Abstract
β-arrestin1 (or arrestin2) and β-arrestin2 (or arrestin3) are ubiquitously expressed cytosolic adaptor proteins that were originally discovered for their inhibitory role in G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins. However, further biochemical characterization revealed that β-arrestins do not just ‘block’ the activated GPCRs, but trigger endocytosis and kinase activation leading to specific signaling pathways that can be localized on endosomes. The signaling pathways initiated by β-arrestins were also found to be independent of G protein activation by GPCRs. The discovery of ligands that blocked G protein activation but promoted β-arrestin binding, or vice-versa, suggested the exciting possibility of selectively activating intracellular signaling pathways. Additionally, it is becoming increasingly evident that β-arrestin-dependent signaling is extremely diverse and provokes distinct cellular responses through different GPCRs even when the same effector kinase is involved. In this review, we summarize various signaling pathways mediated by β-arrestins and highlight the physiologic effects of β-arrestin-dependent signaling.
Keywords: Arrestin, endocytosis, 7TMR, adaptor, GPCR, biased agonist, kinase
INTRODUCTION
G protein-coupled receptors (GPCRs) or 7-transmembrane receptors (7TMRs) are the most diverse and well-represented group of membrane receptors, with approximately 800 members in the human genome. They recognize a variety of stimuli that include light, hormones, and chemokines and convert these signals to relevant intracellular messages. GPCR activation can significantly influence many cellular biochemical reactions, cell structure, cell motility, and gene expression. GPCR signaling regulates almost every aspect of human physiology and not surprisingly, about 40% of currently prescribed medications act on GPCRs either directly or indirectly.1–4
GPCRs have a conserved heptahelical architecture and interact with at least three families of proteins in an agonist-dependent manner. These three families include (1) the heterotrimeric G proteins, (2) GPCR kinases or GRKs and (3) arrestins.1,2 The heterotrimeric G proteins are generally divided into four main classes based on sequence similarity of the Gα subunit: Gαs, Gαi/o, Gαq/11 and Gα12/13.5 In an activated conformation, GPCRs function as efficient guanine nucleotide exchange factors (GEFs) for bound G proteins and promote the activation of Gα and their dissociation from Gβγ subunits. The activated G protein subunits in turn trigger a signal transduction cascade via various effector molecules and kinases. Seven GRKs (GRK 1–7) are expressed in human cells.6,7 While GRKs 1 and 7 are confined to the retina, GRK4 is mainly expressed in the brain, kidney and testes, and GRKs 2, 3, 5 and 6 are expressed ubiquitously. The arrestin family includes two visual (arrestin 1 and 4) and two non-visual, ubiquitously expressed arrestins (arrestins 2 and 3, also known β-arrestin 1 and β-arrestin 2 respectively). β-arrestin 1 and 2 share ~80% amino acid sequence identity and present unique as well as redundant roles in GPCR regulation.8–10 Ablation of both β-arrestin isoforms causes embryonic lethality in mice whereas genetic deletion of a single isoform leads to viable offsprings in which GPCR activation engenders distinct responses, perhaps via individual β-arrestin isoforms.7,10 The GRKs and arrestins were initially identified as inhibitors of GPCR signaling, but their roles in GPCR signal transduction are multifactorial and continuously evolving.
GRKS AND β-ARRESTINS PROMOTE DESENSITIZATION OF GPCRS
Most agonist-activated GPCRs are rapidly uncoupled from G proteins resulting in signal desensitization by an efficient two-step process: (1) phosphorylation by GRK(s) and (2) β-arrestin binding (Figure 1A).4 While β-arrestin binding physically obstructs G protein coupling to the GPCR, additional mechanisms are in play by which β-arrestins ensure efficient blockade of G protein signaling. β-arrestins scaffold cyclic nucleotide phosphodiesterases (PDEs) and diacylglycerol kinases (DGKs) that degrade second messengers downstream of activated receptors (Figure 1A).11,12 PDEs of the PDE4 family terminate cyclic adenosine monophosphate (cAMP) signaling by converting cAMP to AMP. By recruiting PDE4D3 and PDE4D5 to the activated β2-adrenergic receptor (β2AR), β-arrestin2 decreases cAMP levels and cAMP-dependent protein kinase (PKA) activity.13 This in turn attenuates PKA-mediated β2AR phosphorylation, preventing the switching of β2AR coupling from Gs to Gi.14 β-arrestin scaffolding of PDE4D is receptor specific, as constitutive binding of the PDE4D8 isoform to the β1AR does not require β-arrestins and PDE4D8 dissociates from the β1AR upon agonist stimulation of the receptor.13,15 Additionally, β-arrestin can diminish M1 muscarinic receptor-mediated protein kinase C (PKC) activity by recruiting DGKs.16 M1 muscarinic receptors couple to Gq/11 to stimulate phospholipase C (PLC) which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). β-arrestin translocation to the activated receptor leads to the recruitment of DGKs, which decrease DAG-dependent PKC activity by phosphorylating DAG, which is then converted into phosphatidic acid.16 Phosphatidic acid not only serves as an intermediate for phospholipid synthesis, but also plays important roles in signal transduction and membrane dynamics as a second messenger.17 Thus while β-arrestin’s partnership with PDEs can terminate second messenger signaling, β-arrestin’s association with DGKs can initiate an additional wave of signaling via phosphatidic acid production.
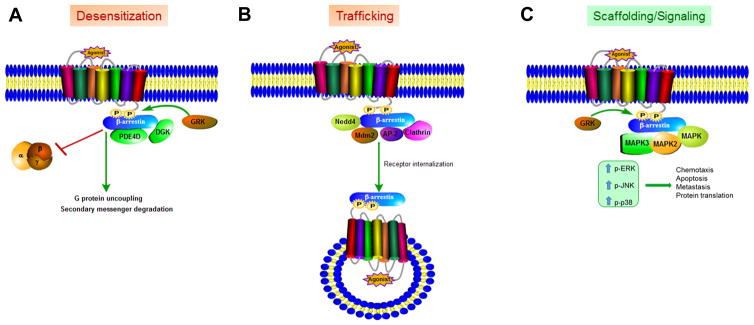
Figure 1. β-arrestins act as multifunctional adaptors for activated GPCRs.
(A) Agonist-occupied GPCRs are rapidly phosphorylated by specific GPCR kinases (GRKs) on target serine(s) and threonine(s) in the intracellular domain(s) of GPCRs. The pattern(s) of phosphorylation dictates the stability and duration of β-arrestin-GPCR association. β-arrestin-binding interdicts G protein coupling and blocks G protein-mediated signaling. This results in desensitization of GPCR signaling. β-arrestins scaffold enzymes namely, phosphodiesterase (PDE) and diacylglycerol kinase (DGK) that degrade second messengers generated by G protein activity. This process boosts the efficiency and fidelity of β-arrestin-mediated desensitization. (B) β-arrestin undergoes conformational rearrangement(s) upon its association with GPCRs; so activated β-arrestin interacts with adaptors of the endocytic machinery as well as with specific E3 ubiquitin ligases that ubiquitinate either β-arrestin itself (Mdm2) or ubiquitinate the GPCR (Nedd4). These and other protein interactions (not shown, see text and cited references) promote specific trafficking itineraries of internalized GPCRs. (C) Activated β-arrestin scaffolds multiple components of a kinase cascade such as the MAPKKK, MAPK thus propagating specific linear pathways of signaling. During this process, β-arrestin-GPCR complex can be localized to endosomes along with the MAPK scaffold allowing compartmentalization of signaling.
BIMODAL SIGNALING OF GPCRS
Although the β-arrestins were considered as inhibitors of GPCR signaling for nearly a decade after their discovery, a large body of work that followed has changed our appreciation about the functional roles of β-arrestins.8 β-arrestins have been shown to function as critical adaptors for agonist-induced endocytosis of GPCRs18,19, and also as important adaptors for agonist-induced ubiquitination of GPCRs20–24, thus governing the trafficking itinerary of internalized GPCRs (Figure 1B). β-arrestins were shown to form signaling scaffolds for mitogen activated protein kinases (MAPKs) such as the extracellular signaling kinases 1 and 2 (ERK), and c-Jun N-terminal kinase 3 (JNK3) on endosomes with internalized GPCRs (Figure 1C).25 Subsequently, β-arrestins were shown to promote some of these pathways even when the G protein activity is disabled.3,26 Thus, GPCR signaling is bimodal, in which the first mode is dependent on G proteins and the second mode dependent on GRK phosphorylation of the GPCR and on β-arrestin-dependent signaling.4,27–29 GPCRs can be activated to favor one of these modes over the other, thus engendering biased signaling (Figure 2). In some cases, complete bias allows signaling via only one mode: either G protein(s) or β-arrestin(s).
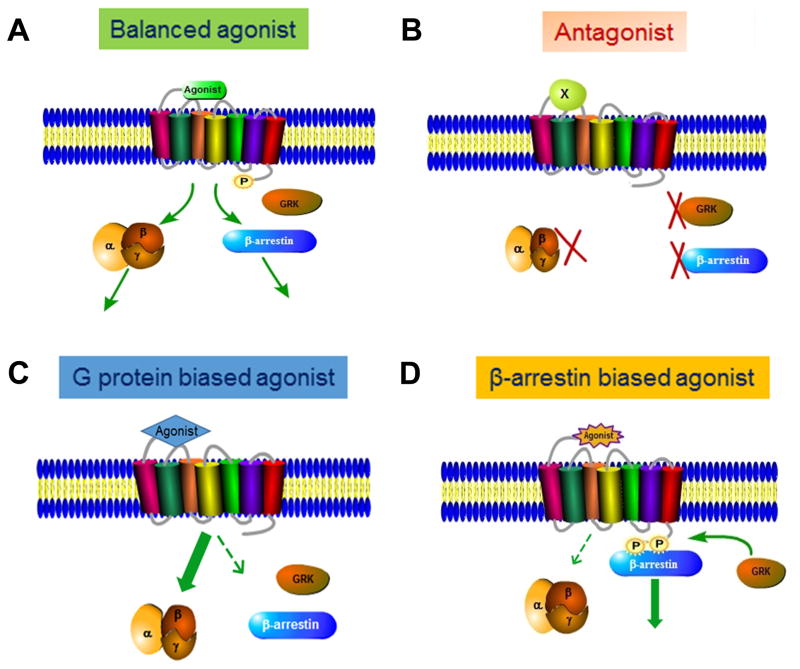
Figure 2. GPCR ligands and their effects.
(A) Balanced agonist stabilizes receptor conformation(s) that induces both G-protein and β-arrestin-dependent signaling with variable efficacies and kinetic displacement. (B) Non-selective antagonist locks the receptor in an inactive state, and neither G protein nor β-arrestins promote signaling. (C and D) Biased agonists promote an active conformation of the receptor that selects either G-proteins or β-arrestins as the transducer for signaling, potentiating (thick arrow) certain signaling events while attenuating (thin arrow) others.
PHOSPHORYLATION BARCODING BY GRKS REGULATES β-ARRESTIN ACTIVITY
Phosphorylation of activated GPCRs by GRKs, originally described as the first step in GPCR desensitization is now known to be a critical regulatory mechanism, which initiates a variety of downstream effects by engaging different conformations of β-arrestins and promoting their various functions.12,30–32 The correlation between the extent of GPCR phosphorylation and the affinity of GPCR-arrestin complex is inferred by the distinct patterns of β-arrestin recruitment dictated by the abundance and distribution of serine/threonine residues in the GPCR intracellular domains.33,34 A stable β-arrestin/GPCR endosomal complex is formed by GPCRs that have clustered serine/threonine motifs in their carboxyl tails (for example, the AT1aR). In contrast, a transient plasma membrane complex is obtained with GPCRs that possess discontinuous or scattered serines/threonines (for example, the β2AR). Eliminating all the phosphorylation sites by mutagenesis either weakens a former strong β-arrestin interaction (e.g. AT1aR)35 or eradicates an original weak β-arrestin binding (e.g. β2AR)8.
Studies carried out with a number of GPCRs (β2AR 36, follicle-stimulating hormone receptor37, somatostatin 2A receptor38, thyrotropin-releasing hormone receptor39, dopamine D1 receptor40, free fatty acid receptor 441, neuropeptide FF2 receptor42 and other GPCRs 11,43) showed that elimination of specific phosphorylation sites in a GPCR has distinct downstream effects on β-arrestin binding and/or signaling. Using mass spectrometry and phospho-site antibodies, differential phosphorylation of M3 muscarinic receptor was demonstrated in different cell and tissue types suggesting that particular patterns of phosphorylation engenders tissue-specific signaling.44 By utilizing quantitative proteomics chemically distinct ligands were shown to produce qualitatively similar mixture of phosphorylated μ-opioid receptors (MORs).45 However, multisite phosphorylation of a specific serine-threonine motif is required for MOR internalization as well as β-arrestin recruitment and quantitatively, phosphorylation of this region is greater when MORs are stimulated with DAMGO than when stimulated with morphine.45 These studies support the idea that distinct phosphorylation motifs may be sensed by GRKs yielding a distinct outcome through subsequent protein interactions, especially via β-arrestin recruitment.
