated cardiac hypertrophy (74–76), but this Gαq-mediated hypertrophy and HF is blocked by cardiac overexpression of RGS4 (77). Furthermore, studies in a Tg mouse line with αMHC-targeted cardiac expression of a Gαq inhibitor (GqI), the last 50 amino acids of murine Gαq that targets the Gαq-coupled GPCR (GqPCR) interface, have demonstrated significantly lessened ventricular hypertrophy and ventricular dysfunction after pressure overload (78–80). Classically, RGS proteins bind to Gα proteins, allosterically increasing their rate of GTP hydrolysis and thereby inhibiting their activity (72, 77). The RGS domain of GRK2 appears to directly interact with Gαq and inhibit signaling without altering its GTPase activity (73). Rather Gαq was found to adopt an effector binding structure with GRK2 (81), and the domain of GRK2 responsible for Gαq binding is distinct from known binding sites for RGS4 and RGS9 (72, 82). Together, these data suggest potential distinct functions of the RGS domain of GRK2, such that a selective interaction of the GRK2 RGS domain with Gαq may alter cardiac hypertrophic responses.
The potential impact of an interaction between the GRK2 RGS domain and Gαq was recently investigated in a mouse model of pressure overload-induced hypertrophy and HF in transgenic mice with αMHC-targeted cardiac expression of this domain (residues 45–185, βARKrgs) (83). In this study, the βARKrgs mice were not only resistant to hypertrophic growth after trans-aortic constriction, providing the same anti-hypertrophic effect as inhibition of Gαq association with GPCRs, but the reduction in fibrosis and molecular remodeling prevented the transition to HF during chronic pressure overload (83). This occurred through an association of βARKrgs, but not full-length GRK2, selectively with Gαq in the heart in a manner that inhibited the activity of the Gαq effector phospholipase C beta (PLCβ) (Figure 1-5). This interaction was enhanced after pressure overload, presumably through Gαq-coupled GPCR activation driving the association of βARKrgs with dissociated Gαq (83). Importantly, unlike βARKrgs, this study revealed that cardiac restricted overexpression (TgGRK2) or peptide inhibition (TgβARKct) of GRK2 did not alter cardiac hypertrophy, demonstrating that loss or gain of function of the full-length enzyme does not alter cardiac hypertrophic responses. In contrast to previous in vitro studies, these data are consistent with βARKrgs inhibiting pressure-overload induced hypertrophy via altered Gαq-mediated signaling in manner independent of kinase activity and encompassing and interaction that is not observed for full-length GRK2.
In addition to mapping the distinct functional domains of GRK2 and their relevant protein interactions, the diverse cellular functions of this enzyme are also due to non-canonical targeting and localization of GRK2. In addition to the classical membrane translocation, GRK2 has been shown to localize to the mitochondrial outer membrane, where phosphorylation of non-GPCR targets causes significant alterations in mitochondrial biogenesis and cell survival (Figure 1-6) (84–86). In a mouse model of ischemia/reperfusion (I/R), mice overexpressing GRK2 exhibited increased infarct size and apoptosis post-injury due to reduced induction of vital cell survival pathways such as Akt and nitric oxide (NO) generation(87). The opposite was observed in mice expressing the βARKct (87). In contrast, GRK2 KO resulted in cardioprotection, wherein mice exhibited reduced mitochondrial-mediated apoptosis and infarct size (88). GRK2 exerts these detrimental effects via translocation to mitochondria, an event dependent on both extracellular-regulated kinase (ERK)-mediated phosphorylation at serine 670 and heat shock protein 90 (Hsp90) chaperone function (89, 90). Elevated mitochondrial GRK2 increased Ca2+-induced opening of the mitochondrial transition pore and was an absolute requirement for stress-induced, mitochondrial-dependent pro-death signaling, while βARKct led to cardioprotection (Figure 1-6) (89). In fibroblasts, GRK2 can localize to the mitochondrial outer membrane through binding sites within its RGS homology and kinase domain (85). This localization is enhanced during ischemic stress, and was found to elevate cellular ATP content by promoting mitochondrial respiration (85). In cardiomyocytes, GRK2 overexpression impaired fatty acid oxidation and increased mitochondrial-based superoxide production, while GRK2 inhibition increased oxygen consumption rates and ATP production (Figure 1-6) (91). These effects were dependent upon GRK2 kinase activity and demonstrated that independent of cardiac injury, GRK2 localization to the mitochondria altered substrate utilization and impaired mitochondrial biogenesis (91). Future studies will be needed to elucidate the protein substrates for GRK2 kinase activity, or GRK2 binding interactions that mediate the clear role of this enzyme in regulating mitochondrial function, as well as how mitochondrial GRK2 activity is regulated.
In addition to regulatory protein-protein binding interactions, GRK activity is controlled by multiple phosphorylation sites within both the N- and C-termini. PKC-mediated phosphorylation of GRK2 alleviates the inhibition induced by calmodulin binding (92). c-Src-mediated phosphorylation of GRK2 at select N-terminal Tyrosine residues (amino acids 13, 86, and 92) enhances its kinase activity (92, 93) and selectively increases binding of GRK2 to Gαq as demonstrated by the use of phospho-tyrosine defective and mimic GRK2 mutants (94). Furthermore, increased tyrosine phosphorylation on GRK2 occurs in parallel with both increased co-immunoprecipitation of GRK2 and Gαq post activation of the Gαq-coupled M1 muscarinic receptor and inhibition of Gαq-mediated activation of phospholipase Cβ signaling in vitro, supporting a physiological role for this GRK2 regulatory mechanism (94). GRK2 is also differentially regulated by phosphorylation at distinct serine residues. MAP kinase phosphorylation at C-terminal residue Ser670 reduces binding with Gβγ, thereby inhibiting GRK2 activity (95), while protein kinase A (PKA)-mediated phosphorylation at Ser685 increases Gβγ association (55), enhancing GRK2 kinase activity at the membrane. In addition to regulatory phosphorylation of GRKs, GRK2 has also been recently shown to undergo post-translational modification in the form of S-nitrosylation at cysteine (Cys) 340 (96). The physiological consequence of this modification is that when endothelial nitric oxide synthase (eNOS)-NO bioavailability is high, S-nitrosylation of GRK Cys340 inhibits GRK2 kinase activity (96, 97). Further, in mice with heterozygous KO of GRK2, de-repression of eNOS led to a preservation of NO bioavailability, supporting resistance to vascular remodeling in response to angiotensin II-induced hypertension (98). Thus, NO can be considered an endogenous GRK2 inhibitor. Together, these data suggest that distinct GRK interactions and their effect on cellular signaling processes may be tightly controlled by post-translational modification of these kinases. This may provide an additional level of regulation beyond the capability for diverse intermolecular interactions by fine-tuning the affinity of these interactions and/or the resulting activity of GRKs at specific targets within the cell. Future studies are needed to fully elucidate in what signaling complexes GRKs participate in at any given time, their localization within the cell, and the full functional consequences of regulatory binding interactions and post-translational modifications on cardiovascular signaling and function.
Based on these canonical and non-canonical activities of GRK2 in the heart and other cells types, GRK2 has emerged as a target of interest for therapeutic intervention in numerous disorders. In fact, the role of GRK2 in diverse pathologies including vascular, immune, cancer, and neurological disorders, and methods targeting GRK2 inhibition have been reviewed in detail (99). Recently, a high-throughput screen for small molecule inhibitors of GRK2 was developed using an RNA aptamer to elucidate compounds that would selectively and potently inhibit GRK2 kinase activity (100). This screen uncovered the FDA approved selective serotonin reuptake inhibitor (SSRI) paroxetine that effectively inhibited GRK2-mediated phosphorylation of cytosolic and membrane receptor targets in vitro, and enhanced contractility and relaxation during in vivo hemodynamics, unlike the chemically unrelated SSRI fluoxetine (100). In a mouse model of myocardial infarction, initiation of paroxetine, but not fluoxetine, treatment after HF development significantly improved left ventricular (LV) structure and function, inhibiting or reversing several hallmarks of HF progression, with beneficial effects greater than β-blocker therapy alone (101). Importantly, no adverse effects on cardiovascular function or gross phenotypic effects were observed in paroxetine treated Sham mice in this study, lending support to the therapeutic potential for moderate, global GRK2 inhibition. While significant limitations exist for the use of paroxetine as such a therapy, this compound served as a starting point for the development of rationally designed analogs with increased efficacy and selectivity for GRK2 and reduced central nervous system activity (102, 103). While it remains to be seen whether these or related compounds will progress through clinical trials to provide a significant advance in the treatment of human HF, these compounds will serve as useful research tools to look at various aspects of GRK2 kinase function in cell culture and animals models of disease. Furthermore, the crystal structure of GRK2 will offer a template on which to not only investigate kinase domain inhibitors, but perhaps target and disrupt protein-protein interactions that represent key aspects of cardiovascular signaling and function or are sources of dysregulation during disease (16, 99, 103).
