Abstract
The function of the meibomian gland in the upper and lower eyelids is critical to maintaining homeostasis at the ocular surface. Highly specialized meibocytes within the gland must differentiate and accumulate intracellular lipid droplets that are released into the tear film following rupture of the cell membrane. Proteases and their inhibitors have been recognized as key players in remodeling extracellular matrices and promoting the normal integrity of glandular tissue. They modulate a wide range of biological processes, such as cell proliferation and differentiation, and can contribute to disease when aberrantly expressed. Deciphering the role of proteolytic activity in the meibomian gland offers an opportunity to gain a more comprehensive and fundamental understanding of the developmental, physiological, and pathological processes associated with this gland.
Keywords: sebocyte, holocrine secretion, meibomian gland, matrix metalloproteinase, CD147
1. Introduction
Meibomian glands are large exocrine glands located in the in the tarsal plate of the eyelids that release their product, known as meibum, by holocrine secretion following disruption of the cell membrane. Lipids within the meibum have been shown to have multiple protective effects at the ocular surface, such as forming a smooth optical surface for the cornea and lowering the surface tension of the tear film (Bron et al., 2004; Millar and Schuett, 2015). Research during the last decade has led to the identification of key regulatory mechanisms critical to maintenance of the integrity and function of the meibomian gland and its alteration in pathological conditions. These advances have been facilitated by the development of novel imaging and analytical methods that allow the structural assessment of the meibomian glands during ocular surface disease and aging (Butovich et al., 2014; Fasanella et al., 2016; Parfitt et al., 2012). Furthermore, a better understanding of the biology of the meibomian gland and the tear lipidome is fueling advances in the identification of biomedical targets and potentially novel therapeutic approaches (Butovich, 2013; Parfitt et al., 2016a; Parfitt et al., 2016b). Proteases have been recognized as key players in a wide range of biological processes critical for the functioning and maintenance of glandular tissues, including control of cell proliferation and differentiation (Hooper, 2002). This review discusses recent findings on the impact of proteases and their regulators in the physiology and pathology of the meibomian gland and outlines directions for future research.
2. Proteases as regulators of biological events
Completion of the sequencing of the human genome has revealed that more than 2% of genes encode for proteases, a group of more than 500 hydrolytic enzymes with a broad range of regulatory functions, as well as their inhibitors (Craik et al., 2011; Puente et al., 2005). The activity of proteases can occur within and outside the cell, and can influence multiple fundamental processes in biology such as developmental patterning, control of homeostatic tissue remodeling, cell cycle regulation and apoptosis (Puente et al., 2003). Dysregulation of protease expression and proteolytic activity have been associated with a wide variety of diseases including cancer and inflammation (Turk, 2006).
The regulation of proteolytic activity is complex and strictly controlled at multiple levels during conditions of homeostasis in a tissue-specific manner. These include regulation at the transcriptional level by differential expression, and at the protein level by activation of the inactive proenzyme as well as binding of inhibitors and cofactors (Turk, 2006). Proteases can be classified in five major groups according to the nature of the amino acid residue or cofactor at the active site that is involved in the hydrolytic reaction, and are thus referred to as aspartic proteases, cysteine proteases, serine proteases, threonine proteases and metalloproteinases (Hooper, 2002). Metalloproteinases have a divalent metal ion at their active site, usually zinc or occasionally cobalt, iron or manganese, which are essential for enzyme activity.
The most abundant human protease genes are metalloproteinases and serine proteases, with 187 and 175 genes, respectively (Puente et al., 2005). Each class of proteinase appears to be related to specific biological processes; for example, metalloproteinases regulate the structural composition of the extracellular matrix whereas serine proteases enable immune responses (Page and Di Cera, 2008). Remodeling of the extracellular matrix by metalloproteinases reciprocally influences cell behavior and therefore allows adaptation to environmental change, as proteins within the extracellular matrix control differentiation, proliferation, survival and migration of cells (Hynes, 2009). In glandular epithelia, matrix metalloproteinases are necessary for cells to sprout from the side of the duct and initiate collective epithelial migration during branching morphogenesis. Indeed, mice carrying genetic defects in matrix metalloproteinases and their inhibitors are characterized by defects in branched organs (Lu et al., 2011).