The differential roles of GRK isoforms in regulating a particular GPCR-activated function of β-arrestin became evident from targeted knockdown of each GRK by RNAi.46,47 GRK2 and/or GRK3-mediated bulk phosphorylation of the AT1aR and of the vasopressin receptor (V2R) enables β-arrestin binding and internalization, but not ERK activation. In contrast, GRK5 and/or GRK6 mediated phosphorylation of the same two GPCRs, leads to β-arrestin-dependent ERK activation but does not provoke robust β-arrestin binding nor internalization. The chemokine receptor CCR7, is activated by two endogenous agonists, namely, CCL19 and CCL12, which engage different GRK isoforms and promote different downstream effects via β-arrestin. CCL19 stimulation promotes GRK3 and GRK6-mediated CCR7 phosphorylation whereas CCL21 engages only GRK6 leading to differential consequences: β-arrestin-dependent ERK activation by both agonists and robust β-arrestin binding and desensitization only by CCL19.48
Specific phosphorylation barcodes were delineated by mapping the GRK phosphorylation sites on activated β2AR and the chemokine receptor CXCR4 by employing combinatorial approaches involving stable isotope labeling, mass spectrometry, site-directed mutagenesis, site-specific phospho-antibodies, and RNAi of individual GRKs.49,50 These studies confirmed the presence of GRK isoform-dependent, phosphorylation bar codes that correspond to the activated conformation of the receptor. Upon CXCL12 treatment, CXCR4 is rapidly phosphorylated on serines 324 and 325 by PKC and GRK6. Additional serines, which are not targeted by GRKs 2, 3 and 5 are also phosphorylated by GRK6 in agonist-activated CXCR4. GRKs 2/3 phosphorylate a small serine cluster at the carboxyl terminus of CXCR4. GRK2, GRK6 phosphorylation and β-arrestin2 binding primarily regulate calcium mobilization triggered by CXCR4 whereas CXCR4-induced ERK activity is dependent on GRK3, GRK6 and β-arrestin1 binding.50
Isoproterenol stimulation of the human β2AR triggers phosphorylation of 13 residues, among which two serines at positions 355 and 356 are phosphorylated by GRK6.49 Interestingly, carvedilol provokes only phosphorylation of these two residues by GRK6. Both isoproterenol and carvedilol-induced β-arrestin-dependent ERK activation requires that GRK6 phosphorylates the β2AR. On the other hand, GRK2 phosphorylation of six sites in the carboxyl tail is required for β-arrestin recruitment and receptor internalization. β2AR phosphorylation at GRK2 as well as GRK6 sites is necessary for efficient desensitization of cAMP signaling.49
In summary, barcoding of GPCRs by GRKs that define specific activities through β-arrestins involve hierarchical molecular mechanisms (Figure 3). As a first tier of regulation, each GPCR has distinct phosphorylation sites or motifs distributed in its intracellular domains. As a second tier of regulation, the recruitment of a GRK is dictated by specific ligand-activated conformation(s) of the GPCR. As a third tier of fine tuning specific β-arrestin-dependent signaling, the expression level of GRK isoforms can be modulated in a cell type or tissue, such that only certain phosphorylation codes are enabled.51 As a fourth tier of regulation, each GRK phosphorylates a set of target sites, which can be either overlapping or non-overlapping with other GRKs or with other serine/threonine kinases. In some cases, GRKs can compete or antagonize each other’s activity suppressing or increasing specific phospho-signatures.46,47 The resulting unique phosphorylation code on a GPCR engages a particular active conformation of β-arrestin facilitating one or more functions of β-arrestin (i.e. desensitization, internalization or signal transduction).
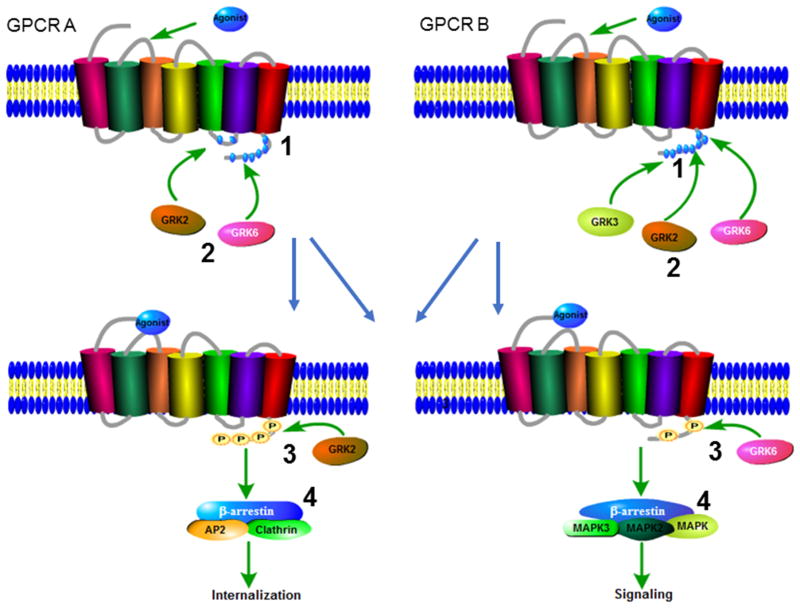
Figure 3. GRK phosphorylation and barcoding of GPCRs.
(1) Individual GPCRs possess unique phosphorylation sites that are targets for GRK phosphorylation. (2) Each GRK phosphorylates specific sites in a GPCR. Expression and activity of each GRK isoform is cell and tissue-specific. Recruitment of a GRK isoform to activated GPCR is ligand-specific. (3) When a GPCR is phosphorylated on particular sites a specific phosphorylation code emerges. (4) This barcode is sensed by the recruited β-arrestin, resulting in specific conformational orientations of β-arrestins. Additional conformational signals are appended by other post-translational modifications such as ubiquitination. The activated β-arrestin then propagates specific signals downstream of a GPCR.
β-arrestins undergo receptor and ligand-specific conformational changes, and distinct activated conformations of β-arrestins correlate with their trafficking and signaling activities.30–32 Recently, the existence of such specific β-arrestin conformations that tune with specific receptor phosphorylation barcodes have been demonstrated by employing fluorine-19 nuclear magnetic resonance.31 These studies were achieved by substituting unnatural amino acid at the 7 sites of β-arrestin1’s amino terminus, which were identified as the phosphate binding sites.31,52 When the phospho-acceptor sites 1, 4, 6 and 7 in β-arrestin1 were involved in engaging GPCR domains, such pattern induced a β-arrestin-1 conformation specific for clathrin recruitment. When the phosphates on the GPCR engaged only the acceptor sites 1 and 5, then the resulting conformation of β-arrestin1 was conducive for c-Src activation.31 In addition to conformations provoked in β-arrestin by specific phosphorylation patterns on the GPCR interface, additional modifications on a pre-activated β-arrestin by differential ubiquitination or by other post-translational modifications can expand the conformational repertoire of each active form of β-arrestin.11,22,53,54 This perhaps allows β-arrestin to possess unlimited conformational plasticity enabling its multi-faceted functions in GPCR signaling.
β-ARRESTIN-DEPENDENT SIGNALING VIA SCAFFOLDING
Soon after the identification of β-arrestins as adaptors for endocytosis, they were linked with the activation of signaling kinases.55 Interestingly, β-arrestins were shown to simultaneously bind and link components of a kinase cascade (i.e. binding to MAPKKK, and MAPK) and act as kinase scaffolds (Figure 1C).56–58 Upon GPCR activation, β-arrestins also scaffold or sequester other non-kinase proteins such as small GTPases, phosphatases or adaptor proteins, promoting the formation of various signalosomes that regulate cellular activities (Figure 4). Accordingly, β-arrestin’s roles as a scaffold in signal transduction can be broadly classified as (A) β-arrestin scaffolding that primarily involves kinases and (B) β-arrestin scaffolding that primarily involves non-kinase proteins with or without a secondary link to a kinase activity.
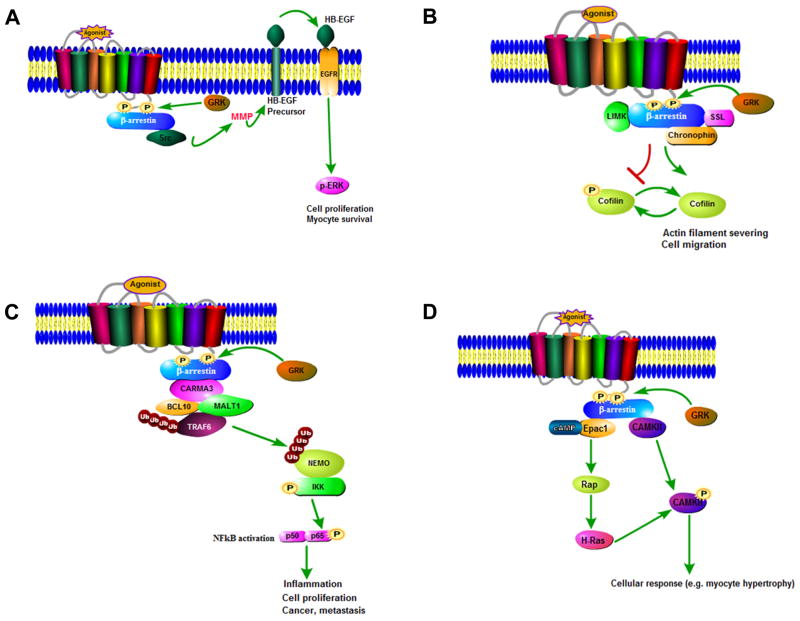
Figure 4. Examples of β-arrestin-dependent signaling mechanisms.
(A) Agonist-stimulated GPCR when phosphorylated by GRK5/6 recruits β-arrestin and bound active c-Src, which activates matrix metalloproteases (MMP) engendering cleavage and shedding of HB-EGF, which in turn binds and activates EGFR and promotes its signal transduction. (B) By binding to LIMK, β-arrestin blocks cofilin phosphorylation by LIMK. By scaffolding phosphatases such as chronophin or slingshot β-arrestin promotes cofilin dephosphorylation. This facilitates cofilin association with actin, and actin filament severing at the leading edge of a migrating cell. (C) β-arrestin2 recruits CARMA3 to activated GPCRs, enabling the CBM signalosome to ubiquitinate the NEMO subunit of IKK via TRAF6 which leads to NFκB activation. (D) β-arrestin forms a complex with CaMKII and exchange protein directly activated by cAMP 1 (Epac1), enabling their translocation to the plasma membrane upon activation of β1ARs. Epac1 binding to cAMP activates the small GTPases of Ras superfamily Rap1 and Rap2 and thence CAMKII activation.