GRK3
GRK3 shares 85% sequence identity with GRK2, with nearly 95% identity in the catalytic domain, and has nearly the same tissue distribution although with lower expression levels (104). Unlike GRK2 or GRK5, myocardial levels of GRK3 are not regulated in HF (105), and cardiac restricted overexpression of GRK3 did not alter βAR signaling or downstream adenylyl cyclase activation (106, 107). In fact, no overt cardiac phenotype was observed in either transgenic mice with cardiac restricted overexpression of GRK3 or GRK3 KO mice (108, 109). While GRK3 overexpression had no effect on angiotensin II receptor activation and mitogen-activated protein (MAP) kinase signaling, it was found to significantly blunt responses to the thrombin receptor agonist SFLLRN (Figure 2-1) (108). This was confirmed in that both GRK3 overexpression and peptide inhibition of GRK3 through expression of the C-terminal pleckstrin homology domain of GRK3 (GRK3ct) were highly potent and efficient at reducing or enhancing endothelin receptor activity, respectively (Figure 2-1) (110). Further, GRK3 was highly potent at inhibiting α1-adrenergic receptor (α1AR) activity, whereas GRK2 was not effective at endothelin or α1ARs even at substantial overexpression levels (Figure 2-1) (110). This is consistent with a primary role for GRK2 in the regulation of cardiac contractility and chronotropy through β1ARs while GRK3 may regulate cardiac growth and hypertrophy through regulation of endothelin and α1ARs (110). Further, proto-oncogene tyrosine kinase Src (c-Src) phosphorylation of GRK3 has been shown to participate in the regulation of α1BAR activity (111); however, the mechanism has not been uncovered. Interestingly, the highest degree of variance between these two GRKs occurs within the approximately 28 amino acid region critical for Gβγ binding (26, 28, 112). This may underlie differences in Gβγ subunit affinity and therefore the divergent receptor selectivity observed in vivo between the otherwise similar GRK2 and GRK3 (108, 113), particularly as Gβγ subunits have also show preferences for distinct GPCRs (114, 115).
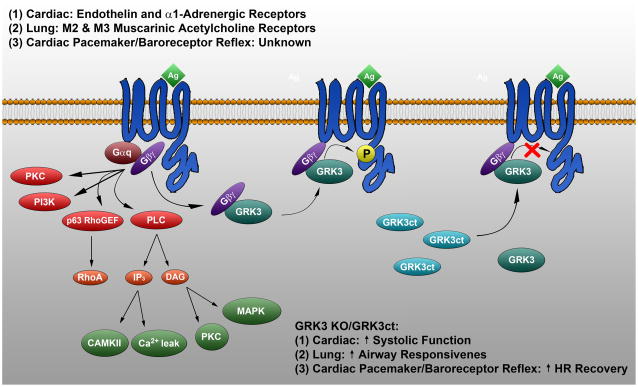
Figure 2.
Summary diagram depicting the current understanding of GRK3 regulation and function in the cardiovascular and respiratory systems. 1) GRK3 may regulate cardiac growth and hypertrophy, since cardiac overexpression potently inhibits endothelin and α1AR signaling, whereas GRK3ct expression enhances these signaling pathways and cardiac systolic function. (2) GRK3 does not contribute to the regulation of cardiac M2 receptors; however, GRK3 homozygous KO mice exhibit an increase in cholinergic airway responsiveness by an undetermined mechanism. (3) GRK3 KO enhances HR recovery from muscarinic acetylcholine-induced bradycardia independent of GPCR signaling, thus GRK3 may play a role in desensitization of the baroreceptor reflex response downstream of MAPK signaling or regulate the chronotropic component by altering activation of adrenergic neurons in this reflex pathway.
Transgenic mice expressing the C-terminal pleckstrin homology domain of GRK3 (TgGRK3ct) demonstrated an increase in diastolic and systolic blood pressure compared to non-transgenic littermate controls (NLC), while heart rate (HR) and activity level were maintained (Figure 2-1) (116). In vivo hemodynamics demonstrated an increase in stroke work and stroke volume in the TgGRK3ct mice, supporting an increase in systolic function (116). Interestingly, this was accompanied by myocardial hyper-responsiveness to α1AR stimulation both in vivo and in vitro. This suggests that the loss of GRK3-mediated desensitization of the cardiac α1ARs in TgGRK3ct, a positive inotropic signaling pathway, may be the mechanism for increased contractility (Figure 2-1) (116). Similar to TgGRK3ct, mice with cardiac restricted overexpression of the α1AAR exhibited hyper-contractile hearts without cardiac hypertrophy (117). More importantly, they demonstrated preserved cardiac function after chronic pressure overload and are protected from LV remodeling after myocardial infarction (118, 119). Consistent with these results, while cardiac hypertrophy was equivalent in TgGRK3ct and NLC hearts following aortic banding, in vivo hemodynamics revealed that the elevated end-diastolic pressure, reduced cardiac output, and reduced dP/dt maximum and minimum in NLC mice were all prevented in the GRK3ct mice (Figure 2-1) (120). Thus, phosphorylation of cardiac α1ARs by GRK3 may contribute to the detrimental molecular alterations that occur during cardiac disease, such that inhibition by GRK3ct preserves cardiac function and prevents the transition to HF during chronic pressure overload.
In addition to an assessment of the classical activity of GRK3 in regulating GPCR signaling in the heart, a study of GRK3 KO mice observed an unexpected and potentially non-canonical effect of this GRK on the baroreceptor reflex (109). GRK2 and GRK3 have been demonstrated to phosphorylate and desensitize human M2 and M3 muscarinic acetylcholine receptors in vitro, and given their expression in airway smooth muscle and cardiac pacemaker cells, these GRKs may play a role in regulating airway narrowing and bradycardia (121–123). Unlike GRK2 heterozygous KO mice that displayed no alteration in the respiratory or cardiac parameters tested, GRK3 homozygous KO mice exhibited an increase in cholinergic airway responsiveness and an enhanced HR recovery from muscarinic acetylcholine-induced bradycardia (Figure 2-2 and 2-3) (109). Importantly, direct vagal nerve stimulation revealed no significant difference in the absolute decrease in HR or in the time course of HR recovery between GRK3 KO and wild-type mice, nor was any alteration observed in the dose-response to the selective M2 agonist gallamine during vagal stimulation, suggesting that GRK3 does not contribute to the regulation of cardiac M2 receptors (109). Further, treatment with the βAR antagonist propranolol had no effect on resting HR in the GRK3 KO mice, and normalized the HR recovery in these mice following vagal stimulation, suggesting that βAR regulation by GRK3 is also not the mechanism for enhanced HR recovery in the GRK3 KO mice. In combination with the lower resting HR in these mice, these data indicate that tonic sympathetic outflow may be diminished (109, 124). Data suggests that the HR recovery in GRK3 KO mice is not due to any adaptation of cardiac GPCR signaling, but rather that GRK3 may play a role in desensitization of the baroreceptor reflex response to the vasodilation induced changes in MAP kinase signaling following muscarinic acetylcholine injection (Figure 2-3) (109). Further, since the baroreceptor reflex response is mediated through multiple central nervous system structures, GRK3 may regulate the chronotropic component of the baroreceptor reflex in mice by altering activation of adrenergic neurons in this reflex pathway (Figure 2-3) (109, 125). Importantly, while the significance of alterations in GRK3 activity on human pathophysiological conditions remains to be elucidated, given these data regarding GPCR selectivity and the baroreflex, it is interesting to note that the GRK3 gene locus corresponds with a position on chromosome 22 that has been associated with hypertension (126). Ultimately, the mechanism by which GRK3 alters airway reactivity and the cardiac component of the baroreceptor reflex, and whether this occurs through regulation of GPCR signaling or alternative, non-canonical pathways, remains to be determined.