3. Sebaceous glands and their secretion
3.1 Structure and differentiation of the sebaceous gland
Sebaceous glands are holocrine glands with lobulated structures connected to a common secretory duct found over most surfaces of the human body. They can be associated with the upper portion of the hair follicle within the pilosebaceous unit (Smith and Thiboutot, 2008). Free or ectopic sebaceous glands are not associated with hair follicles and can be found in areas such as the eyelids, the areola of the nipples, and around the genitals. Free sebaceous glands in the eyelid are known as meibomian glands. The primary function of a sebaceous gland is to secrete sebum and to contribute to the formation of a protective film that waterproofs and lubricates the skin. Several other functions have been proposed that include antimicrobial and antioxidative properties, since the sebum contains a range of fatty acids with self-sterilizing properties and vitamin E (Smith and Thiboutot, 2008).
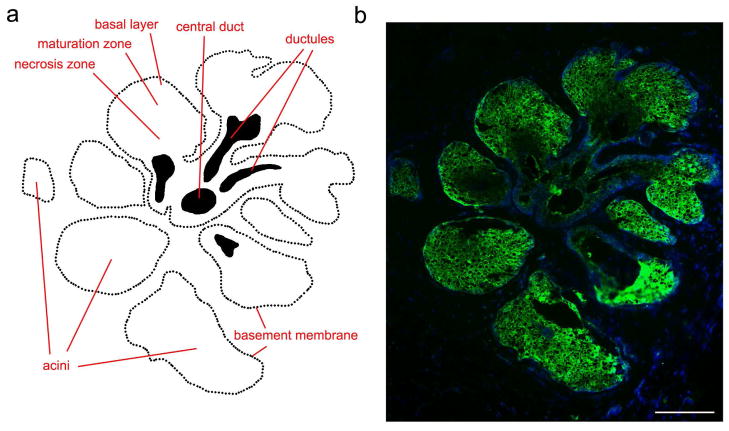
Sebocytes can be distinguished according to their differentiation state (Figure 1a). Within the basal layer or peripheral zone of the acinus surrounded by a basement membrane, there are mitotically active cells that continuously proliferate through life (Niemann and Horsley, 2012). As cells prepare for secretion, they enter another compartment, the maturation zone, characterized by enlarged sebocytes that accumulate large numbers of cytoplasmic lipid droplets. These are gradually pushed towards the center of the gland into the necrosis zone, where cell disruption and the release of lipids and cellular debris occur. Finally, the lipid-containing sebum passes a specialized secretory duct composed of stratified squamous epithelium before reaching the surface. The relative amount of each lipid within the sebum varies between different types of sebaceous glands and among mammalian species (Niemann and Horsley, 2012).
Figure 1. Proteolytic activity in the normal human meibomian gland.
(a) Schematic representation of the structure of the meibomian gland. Cells at varying degrees of differentiation are present within the secretory acini, corresponding to proliferating cells in the basal layer and differentiating cells that accumulate intracellular lipid droplets in the maturation zone. Fully differentiated cells are present in the necrosis zone, where the meibum is produced and then released through small ductules into a long central duct. (b) In situ gelatin zymography (green) showing localization of gelatinolytic activity in secretory acini of a meibomian gland, shown schematically in (a). Within the acinus, activity was detected in proliferating and differentiating cells, and decreased towards the necrosis zone. Nuclei were counterstained using DAPI (blue). Scale bar, 100 μm. Adapted from Mauris et al. (Mauris et al., 2015).
3.2 The meibomian gland
Meibomian glands consist of clusters of secretory acini located throughout the length of the tarsal plates in the upper and lower lids. Small ductules connect these acini to a long central duct in each gland, whose terminal part opens as an orifice in the free margin of the eyelids (Knop et al., 2011). There are 25–40 glands in the upper lid with a length of approximately 5.5 mm at the center, whereas the lower lid contains 20–30 glands approximately 2 mm in length. Each gland contains between 10 and 15 secretory acini, with the highest density found in the upper lid. Secretions from the meibomian gland are known to play important roles in promoting stability and reducing the evaporation of the tear film (Mathers, 1993). Several age-related changes have been observed in the meibomian gland, including decreased number of active glands and altered cell differentiation and lipid secretion (Nien et al., 2011; Norn, 1985; Sullivan et al., 2006).