A) β-arrestin scaffolding that primarily involves kinases
Non-receptor Tyrosine Kinases
Soon after the identification of β-arrestins’ roles as endocytic adaptors for activated GPCRs, they were shown to trigger MAPK signaling by binding and recruiting several members of the Src family non-receptor tyrosine kinases.25 β-arrestin-dependent Src activity in turn regulates GRK2 levels, providing a negative feedback on GPCR desensitization.59 Additionally, β-arrestin-Src complexes phosphorylate and modulate the activity of the endocytic proteins, dynamin I and β2-adaptin and regulate clathrin-dependent internalization of GPCRs including AT1aR, β2AR, V2R and bradykinin B2 receptor.25 β-arrestin-Src kinase association can also facilitate exocytosis and granule release as shown for the interleukin 8 receptor CXCR1.60 Additionally, β-arrestin-Src interaction acts as a critical step upstream of MAPK signaling via select GPCRs.25
Mitogen-activated protein kinases
Among the MAPKs, the role of β-arrestins has been extensively characterized for ERK signaling. Most GPCRs activate ERK via both β-arrestins and G proteins; however, some GPCRs primarily employ β-arrestins for ERK activation (Figures 1, 2). G protein-mediated ERK activation is transient, localized in the nucleus and promotes gene transcription, whereas β-arrestin-dependent ERK signaling is sustained, localized in clathrin-coated structures (CCSs) and stabilized in endosomes.8,61–63 The AT1aR and the V2R which form a stable complex with either β-arrestin isoform, promote sustained ERK signaling in endosomes, whereas the β2AR forms a transient complex with β-arrestins and induces transient ERK activity.64 Interestingly, replacement of the C tail of the β2AR with that of V2R promotes ERK signaling in endosomes, while the substitution of V2R C tail with that of β2AR eliminates ERK signaling in endosomes.64 These reciprocal effects caused by switching of C tails also correlate with the kinetics of de-ubiquitination of β-arrestins induced by the β2AR and V2R.65,66 Accordingly, β2AR C tail promotes transient ubiquitination (rapid deubiquitination) of β-arrestin2, while V2R C tail provokes sustained ubiquitination.65 Ubiquitinated β-arrestins also display greater binding affinity than non-ubiquitinated β-arrestins with (a) activated GPCRs, (b) clathrin subunits and (c) components of ERK signaling (c-Raf and ERK), which suggests a tight relationship between β-arrestin ubiquitination status and the transmission of β-arrestin-dependent signaling.11,62,63
β-arrestins scaffold components of the JNK cascade: apoptosis signal-regulating kinase (ASK1), MKK4, MKK7 as well as JNKs 1, 2 and 3.58,67 JNK signaling regulates growth arrest, apoptosis and activation of immune cells in response to environmental and hormonal stresses. While all visual and non-visual arrestins bind to JNK3, only β-arrestin2 facilitates JNK3 phosphorylation and activation. Upon AT1aR agonist-stimulation, β-arrestin2 specifically localizes activated JNK3 on endosomes, analogous to its ability to scaffold activated ERK on endosomes.58 On the other hand, for the metabotropic glutamate 7 (mGlu7) receptor68, β-arrestin-mediated JNK signaling is regulated by specific GRKs and β-arrestin isoforms: GRK4 activates JNK, whereas GRK2 promotes ERK signaling; β-arrestin2 enables JNK activation, but inhibits ERK, whereas β-arrestin1 activates ERK while inhibiting JNK.68
In addition to promoting ERK and JNK signaling, β-arrestins also regulate p38 MAPK activation. CXCR4-induced p38 activity promotes cellular chemotaxis and is primarily mediated by β-arrestins.69 β2AR provokes biphasic p38 activation via G protein and β-arrestin-dependent mechanisms. While the late p38 activation is Gs/cAMP-mediated, early p38 activation is dependent on β-arrestin1/Rac1-GTPase/NADPH oxidase signaling and is solely responsible for agonist induced F-actin rearrangement.70 For the κ-opioid receptors (KORs), β-arrestin-dependent signaling and p38 activation requires phosphorylation of the receptors by GRK3.71 While the analgesic effects of kappa opioids are G protein-mediated, dysphoria and addiction is linked with β-arrestin-dependent p38 activation.72
PI3K, Akt and GSK3β signaling
The activity of phosphatidylinositol-3 kinase (PI3K) is modulated by β-arrestin as well. However, while β-arrestins inhibit Gαq/Ca2+-dependent PI3K activation downstream of the protease activated receptor 2 (PAR2), they facilitate PI3K activity for the chemokine receptor CCR5.73,74 CCR5 stimulation by the chemokine macrophage inflammatory protein-1β (MIP-1β) promotes macrophage chemotaxis by Gi-dependent as well as by Gi-independent/β-arrestin-dependent signaling mechanisms. The latter signaling involves concurrent activation of ERK, Protein tyrosine kinase 2B (Pyk2), PI3K p85 regulatory subunit and the tyrosine-protein kinase Lyn in a multimolecular complex that is scaffolded by β-arrestins and recruited to the activated CCR5.74
β-arrestin induces the activation of the serine/threonine kinase Akt (also known as protein kinase B) downstream of ghrelin receptor GHS-R1a and α-thrombin receptor.75,76 Generally, Akt affects components of the apoptotic machinery either directly or indirectly, promotes cell survival, regulates cell proliferation and myocardial angiogenesis.77 β-arrestin scaffolding of GHS-R1a, c-Src and Akt into a multiprotein complex is necessary for prolonged Akt signaling.76 In contrast, G protein-mediated Akt activation by ghrelin involves Gβγ triggered-PI3K activation, Akt phosphorylation by c-Src and subsequently by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and mammalian rapamycin-insensitive complex 2 (mTORC2).76 In the case of the thrombin receptor PAR4, formation of a heterodimer with the purinergic receptor P2Y12 promotes sustained β-arrestin-mediated Akt activation.78 AT1R stimulation also leads to β-arrestin-dependent PI3K/Akt activation which contributes to phosphorylation and inactivation of Bcl-2 associated death promoter (BAD), a pro-apoptotic protein of the Bcl-2 family.79 Akt signaling via AT1R and β-arrestin induces phosphorylation of mTOR and its downstream effector p70/p85 ribosomal S6 kinase (p70/85 S6K).80 β-arrestin-mediated PI3K/Akt and ERK/p90RSK signaling promotes AT1R-triggered cell growth and hypertrophy. Chronic catecholamine stimulation of the β2AR results in the accumulation of chromosomal DNA damage in both somatic and germ cells via β-arrestin1-dependent mechanisms that include: PI3K/AKT activation, AKT mediated phosphorylation and activation of the E3 ubiquitin ligase Mdm2, Mdm2-mediated ubiquitination and proteasomal degradation of p53.81
β-arrestin forms a complex with Akt, glycogen synthase kinase 3β (GSK3β) and the protein phosphatase PP2A, upon D2R activation.82 Phosphorylation of GSK3β on serine 9 by Akt inhibits GSK3β activity. Akt itself is activated upon phosphorylation of threonine 308 and serine 473 by the phosphatidyl-dependent kinases PDK1 and PDK2/rictor-mTOR, in a PI3K-dependent manner. β-arrestin2, when recruited to the D2R enhances Akt and PP2A association, leading to Akt dephosphorylation and inactivation.82 Lithium compounds used as a mood stabilizing medication, inhibit GSK3β activity directly or as shown in vivo, by disrupting β-arrestin-PP2A-Akt complexes, which then promotes Akt activation and GSK3 inactivation.83,84 β-Arrestin–mediated Akt/PP2A/GSK3β signaling cascade presents as a target for antipsychotic drugs aimed at treating schizophrenia.83,84 On the other hand, β-arrestin2 does not associate with Akt downstream of PAR2, but forms a complex with PP2A/GSK3β in a Rho kinase dependent manner.85 β-arrestin-PP2A-GSK3β signaling facilitates stem/progenitor cell survival in normal colon crypts and colon cancer. 85
Transactivation of receptor tyrosine kinases
GPCR-triggered transactivation of receptor tyrosine kinases (RTKs), namely, epidermal growth factor receptor (EGFR), insulin-like growth factor type 1 receptor (IGF-1R) and vascular endothelial growth factor receptor (VEGFR) are modulated by both β-arrestin and G protein-dependent mechanisms (Figure 4A).25,29,86 β-arrestin2 inhibits EGFR transactivation by CXCR7 and counteracts mitogenic signaling and cell proliferation.87 On the other hand, β-arrestins promote sphingosine-1-phosphate-induced transactivation of Fetal Liver Kinase 1 (Flk-1) through S1P1/S1P3 receptors, resulting in mouse embryonic stem cell proliferation.88 β-arrestins also mediate EGFR transactivation by the β1AR,89 a genetic variant of α1AR,90 ET1AR,91 urotensin II receptor (UTR),92 GPR39 93 and GPR54.94 β-arrestin mediated EGFR transactivation may induce diverse cellular responses such as cardioprotection when provoked by β1AR and UTR, cardiomyoblast hyperproliferation and hypertrophy in the case of α1AR, myogenesis for GPR39 and cancer cell invasiveness for ET1AR and GPR54.89–94
β-arrestin2 and AT1R-dependent EGFR transactivation and ERK signaling is also evoked by mechanical stimuli such as membrane stretch and leads to load-induced cardiac hypertrophy.95–97 Hearts from mice lacking either β-arrestin2 or AT1Rs fail to promote EGFR transactivation and ERK phosphorylation in response to mechanical stretch, while they do promote enhanced myocyte apoptosis.97 Besides β-arrestin2, Src kinase is also required for the pathologic effects of stretch since Src inhibition prevents stretch-induced activation of ERK, and significantly reduces pressure overload-induced hypertrophy and improves cardiac function.98
AngII-induced β-arrestin2 mediated EGFR transactivation contributes to the development of aortic aneurysms, a vascular disease characterized by the dilation, degeneration and eventual rupture of the wall of the aorta. The proaneurysmal effects of β-arrestin2 are independent of TGF-β and of AT1aR-induced Gq signaling.99 However, AngII-induced abdominal aortic aneurysms (AAAs) and macrophage infiltration into the abdominal aorta are attenuated in the absence of β-arrestin2.100 This outcome coincides with reduced expression of the inflammatory markers cyclooxygenase-2 (COX-2), monocyte chemoattractant protein-1, and macrophage inflammatory protein 1α.100 The matrix metalloproteases, MMP2 and MMP9, which disrupt extracellular matrix components and provoke vascular remodeling in aortic aneurysms, are also downregulated in AngII-treated mice lacking β-arrestin2. However, β-arrestin2 deficiency doesn’t reverse the increase in blood pressure upon chronic AngII treatment, indicating that β-arrestin2’s effects on AAAs are independent of blood pressure. The proaneurysmal effects of β-arrestin2 are also demonstrated in a murine model of Marfan syndrome (MFS).99,101 MFS is a systemic, inherited disease of connective tissue that is associated with mutations in the fibrillin-1 gene. MFS is responsible for various ocular, skeletal and cardiovascular pathologies and often results in the development of thoracic aortic aneurysms (TAAs). β-arrestin2 deficiency attenuates TAAs as well as the expression of proaneurysmal proteins, MMP-2 and -9 and collagen type 1a1 in aortic tissue of MFS mice carrying the fibrillin-1 C1039G mutation.99
(B) β-arrestin scaffolding that primarily involves non-kinases
Rho GTPase and cofilin signaling pathways
GPCRs activate the small GTPase RhoA mainly via Gα12/13 coupling to Rho guanine nucleotide exchange factors; however, both β-arrestin isoforms also modulate RhoA activity, affecting actin cytoskeletal organization, cell proliferation and cell cycle progression. The growth hormone secretagogue receptor GHSR-1a activates RhoA through G12/13, Gq/11 and β-arrestin2-dependent mechanisms.102 β-arrestin2 also mediates Gs-independent RhoA activation by the β2AR: β-arrestin2 forms a complex with a guanine nucleotide exchange factor, p115RhoGEF,103 enabling p115RhoGEF translocation to the plasma membrane, and concomitant RhoA activation, which promotes focal adhesion remodeling and stress fiber formation.103 Formyl-Met-Leu-Phe (fMLP) receptor-triggered membrane ruffling is regulated by agonist-induced disassembly of cytosolic β-arrestin- Ral guanine nucleotide dissociation stimulator (RalGDS) complexes, which allows RalGDS to translocate to membrane domains and promote Ras-independent Ral signaling and Rho activation.104
RhoA activation and stress fiber formation by the AT1R are dependent on both Gαq/11 and β-arrestin1.102,105 Angiotensin-stimulation promotes β-arrestin1’s interaction with Rho GTPase activating protein, ARHGAP21, inhibiting the conversion of RhoA to its inactive GDP-bound state.106 In addition, β-arrestin2 is required for membrane bleb formation following AT1R stimulation in a signaling pathway that involves RhoA and its downstream effectors Rho-associated kinase (ROCK) and myosin light chain kinase (MLCK).107 Alternatively, β-arrestins 1 and 2 can promote angiotensin-induced cell contraction and migration as they interact with myosin light chain phosphatase targeting subunit (MYPT-1) and reciprocally regulate myosin light chain phosphatase (MLCP) activity and myosin light chain (MLC) phosphorylation status.108
PAR2-induced β-arrestin-dependent scaffolding of LIM kinase (LIMK), the actin severing protein cofilin, and the phosphatase chronophin decreases phosphorylated-cofilin and increases unphosphorylated cofilin, regulating actin assembly and cell migration (Figure 4B).109 β-arrestin1 also regulates the phosphorylation-dependent actin association of cofilin by scaffolding ROCK, LIMK and cofilin phosphatases such as slingshot (SSL) following stimulation of the delta opioid receptors (DORs) with the agonist SNC80.110 β-arrestin1 and PDZ-RhoGEF (postsynaptic density protein 95/disc-large/zonula occludens-RhoGEF) mediate ROCK-LIMK-cofilin signaling upon endothelin-1 (ET-1)/ETA receptor (ETAR) activation.111 β-arrestin1 PDZ-RhoGEF interaction facilitates the activation of RhoA and RhoC and subsequent ROCK kinase activity, resulting in enhanced invadopodia and cell invasion of ovarian carcinoma cells.111
Wnt and β-catenin signaling pathways