GRK5
GRK5 has been shown to be highly expressed in the heart and other muscle types, as well as the respiratory, nervous, and immune systems (12, 127, 128). While homozygous KO of GRK5 is not embryonic lethal (13), this enzyme is believed to play a role in cardiac development based upon studies in zebrafish, and it may be compensated for by GRK6 as double KO of GRK5 and GRK6 is embryonic lethal (129, 130). Similar to GRK2, GRK5 expression is elevated in patients with various cardiovascular diseases (131–134), and participates in desensitization of multiple GPCRs including β1-adrenergic receptors (β1AR) (Figure 3-1) (107). PKC-mediated phosphorylation of GRK5 at Ser572 and either Ser566 or Ser568, or at the auto-regulatory sites Ser484 or Thr485, dramatically inhibits GRK5 affinity and activity at agonist-bound GPCRs (135). The role of GRK5 in the regulation cardiac signaling in the healthy and diseased myocardium and the pursuit for therapeutic inhibition of GRK5 has recently been reviewed in detail (136). Interestingly, mice with cardiac-restricted overexpression of GRK5 demonstrated increased sensitivity to pressure overload, marked by enhanced hypertrophy and a more rapid progression to HF (137). Further analysis demonstrated that this exaggerated hypertrophy was caused by a unique, non-canonical activity of GRK5 in the nucleus. This was facilitated by the presence of a nuclear localization sequence (NLS) and nuclear export sequence (NES), both within the catalytic domain, with the NLS sequence homologous to that of homeobox containing transcription factors (41, 138, 139). Following Gαq-coupled receptor signaling, GRK5 translocated to the nucleus where it acted as a class II histone deacetylase (HDAC) kinase, phosphorylating HDAC5 and leading to de-repression of the hypertrophic gene transcription factor myocyte enhancer factor-2 (MEF2) (Figure 3-2a) (137, 140, 141). Activation of α1-adrenergic and angiotensin II receptors, neither of which is desensitized by GRK5, induced this translocation through calmodulin binding to the N-terminus of GRK5 (140), and was dependent on the intact nuclear localization sequence (NLS) (Figure 3-2a). Calmodulin binding to GRK5 led to C-terminal auto-phosphorylation, reducing activity at GPCRs and enhancing phosphorylation of non-receptor substrates (Figure 3-2a) (142). In contrast, Gαq-coupled endothelin receptor activation, a GPCR that is desensitized by GRK5, did not cause nuclear translocation or transcriptional regulation by GRK5 (Figure 3-3) (140). Reciprocally, cardiac KO of GRK5 protected the heart during pressure overload, reducing hypertrophy and fetal gene induction (143). Together, these data demonstrate a non-canonical role for GRK5 through GPCR-mediated targeting of this GRK to the nucleus followed by kinase-dependent phosphorylation of the non-GPCR target HDAC5 (Figure 3-2a).
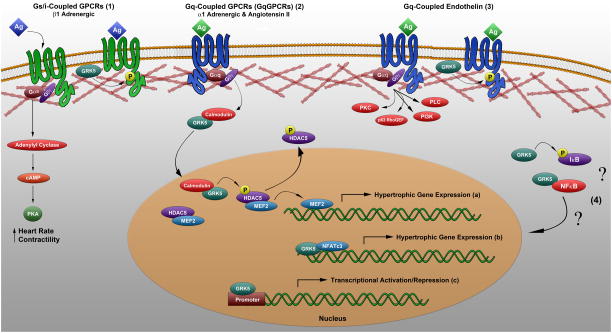
Figure 3.
Summary diagram of the predominant canonical and non-canonical activities of GRK5 in modulating GPCR signaling and gene transcription during cardiac hypertrophy and HF. (1) GRK5 expression is elevated in patients with various cardiovascular diseases and participates in desensitization of multiple GPCRs including β1ARs. (2a) GRK5 enhances hypertrophy through a unique, non-canonical activity in the nucleus. Following Gαq-coupled α1AR or angiotensin receptor signaling, calmodulin binds the N-terminus of GRK5 to facilitate nuclear translocation. There, GRK5 acts as an HDAC kinase, phosphorylating HDAC5 and leading to de-repression of the hypertrophic gene transcription factor MEF2. (2b) GRK5 directly binds to DNA and activates the hypertrophic transcription factor NFATc3, further exaggerating the response to pressure overload. (2c) GRK5 may also be involved in transcriptional regulation in cardiac and other tissues through direct DNA binding at target promoter sequences. (3) The Gαq-coupled endothelin receptor is desensitized by GRK5, and does not cause nuclear translocation or transcriptional regulation by GRK5. (4) GRK5 regulates NFκB signaling through phosphorylation of IκB and alterations NFκB subunit expression in various cell models, with no overall consensus on the mechanism or impact on cardiac hypertrophy or inflammation.
In addition to this non-canonical regulation of transcriptional activity, GRK5 has also been shown to directly bind to DNA, wherein activity of the hypertrophic transcription factor NFAT directly correlated with GRK5 expression levels, and deletion of NFATc3 protected mice from the rapid progression to HF seen in GRK5 overexpressing mice after pressure overload (Figure 3-2b) (144). Further, chromatin immunoprecipitation assays demonstrated direct binding of GRK5 to the Bcl-2 promoter and transcriptional repression in the brain, suggesting that GRK5 may also be involved in transcriptional regulation in other tissues through direct DNA binding at target promoter sequences (Figure 3-2c) (145). These data demonstrate a kinase-independent nuclear role for GRK5 in facilitating maladaptive cardiac hypertrophy and HF. Beyond the demonstrated role for GRK5 in transcriptional regulation, it has also been shown to be involved in the regulation of NFκB signaling, although without a consensus on the overall effect of this regulation on cardiac hypertrophy or inflammation (Figure 3-4). Studies using RNA interference in Drosophila cells report GRK5-mediated phosphorylation of IκB, leading to increased inflammation, as well as an evolutionarily conserved association of GRK5 with NFκB (Figure 3-4) (146). Conflicting reports also suggest both activation of NFκB through upregulation of the p50 and p65 subunits in neonatal rat ventricular cardiomyocytes, alterations in p105 subunit control in macrophages, and inhibition of NFκB signaling in bovine aorta endothelial cells (Figure 3-4) (147–150). These studies indicate that the overall impact of GRK5 levels and activity on NFκB signaling may significantly depend on the particular stimuli and cell type in consideration. Thus, the overall role of GRK5 in moderating NFκB signaling, the mechanism by which it occurs, and the physiological outcome of this regulation for cardiovascular function remains poorly understood and may encompass additional, non-canonical protein interactions for GRK5 in the heart.
A growing area of research is the study of biased agonists that can bias receptor signaling through either G protein or β-arrestin signaling pathways. GRKs have been implicated in this signaling due to their selectivity for distinct GPCR phosphorylation sites that may serve as a molecular “barcode” to regulate the activation of downstream signaling pathways (151). For example, mechanical stretch in cardiomyocytes has been shown to alter the conformation of the angiotensin receptor (AT1R) in a manner that selectively activates β-arrestin-mediated cell survival signaling, G protein signaling, and this signaling was lost in GRK5 KO hearts (152). GRK5 also plays a role in the transactivation of β1ARs and EGFR in a manner that confers cardioprotection (153). Tg mice with cardiac-specific overexpression of the wild-type murine β1AR or β1ARs lacking PKA phosphorylation sites exhibited no deleterious effect after 7 days of isoproterenol, while mice overexpressing β1ARs lacking GRK phosphorylation sites exhibited significant cardiac dysfunction and left ventricular dilation (Figure 3-1) (153). The mechanism for transactivation required GRK5-mediated phosphorylation of the β1AR and β-arrestin 1 and 2 recruitment in the mouse heart in vivo, with downstream Src, matrix metalloproteinase (MMP) activation and heparin-binding EGF-like growth factor (HB-EGF) stimulation of EGFR demonstrated in HEK293 cells in vitro (Figure 3-1) (152). These data imply that in contrast to the pathological signaling triggered by nuclear translocation of GRK5, phosphorylation of GPCRs at the membrane may be protective, highlighting a potential dichotomy for the canonical and non-canonical mechanisms of GRK5 signaling in cardiomyocytes (Figure 3). These data suggest that modulating the cellular localization of GRK5, perhaps by targeting the nuclear localization sequence (139), may be a more effective strategy to reduce the deleterious effects of GRK5 while allowing its known cardioprotective effects at the membrane.