4. Proteolytic activity in the meibomian gland
4.1 Localization
Critical to maintaining normal function in tissues where radical remodeling occurs is the extracellular matrix, a major constituent of the cellular microenvironment composed primarily of proteoglycans and fibrous proteins (Lu et al., 2011). The remodeling of the extracellular matrix during homeostatic conditions is mediated by the coordinated secretion of metalloproteinases, the counterbalance action of tissue inhibitors of metalloproteinases, and other enzymes, such transglutaminases that crosslink and stiffen the extracellular matrix (Frantz et al., 2010). There is a growing body of evidence indicating that, similarly to other glandular epithelia such as the mammary gland (Fata et al., 2004; Page-McCaw et al., 2007), proteolytic activity controls the cellular behavior in the meibomian gland.
The gelatinases MMP2 and MMP9 are two members of the metalloproteinase family that have been extensively studied because of their consistent association with pathological processes such as tumor invasion and metastasis (Toth et al., 2012). They can degrade a broad spectrum of extracellular matrix components such as collagens, but also other molecules including proinflammatory mediators and growth factors (Fanjul-Fernandez et al., 2010). The distribution of gelatinolytic activity has been recently evaluated in the normal human meibomian gland using in situ gelatin zymography (Mauris et al., 2015). These analyses demonstrated localization of gelatinases primarily in secretory acinar structures (Figure 1b). The gelatinolytic activity was evident in the basal cell layer and the maturation zone, representing proliferating and mature meibocytes containing cytoplasmic lipid droplets, respectively. Interestingly, the activity decreased towards the necrosis zone where the meibum is formed and secreted.
Basal levels of matrix metalloproteinases that could potentially mediate biological responses at the ocular surface have been found in the normal human tear film (Acera et al., 2008; Leonardi et al., 2003); however, it is not clear to what degree, if any, meibum contributes to the proteolytic activity detected in tears. Using a combination of gelatin zymography, immunoblotting and ELISA techniques, investigators have demonstrated that sebum contains a range of matrix metalloproteinases and their inhibitors, including latent MMP9, MMP1, MMP13, TIMP1, and TIMP2 (Papakonstantinou et al., 2005). The cellular origin of these compounds has been attributed to both keratinocytes and sebocytes. In the meibomian gland, mature meibocytes robustly stained for gelatinase activity (Figure 1b), but the signal decreased towards the multilayered stratified epithelium and the luminal orifices of the ductules and the central duct, suggesting that meibum may have little or no contribution towards the basal gelatinolytic activity detected in the tear film. Unfortunately, whereas the lipid composition of meibum has been extensively characterized, information on the protein content remains scarce.
4.2 Regulation
Regulators of matrix metalloproteinase activity include CD147/EMMPRIN, also known as basigin in the mouse. CD147 is a glycosylated type I transmembrane protein initially discovered in a series of studies showing that co-culture of tumor cells and fibroblasts leads to induction of interstitial collagenase production (Biswas, 1982, 1984). CD147 allows modulation of matrix metalloproteinase expression during development and cell differentiation, and is strongly induced in basal and suprabasal cells during skin organogenesis (Chen et al., 2001; Gabison et al., 2005). In the human meibomian gland, CD147 localizes to basal cells at the peripheral margin of secretory acini and within discrete membrane domains of differentiated meibocytes (Mauris et al., 2015). Experiments using small interfering RNA have revealed that CD147 regulates MMP9 secretion in human meibocytes in vitro, as well as meibocyte proliferation and cytoplasmic lipid content. Indeed, meibomian glands of CD147 knockout mice have a lower number of acini in both the superior and inferior tarsal plates of the eyelids, suggesting that CD147 promotes meibomian gland turnover by regulating proteolytic activity and meibocyte maturation (Mauris et al., 2015).