Wnts are secreted lipoglycoproteins that trigger signaling via the 7-transmembrane Frizzled (Fzd) receptors, which function in concert with their single transmembrane co-receptors called low density lipoprotein receptor-related protein 5/6 (Lrp5/6). Wnt signaling is essential for embryonic development; it also regulates cell adhesion, polarity and migration and contributes to cardiac fibrosis.112,113 Canonical Wnt signaling involves inhibition of GSK3 downstream of Fzd-LRP5/6 and stabilization of β-catenin followed by induction or repression of target genes. Wnts can also trigger β-catenin-independent signaling via the activation of Rho, Rac-1 or an atypical receptor tyrosine kinase called ROR2.114 β-arrestins have been identified as important scaffolds in both aforementioned Wnt signaling pathways. β-arrestin1 as well as its association with the Fzd adaptor disheveled (Dvl) are required for Wnt3a-induced, β-catenin-dependent TCF/LEF-mediated transcription. β-arrestins are necessary for maximal Wnt3a-induced phosphorylation of the Dvl by casein kinase 1δ/ε (CK1δ/ε), which is critical for Fzd internalization and for endosomal signaling of internalized Fzd complexes.115 β-arrestin2 interacts with two Dvl-associated kinases PI4KIIα and PIP5KIβ which stimulate PIP2 production and provoke β-arrestin2 interaction with PIP2-binding protein Amer1 (APC membrane recruitment protein 1). β-arrestin2’s scaffolding of Amer1 facilitates Wnt3a-induced Lrp6 phosphorylation, which stabilizes β-catenin.116 β-arrestin also forms a trimeric complex with phosphorylated Dvl and axis inhibition protein 1 (Axin1) and associated GSK3, thus blocking the phosphorylation and degradation of β-catenin114
Alternatively, β-arrestin regulates Wnt-activated, β-catenin-independent signaling cascades such as Wnt/Planar Cell Polarity (PCP) pathway and Wnt/Ca2+ pathway. β-arrestin dependent Wnt/PCP signaling induces intracellular cytoskeletal rearrangements by scaffolding phosphorylated Dvl and dishevelled-associated activator of morphogenesis 1 (DAAM1).117 β-arrestin connects the C-terminal diaphanous autoregulatory domain (DAD) in DAAM1 with the PDZ domain of Dvl engendering RhoA activation.117 β-Arrestin forms a multiprotein signaling complex with Dvl and Gβγ to promote Wnt/Ca2+ signaling and provoke convergent extension movements during Xenopus gastrulation.114,118,119
β-arrestin2 also associates with Dvl-2 downstream of PAR1 upon binding of activated protein C to a coreceptor called endothelial protein C receptor (EPCR).120 The binding of activated protein C with EPCR facilitates the cleavage of PAR1 at a site different from the thrombin cleavage site and stimulates PAR1 signaling.121 While the EPCR/PAR1 signaling has anticoagulant and anti-inflammatory effects in endothelial cells, thrombin-induced activation of PAR1 promotes coagulation and inflammation.122–124 β-arrestin2 enables activated protein C-triggered PAR1 signaling via a mechanism that involves 1) EPCR-dependent recruitment of GRK5 to the cytoplasmic domain of PAR1, 2) β-arrestin2 scaffolding of Dvl-2, and 3) induction of Rac1 signaling, which confers protection against thrombin-induced endothelial barrier permeability.120,125
β-arrestin1 forms two trimeric signaling complexes following ETAR activation to induce β-catenin signaling pathways and promote cell invasion. First, β-arrestin activates Src, leading to EGFR transactivation, MAPK signaling and β-catenin stabilization via Tyr phosphorylation. Second, β-arrestin inhibits the “destruction complex” formed by GSK3β, Axin1, adenomatous polyposis coli (APC) and the E3 ligase β-transducin repeat containing protein (β-TrCP), preventing Ser/Thr phosphorylation and subsequent degradation of β-catenin.91 By acting as a transcriptional regulator of endothelin-1-induced β-catenin signaling, β-arrestin1 promotes epithelial to mesenchymal transition (EMT), cancer invasion and metastasis.126,127
Hedgehog signaling
Hedgehog (Hh) signaling regulates stem cell maintenance, embryonic development and adult tissue integrity; dysregulation of this signaling is associated with congenital birth defects and various types of cancers.128 β-arrestin recruitment to Smoothened (Smo), a GPCR of the frizzled family is essential for Hh signaling and for the subsequent activation the Gli family of transcription factors in vertebrates.129,130 In the absence of Hh, Smo activity is inhibited by the 12-pass transmembrane protein, Patched (Ptch). Binding of Hh to Ptch relieves Smo inhibition by provoking not only Ptch dissociation from Smo, but also Ptch internalization and degradation.128 β-arrestin2 preferentially interacts with activated Smo in a GRK2-dependent manner, and promotes Smo endocytosis into clathrin-coated pits.131 Activated Smo also translocates to the primary cilium, which promotes Gli-mediated transcription and upregulation of Hh-responsive genes.132 Chemical agonists that directly bind to Smo such as SAG (Smoothened agonist) or 20(S)-hydroxycholesterol (20-OHC) provoke Smo accumulation in the cilium independently of Hh and Ptch.133,134 β-arrestins translocate to the primary cilia and associate with KIF3A and with integrin-linked kinase (ILK) which are both linked to Smo ciliary translocation.132,135 Both β-arrestin isoforms interact with a recently identified negative regulator of Smo, the orphan GPCR, GPR161, which represses Hh signaling by constitutively activating cAMP.136,137 In a Smo, GRK2 and clathrin-dependent manner, β-arrestin binds to GPR161 and promotes GPR161 clearance from the cilium.136
NFκB and inflammatory signaling
The transcription factor nuclear factor kappa B (NF-κB) regulates the expression of target genes involved in immune and inflammatory responses, cell proliferation, and tumorigenesis. The lysophosphatidic acid receptor (LPAR) activates NF-κB via a G protein-mediated, PKCα-dependent mechanism that induces IκB kinase (IKK) phosphorylation, or via an alternative pathway in which β-arrestin2 promotes IKK activation by recruiting the adaptor protein CARD and MAGUK domain-containing protein 3 (CARMA3) (Figure 4C).138 Upon LPAR activation, CARMA3 forms a complex with B-cell chronic lymphocytic leukemia-lymphoma 10 (Bcl10), mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1), and the E3 ligase tumor necrosis factor receptor-associated factor 6 (TRAF6) to form the CBM signalosome, which associates with IKK to ubiquitinate the regulatory subunit of the IKK complex called NF-κB essential modulator (NEMO).138 NF-κB activation elicited by GPCRs such as AT1aR, ET1R, CXCR4 and PAR1 or by RTKs like EGFR and VEGFR2 requires CARMA3; however, NF-κB activated by cytokine receptors such as the tumor necrosis factor receptor (TNFR) is not dependent on CARMA3.138 β-arrestin2, and not β-arrestin1, is required for LPAR and PAR1-mediated NF-κB signaling and CARMA3-containing CBM signalosome assembly, as it recruits CARMA3 to the activated GPCR complexes (Figure 4C).138 On the other hand, both β-arrestin isoforms are known to interact with TRAF6, the NFκB inhibitor IκBα, and regulatory kinases such as IKKs α and β and NFκB-inducing kinase (NIK).139–141
Interestingly, β-arrestin isoforms reciprocally regulate NFκB signaling, inflammation and consequently atherosclerosis. For instance, β-arrestin2 promotes aortic smooth muscle cell (SMC) proliferation and migration whereas β-arrestin1 inhibits these processes.142 Moreover, although β-arrestin1 inhibits NFκB signaling by preventing IκB degradation and TRAF6 ubiquitination downstream of TNFR and the Toll-like receptor/interleukin-1 receptor respectively, it promotes NFκB signaling in endothelin-1 stimulated epithelial ovarian cancer (EOC) cells by forming a complex with the p65 subunit of NF-κB.139,141,143 On the other hand, β2AR stimulation inhibits NFκB signaling by promoting β-arrestin2 interaction with IκB.140 β-arrestin2 effects on NF-κB signaling are closely associated with its ubiquitination status.54 Ubiquitinated β-arrestin2 promotes NF-κB signaling in SMCs, whereas deubiquitinated β-arrestin2 attenuates NF-κB signaling by scaffolding the deubiquitinase USP20, that in turn deubiquitinates TRAF6, which blocks the NF-κB signaling cascade.54 Although these effects of reversible β-arrestin ubiquitination are demonstrated for the Toll-like receptor pathway, these mechanisms may potentially regulate NFκB signaling downstream of GPCRs.
cAMP/Epac/CaMKII pathway and hypertrophic signaling
Both β-arrestins 1 and 2 are required for β1AR-induced CaMKII activation (Figure 4D).144 β-arrestin-mediated CaMKII activation by the β1AR increases cardiac excitation-contraction coupling via phosphorylation of ryanodine receptors, phospholamban, cardiac troponin I and myosin-binding protein C and induces myocardial hypertrophy following pressure overload and chronic isoproterenol stimulation.145–147 β-arrestins form a complex with CaMKII and the cAMP sensor protein exchange protein directly activated by cAMP 1 (Epac1), enabling their translocation to the plasma membrane upon β1AR activation. CaMKII activation via Epac involves Rap–PLC-ε–PKC-ε signaling and is independent of cAMP-dependent protein kinase A (PKA).144 Epac1 binding to cAMP activates the small GTPases of Ras superfamily Rap1 and Rap2. Epac1 also couples to H-Ras in a β-arrestin2 dependent manner, promoting H-Ras activation via Rap2B and subsequent hypertrophic signaling.146 Interestingly, CaMKII is activated only by the β1AR and not by the β2AR, even though both βARs stimulate cAMP production. This specificity rests on the C terminal domain of the β1AR and constitutive interaction of β-arrestin2 with PDE4D5.144,146 PDE4D5 and Epac1 share common β-arrestin2 binding sites and compete for interaction with β-arrestin2. Disruption of PDE4D5–β-arrestin2 interaction allows the recruitment of Epac1 to β2AR, causing a switch in β2AR signaling from non-hypertrophic to pro-hypertrophic mode. β-arrestin2–induced CaMKII signaling also provokes the nuclear export of histone deacetylase 4 (HDAC4) which is critical for the development of cardiac hypertrophy.146
NON-CANONICAL SIGNALING VIA β-ARRESTINS
β-arrestin and G protein signaling at early endosomes
Paradoxically, β-arrestins can prolong G protein-mediated signaling instead of blocking it for a subset of GPCRs.148,149 This newly identified function of β-arrestins appears to challenge their conventional role in inhibiting G protein coupling and in promoting receptor desensitization. However, because β-arrestin’s binding with activated GPCRs causes dynamic changes in its structure, it is likely that certain conformations of β-arrestins may inhibit G protein coupling, while other conformations of β-arrestins may fail to uncouple G proteins from GPCRs.
Prolonged G protein activity and cAMP response have been observed in early endosomes for the following GPCRs: thyroid-stimulating hormone receptor (TSHR), parathyroid hormone receptor type 1 (PTH1R), and V2R which couple with Gs and for the sphingosine-1-phosphate receptor (S1PR) which couples to Gi.149–153 The aforementioned GPCRs belong to the group termed ‘class B’, since they have equal affinity for either β-arrestin isoform and promote stable β-arrestin interaction and sustained ERK signaling in endosomes.34,64 In contrast, the class A receptor β2AR,34 which prefers to bind β-arrestin2, forms transient complexes with β-arrestin2 and fails to induce cAMP signaling in endosomes unless the β2AR is forced to have the carboxyl tail of V2R (β2V2R chimera).148 For the S1P receptor, sustained endosomal signaling after washout is ligand-specific and is triggered by FTY720-phosphate but not by the native ligand SIP.153 Similar ligand dependence is observed for the V2R since vasopressin, but not oxytocin induces β-arrestin recruitment to endosomes, as well as prolonged cAMP signaling in endosomes.150 β-arrestins, by sequestering the activated receptors away from phosphodiesterases at the cell surface or by inhibiting PDE4 activity through ERK activation in endosomes, may promote sustained cAMP production.154,155 Intriguingly, desensitization of vasopressin-mediated cAMP generation involves the retromer complex (VPS26/29/35) that regulates trafficking of internalized receptor from endosomes to the Golgi; of these retromer subunits, VPS26 crystal structures have shown a strong structural homology with β-arrestins.156
Super complexes, termed “megaplexes” consisting of the activated receptor, β-arrestin, Gα and Gβγ subunits have been characterized by cryo-electron microscopy (EM) and bioluminescence resonance energy transfer, suggesting that G protein and β-arrestin interactions with activated GPCRs are not mutually exclusive.148 A ternary complex containing PTH1R, β-arrestin and the Gβγ dimer was observed in endosomes in response to PTH stimulation.152 β-arrestin is known to embrace two different conformations when bound to GPCRs as observed in negative-stain EM study of the β2V2R chimera.52,157 Of the two detected β-arrestin conformations, the “core” conformation involves tight interaction with the activated receptor via full integration of β-arrestin into the transmembrane core of the receptor while concurrently binding with the phosphorylated C-terminal tail of the receptor. The other conformation involves loose receptor interaction as β-arrestin “hangs” from the phosphorylated C-terminal tail of the activated receptor (“tail” conformation).157 β-arrestin1 configuration in the megaplex of signaling endosomes assumes the ‘tail’ conformation of the receptor complex which perhaps enables concurrent interaction of the heterotrimeric Gs with the transmembrane core of the receptor for signal transduction.148 The core interaction of β-arrestin in the β2V2R-β-arrestin complex hinders G protein coupling to the receptor but is also found to be expendable for agonist-induced receptor internalization as well as for β–arrestin binding and activation of ERK.148,158 Thus, the tail conformation by simultaneously enabling G protein coupling to the internalized receptor-β–arrestin complex and β–arrestin association with ERK may allow concurrent cAMP production and ERK signaling in endosomes.