This potential dichotomy is particularly important given the ongoing development of selective GRK inhibitors. While seeking to develop more specific small molecular inhibitors of GRK2, the Tesmer lab generated the rationally designed inhibitor CCG215022, and found through in vitro phosphorylation and cardiomyocyte contractility assays that it exhibited nanomolar potency at both GRK2 and GRK5 (154). This compound significantly stabilized GRK5 during a heat denaturation assay; therefore, it was utilized to optimize crystallization conditions for bovine GRK5, producing a 2.4 Å structure with the inhibitor bound within the hydrophobic region of the kinase domain (154). This solved crystal structure of GRK5 will facilitate investigation not only into the design of kinase inhibitors, but also a more thorough investigation of other protein interactions and a potential means for their allosteric modulation, that may prove useful in targeting the nuclear translocation of this enzyme.
Summary
It has become clear from all of these studies that GRK2, GRK3, and GRK5 play distinct and important roles in regulating cardiovascular physiology and in the development of pathophysiology during cardiac disease. Furthermore, one can appreciate that this GRK-mediated regulation does not occur strictly through the classic activity of GPCR phosphorylation, but through phosphorylation of non-GCPR targets and kinase-independent protein-protein and protein-DNA interactions. The diverse expression patterns of these kinases, as well as their unique functions within each cell type, underlie their diverse biological functions. While some of these have been elucidated as seen above, in general little is still known regarding several key aspects of GRK function. For example, work outlined in this article hints at Gβγ subunit selectivity as a mechanism for GPCR selectivity of GRK2 and GRK3; however, it is also known that GRKs contain GPCR recognitions sequences within their N-termini and these may also play a role in receptor targeting. Nonetheless, these studies support the idea of a dynamic “GRK interactome” in which novel interacting partners may associate with functionally distinct domains of GRKs and thereby participate in GRK-mediated regulation of cell signaling and function (43, 59). GRKs are known to regulate diverse cellular processes including cell migration (155), metabolism (156–158), and apoptosis (89), often through translocation to distinct intracellular compartments such as the mitochondria for GRK2 and the nucleus for GRK5. Therefore, elucidating the identity and any GRK selectivity for these intermolecular interactions and how they modulate various cellular processes may provide novel insight into the canonical and non-canonical mechanisms of action of GRKs in cardiac physiology and pathophysiology. In general, much remains to be elucidated regarding the mechanisms that regulate GRK expression, intermolecular interactions, localization and activity and how these mechanisms translate to alterations in cardiovascular signaling and function.
As mentioned above, cardiac expression levels of both GRK2 and GRK5 are elevated during human HF. Thus, an important element of GRK activity that remains to be elucidated is the mechanism governing GRK transcriptome regulation. Circulating catecholamines and βAR activity correlate with increased GRK2 mRNA (159), and pharmacological stimulation of mitogenic T cells enhances GRK2 mRNA levels, while neither effects GRK5 or GRK6 mRNA expression (160). Further, analysis of the human GRK2 gene promoter in aortic smooth muscle cells uncovered increased GRK2 promoter activity in response to vasoconstriction and hypertrophy, while inflammatory cytokine exposure led to transcriptional repression (161). One potential mechanism for these alterations in GRK transcript levels may be microRNA regulation, as these approximately 22 nucleotide transcripts bind to the 3′ untranslated region (3′UTR) of mRNA transcripts repress protein translation by decapping and deadenylation (162). Importantly, changes in microRNA expression are dynamic, and the expression of several microRNAs has recently been shown to be altered in both murine and human HF (162–165). Analysis of the 3′ UTR of both GRKs has revealed potential microRNA binding sites that may regulate the expression of GRK2 and GRK5, and Kaposi’s sarcoma-associated herpes virus microRNA has recently been shown to directly target GRK2, altering migration and infiltration of endothelial cells (166). This raises an interesting question of whether downregulation of GRK-targeting microRNAs during HF may relieve translational regulation and allow for their increased levels during disease. This would open new avenues of therapeutic intervention wherein microRNA mimics could be delivered to restrict the elevation in GRK levels and thereby prevent the pathologic activity of these enzymes in cardiac remodeling. This would require an elucidation of the microRNA species that target GRKs and whether they demonstrate specificity in their targeting.
Over the last 20+ years GRKs have been a focus of research investigations into the mechanism by which they coordinate both the activity of diverse GPCRs in numerous cells types throughout the body, and how they regulate additional, non GPCR activities from cell motility to metabolism. This research has uncovered mechanisms for receptor selectivity, identified non-receptor targets, and uncovered regulatory post-translational modifications and trafficking that all contribute to the complex function of GRKs. Throughout these studies GRK2 and GRK5, in particular, have arisen as major contributors not only in the regulation of cardiovascular signaling and function in a normal heart, but as driving forces in the molecular alterations occurring during cardiac disease (167). The role of GRK2 in downregulating βAR density and facilitating sympathetic overdrive during systolic HF and the role of GRK5 in mediating the increase in hypertrophic gene transcription during pressure overload have now been well characterized. The role of both GRK2 and GRK5 in metabolism and cell survival signaling are not completely understood, but an appreciation that their upregulation may be detrimental has emerged. Overall, GRK2 and GRK5 have become viable targets for the development of human HF therapies, and research has begun to achieve selectivity and potency for small molecule inhibition of these enzymes. As research continues to define the regulation of GRK2 and GRK5 and their mechanism of action during canonical and non-canonical interactions, more informed decisions may be made regarding the targeting of their kinase domain or other allosteric sites for the inhibition of these kinases in the treatment of human disease.
Acknowledgments
Disclosure of Funding
W. J. Koch is the W. W. Smith Chair in Cardiovascular Medicine and is funded by National Heart, Lung, and Blood Institute Grants R37 HL061690, R01 HL085503, P01 HL091799, P01 HL75443 (Project 2), and P01 HL108806 (Project 3). S. M. Schumacher was funded by a postdoctoral Brody Family Medical Trust Fund Fellowship and is now funded by National Heart, Lung, and Blood Institute Grant 1K99HL132882.
References
- 1.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta physiologica. 2007 May;190(1):9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 2.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annual review of pharmacology and toxicology. 2013;53:531–56. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ. Structure and mechanism of the G protein-coupled receptor kinases. The Journal of biological chemistry. 1993 Nov 15;268(32):23735–8. [PubMed] [Google Scholar]
- 4.Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends in cardiovascular medicine. 2000 Feb;10(2):81–9. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 5.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nature reviews Molecular cell biology. 2002 Sep;3(9):639–50. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 6.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annual review of biochemistry. 1998;67:653–92. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 7.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annual review of physiology. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nature reviews Drug discovery. 2010 May;9(5):373–86. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jun 16;106(24):9649–54. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nature reviews Molecular cell biology. 2010 Jan;11(1):9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 11.Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiological reviews. 2015 Apr;95(2):377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annual review of physiology. 2007;69:511–34. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 13.Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999 Dec;24(4):1029–36. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 14.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circulation research. 2006 Sep 15;99(6):570–82. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Villar VA, Armando I, Jose PA, Zeng C. G Protein-Coupled Receptor Kinases: Crucial Regulators of Blood Pressure. Journal of the American Heart Association. 2016 Jul 07;5(7) doi: 10.1161/JAHA.116.003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homan KT, Tesmer JJ. Structural insights into G protein-coupled receptor kinase function. Current opinion in cell biology. 2014 Apr;27:25–31. doi: 10.1016/j.ceb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang ZM, Gold JI, Koch WJ. G protein-coupled receptor kinases in normal and failing myocardium. Frontiers in bioscience. 2011;16:3047–60. doi: 10.2741/3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisatomi O, Matsuda S, Satoh T, Kotaka S, Imanishi Y, Tokunaga F. A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS letters. 1998 Mar 13;424(3):159–64. doi: 10.1016/s0014-5793(98)00162-8. [DOI] [PubMed] [Google Scholar]