At the transcriptional level, there are several regulators of meibomian gland development and maturation, such as the Klf5 transcription factor (Kenchegowda et al., 2011) and the Notch1 receptor (Gidfar et al., 2016; Vauclair et al., 2007). One of them, the homeodomain transcription factor Barx2, has been proposed to control meibomian gland morphogenesis by inducing expression of matrix metalloproteinases (Tsau et al., 2011). In this report, the authors show that Barx2 is expressed in epithelial cords between the eyelash follicles in the mouse embryo, whereas in the adult, it is mainly present in ductal but not acinar regions. They further demonstrate that Barx2 regulates expression of matrix metalloproteinases, and that it facilitates the formation of the invading epithelial bud and the development of acinar structures through a mechanism that could potentially involve the degradation of the extracellular matrix and the transduction of growth factor signals. Interestingly, Klf5 is also a transcriptional inductor of MMP9 expression (Shinoda et al., 2008), suggesting that multiple pathways may lead directly or indirectly to activation of promoter regions within protease genes to finely regulate meibomian gland development and maintenance.
5. Proteases in aging and meibomian gland pathology
Most research describing abnormal protease activity in holocrine glands during pathological states originate from studies investigating the pilosebaceous unit of the skin. Acne, in particular, has received special attention as it is one of the most common skin disorders (Bickers et al., 2006). Pathogenesis occurs at the level of the pilosebaceous unit and involves inflammation and abnormal hyperkeratinization that results in alterations in sebum composition and formation of a keratin plug. Matrix metalloproteinases have been shown to play important roles in dermal matrix destruction (Das and Reynolds, 2014). These proteases have been detected in facial sebum specimens of acne patients (Papakonstantinou et al., 2005) and are induced by multiple signals such as the transcription factors nuclear factor-κB and activator protein-1 (Kang et al., 2005; Trivedi et al., 2006). In addition to acne, there is additional evidence using a tissue microarray showing overexpression of MMP1 in sebaceous gland carcinomas, which may suggest a role for proteases in regulating the tumor microenvironment in the gland (Erovic et al., 2013).
Little information is available as to whether protease activity is altered or does it regulate pathological responses in the meibomian gland. Impairment in meibomian gland function occurs with aging and dry eye, a disease affecting millions of people worldwide (Ding and Sullivan, 2012). The condition is a consequence of changes within the gland that includes keratinization of the ducts, gland dropout, and alteration in lipid composition of the meibum (Gipson, 2013). It is possible to speculate that potential alteration of protease activity in meibomian gland disease may contribute to an anomalous arrangement of the extracellular matrix, therefore affecting the terminal differentiation of the gland as well as its secretory product. It is now clear that, as tissue ages, matrix metalloproteinases degrade the extracellular matrix and alter the function of epithelial cells (Frantz et al., 2010). In this context, transcriptome analyses in mice have shown differential expression levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the eyelids of 3-month- and 2-year-old mice (Parfitt et al., 2016a), suggesting that aging affects the proteolytic activity occurring within the meibomian gland.
Of interest is the clinical finding that isotretinoin, a retinoic acid derivative used to reduce sebum secretion in patients with acne, promotes meibomian gland atrophy and alters the composition of the meibum (Moy et al., 2015). Interestingly, the therapeutic effects of the isotretinoin in acne involve down-regulation of matrix metalloproteinases (Papakonstantinou et al., 2005). Furthermore, complete absence of meibomian glands has been reported in patients with ectodermal dysplasia, ectrodactyly, and clefting syndrome and anhidrotic ectodermal dysplasia, rare congenital conditions characterized by abnormal ectoderm development (Bonnar et al., 1996). However, there is limited information to suggest that proteolytic activity plays a role in the pathogenesis of this disorder other than the known role of matrix metalloproteinases and their inhibitors in controlling tissue development and morphogenesis (Stanier and Moore, 2004).
6. Conclusions and future directions
The past decade has witnessed a plethora of developments that have contributed to our better understanding of the physiology and pathophysiology of the meibomian gland. The knowledge gained has begun to uncover molecules involved in cellular, developmental and metabolic processes associated to this gland (Knop et al., 2011). Still, the role of proteases and their regulators in controlling these processes is in its infancy.