β-arrestin signaling distant from GPCRs
Intriguingly, sustained interaction with the activated receptor is not mandatory for β-arrestin-mediated signal transduction. When β-arrestin2 was forced to translocate to the plasma membrane, it triggered ERK activation even in the absence of GPCR activity.159 β-arrestin2 was recently found to undergo receptor-specific conformational changes that outlast β-arrestin/receptor interaction and enable β-arrestin signaling activity after dissociation from the activated receptor.32 The receptor-specific activation/deactivation cycles of β-arrestins can optimize signaling by freeing the receptor to allow activation of additional β-arrestins and by permitting β-arrestin trafficking for activation of effectors located at distal sites from the receptor.32 β-arrestin activation at distance was also demonstrated for β1AR-induced signaling.61 While both β1AR and β2AR form transient complexes with β-arrestin2 and trigger equal β-arrestin2 recruitment to clathrin-coated structures (CCSs), only β2AR internalizes readily and accumulates in CCSs in a complex with β-arrestin. Although β1AR does not traffic with β-arrestin, it promotes β-arrestin accumulation in the CCSs to elicit clathrin-dependent ERK activation, which persists in endosomes.61
β-arrestin-mediated transfer of functional GPCRs to exosomes
Exosomes are extracellular vesicles that play an important role as intercellular messengers, influencing the cellular activities of neighboring cells via the transfer of bioactive molecules such as lipids, proteins, RNAs and microRNAs.160 Intriguingly, β-arrestin regulates the intercellular trafficking of functional GPCRs via exosomes. Mice subjected to cardiac pressure overload, release functional AT1Rs into circulation and these receptor are packaged into exosomes in a β-arrestin2-dependent manner.161 Although β-arrestin2 is not required for the intracellular maturation and exocytosis of exosomes, it is necessary for the sorting and packaging of the AT1R and possibly other GPCRs into these vesicular bodies. The exosomes released from cardiomyocytes during cardiac pressure overload are transferred to recipient cells that include cardiomyocytes, skeletal myocytes, and mesenteric resistance vessels and could serve as reservoirs of functional AT1Rs to preserve cardiovascular homeostasis.161
β-ARRESTIN-BIASED AGONISTS
The therapeutic potential of β-arrestin-dependent signaling has attracted tremendous research interests in academia as well as pharmaceutical companies and a number of ligands with bias for β-arrestin have been discovered and their biological properties described (TABLE 1). 27,29 The following sections include some of the well-studied GPCR examples for β-arrestin-biased agonism highlighting the biological effects and therapeutic potential of β-arrestin-biased signaling.
TABLE 1.
LIST OF β-ARRESTIN-BIASED LIGANDS
| GPCR | LIGAND | EFFECT | THERAPEUTIC AREA | References |
|---|---|---|---|---|
| α2-Adrenergic Receptor (α2AR) | Desipramine | Blockade of vasoconstriction via α2AR down-regulation | Hypertension | 200 |
| Angiotensin Receptor (AT1R) | Sar1,Ile4,Ile8-AngII (SII) | β-arrestin2-dependent signaling and
protein synthesis. Increased circulating aldosterone levels via β-arrestin1 |
Heart Failure | 162 |
| Sar1,Gly4,Gly8- AngII (SGG) | β-arrestin2-dependent signaling | 201 | ||
| TRV023,TRV027 | Reduction of blood pressure and increase in cardiac contraction | Hypertension & Acute heart failure | 169 | |
| Ang-(1–7) | Attenuation of phenylephrine-induced aorta contraction | Hypertension | 171 | |
| CORML: [Met5, Leu8]-AngII, CORSML: [Sar1, Met5, Leu8]-AngII, and CORET: [Sar1, Cys(Et)5, Leu8]-AngII | Post-MI hyperaldosteronism | Heart Failure | 168 | |
| β-Adrenergic Receptors (βARs) | Carvedilol | Cardioprotection, anti-oxidative, anti-inflammatory and anti-proliferative, anti- apoptotic. | Congestive Heart Failure Hypertension | 179,181,202 |
| Nebivolol | Vasodilation by inducing nitric oxide (NO) production | Hypertension | 203 | |
| β2-Adrenergic Receptors (β2AR) | ICL1–9 (pepducin) | Cardiomyocyte contraction | Congestive Heart Failure | 194 |
| CXCR3 (Chemokine Receptor) | FAUC1036 | Weak chemotaxis, β-arrestin2 recruitment and receptor internalization without G protein activation | Rheumatoid Arthritis, Multiple Sclerosis, Psoriasis, Cancer | 204,205 |
| Dopamine D2 Receptor (D2R) | UNC9975, UNC0006, and UNC9994 | Reduction of psychotic responses in the brain | Schizophrenia | 195 |
| Free Fatty Acid Receptor 1 FFAR1/GPR40 | TAK-875 | Increased glucose-stimulated insulin secretion | Type 2 Diabetes | 206 |
| Histamine H4 Receptor | 1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methyl-piperazine (JNJ7777120) | Unknown | Chronic inflammatory diseases | 207 |
| Metabotropic Glutamate 1a (mGlu1aR) | Glutaric acid; Succinic acid | Cytoprotective signaling | Schizophrenia Melanoma | 208 |
| Neurotensin 1 Receptor NTR1 | 2-cyclopropyl-6,7-dimethoxy-4-(4-(2-methoxyphenyl)- piperazin-1-yl)quinazoline, 32(ML314) | Attenuation of methamphetamine-induced hyper-locomotion, and associated conditioned place preference. | Drug abuse, addiction | 209,210 |
| κ-Opioid Receptor (KOR) | GR89696, ICI 199,441 | Sedation, dysphoria, hallucination | Psychotic disorders | 211 |
| Protease Activated Receptor 1 (PAR1) | Anticoagulant protease-activated protein C (APC) | Endothelial barrier protection | Thrombosis, Wound healing | 120,121,125 |
| Parathyroid Hormone Receptor (PTH1R) | [d-Trp12,Tyr34]-bPTH (7–34) | Stimulate anabolic bone formation with limited bone resorption | Osteoporosis | 197 |
AT1R
AT1R and the renin-angiotensin-aldosterone system (RAAS) are targeted for renal and hypertensive vascular diseases because of their predominant role in stimulating vasoconstriction. Angiotensin receptor blockers (ARBs) are commonly used to alleviate hypertension, diabetic nephropathy, cardiac hypertrophy and congestive heart failure.
An AngII analog, SII ([Sar1,Ile4,Ile8]Ang II) is a modified AngII peptide, which does not activate Gq-dependent calcium influx and IP3 production.162 However, SII functions as a β-arrestin-biased agonist (Figure 2) and triggers AT1R phosphorylation, β-arrestin recruitment as well as β-arrestin-mediated receptor internalization and signaling. Elegant phosphoproteomics studies have revealed that while AngII and SII provoke matching phosphorylation of common proteins, SII also triggers unique phosphorylation patterns (based on phospho-peptide identification).163,164 SII elicits GRK6 and β-arrestin2-dependent ERK activation in endosomes and promotes β-arrestin-regulated Akt activity and mTOR phosphorylation to stimulate protein synthesis.80,165
β-arrestin1-dependent signaling triggered by the AT1aR increases aldosterone production and also upregulates steroidogenic acute regulatory protein (StAR), which catalyzes mitochondrial cholesterol uptake in adrenocortical zona glomerulosa (AZG) cells.166 β-arrestin1 overexpression or AT1aR stimulation by the β-arrestin-biased agonist SII increases circulating aldosterone levels. β-arrestin1-mediated aldosterone elevation worsens cardiac function after myocardial infarction and accelerates cardiac remodeling. Inhibition of adrenal β-arrestin1 is cardioprotective because it decreases circulating aldosterone levels and regresses remodeling of the infarcted heart.167 Three novel AngII peptide analogs: CORML: [Met5, Leu8]-AngII, CORSML: [Sar1, Met5, Leu8]-AngII, and CORET: [Sar1, Cys(Et)5, Leu8]-AngII are more potent than SII at inducing β-arrestin coupling and provoking AT1R-triggered aldosterone secretion.168 CORET, the most potent and efficient out of the three, significantly increases aldosterone levels after post-myocardial infarction in mice. While the above β-arrestin1-dependent signaling triggered by elevated aldosterone leads to adverse cardiac remodeling downstream of the AT1R, whether β-arrestin2 has a reciprocal effect through these biased ligands remains to be defined.
TRV023 (Sar-Arg-Val-Tyr-Lys-His-Pro-Ala-OH) and TRV027 (Sar-Arg-Val-Tyr-Ile-His-Pro-D-Ala-OH) are additional β-arrestin-biased agonists of AT1R that have cardioprotective effects.169 They bind AT1R with higher affinity than SII and antagonize AngII-stimulated G-protein signaling. Binding of these peptide ligands to the AT1aR also promotes β-arrestin recruitment but with less efficiency than AngII. Nevertheless, TRV023 and TRV027 provoke receptor internalization more efficiently than AngII and promote ERK, Src, and endothelial nitric-oxide synthase phosphorylation in a β-arrestin-dependent manner.170 These compounds have cardiovascular benefits of reducing blood pressure and promoting cardiac contraction. Thus, they provide an alternative for angiotensin converting enzyme (ACE) inhibitors and ARBs which, despite their vasodilative effects, are contraindicated in acute heart failure as they may induce hypotension and reduce cardiac output.170
Functional selectivity has recently been investigated in natural renin-angiotensin-aldosterone system peptides which include AngII, AngIII, AngIV, and Ang-(1–7).171 Interestingly, Ang-(1–7) presents β-arrestin biased agonism and acts as a competitive antagonist for AngII as it fails to stimulate G protein signaling. Ang-(1–7) also negatively regulates vasoconstriction in vivo as its binding to the AT1R attenuates phenylephrine-induced aorta contraction.