- 19.Inglese J, Koch WJ, Caron MG, Lefkowitz RJ. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992 Sep 10;359(6391):147–50. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Benovic JL, Wedegaertner PB. Plasma membrane and nuclear localization of G protein coupled receptor kinase 6A. Molecular biology of the cell. 2007 Aug;18(8):2960–9. doi: 10.1091/mbc.E07-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. The Journal of biological chemistry. 1996 Mar 15;271(11):6403–10. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 22.Stoffel RH, Randall RR, Premont RT, Lefkowitz RJ, Inglese J. Palmitoylation of G protein-coupled receptor kinase, GRK6. Lipid modification diversity in the GRK family. The Journal of biological chemistry. 1994 Nov 11;269(45):27791–4. [PubMed] [Google Scholar]
- 23.Thiyagarajan MM, Stracquatanio RP, Pronin AN, Evanko DS, Benovic JL, Wedegaertner PB. A predicted amphipathic helix mediates plasma membrane localization of GRK5. The Journal of biological chemistry. 2004 Apr 23;279(17):17989–95. doi: 10.1074/jbc.M310738200. [DOI] [PubMed] [Google Scholar]
- 24.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacology & therapeutics. 2012 Jan;133(1):40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mumby SM, Casey PJ, Gilman AG, Gutowski S, Sternweis PC. G protein gamma subunits contain a 20-carbon isoprenoid. Proceedings of the National Academy of Sciences of the United States of America. 1990 Aug;87(15):5873–7. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the beta gamma subunits of heterotrimeric G proteins on the beta-adrenergic receptor kinase. The Journal of biological chemistry. 1993 Apr 15;268(11):8256–60. [PubMed] [Google Scholar]
- 27.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992 Aug 28;257(5074):1264–7. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 28.Touhara K, Koch WJ, Hawes BE, Lefkowitz RJ. Mutational analysis of the pleckstrin homology domain of the beta-adrenergic receptor kinase. Differential effects on G beta gamma and phosphatidylinositol 4,5-bisphosphate binding. The Journal of biological chemistry. 1995 Jul 14;270(28):17000–5. doi: 10.1074/jbc.270.28.17000. [DOI] [PubMed] [Google Scholar]
- 29.DebBurman SK, Ptasienski J, Benovic JL, Hosey MM. G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein betagamma subunits. The Journal of biological chemistry. 1996 Sep 13;271(37):22552–62. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- 30.Yang P, Homan KT, Li Y, Cruz-Rodriguez O, Tesmer JJ, Chen Z. Effect of Lipid Composition on the Membrane Orientation of the G Protein-Coupled Receptor Kinase 2-Gbeta1gamma2 Complex. Biochemistry. 2016 May 24;55(20):2841–8. doi: 10.1021/acs.biochem.6b00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapham DE, Neer EJ. G protein beta gamma subunits. Annual review of pharmacology and toxicology. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 32.Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annual review of pharmacology and toxicology. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hebert TE. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacological reviews. 2013 Apr;65(2):545–77. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 34.Huang CC, Tesmer JJ. Recognition in the face of diversity: interactions of heterotrimeric G proteins and G protein-coupled receptor (GPCR) kinases with activated GPCRs. The Journal of biological chemistry. 2011 Mar 11;286(10):7715–21. doi: 10.1074/jbc.R109.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nature reviews Neuroscience. 2001 Oct;2(10):727–33. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 36.Dorn GW., 2nd GRK mythology: G-protein receptor kinases in cardiovascular disease. Journal of molecular medicine. 2009 May;87(5):455–63. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 37.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993 Feb;87(2):454–63. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 38.Cho MC, Rapacciuolo A, Koch WJ, Kobayashi Y, Jones LR, Rockman HA. Defective beta-adrenergic receptor signaling precedes the development of dilated cardiomyopathy in transgenic mice with calsequestrin overexpression. The Journal of biological chemistry. 1999 Aug 6;274(32):22251–6. doi: 10.1074/jbc.274.32.22251. [DOI] [PubMed] [Google Scholar]
- 39.Maurice JP, Shah AS, Kypson AP, Hata JA, White DC, Glower DD, Koch WJ. Molecular beta-adrenergic signaling abnormalities in failing rabbit hearts after infarction. The American journal of physiology. 1999 Jun;276(6 Pt 2):H1853–60. doi: 10.1152/ajpheart.1999.276.6.H1853. [DOI] [PubMed] [Google Scholar]
- 40.Ping P, Anzai T, Gao M, Hammond HK. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. The American journal of physiology. 1997 Aug;273(2 Pt 2):H707–17. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 41.Yi XP, Gerdes AM, Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002 Jun;39(6):1058–63. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- 42.Hullmann J, Traynham CJ, Coleman RC, Koch WJ. The expanding GRK interactome: Implications in cardiovascular disease and potential for therapeutic development. Pharmacological research. 2016 Aug;110:52–64. doi: 10.1016/j.phrs.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. British journal of pharmacology. 2010 Jun;160(4):821–32. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterne-Marr R, Dhami GK, Tesmer JJ, Ferguson SS. Characterization of GRK2 RH domain-dependent regulation of GPCR coupling to heterotrimeric G proteins. Methods in enzymology. 2004;390:310–36. doi: 10.1016/S0076-6879(04)90020-1. [DOI] [PubMed] [Google Scholar]
- 45.Pao CS, Benovic JL. Phosphorylation-independent desensitization of G protein-coupled receptors? Science’s STKE : signal transduction knowledge environment. 2002 Oct 8;2002(153):pe42. doi: 10.1126/stke.2002.153.pe42. [DOI] [PubMed] [Google Scholar]
- 46.Willets JM, Nahorski SR, Challiss RA. Roles of phosphorylation-dependent and -independent mechanisms in the regulation of M1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 in hippocampal neurons. The Journal of biological chemistry. 2005 May 13;280(19):18950–8. doi: 10.1074/jbc.M412682200. [DOI] [PubMed] [Google Scholar]
- 47.Dhami GK, Anborgh PH, Dale LB, Sterne-Marr R, Ferguson SS. Phosphorylation-independent regulation of metabotropic glutamate receptor signaling by G protein-coupled receptor kinase 2. The Journal of biological chemistry. 2002 Jul 12;277(28):25266–72. doi: 10.1074/jbc.M203593200. [DOI] [PubMed] [Google Scholar]
- 48.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovascular research. 2006 Jan;69(1):46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Lafarga V, Mayor F, Jr, Penela P. The interplay between G protein-coupled receptor kinase 2 (GRK2) and histone deacetylase 6 (HDAC6) at the crossroads of epithelial cell motility. Cell adhesion & migration. 2012 Nov-Dec;6(6):495–501. doi: 10.4161/cam.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penela P, Nogues L, Mayor F., Jr Role of G protein-coupled receptor kinases in cell migration. Current opinion in cell biology. 2014 Apr;27:10–7. doi: 10.1016/j.ceb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, Murga C, Fernandez-Veledo S, Mayor F, Jr, Lorenzo M. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010 Oct;59(10):2407–17. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Molecular endocrinology. 2005 Nov;19(11):2760–8. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 53.Mayor F, Jr, Lucas E, Jurado-Pueyo M, Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Fernandez-Veledo S, Murga C. G Protein-coupled receptor kinase 2 (GRK2): A novel modulator of insulin resistance. Archives of physiology and biochemistry. 2011 Jul;117(3):125–30. doi: 10.3109/13813455.2011.584693. [DOI] [PubMed] [Google Scholar]