To understand how this extensive group of hydrolytic enzymes controls the function of the meibocyte at different stages of differentiation, it will be necessary to obtain more precise information using genetic and enzymological approaches. It has already become apparent that the meibomian gland acinus displays gelatinolytic activity and that CD147 acts an upstream modulator of matrix metalloproteinase production necessary for the differentiation and structural integrity of the meibomian gland. However, it is also clear from transcriptional studies in rodents, that proteases other than gelatinases, such as matrisilyn, metalloellatase and adamalysins, as well as their inhibitors, are present in the tarsal plates and differentially expressed in aging animals (Parfitt et al., 2016a). Elucidating the key mechanisms that maintain a proper balance of proteolytic activity and substrate degradation in the meibomian gland should lead to a better understanding of its development and homeostatic maintenance in the adult.
Several classes of meibomian lipids are altered in ocular surface disease, with some of them, such as hydroxy fatty acids, correlating with disease severity (Pucker and Nichols, 2012). How proteolytic activity affects the maturation process of sebocytes and, consequently, the composition and accumulation of cytoplasmic lipids, is still poorly understood. At the protein level, large quantities of nonlipid, protein-like inclusions have been detected in the meibum of patients with dry eye (Butovich et al., 2014). However, it is unclear whether proteases, reported in the facial sebum of patients with acne, are present in meibum, and whether they play a role in the pathophysiology of ocular surface disease. Insights into these questions may lead to the discovery of important pathways with respect to the basic biology of the meibomian gland and novel strategies for therapeutic interventions.
Proteases are involved in many biological functions in glandular epithelia.
Secretory acini of human meibomian glands display proteolytic activity.
Proper regulation of proteolytic activity is necessary for meibocyte maintenance.
Acknowledgments
Supported by NIH/NEI R01EY024031.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acera A, Rocha G, Vecino E, Lema I, Duran JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40:315–321. doi: 10.1159/000150445. [DOI] [PubMed] [Google Scholar]
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T American Academy of Dermatology A, Society for Investigative D. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982;109:1026–1034. doi: 10.1016/0006-291x(82)92042-3. [DOI] [PubMed] [Google Scholar]
- Biswas C. Collagenase stimulation in cocultures of human fibroblasts and human tumor cells. Cancer Lett. 1984;24:201–207. doi: 10.1016/0304-3835(84)90137-x. [DOI] [PubMed] [Google Scholar]
- Bonnar E, Logan P, Eustace P. Absent meibomian glands: a marker for EEC syndrome. Eye (Lond) 1996;10(Pt 3):355–361. doi: 10.1038/eye.1996.73. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27. doi: 10.1016/j.exer.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Lu H, McMahon A, Ketelson H, Senchyna M, Meadows D, Campbell E, Molai M, Linsenbardt E. Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Invest Ophthalmol Vis Sci. 2014;55:87–101. doi: 10.1167/iovs.13-13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kanekura T, Kanzaki T. Expression of Basigin in human fetal, infantile and adult skin and in basal cell carcinoma. J Cutan Pathol. 2001;28:184–190. doi: 10.1034/j.1600-0560.2001.028004184.x. [DOI] [PubMed] [Google Scholar]
- Craik CS, Page MJ, Madison EL. Proteases as therapeutics. Biochem J. 2011;435:1–16. doi: 10.1042/BJ20100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Reynolds RV. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol. 2014;15:479–488. doi: 10.1007/s40257-014-0099-z. [DOI] [PubMed] [Google Scholar]
- Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012;47:483–490. doi: 10.1016/j.exger.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erovic BM, Al Habeeb A, Harris L, Goldstein DP, Kim D, Ghazarian D, Irish JC. Identification of novel target proteins in sebaceous gland carcinoma. Head Neck. 2013;35:642–648. doi: 10.1002/hed.23021. [DOI] [PubMed] [Google Scholar]
- Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Fasanella V, Agnifili L, Mastropasqua R, Brescia L, Di Staso F, Ciancaglini M, Mastropasqua L. In Vivo Laser Scanning Confocal Microscopy of Human Meibomian Glands in Aging and Ocular Surface Diseases. Biomed Res Int. 2016;2016:7432131. doi: 10.1155/2016/7432131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87:361–368. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Gidfar S, Afsharkhamseh N, Sanjari S, Djalilian AR. Notch Signaling in Meibomian Gland Epithelial Cell Differentiation. Invest Ophthalmol Vis Sci. 2016;57:859–865. doi: 10.1167/iovs.15-18319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK. Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 2013;54:ORSF48–53. doi: 10.1167/iovs.13-12840. [DOI] [PubMed] [Google Scholar]