βAR
β1AR is the dominant βAR in the heart, which promotes catecholamine-induced cardiac inotropy, lusitropy and chronotropy, and represents 75–80% of the total cardiac βARs. On the other hand, β2AR expression contributes 20–25% of the total cardiac βARs in normal hearts.172 In failing human hearts, β1ARs are substantially downregulated, whereas β2AR levels remain unaltered.173,174 Chronic induction of G protein signaling via the β1AR promotes harmful apoptotic signaling whereas β2AR activation in failing hearts promotes cell survival.175 On the other hand, β-arrestin isoforms may regulate cardiac function and βAR-induced cardiac contractility differentially. While βarrestin1 seems to be mainly responsible for βAR desensitization, β-arrestin 2 is cardioprotective with beneficial effects on β1AR-dependent contractility.29,176,177
βAR-signaling via β-arrestin-dependent mechanism is induced by carvedilol, a nonselective βAR antagonist that also blocks α1-adrenergic receptors. Carvedilol is more effective than most traditional β-blockers in reducing mortality and morbidity of patients with congestive heart failure as it presents additional cardiovascular benefits associated with its anti-oxidative, anti-inflammatory and anti-proliferative properties.178 While both carvedilol and the β-blocker propranolol act as inverse agonists for Gs-dependent adenylyl cyclase activation by the β2AR, only carvedilol activates ERK signaling in a β-arrestin-dependent manner.49,179 Carvedilol-induced effects are insensitive to pertussis toxin pretreatment and hence are independent of β2AR coupling to Gi.179,180 Carvedilol induces EGFR transactivation by the β1AR in a GRK5/6 and β-arrestin-dependent manner.89,181 β-arrestins recruit the tyrosine-protein kinase Src to carvedilol-activated β1AR, promoting membrane-bound MMP activation, heparin-binding EGF (HB-EGF) cleavage and release into the extracellular space for EGFR binding, resulting in EGFR tyrosine phosphorylation, EGFR internalization and subsequent ERK signaling. β-arrestin-mediated β1AR transactivation of EGFR is cardioprotective in mice, highlighting the therapeutic benefits of carvedilol.89
β1AR activation by carvedilol also promotes microRNA (miR) maturation in a β-arrestin-dependent fashion.182 miRs are small noncoding RNAs that regulate post-transcriptional gene expression bearing a variety of effects on cardiac physiology and function. Irregularities in their expression are associated with myocardial infarction, hypertrophy, and cardiac remodeling. As a result, miRs are emerging as biomarkers and therapeutic targets for cardiovascular diseases and heart failure.183,184 Carvedilol upregulates a subset of mature and pre-miRs that includes miRs 125a-5p, 125b-5p, 150, 190, 199a-3p and 214.182,185 Among them, miR-150 has cardio-protective effects and is down-regulated during acute myocardial infarction, atrial fibrillation, dilated cardiomyopathy, ischemic injury and heart failure.185–190 miR-199a-3p and miR-214 are activated by carvedilol in cardiomyocytes subjected to ischemia/reperfusion and promote cardiomyocyte survival by activating p-Akt survival signaling, inducing the expression SRY (sex determining region Y)-box 2 (Sox2), a transcription factor that promotes pluripotency and also by repressing the apoptosis-associated genes ddit4 (DNA damage-inducible transcript 4) and ing4 (inhibitor of growth family member 4).191 miR-190 upregulation by carvedilol occurs via the β1AR and is independent of β2AR and α1AR activation. It also requires specific activated conformations of the receptor, β1AR’s phosphorylation by GRKs 5 or 6 and the recruitment of β-arrestin1 to the carvedilol bound-receptor complex. Ablation of β1AR or knockdown of GRK 5, 6 or β-arrestin1 blocks the effects of carvedilol on miR-190 expression.182 β-arrestin’s effects on miR maturation are attributed to its ability to interact with enzymes that regulate miR processing such as Drosha and hnRNPA1.182
Recent studies have shown that pepducins, which are cell-permeable lipidated peptides representing the intracellular loop regions of GPCRs can trigger signaling by acting as agonists or allosteric modulators although in general they act as inhibitors of GPCR activity.192,193 Of the various pepducins generated from the β2AR intracellular loop sequences, the pepducin called ICL1-9 surprisingly elicits β-arrestin-biased signaling via the β2AR.194 Like carvedilol, ICL1–9 promotes β2AR phosphorylation by GRKs, β-arrestin recruitment, receptor internalization, ERK activation, and EGFR transactivation. Nevertheless, unlike carvedilol, ICL1–9 does not block the orthosteric-binding site of β2AR and does not induce β1AR signaling and trafficking. Moreover, ICL1–9 also provokes cardiomyocyte contraction mediated by β-arrestin1. The β-arrestin-bias and inotropic abilities of ICL1–9 offer a new therapeutic option for cardiac dysfunction.194
D2R
Three β-arrestin-biased D2R ligands designated as UNC9975, UNC0006 and UNC9994 were identified from a small library of compounds generated based on the chemical structure of aripiprazole, which is an FDA-approved antipsychotic drug.195 Aripiprazole was originally defined as a partial D2 selective agonist, but later studies showed that based on the assay platform utilized, it had a wide range of efficacy (partial agonist, full agonist and antagonist) as a D2R ligand.196 UNC9975, UNC0006 and UNC9994 antagonize D2R/Gi coupling, but serve as partial agonists for D2R/β-arrestin2 association.195 Interestingly, when administered to mice, UNC9975 and UNC9994 displayed potent antipsychotic-like activity, which required β-arrestin2 expression. When mice lacked β-arrestin2, UNC9975 had little antipsychotic actions, but instead provoked catalepsy or motor dysfunction, that is generally observed as a side effect of antipsychotic drugs.195 These studies convey that β-arrestin2-D2R interactions provoke signaling that not only reduces psychotic responses in the brain, but also suppress motoric side effects of D2R activation. Accordingly, β-arrestin-biased D2R agonists could be valuable in the treatment of schizophrenia and related disorders.
PTH1R
Biased ligands have been characterized for the PTH1R, a GPCR mainly expressed in kidneys and bones. PTH1R stimulation activates G protein and β-arrestin-mediated signaling pathways to regulate calcium homeostasis and/or bone formation.197–199 Binding of conventional, nonselective agonist PTH(1–34) to PTH1R triggers G protein activation, and also induces β-arrestin recruitment, resulting in receptor internalization and ERK signaling. On the other hand, β-arrestin-biased agonist, D-Trp(12), Tyr(34)-bPTH(7–34), antagonizes G protein coupling and triggers β-arrestin-mediated ERK activity and PTH1R internalization. These ligands also present distinct effects on bone formation. While both PTH(1–34) and PTH(7–34) stimulate anabolic bone formation in mice, only PTH(1–34) increases markers of bone resorption and promote hypercalcemia.197 PTH(7–34)-triggered β-arrestin signaling, by its limiting effects on hypercalcemia, is a potential therapeutic target for the treatment of metabolic bone diseases like osteoporosis.
CONCLUSIONS
GPCRs are major therapeutic targets because their signal activation or inactivation affects almost all physiological pathways. β-arrestins are at the nexus of a variety of GPCR signal transduction modules and largely influence the signaling activities, vesicular trafficking and fate of GPCRs by scaffolding a wide range of proteins to the activated receptor complex. It has become evident that β-arrestin molecules embrace different activated conformations upon association with the activated GPCR.30,32 These conformations of β-arrestins are dictated by ligand-triggered “barcoding” of the receptor tail by GRKs or by agonist-induced posttranslational modifications of β-arrestins. Biased agonists present an unmatched opportunity to selectively target one mode of signaling (via a G protein or β-arrestin); however, such compounds can have unique pharmacological and biological profiles than those observed when the particular signaling mode is stimulated by a ‘balanced’ agonist. Therefore, in order to fully harness the potential benefits of biased agonism, it becomes critical to chart the signal transduction network(s) induced by the biased agonist in a cell-specific manner and further uncover the molecular mechanisms that regulate (or desensitize) biased signaling.
Acknowledgments
The authors acknowledge funding from the NIH (HL080525 and HL118369 to SKS), and 15GRNT25550051 from the American Heart Association (SKS). We also acknowledge support from the Duke O’Brien Center for Kidney Research (NIH/NIDDK, Award Number P30-DK096493).
References
- 1.Lefkowitz RJ. The superfamily of heptahelical receptors. Nat Cell Biol. 2000;2:E133–136. doi: 10.1038/35017152. [DOI] [PubMed] [Google Scholar]
- 2.Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta. 2007;1768:794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 5.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 7.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 8.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A, Gupta B, Gupta C, Shukla AK. Emerging Functional Divergence of beta-Arrestin Isoforms in GPCR Function. Trends Endocrinol Metab. 2015;26:628–642. doi: 10.1016/j.tem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 14.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 2008;27:384–393. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- 17.Kooijman EE, Burger KN. Biophysics and function of phosphatidic acid: a molecular perspective. Biochim Biophys Acta. 2009;1791:881–888. doi: 10.1016/j.bbalip.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 19.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 20.Han SO, Kommaddi RP, Shenoy SK. Distinct roles for beta-arrestin2 and arrestin-domain-containing proteins in beta2 adrenergic receptor trafficking. EMBO Rep. 2013;14:164–171. doi: 10.1038/embor.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean-Charles PY, Snyder JC, Shenoy SK. Chapter One - Ubiquitination and Deubiquitination of G Protein-Coupled Receptors. Prog Mol Biol Transl Sci. 2016;141:1–55. doi: 10.1016/bs.pmbts.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Kommaddi RP, Shenoy SK. Arrestins and protein ubiquitination. Prog Mol Biol Transl Sci. 2013;118:175–204. doi: 10.1016/B978-0-12-394440-5.00007-3. [DOI] [PubMed] [Google Scholar]
- 23.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 24.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luttrell LM, Miller WE. Arrestins as regulators of kinases and phosphatases. Prog Mol Biol Transl Sci. 2013;118:115–147. doi: 10.1016/B978-0-12-394440-5.00005-X. [DOI] [PubMed] [Google Scholar]
- 26.Han SO, Xiao K, Kim J, Wu JH, Wisler JW, Nakamura N, Freedman NJ, Shenoy SK. MARCH2 promotes endocytosis and lysosomal sorting of carvedilol-bound beta(2)-adrenergic receptors. J Cell Biol. 2012;199:817–830. doi: 10.1083/jcb.201208192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Luttrell LM. Minireview: More than just a hammer: ligand “bias” and pharmaceutical discovery. Mol Endocrinol. 2014;28:281–294. doi: 10.1210/me.2013-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodavance SY, Gareri C, Torok RD, Rockman HA. G Protein-coupled Receptor Biased Agonism. J Cardiovasc Pharmacol. 2016;67:193–202. doi: 10.1097/FJC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MH, Appleton KM, Strungs EG, Kwon JY, Morinelli TA, Peterson YK, Laporte SA, Luttrell LM. The conformational signature of beta-arrestin2 predicts its trafficking and signalling functions. Nature. 2016;531:665–668. doi: 10.1038/nature17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang F, Yu X, Liu C, Qu CX, Gong Z, Liu HD, Li FH, Wang HM, He DF, Yi F, Song C, Tian CL, Xiao KH, Wang JY, Sun JP. Phospho-selective mechanisms of arrestin conformations and functions revealed by unnatural amino acid incorporation and (19)F-NMR. Nat Commun. 2015;6:8202. doi: 10.1038/ncomms9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuber S, Zabel U, Lorenz K, Nuber A, Milligan G, Tobin AB, Lohse MJ, Hoffmann C. beta-Arrestin biosensors reveal a rapid, receptor-dependent activation/deactivation cycle. Nature. 2016;531:661–664. doi: 10.1038/nature17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 34.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 35.Wei H, Ahn S, Barnes WG, Lefkowitz RJ. Stable interaction between beta-arrestin 2 and angiotensin type 1A receptor is required for beta-arrestin 2-mediated activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 2004;279:48255–48261. doi: 10.1074/jbc.M406205200. [DOI] [PubMed] [Google Scholar]
- 36.Krasel C, Zabel U, Lorenz K, Reiner S, Al-Sabah S, Lohse MJ. Dual role of the beta2-adrenergic receptor C terminus for the binding of beta-arrestin and receptor internalization. J Biol Chem. 2008;283:31840–31848. doi: 10.1074/jbc.M806086200. [DOI] [PubMed] [Google Scholar]
- 37.Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol Endocrinol. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- 38.Kao YJ, Ghosh M, Schonbrunn A. Ligand-dependent mechanisms of sst2A receptor trafficking: role of site-specific phosphorylation and receptor activation in the actions of biased somatostatin agonists. Mol Endocrinol. 2011;25:1040–1054. doi: 10.1210/me.2010-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BW, Hinkle PM. Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol Pharmacol. 2008;74:195–202. doi: 10.1124/mol.108.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim OJ, Gardner BR, Williams DB, Marinec PS, Cabrera DM, Peters JD, Mak CC, Kim KM, Sibley DR. The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J Biol Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prihandoko R, Alvarez-Curto E, Hudson BD, Butcher AJ, Ulven T, Miller AM, Tobin AB, Milligan G. Distinct Phosphorylation Clusters Determine the Signaling Outcome of Free Fatty Acid Receptor 4/G Protein-Coupled Receptor 120. Mol Pharmacol. 2016;89:505–520. doi: 10.1124/mol.115.101949. [DOI] [PubMed] [Google Scholar]