- 54.Woodall MC, Ciccarelli M, Woodall BP, Koch WJ. G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism. Circulation research. 2014 May 9;114(10):1661–70. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penela P, Ribas C, Mayor F., Jr Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003 Nov;15(11):973–81. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 56.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003 Dec 04;426(6966):574–9. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- 57.Jimenez-Sainz MC, Murga C, Kavelaars A, Jurado-Pueyo M, Krakstad BF, Heijnen CJ, Mayor F, Jr, Aragay AM. G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Molecular biology of the cell. 2006 Jan;17(1):25–31. doi: 10.1091/mbc.E05-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nature medicine. 2005 Sep;11(9):952–8. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 59.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochimica et biophysica acta. 2007 Apr;1768(4):913–22. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 60.Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proceedings of the National Academy of Sciences of the United States of America. 1998 Nov 24;95(24):14082–7. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. The Journal of biological chemistry. 2001 Jun 1;276(22):18953–9. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- 62.Carman CV, Lisanti MP, Benovic JL. Regulation of G protein-coupled receptor kinases by caveolin. The Journal of biological chemistry. 1999 Mar 26;274(13):8858–64. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- 63.Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. The Journal of biological chemistry. 1997 Jul 18;272(29):18273–80. doi: 10.1074/jbc.272.29.18273. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacological reviews. 2001 Mar;53(1):1–24. [PubMed] [Google Scholar]
- 65.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. The Journal of biological chemistry. 2000 Dec 29;275(52):41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 66.Steinberg SF. The molecular basis for distinct beta-adrenergic receptor subtype actions in cardiomyocytes. Circulation research. 1999 Nov 26;85(11):1101–11. doi: 10.1161/01.res.85.11.1101. [DOI] [PubMed] [Google Scholar]
- 67.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira de Souza A, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. American journal of physiology Cell physiology. 2003 Feb;284(2):C457–74. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 68.Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M, Shirani J, Razani B, Tang B, Jelicks LA, Factor SM, Weiss LM, Tanowitz HB, Lisanti MP. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. The American journal of pathology. 2002 Jun;160(6):2207–17. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. The Journal of biological chemistry. 1999 Dec 3;274(49):34531–4. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- 70.Carman CV, Parent JL, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. The Journal of biological chemistry. 1999 Nov 26;274(48):34483–92. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 71.Sallese M, Mariggio S, D’Urbano E, Iacovelli L, De Blasi A. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated galphaq. Molecular pharmacology. 2000 Apr;57(4):826–31. [PubMed] [Google Scholar]
- 72.Sterne-Marr R, Tesmer JJ, Day PW, Stracquatanio RP, Cilente JA, O’Connor KE, Pronin AN, Benovic JL, Wedegaertner PB. G protein-coupled receptor Kinase 2/G alpha q/11 interaction. A novel surface on a regulator of G protein signaling homology domain for binding G alpha subunits. The Journal of biological chemistry. 2003 Feb 21;278(8):6050–8. doi: 10.1074/jbc.M208787200. [DOI] [PubMed] [Google Scholar]
- 73.Usui H, Nishiyama M, Moroi K, Shibasaki T, Zhou J, Ishida J, Fukamizu A, Haga T, Sekiya S, Kimura S. RGS domain in the amino-terminus of G protein-coupled receptor kinase 2 inhibits Gq-mediated signaling. International journal of molecular medicine. 2000 Apr;5(4):335–40. doi: 10.3892/ijmm.5.4.335. [DOI] [PubMed] [Google Scholar]
- 74.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., 2nd Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proceedings of the National Academy of Sciences of the United States of America. 1997 Jul 22;94(15):8121–6. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dorn GW, 2nd, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proceedings of the National Academy of Sciences of the United States of America. 1999 May 25;96(11):6400–5. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 2nd Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proceedings of the National Academy of Sciences of the United States of America. 1998 Aug 18;95(17):10140–5. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogers JH, Tsirka A, Kovacs A, Blumer KJ, Dorn GW, 2nd, Muslin AJ. RGS4 reduces contractile dysfunction and hypertrophic gene induction in Galpha q overexpressing mice. Journal of molecular and cellular cardiology. 2001 Feb;33(2):209–18. doi: 10.1006/jmcc.2000.1307. [DOI] [PubMed] [Google Scholar]
- 78.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998 Apr 24;280(5363):574–7. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 79.Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001 Mar 13;103(10):1453–8. doi: 10.1161/01.cir.103.10.1453. [DOI] [PubMed] [Google Scholar]
- 80.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002 Jan 1;105(1):85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 81.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005 Dec 9;310(5754):1686–90. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 82.Day PW, Tesmer JJ, Sterne-Marr R, Freeman LC, Benovic JL, Wedegaertner PB. Characterization of the GRK2 binding site of Galphaq. The Journal of biological chemistry. 2004 Dec 17;279(51):53643–52. doi: 10.1074/jbc.M401438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schumacher SM, Gao E, Cohen M, Lieu M, Chuprun JK, Koch WJ. A peptide of the RGS domain of GRK2 binds and inhibits Galpha(q) to suppress pathological cardiac hypertrophy and dysfunction. Science signaling. 2016 Mar 22;9(420):ra30. doi: 10.1126/scisignal.aae0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eichmann T, Lorenz K, Hoffmann M, Brockmann J, Krasel C, Lohse MJ, Quitterer U. The amino-terminal domain of G-protein-coupled receptor kinase 2 is a regulatory Gbeta gamma binding site. The Journal of biological chemistry. 2003 Mar 07;278(10):8052–7. doi: 10.1074/jbc.M204795200. [DOI] [PubMed] [Google Scholar]
- 85.Fusco A, Santulli G, Sorriento D, Cipolletta E, Garbi C, Dorn GW, 2nd, Trimarco B, Feliciello A, Iaccarino G. Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cellular signalling. 2012 Feb;24(2):468–75. doi: 10.1016/j.cellsig.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obrenovich ME, Palacios HH, Gasimov E, Leszek J, Aliev G. The GRK2 Overexpression Is a Primary Hallmark of Mitochondrial Lesions during Early Alzheimer Disease. Cardiovascular psychiatry and neurology. 2009;2009:327360. doi: 10.1155/2009/327360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circulation research. 2010 Oct 29;107(9):1140–9. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fan Q, Chen M, Zuo L, Shang X, Huang MZ, Ciccarelli M, Raake P, Brinks H, Chuprun KJ, Dorn GW, 2nd, Koch WJ, Gao E. Myocardial Ablation of G Protein-Coupled Receptor Kinase 2 (GRK2) Decreases Ischemia/Reperfusion Injury through an Anti-Intrinsic Apoptotic Pathway. PloS one. 2013;8(6):e66234. doi: 10.1371/journal.pone.0066234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circulation research. 2013 Apr 12;112(8):1121–34. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo J, Benovic JL. G protein-coupled receptor kinase interaction with Hsp90 mediates kinase maturation. The Journal of biological chemistry. 2003 Dec 19;278(51):50908–14. doi: 10.1074/jbc.M307637200. [DOI] [PubMed] [Google Scholar]
- 91.Sato PY, Chuprun JK, Ibetti J, Cannavo A, Drosatos K, Elrod JW, Koch WJ. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. Journal of molecular and cellular cardiology. 2015 Dec;89(Pt B):360–4. doi: 10.1016/j.yjmcc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Penela P, Elorza A, Sarnago S, Mayor F., Jr Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. The EMBO journal. 2001 Sep 17;20(18):5129–38. doi: 10.1093/emboj/20.18.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sarnago S, Elorza A, Mayor F., Jr Agonist-dependent phosphorylation of the G protein-coupled receptor kinase 2 (GRK2) by Src tyrosine kinase. The Journal of biological chemistry. 1999 Nov 26;274(48):34411–6. doi: 10.1074/jbc.274.48.34411. [DOI] [PubMed] [Google Scholar]
- 94.Mariggio S, Garcia-Hoz C, Sarnago S, De Blasi A, Mayor F, Jr, Ribas C. Tyrosine phosphorylation of G-protein-coupled-receptor kinase 2 (GRK2) by c-Src modulates its interaction with Galphaq. Cellular signalling. 2006 Nov;18(11):2004–12. doi: 10.1016/j.cellsig.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Elorza A, Sarnago S, Mayor F., Jr Agonist-dependent modulation of G protein-coupled receptor kinase 2 by mitogen-activated protein kinases. Molecular pharmacology. 2000 Apr;57(4):778–83. doi: 10.1124/mol.57.4.778. [DOI] [PubMed] [Google Scholar]