- Hooper NM. Proteases: a primer. Essays Biochem. 2002;38:1–8. doi: 10.1042/bse0380001. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett J, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011;356:5–18. doi: 10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A, Brun P, Abatangelo G, Plebani M, Secchi AG. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052–3058. doi: 10.1167/iovs.02-0766. [DOI] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- Mauris J, Dieckow J, Schob S, Pulli B, Hatton MP, Jeong S, Bauskar A, Gabison E, Nowak R, Argueso P. Loss of CD147 results in impaired epithelial cell differentiation and malformation of the meibomian gland. Cell Death Dis. 2015;6:e1726. doi: 10.1038/cddis.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film - A review. Exp Eye Res. 2015;137:125–138. doi: 10.1016/j.exer.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Moy A, McNamara NA, Lin MC. Effects of Isotretinoin on Meibomian Glands. Optom Vis Sci. 2015;92:925–930. doi: 10.1097/OPX.0000000000000656. [DOI] [PubMed] [Google Scholar]
- Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol. 2012;23:928–936. doi: 10.1016/j.semcdb.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nien CJ, Massei S, Lin G, Nabavi C, Tao J, Brown DJ, Paugh JR, Jester JV. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol. 2011;129:462–469. doi: 10.1001/archophthalmol.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norn M. Meibomian orifices and Marx’s line. Studied by triple vital staining. Acta Ophthalmol (Copenh) 1985;63:698–700. doi: 10.1111/j.1755-3768.1985.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Page MJ, Di Cera E. Evolution of peptidase diversity. J Biol Chem. 2008;283:30010–30014. doi: 10.1074/jbc.M804650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, Fimmel S, Gouvousis P, Herwig R, Lehrach H, Zouboulis CC, Karakiulakis G. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol. 2005;125:673–684. doi: 10.1111/j.0022-202X.2005.23848.x. [DOI] [PubMed] [Google Scholar]
- Parfitt GJ, Brown DJ, Jester JV. Transcriptome analysis of aging mouse meibomian glands. Mol Vis. 2016a;22:518–527. [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Lewis PN, Young RD, Richardson A, Lyons JG, Di Girolamo N, Jester JV. Renewal of the Holocrine Meibomian Glands by Label-Retaining, Unipotent Epithelial Progenitors. Stem Cell Reports. 2016b;7:399–410. doi: 10.1016/j.stemcr.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Xie Y, Reid KM, Dervillez X, Brown DJ, Jester JV. A novel immunofluorescent computed tomography (ICT) method to localise and quantify multiple antigens in large tissue volumes at high resolution. PLoS One. 2012;7:e53245. doi: 10.1371/journal.pone.0053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucker AD, Nichols JJ. Analysis of meibum and tear lipids. Ocul Surf. 2012;10:230–250. doi: 10.1016/j.jtos.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Puente XS, Sanchez LM, Gutierrez-Fernandez A, Velasco G, Lopez-Otin C. A genomic view of the complexity of mammalian proteolytic systems. Biochem Soc Trans. 2005;33:331–334. doi: 10.1042/BST0330331. [DOI] [PubMed] [Google Scholar]
- Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Ogata N, Higashikawa A, Manabe I, Shindo T, Yamada T, Kugimiya F, Ikeda T, Kawamura N, Kawasaki Y, Tsushima K, Takeda N, Nagai R, Hoshi K, Nakamura K, Chung UI, Kawaguchi H. Kruppel-like factor 5 causes cartilage degradation through transactivation of matrix metalloproteinase 9. J Biol Chem. 2008;283:24682–24689. doi: 10.1074/jbc.M709857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- Stanier P, Moore GE. Genetics of cleft lip and palate: syndromic genes contribute to the incidence of non-syndromic clefts. Hum Mol Genet. 2004;13(Spec No 1):R73–81. doi: 10.1093/hmg/ddh052. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol. 2012;878:121–135. doi: 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126:1071–1079. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- Tsau C, Ito M, Gromova A, Hoffman MP, Meech R, Makarenkova HP. Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling. Development. 2011;138:3307–3317. doi: 10.1242/dev.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13:242–253. doi: 10.1016/j.devcel.2007.06.012. [DOI] [PubMed] [Google Scholar]