- 42.Bray L, Froment C, Pardo P, Candotto C, Burlet-Schiltz O, Zajac JM, Mollereau C, Mouledous L. Identification and functional characterization of the phosphorylation sites of the neuropeptide FF2 receptor. J Biol Chem. 2014;289:33754–33766. doi: 10.1074/jbc.M114.612614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobin AB. G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol. 2008;153(Suppl 1):S167–176. doi: 10.1038/sj.bjp.0707662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, Bottrill A, Mistry S, Tobin AB. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal. 2011;4:ra52. doi: 10.1126/scisignal.2001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem. 2010;285:7805–7817. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urs NM, Gee SM, Pack TF, McCorvy JD, Evron T, Snyder JC, Yang X, Rodriguiz RM, Borrelli E, Wetsel WC, Jin J, Roth BL, O’Donnell P, Caron MG. Distinct cortical and striatal actions of a beta-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1614347113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, Paduch M, Tripathi-Shukla P, Koide A, Koide S, Weis WI, Kossiakoff AA, Kobilka BK, Lefkowitz RJ. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jean-Charles PY, Rajiv V, Shenoy SK. Ubiquitin-Related Roles of beta-Arrestins in Endocytic Trafficking and Signal Transduction. J Cell Physiol. 2016;231:2071–2080. doi: 10.1002/jcp.25317. [DOI] [PubMed] [Google Scholar]
- 54.Jean-Charles PY, Zhang L, Wu JH, Han SO, Brian L, Freedman NJ, Shenoy SK. Ubiquitin-specific Protease 20 Regulates the Reciprocal Functions of beta-Arrestin2 in Toll-like Receptor 4-promoted Nuclear Factor kappaB (NFkappaB) Activation. J Biol Chem. 2016;291:7450–7464. doi: 10.1074/jbc.M115.687129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 56.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 59.Penela P. Chapter Three - Ubiquitination and Protein Turnover of G-Protein-Coupled Receptor Kinases in GPCR Signaling and Cellular Regulation. Prog Mol Biol Transl Sci. 2016;141:85–140. doi: 10.1016/bs.pmbts.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ. Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol. 2000;1:227–233. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- 61.Eichel K, Jullie D, von Zastrow M. beta-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat Cell Biol. 2016;18:303–310. doi: 10.1038/ncb3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shenoy SK, Lefkowitz RJ. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem. 2005;280:15315–15324. doi: 10.1074/jbc.M412418200. [DOI] [PubMed] [Google Scholar]
- 64.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 65.Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 66.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci U S A. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhan X, Kook S, Gurevich EV, Gurevich VV. Arrestin-dependent activation of JNK family kinases. Handb Exp Pharmacol. 2014;219:259–280. doi: 10.1007/978-3-642-41199-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iacovelli L, Felicioni M, Nistico R, Nicoletti F, De Blasi A. Selective regulation of recombinantly expressed mGlu7 metabotropic glutamate receptors by G protein-coupled receptor kinases and arrestins. Neuropharmacology. 2014;77:303–312. doi: 10.1016/j.neuropharm.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 70.Gong K, Li Z, Xu M, Du J, Lv Z, Zhang Y. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J Biol Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang P, Jiang Y, Wang Y, Shyy JY, DeFea KA. Beta-arrestin inhibits CAMKKbeta-dependent AMPK activation downstream of protease-activated-receptor-2. BMC Biochem. 2010;11:36. doi: 10.1186/1471-2091-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheung R, Malik M, Ravyn V, Tomkowicz B, Ptasznik A, Collman RG. An arrestin-dependent multi-kinase signaling complex mediates MIP-1beta/CCL4 signaling and chemotaxis of primary human macrophages. J Leukoc Biol. 2009;86:833–845. doi: 10.1189/jlb.0908551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goel R, Baldassare JJ. beta-Arrestin 1 couples thrombin to the rapid activation of the Akt pathway. Ann N Y Acad Sci. 2002;973:138–141. doi: 10.1111/j.1749-6632.2002.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 76.Lodeiro M, Theodoropoulou M, Pardo M, Casanueva FF, Camina JP. c-Src regulates Akt signaling in response to ghrelin via beta-arrestin signaling-independent and -dependent mechanisms. PLoS One. 2009;4:e4686. doi: 10.1371/journal.pone.0004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan A, Li D, Ibrahim S, Smyth E, Woulfe DS. The physical association of the P2Y12 receptor with PAR4 regulates arrestin-mediated Akt activation. Mol Pharmacol. 2014;86:1–11. doi: 10.1124/mol.114.091595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kendall RT, Lee MH, Pleasant DL, Robinson K, Kuppuswamy D, McDermott PJ, Luttrell LM. Arrestin-dependent angiotensin AT1 receptor signaling regulates Akt and mTor-mediated protein synthesis. J Biol Chem. 2014;289:26155–26166. doi: 10.1074/jbc.M114.595728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, Shenoy SK, Gregory SG, Ahn S, Duckett DR, Lefkowitz RJ. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature. 2011;477:349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 83.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 84.O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT, Klein PS. Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest. 2011;121:3756–3762. doi: 10.1172/JCI45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nasri I, Bonnet D, Zwarycz B, d’Aldebert E, Khou S, Mezghani-Jarraya R, Quaranta M, Rolland C, Bonnart C, Mas E, Ferrand A, Cenac N, Magness S, Van Landeghem L, Vergnolle N, Racaud-Sultan C. PAR2-dependent activation of GSK3beta regulates the survival of colon stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2016;311:G221–236. doi: 10.1152/ajpgi.00328.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lecat S, Belemnaba L, Galzi JL, Bucher B. Neuropeptide Y receptor mediates activation of ERK1/2 via transactivation of the IGF receptor. Cell Signal. 2015;27:1297–1304. doi: 10.1016/j.cellsig.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 87.Kallifatidis G, Munoz D, Singh RK, Salazar N, Hoy JJ, Lokeshwar BL. beta-Arrestin-2 Counters CXCR7-Mediated EGFR Transactivation and Proliferation. Mol Cancer Res. 2016;14:493–503. doi: 10.1158/1541-7786.MCR-15-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryu JM, Baek YB, Shin MS, Park JH, Park SH, Lee JH, Han HJ. Sphingosine-1-phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P1/S1P3-dependent beta-arrestin/c-Src pathways. Stem Cell Res. 2014;12:69–85. doi: 10.1016/j.scr.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gradinaru I, Babaeva E, Schwinn DA, Oganesian A. Alpha1a-Adrenoceptor Genetic Variant Triggers Vascular Smooth Muscle Cell Hyperproliferation and Agonist Induced Hypertrophy via EGFR Transactivation Pathway. PLoS One. 2015;10:e0142787. doi: 10.1371/journal.pone.0142787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci U S A. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Esposito G, Perrino C, Cannavo A, Schiattarella GG, Borgia F, Sannino A, Pironti G, Gargiulo G, Di Serafino L, Franzone A, Scudiero L, Grieco P, Indolfi C, Chiariello M. EGFR trans-activation by urotensin II receptor is mediated by beta-arrestin recruitment and confers cardioprotection in pressure overload-induced cardiac hypertrophy. Basic Res Cardiol. 2011;106:577–589. doi: 10.1007/s00395-011-0163-2. [DOI] [PubMed] [Google Scholar]
- 93.Santos-Zas I, Gurriaran-Rodriguez U, Cid-Diaz T, Figueroa G, Gonzalez-Sanchez J, Bouzo-Lorenzo M, Mosteiro CS, Senaris J, Casanueva FF, Casabiell X, Gallego R, Pazos Y, Mouly V, Camina JP. beta-Arrestin scaffolds and signaling elements essential for the obestatin/GPR39 system that determine the myogenic program in human myoblast cells. Cell Mol Life Sci. 2016;73:617–635. doi: 10.1007/s00018-015-1994-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zajac M, Law J, Cvetkovic DD, Pampillo M, McColl L, Pape C, Di Guglielmo GM, Postovit LM, Babwah AV, Bhattacharya M. GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS One. 2011;6:e21599. doi: 10.1371/journal.pone.0021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 96.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 97.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S, Gong H, Jiang G, Ye Y, Wu J, You J, Zhang G, Sun A, Komuro I, Ge J, Zou Y. Src is required for mechanical stretch-induced cardiomyocyte hypertrophy through angiotensin II type 1 receptor-dependent beta-arrestin2 pathways. PLoS One. 2014;9:e92926. doi: 10.1371/journal.pone.0092926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wisler JW, Harris EM, Raisch M, Mao L, Kim J, Rockman HA, Lefkowitz RJ. The role of beta-arrestin2-dependent signaling in thoracic aortic aneurysm formation in a murine model of Marfan syndrome. Am J Physiol Heart Circ Physiol. 2015;309:H1516–1527. doi: 10.1152/ajpheart.00291.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trivedi DB, Loftin CD, Clark J, Myers P, DeGraff LM, Cheng J, Zeldin DC, Langenbach R. beta-Arrestin-2 deficiency attenuates abdominal aortic aneurysm formation in mice. Circ Res. 2013;112:1219–1229. doi: 10.1161/CIRCRESAHA.112.280399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Canadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. Part 1: pathophysiology and diagnosis. Nat Rev Cardiol. 2010;7:256–265. doi: 10.1038/nrcardio.2010.30. [DOI] [PubMed] [Google Scholar]
- 102.Evron T, Peterson SM, Urs NM, Bai Y, Rochelle LK, Caron MG, Barak LS. G Protein and beta-arrestin signaling bias at the ghrelin receptor. J Biol Chem. 2014;289:33442–33455. doi: 10.1074/jbc.M114.581397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma X, Zhao Y, Daaka Y, Nie Z. Acute activation of beta2-adrenergic receptor regulates focal adhesions through betaArrestin2- and p115RhoGEF protein-mediated activation of RhoA. J Biol Chem. 2012;287:18925–18936. doi: 10.1074/jbc.M112.352260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhattacharya M, Anborgh PH, Babwah AV, Dale LB, Dobransky T, Benovic JL, Feldman RD, Verdi JM, Rylett RJ, Ferguson SS. Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat Cell Biol. 2002;4:547–555. doi: 10.1038/ncb821. [DOI] [PubMed] [Google Scholar]
- 105.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 106.Anthony DF, Sin YY, Vadrevu S, Advant N, Day JP, Byrne AM, Lynch MJ, Milligan G, Houslay MD, Baillie GS. beta-Arrestin 1 inhibits the GTPase-activating protein function of ARHGAP21, promoting activation of RhoA following angiotensin II type 1A receptor stimulation. Mol Cell Biol. 2011;31:1066–1075. doi: 10.1128/MCB.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Godin CM, Ferguson SS. The angiotensin II type 1 receptor induces membrane blebbing by coupling to Rho A, Rho kinase, and myosin light chain kinase. Mol Pharmacol. 2010;77:903–911. doi: 10.1124/mol.110.063859. [DOI] [PubMed] [Google Scholar]
- 108.Simard E, Kovacs JJ, Miller WE, Kim J, Grandbois M, Lefkowitz RJ. beta-Arrestin regulation of myosin light chain phosphorylation promotes AT1aR-mediated cell contraction and migration. PLoS One. 2013;8:e80532. doi: 10.1371/journal.pone.0080532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zoudilova M, Min J, Richards HL, Carter D, Huang T, DeFea KA. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem. 2010;285:14318–14329. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mittal N, Roberts K, Pal K, Bentolila LA, Fultz E, Minasyan A, Cahill C, Pradhan A, Conner D, DeFea K, Evans C, Walwyn W. Select G-protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK, and beta-arrestin 1 pathway. Cell Rep. 2013;5:1010–1021. doi: 10.1016/j.celrep.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Semprucci E, Tocci P, Cianfrocca R, Sestito R, Caprara V, Veglione M, Castro VD, Spadaro F, Ferrandina G, Bagnato A, Rosano L. Endothelin A receptor drives invadopodia function and cell motility through the beta-arrestin/PDZ-RhoGEF pathway in ovarian carcinoma. Oncogene. 2016;35:3432–3442. doi: 10.1038/onc.2015.403. [DOI] [PubMed] [Google Scholar]
- 112.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 113.Tao H, Yang JJ, Shi KH, Li J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism. 2016;65:30–40. doi: 10.1016/j.metabol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Schulte G, Schambony A, Bryja V. beta-Arrestins - scaffolds and signalling elements essential for WNT/Frizzled signalling pathways? Br J Pharmacol. 2010;159:1051–1058. doi: 10.1111/j.1476-5381.2009.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 116.Kriz V, Pospichalova V, Masek J, Kilander MB, Slavik J, Tanneberger K, Schulte G, Machala M, Kozubik A, Behrens J, Bryja V. beta-arrestin promotes Wnt-induced low density lipoprotein receptor-related protein 6 (Lrp6) phosphorylation via increased membrane recruitment of Amer1 protein. J Biol Chem. 2014;289:1128–1141. doi: 10.1074/jbc.M113.498444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gentzel M, Schille C, Rauschenberger V, Schambony A. Distinct functionality of dishevelled isoforms on Ca2+/calmodulin-dependent protein kinase 2 (CamKII) in Xenopus gastrulation. Mol Biol Cell. 2015;26:966–977. doi: 10.1091/mbc.E14-06-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seitz K, Dursch V, Harnos J, Bryja V, Gentzel M, Schambony A. beta-Arrestin interacts with the beta/gamma subunits of trimeric G-proteins and dishevelled in the Wnt/Ca(2+) pathway in xenopus gastrulation. PLoS One. 2014;9:e87132. doi: 10.1371/journal.pone.0087132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A. 2011;108:E1372–1380. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 123.Vergnolle N, Hollenberg MD, Wallace JL. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR1) Br J Pharmacol. 1999;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schuepbach RA, Feistritzer C, Fernandez JA, Griffin JH, Riewald M. Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb Haemost. 2009;101:724–733. doi: 10.1160/th08-10-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roy RV, Ardeshirylajimi A, Dinarvand P, Yang L, Rezaie AR. Occupancy of human EPCR by protein C induces beta-arrestin-2 biased PAR1 signaling by both APC and thrombin. Blood. 2016 doi: 10.1182/blood-2016-06-720581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosano L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Spadaro F, Salvati E, Biroccio AM, Natali PG, Bagnato A. beta-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced beta-catenin signaling. Oncogene. 2013;32:5066–5077. doi: 10.1038/onc.2012.527. [DOI] [PubMed] [Google Scholar]