- 96.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007 May 4;129(3):511–22. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 97.Huang ZM, Gao E, Fonseca FV, Hayashi H, Shang X, Hoffman NE, Chuprun JK, Tian X, Tilley DG, Madesh M, Lefer DJ, Stamler JS, Koch WJ. Convergence of G protein-coupled receptor and S-nitrosylation signaling determines the outcome to cardiac ischemic injury. Science signaling. 2013 Oct 29;6(299):ra95. doi: 10.1126/scisignal.2004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avendano MS, Lucas E, Jurado-Pueyo M, Martinez-Revelles S, Vila-Bedmar R, Mayor F, Jr, Salaices M, Briones AM, Murga C. Increased nitric oxide bioavailability in adult GRK2 hemizygous mice protects against angiotensin II-induced hypertension. Hypertension. 2014 Feb;63(2):369–75. doi: 10.1161/HYPERTENSIONAHA.113.01991. [DOI] [PubMed] [Google Scholar]
- 99.Guccione M, Ettari R, Taliani S, Da Settimo F, Zappala M, Grasso S. G-Protein-Coupled Receptor Kinase 2 (GRK2) Inhibitors: Current Trends and Future Perspectives. Journal of medicinal chemistry. 2016 Oct 27;59(20):9277–94. doi: 10.1021/acs.jmedchem.5b01939. [DOI] [PubMed] [Google Scholar]
- 100.Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, Sklar LA, Koch WJ, Tesmer JJ. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS chemical biology. 2012 Nov 16;7(11):1830–9. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, JJGT, Koch WJ. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Science translational medicine. 2015 Mar 4;7(277):277ra31. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Homan KT, Wu E, Wilson MW, Singh P, Larsen SD, Tesmer JJ. Structural and functional analysis of g protein-coupled receptor kinase inhibition by paroxetine and a rationally designed analog. Molecular pharmacology. 2014 Feb;85(2):237–48. doi: 10.1124/mol.113.089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waldschmidt HV, Homan KT, Cruz-Rodriguez O, Cato MC, Waninger-Saroni J, Larimore KM, Cannavo A, Song J, Cheung JY, Kirchhoff PD, Koch WJ, Tesmer JJ, Larsen SD. Structure-Based Design, Synthesis, and Biological Evaluation of Highly Selective and Potent G Protein-Coupled Receptor Kinase 2 Inhibitors. Journal of medicinal chemistry. 2016 Apr 28;59(8):3793–807. doi: 10.1021/acs.jmedchem.5b02000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benovic JL, Onorato JJ, Arriza JL, Stone WC, Lohse M, Jenkins NA, Gilbert DJ, Copeland NG, Caron MG, Lefkowitz RJ. Cloning, expression, and chromosomal localization of beta-adrenergic receptor kinase 2. A new member of the receptor kinase family. The Journal of biological chemistry. 1991 Aug 15;266(23):14939–46. [PubMed] [Google Scholar]
- 105.Vinge LE, Oie E, Andersson Y, Grogaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and beta-arrestin isoforms in congestive heart failure in rats. American journal of physiology Heart and circulatory physiology. 2001 Dec;281(6):H2490–9. doi: 10.1152/ajpheart.2001.281.6.H2490. [DOI] [PubMed] [Google Scholar]
- 106.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995 Jun 2;268(5215):1350–3. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 107.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1996 Sep 3;93(18):9954–9. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iaccarino G, Rockman HA, Shotwell KF, Tomhave ED, Koch WJ. Myocardial overexpression of GRK3 in transgenic mice: evidence for in vivo selectivity of GRKs. The American journal of physiology. 1998 Oct;275(4 Pt 2):H1298–306. doi: 10.1152/ajpheart.1998.275.4.H1298. [DOI] [PubMed] [Google Scholar]
- 109.Walker JK, Peppel K, Lefkowitz RJ, Caron MG, Fisher JT. Altered airway and cardiac responses in mice lacking G protein-coupled receptor kinase 3. The American journal of physiology. 1999 Apr;276(4 Pt 2):R1214–21. doi: 10.1152/ajpregu.1999.276.4.R1214. [DOI] [PubMed] [Google Scholar]
- 110.Vinge LE, Andressen KW, Attramadal T, Andersen GO, Ahmed MS, Peppel K, Koch WJ, Freedman NJ, Levy FO, Skomedal T, Osnes JB, Attramadal H. Substrate specificities of g protein-coupled receptor kinase-2 and -3 at cardiac myocyte receptors provide basis for distinct roles in regulation of myocardial function. Molecular pharmacology. 2007 Sep;72(3):582–91. doi: 10.1124/mol.107.035766. [DOI] [PubMed] [Google Scholar]
- 111.Alcantara-Hernandez R, Casas-Gonzalez P, Garcia-Sainz JA. Roles of c-Src in alpha1B-adrenoceptor phosphorylation and desensitization. Autonomic & autacoid pharmacology. 2008 Jan;28(1):29–39. doi: 10.1111/j.1474-8673.2007.00414.x. [DOI] [PubMed] [Google Scholar]
- 112.Chuang TT, Pompili E, Paolucci L, Sallese M, De Gioia L, Salmona M, De Blasi A. Identification of a short sequence highly divergent between beta-adrenergic-receptor kinases 1 and 2 that determines the affinity of binding to betagamma subunits of heterotrimeric guanine-nucleotide-binding regulatory proteins. European journal of biochemistry / FEBS. 1997 May 1;245(3):533–40. doi: 10.1111/j.1432-1033.1997.00533.x. [DOI] [PubMed] [Google Scholar]
- 113.Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proceedings of the National Academy of Sciences of the United States of America. 1997 Mar 18;94(6):2180–5. doi: 10.1073/pnas.94.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992 Jul 30;358(6385):424–6. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 115.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993 Feb 5;259(5096):832–4. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 116.Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, Edvardsen T, Bjornerheim R, Ahmed MS, Mikkelsen BW, Oie E, Attramadal T, Skomedal T, Smiseth OA, Koch WJ, Larsen TS, Attramadal H. Cardiac-restricted expression of the carboxyl-terminal fragment of GRK3 Uncovers Distinct Functions of GRK3 in regulation of cardiac contractility and growth: GRK3 controls cardiac alpha1-adrenergic receptor responsiveness. The Journal of biological chemistry. 2008 Apr 18;283(16):10601–10. doi: 10.1074/jbc.M708912200. [DOI] [PubMed] [Google Scholar]
- 117.Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, Woodcock EA, Feneley MP, Graham RM. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circulation research. 2001 Aug 17;89(4):343–50. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- 118.Du XJ, Fang L, Gao XM, Kiriazis H, Feng X, Hotchkin E, Finch AM, Chaulet H, Graham RM. Genetic enhancement of ventricular contractility protects against pressure-overload-induced cardiac dysfunction. Journal of molecular and cellular cardiology. 2004 Nov;37(5):979–87. doi: 10.1016/j.yjmcc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 119.Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, Finch AM, Hannan RA, Dart AM, Graham RM. Transgenic alpha1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovascular research. 2006 Sep 1;71(4):735–43. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 120.von Lueder TG, Gravning J, How OJ, Vinge LE, Ahmed MS, Krobert KA, Levy FO, Larsen TS, Smiseth OA, Aasum E, Attramadal H. Cardiomyocyte-restricted inhibition of G protein-coupled receptor kinase-3 attenuates cardiac dysfunction after chronic pressure overload. American journal of physiology Heart and circulatory physiology. 2012 Jul;303(1):H66–74. doi: 10.1152/ajpheart.00724.2011. [DOI] [PubMed] [Google Scholar]
- 121.Barnes PJ. Muscarinic receptors in airways: recent developments. Journal of applied physiology. 1990 May;68(5):1777–85. doi: 10.1152/jappl.1990.68.5.1777. [DOI] [PubMed] [Google Scholar]
- 122.Cabezas GA, Graf PD, Nadel JA. Sympathetic versus parasympathetic nervous regulation of airways in dogs. J Appl Physiol. 1971 Nov;31(5):651–5. doi: 10.1152/jappl.1971.31.5.651. [DOI] [PubMed] [Google Scholar]
- 123.Garssen J, Van Loveren H, Gierveld CM, Van der Vliet H, Nijkamp FP. Functional characterization of muscarinic receptors in murine airways. British journal of pharmacology. 1993 May;109(1):53–60. doi: 10.1111/j.1476-5381.1993.tb13530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. The American journal of physiology. 1997 Feb;272(2 Pt 2):H1053–61. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- 125.Head GA. Central monoamine neurons and cardiovascular control. Kidney international Supplement. 1992 Jun;37:S8–13. [PubMed] [Google Scholar]
- 126.Arnett DK, Devereux RB, Kitzman D, Oberman A, Hopkins P, Atwood L, Dewan A, Rao DC Hypertension Genetic Epidemiology Network Study G. Linkage of left ventricular contractility to chromosome 11 in humans: The HyperGEN Study. Hypertension. 2001 Oct;38(4):767–72. doi: 10.1161/hy1001.092650. [DOI] [PubMed] [Google Scholar]
- 127.Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. The Journal of biological chemistry. 1994 Mar 4;269(9):6832–41. [PubMed] [Google Scholar]
- 128.Kunapuli P, Benovic JL. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proceedings of the National Academy of Sciences of the United States of America. 1993 Jun 15;90(12):5588–92. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burkhalter MD, Fralish GB, Premont RT, Caron MG, Philipp M. Grk5l controls heart development by limiting mTOR signaling during symmetry breaking. Cell reports. 2013 Aug 29;4(4):625–32. doi: 10.1016/j.celrep.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Philipp M, Berger IM, Just S, Caron MG. Overlapping and opposing functions of G protein-coupled receptor kinase 2 (GRK2) and GRK5 during heart development. The Journal of biological chemistry. 2014 Sep 19;289(38):26119–30. doi: 10.1074/jbc.M114.551952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yi XP, Zhou J, Baker J, Wang X, Gerdes AM, Li F. Myocardial expression and redistribution of GRKs in hypertensive hypertrophy and failure. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2005 Jan;282(1):13–23. doi: 10.1002/ar.a.20143. [DOI] [PubMed] [Google Scholar]
- 132.Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. European journal of pharmacology. 2004 Apr 12;489(3):167–77. doi: 10.1016/j.ejphar.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 133.Monto F, Oliver E, Vicente D, Rueda J, Aguero J, Almenar L, Ivorra MD, Barettino D, D’Ocon P. Different expression of adrenoceptors and GRKs in the human myocardium depends on heart failure etiology and correlates to clinical variables. American journal of physiology Heart and circulatory physiology. 2012 Aug 1;303(3):H368–76. doi: 10.1152/ajpheart.01061.2011. [DOI] [PubMed] [Google Scholar]
- 134.Aguero J, Almenar L, Monto F, Oliver E, Sanchez-Lazaro I, Vicente D, Martinez-Dolz L, D’Ocon P, Rueda J, Salvador A. Myocardial G protein receptor-coupled kinase expression correlates with functional parameters and clinical severity in advanced heart failure. Journal of cardiac failure. 2012 Jan;18(1):53–61. doi: 10.1016/j.cardfail.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Pronin AN, Benovic JL. Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. The Journal of biological chemistry. 1997 Feb 7;272(6):3806–12. doi: 10.1074/jbc.272.6.3806. [DOI] [PubMed] [Google Scholar]
- 136.Traynham CJ, Hullmann J, Koch WJ. Canonical and non-canonical actions of GRK5 in the heart. Journal of molecular and cellular cardiology. 2016 Mar;92:196–202. doi: 10.1016/j.yjmcc.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martini JS, Raake P, Vinge LE, DeGeorge BR, Jr, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America. 2008 Aug 26;105(34):12457–62. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Johnson LR, Robinson JD, Lester KN, Pitcher JA. Distinct structural features of G protein-coupled receptor kinase 5 (GRK5) regulate its nuclear localization and DNA-binding ability. PloS one. 2013;8(5):e62508. doi: 10.1371/journal.pone.0062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson LR, Scott MG, Pitcher JA. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Molecular and cellular biology. 2004 Dec;24(23):10169–79. doi: 10.1128/MCB.24.23.10169-10179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, Tilley DG, Rabinowitz JE, Bossuyt J, Bers DM, Koch WJ. Nuclear translocation of cardiac G protein-Coupled Receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PloS one. 2013;8(3):e57324. doi: 10.1371/journal.pone.0057324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Matkovich SJ, Duan X, Gold JI, Koch WJ, Dorn GW., 2nd Nuclear effects of G-protein receptor kinase 5 on histone deacetylase 5-regulated gene transcription in heart failure. Circulation Heart failure. 2011 Sep;4(5):659–68. doi: 10.1161/CIRCHEARTFAILURE.111.962563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. The Journal of biological chemistry. 2000 Aug 25;275(34):26515–22. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 143.Gold JI, Gao E, Shang X, Premont RT, Koch WJ. Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circulation research. 2012 Sep 28;111(8):1048–53. doi: 10.1161/CIRCRESAHA.112.273367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hullmann JE, Grisanti LA, Makarewich CA, Gao E, Gold JI, Chuprun JK, Tilley DG, Houser SR, Koch WJ. GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circulation research. 2014 Dec 5;115(12):976–85. doi: 10.1161/CIRCRESAHA.116.304475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu P, Wang X, Gao N, Zhu H, Dai X, Xu Y, Ma C, Huang L, Liu Y, Qin C. G protein-coupled receptor kinase 5, overexpressed in the alpha-synuclein up-regulation model of Parkinson’s disease, regulates bcl-2 expression. Brain research. 2010 Jan 11;1307:134–41. doi: 10.1016/j.brainres.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 146.Valanne S, Myllymaki H, Kallio J, Schmid MR, Kleino A, Murumagi A, Airaksinen L, Kotipelto T, Kaustio M, Ulvila J, Esfahani SS, Engstrom Y, Silvennoinen O, Hultmark D, Parikka M, Ramet M. Genome-wide RNA interference in Drosophila cells identifies G protein-coupled receptor kinase 2 as a conserved regulator of NF-kappaB signaling. Journal of immunology. 2010 Jun 1;184(11):6188–98. doi: 10.4049/jimmunol.1000261. [DOI] [PubMed] [Google Scholar]
- 147.Islam KN, Bae JW, Gao E, Koch WJ. Regulation of nuclear factor kappaB (NF-kappaB) in the nucleus of cardiomyocytes by G protein-coupled receptor kinase 5 (GRK5) The Journal of biological chemistry. 2013 Dec 13;288(50):35683–9. doi: 10.1074/jbc.M113.529347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patial S, Shahi S, Saini Y, Lee T, Packiriswamy N, Appledorn DM, Lapres JJ, Amalfitano A, Parameswaran N. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. Journal of cellular physiology. 2011 May;226(5):1323–33. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, Benovic JL. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. The Journal of biological chemistry. 2006 Nov 10;281(45):34159–70. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 150.Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, Galasso G, Astone D, Piscione F, Pastore L, Trimarco B, Iaccarino G. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. Proceedings of the National Academy of Sciences of the United States of America. 2008 Nov 18;105(46):17818–23. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Science signaling. 2011 Aug 9;4(185):ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Science signaling. 2010 Jun 08;3(125):ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. The Journal of clinical investigation. 2007 Sep;117(9):2445–58. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Homan KT, Waldschmidt HV, Glukhova A, Cannavo A, Song J, Cheung JY, Koch WJ, Larsen SD, Tesmer JJ. Crystal Structure of G Protein-coupled Receptor Kinase 5 in Complex with a Rationally Designed Inhibitor. The Journal of biological chemistry. 2015 Aug 21;290(34):20649–59. doi: 10.1074/jbc.M115.647370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Penela P, Ribas C, Aymerich I, Eijkelkamp N, Barreiro O, Heijnen CJ, Kavelaars A, Sanchez-Madrid F, Mayor F., Jr G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. The EMBO journal. 2008 Apr 23;27(8):1206–18. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, Dorn GW, 2nd, Trimarco B, Iaccarino G, Koch WJ. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011 May 10;123(18):1953–62. doi: 10.1161/CIRCULATIONAHA.110.988642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovascular research. 2009 Dec 1;84(3):407–15. doi: 10.1093/cvr/cvp252. [DOI] [PubMed] [Google Scholar]
- 158.Usui I, Imamura T, Satoh H, Huang J, Babendure JL, Hupfeld CJ, Olefsky JM. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. The EMBO journal. 2004 Jul 21;23(14):2821–9. doi: 10.1038/sj.emboj.7600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998 Oct 27;98(17):1783–9. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 160.De Blasi A, Parruti G, Sallese M. Regulation of G protein-coupled receptor kinase subtypes in activated T lymphocytes. Selective increase of beta-adrenergic receptor kinase 1 and 2. The Journal of clinical investigation. 1995 Jan;95(1):203–10. doi: 10.1172/JCI117641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramos-Ruiz R, Penela P, Penn RB, Mayor F., Jr Analysis of the human G protein-coupled receptor kinase 2 (GRK2) gene promoter: regulation by signal transduction systems in aortic smooth muscle cells. Circulation. 2000 May 2;101(17):2083–9. doi: 10.1161/01.cir.101.17.2083. [DOI] [PubMed] [Google Scholar]
- 162.Condorelli G, Latronico MV, Dorn GW., 2nd microRNAs in heart disease: putative novel therapeutic targets? European heart journal. 2010 Mar;31(6):649–58. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW., 2nd MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circulation research. 2010 Jan 8;106(1):166–75. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2006 Nov 28;103(48):18255–60. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. Journal of molecular and cellular cardiology. 2008 Aug;45(2):185–92. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu M, Wang C, Li W, Lu W, Bai Z, Qin D, Yan Q, Zhu J, Krueger BJ, Renne R, Gao SJ, Lu C. A KSHV microRNA Directly Targets G Protein-Coupled Receptor Kinase 2 to Promote the Migration and Invasion of Endothelial Cells by Inducing CXCR2 and Activating AKT Signaling. PLoS pathogens. 2015 Sep;11(9):e1005171. doi: 10.1371/journal.ppat.1005171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cannavo A, Liccardo D, Eguchi A, Elliott KJ, Traynham CJ, Ibetti J, Eguchi S, Leosco D, Ferrara N, Rengo G, Koch WJ. Myocardial pathology induced by aldosterone is dependent on non-canonical activities of G protein-coupled receptor kinases. Nature communications. 2016;7:10877. doi: 10.1038/ncomms10877. [DOI] [PMC free article] [PubMed] [Google Scholar]