- 127.Rosano L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG, Bagnato A. Endothelin A receptor/beta-arrestin signaling to the Wnt pathway renders ovarian cancer cells resistant to chemotherapy. Cancer Res. 2014;74:7453–7464. doi: 10.1158/0008-5472.CAN-13-3133. [DOI] [PubMed] [Google Scholar]
- 128.Arensdorf AM, Marada S, Ogden SK. Smoothened Regulation: A Tale of Two Signals. Trends Pharmacol Sci. 2016;37:62–72. doi: 10.1016/j.tips.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 130.Philipp M, Evron T, Caron MG. The role of arrestins in development. Prog Mol Biol Transl Sci. 2013;118:225–242. doi: 10.1016/B978-0-12-394440-5.00009-7. [DOI] [PubMed] [Google Scholar]
- 131.Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 132.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barakat B, Yu L, Lo C, Vu D, De Luca E, Cain JE, Martelotto LG, Dedhar S, Sadler AJ, Wang D, Watkins DN, Hannigan GE. Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling. EMBO Rep. 2013;14:837–844. doi: 10.1038/embor.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pal K, Hwang SH, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S. Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol. 2016;212:861–875. doi: 10.1083/jcb.201506132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 138.Sun W, Yang J. Molecular basis of lysophosphatidic acid-induced NF-kappaB activation. Cell Signal. 2010;22:1799–1803. doi: 10.1016/j.cellsig.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 140.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 141.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ. Beta-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cianfrocca R, Tocci P, Semprucci E, Spinella F, Di Castro V, Bagnato A, Rosano L. beta-Arrestin 1 is required for endothelin-1-induced NF-kappaB activation in ovarian cancer cells. Life Sci. 2014;118:179–184. doi: 10.1016/j.lfs.2014.01.078. [DOI] [PubMed] [Google Scholar]
- 144.Mangmool S, Shukla AK, Rockman HA. beta-Arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J Cell Biol. 2010;189:573–587. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1377–1386. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berthouze-Duquesnes M, Lucas A, Sauliere A, Sin YY, Laurent AC, Gales C, Baillie G, Lezoualc’h F. Specific interactions between Epac1, beta-arrestin2 and PDE4D5 regulate beta-adrenergic receptor subtype differential effects on cardiac hypertrophic signaling. Cell Signal. 2013;25:970–980. doi: 10.1016/j.cellsig.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 147.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thomsen AR, Plouffe B, Cahill TJ, 3rd, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, Huang L, Breton B, Heydenreich FM, Sunahara RK, Skiniotis G, Bouvier M, Lefkowitz RJ. GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell. 2016;166:907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vilardaga JP, Gardella TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends Pharmacol Sci. 2012;33:423–431. doi: 10.1016/j.tips.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Jr, Hallows KR, Brown D, Bouley R, Vilardaga JP. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288:27849–27860. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G, Vilardaga JP. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gbetagamma complex. Proc Natl Acad Sci U S A. 2013;110:1530–1535. doi: 10.1073/pnas.1205756110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 154.Vilardaga JP, Jean-Alphonse FG, Gardella TJ. Endosomal generation of cAMP in GPCR signaling. Nat Chem Biol. 2014;10:700–706. doi: 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aubry L, Klein G. True arrestins and arrestin-fold proteins: a structure-based appraisal. Prog Mol Biol Transl Sci. 2013;118:21–56. doi: 10.1016/B978-0-12-394440-5.00002-4. [DOI] [PubMed] [Google Scholar]
- 157.Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, Dosey AM, Su M, Liang CR, Gu LL, Shan JM, Chen X, Hanna R, Choi M, Yao XJ, Klink BU, Kahsai AW, Sidhu SS, Koide S, Penczek PA, Kossiakoff AA, Woods VL, Jr, Kobilka BK, Skiniotis G, Lefkowitz RJ. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512:218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumari P, Srivastava A, Banerjee R, Ghosh E, Gupta P, Ranjan R, Chen X, Gupta B, Gupta C, Jaiman D, Shukla AK. Functional competence of a partially engaged GPCR-beta-arrestin complex. Nat Commun. 2016;7:13416. doi: 10.1038/ncomms13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Terrillon S, Bouvier M. Receptor activity-independent recruitment of betaarrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126:1173–1180. doi: 10.1172/JCI81131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation. 2015;131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Christensen GL, Kelstrup CD, Lyngso C, Sarwar U, Bogebo R, Sheikh SP, Gammeltoft S, Olsen JV, Hansen JL. Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol Cell Proteomics. 2010;9:1540–1553. doi: 10.1074/mcp.M900550-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma’ayan A, Gygi SP, Lefkowitz RJ. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci U S A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.DeWire SM, Kim J, Whalen EJ, Ahn S, Chen M, Lefkowitz RJ. Beta-arrestin-mediated signaling regulates protein synthesis. J Biol Chem. 2008;283:10611–10620. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:5825–5830. doi: 10.1073/pnas.0811706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bathgate-Siryk A, Dabul S, Pandya K, Walklett K, Rengo G, Cannavo A, De Lucia C, Liccardo D, Gao E, Leosco D, Koch WJ, Lymperopoulos A. Negative impact of beta-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404–412. doi: 10.1161/HYPERTENSIONAHA.113.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Valero TR, Sturchler E, Jafferjee M, Rengo G, Magafa V, Cordopatis P, McDonald P, Koch WJ, Lymperopoulos A. Structure-activity relationship study of angiotensin II analogs in terms of beta-arrestin-dependent signaling to aldosterone production. Pharmacol Res Perspect. 2016;4:e00226. doi: 10.1002/prp2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ Res. 2011;109:205–216. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 170.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 171.Galandrin S, Denis C, Boularan C, Marie J, M’Kadmi C, Pilette C, Dubroca C, Nicaise Y, Seguelas MH, N’Guyen D, Baneres JL, Pathak A, Senard JM, Gales C. Cardioprotective Angiotensin-(1–7) Peptide Acts as a Natural-Biased Ligand at the Angiotensin II Type 1 Receptor. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.08118. [DOI] [PubMed] [Google Scholar]
- 172.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 173.Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, Feldman AM. Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation. 1990;82:I12–25. [PubMed] [Google Scholar]
- 174.Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- 175.Whelan RS, Konstantinidis K, Xiao RP, Kitsis RN. Cardiomyocyte life-death decisions in response to chronic beta-adrenergic signaling. Circ Res. 2013;112:408–410. doi: 10.1161/CIRCRESAHA.113.300805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lymperopoulos A, Negussie S. betaArrestins in cardiac G protein-coupled receptor signaling and function: partners in crime or “good cop, bad cop”? Int J Mol Sci. 2013;14:24726–24741. doi: 10.3390/ijms141224726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lymperopoulos A, Bathgate A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci. 2013;118:297–334. doi: 10.1016/B978-0-12-394440-5.00012-7. [DOI] [PubMed] [Google Scholar]
- 178.Ruffolo RR, Jr, Feuerstein GZ. Pharmacology of carvedilol: rationale for use in hypertension, coronary artery disease, and congestive heart failure. Cardiovasc Drugs Ther. 1997;11(Suppl 1):247–256. doi: 10.1023/a:1007735729121. [DOI] [PubMed] [Google Scholar]
- 179.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 181.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim IM, Wang Y, Park KM, Tang Y, Teoh JP, Vinson J, Traynham CJ, Pironti G, Mao L, Su H, Johnson JA, Koch WJ, Rockman HA. beta-arrestin1-biased beta1-adrenergic receptor signaling regulates microRNA processing. Circ Res. 2014;114:833–844. doi: 10.1161/CIRCRESAHA.114.302766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Duggal B, Gupta MK, Naga Prasad SV. Potential role of MicroRNAs in cardiovascular disease: Are they up to their hype? Curr Cardiol Rev. 2016 doi: 10.2174/1573403X12666160301120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Naga Prasad SV, Duan ZH, Gupta MK, Surampudi VS, Volinia S, Calin GA, Liu CG, Kotwal A, Moravec CS, Starling RC, Perez DM, Sen S, Wu Q, Plow EF, Croce CM, Karnik S. Unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. J Biol Chem. 2009;284:27487–27499. doi: 10.1074/jbc.M109.036541. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 185.Tang Y, Wang Y, Park KM, Hu Q, Teoh JP, Broskova Z, Ranganathan P, Jayakumar C, Li J, Su H, Tang Y, Ramesh G, Kim IM. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc Res. 2015;106:387–397. doi: 10.1093/cvr/cvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deng P, Chen L, Liu Z, Ye P, Wang S, Wu J, Yao Y, Sun Y, Huang X, Ren L, Zhang A, Wang K, Wu C, Yue Z, Xu X, Chen M. MicroRNA-150 Inhibits the Activation of Cardiac Fibroblasts by Regulating c-Myb. Cell Physiol Biochem. 2016;38:2103–2122. doi: 10.1159/000445568. [DOI] [PubMed] [Google Scholar]
- 187.Zhu XH, Yuan YX, Rao SL, Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci. 2016;20:3653–3660. [PubMed] [Google Scholar]
- 188.Liu Z, Ye P, Wang S, Wu J, Sun Y, Zhang A, Ren L, Cheng C, Huang X, Wang K, Deng P, Wu C, Yue Z, Xia J. MicroRNA-150 protects the heart from injury by inhibiting monocyte accumulation in a mouse model of acute myocardial infarction. Circ Cardiovasc Genet. 2015;8:11–20. doi: 10.1161/CIRCGENETICS.114.000598. [DOI] [PubMed] [Google Scholar]
- 189.Liu W, Liu Y, Zhang Y, Zhu X, Zhang R, Guan L, Tang Q, Jiang H, Huang C, Huang H. MicroRNA-150 Protects Against Pressure Overload-Induced Cardiac Hypertrophy. J Cell Biochem. 2015;116:2166–2176. doi: 10.1002/jcb.25057. [DOI] [PubMed] [Google Scholar]
- 190.Goren Y, Meiri E, Hogan C, Mitchell H, Lebanony D, Salman N, Schliamser JE, Amir O. Relation of reduced expression of MiR-150 in platelets to atrial fibrillation in patients with chronic systolic heart failure. Am J Cardiol. 2014;113:976–981. doi: 10.1016/j.amjcard.2013.11.060. [DOI] [PubMed] [Google Scholar]
- 191.Park KM, Teoh JP, Wang Y, Broskova Z, Bayoumi AS, Tang Y, Su H, Weintraub NL, Kim IM. Carvedilol-responsive microRNAs, miR-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2016;311:H371–383. doi: 10.1152/ajpheart.00807.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kuliopulos A, Covic L. Blocking receptors on the inside: pepducin-based intervention of PAR signaling and thrombosis. Life Sci. 2003;74:255–262. doi: 10.1016/j.lfs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 193.O’Callaghan K, Kuliopulos A, Covic L. Turning receptors on and off with intracellular pepducins: new insights into G-protein-coupled receptor drug development. J Biol Chem. 2012;287:12787–12796. doi: 10.1074/jbc.R112.355461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carr R, 3rd, Schilling J, Song J, Carter RL, Du Y, Yoo SM, Traynham CJ, Koch WJ, Cheung JY, Tilley DG, Benovic JL. beta-arrestin-biased signaling through the beta2-adrenergic receptor promotes cardiomyocyte contraction. Proc Natl Acad Sci U S A. 2016;113:E4107–4116. doi: 10.1073/pnas.1606267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, Jensen NH, Che X, Bai X, Frye SV, Wetsel WC, Caron MG, Javitch JA, Roth BL, Jin J. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gesty-Palmer D, Yuan L, Martin B, Wood WH, 3rd, Lee MH, Janech MG, Tsoi LC, Zheng WJ, Luttrell LM, Maudsley S. beta-arrestin-selective G protein-coupled receptor agonists engender unique biological efficacy in vivo. Mol Endocrinol. 2013;27:296–314. doi: 10.1210/me.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pi M, Oakley RH, Gesty-Palmer D, Cruickshank RD, Spurney RF, Luttrell LM, Quarles LD. Beta-arrestin- and G protein receptor kinase-mediated calcium-sensing receptor desensitization. Mol Endocrinol. 2005;19:1078–1087. doi: 10.1210/me.2004-0450. [DOI] [PubMed] [Google Scholar]
- 200.Cottingham C, Chen Y, Jiao K, Wang Q. The antidepressant desipramine is an arrestin-biased ligand at the alpha(2A)-adrenergic receptor driving receptor down-regulation in vitro and in vivo. J Biol Chem. 2011;286:36063–36075. doi: 10.1074/jbc.M111.261578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Teoh JP, Park KM, Broskova Z, Jimenez FR, Bayoumi AS, Archer K, Su H, Johnson J, Weintraub NL, Tang Y, Kim IM. Identification of gene signatures regulated by carvedilol in mouse heart. Physiol Genomics. 2015;47:376–385. doi: 10.1152/physiolgenomics.00028.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Erickson CE, Gul R, Blessing CP, Nguyen J, Liu T, Pulakat L, Bastepe M, Jackson EK, Andresen BT. The beta-blocker Nebivolol Is a GRK/beta-arrestin biased agonist. PLoS One. 2013;8:e71980. doi: 10.1371/journal.pone.0071980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Milanos L, Brox R, Frank T, Poklukar G, Palmisano R, Waibel R, Einsiedel J, Durr M, Ivanovic-Burmazovic I, Larsen O, Hjorto GM, Rosenkilde MM, Tschammer N. Discovery and Characterization of Biased Allosteric Agonists of the Chemokine Receptor CXCR3. J Med Chem. 2016;59:2222–2243. doi: 10.1021/acs.jmedchem.5b01965. [DOI] [PubMed] [Google Scholar]
- 205.Milanos L, Saleh N, Kling RC, Kaindl J, Tschammer N, Clark T. Identification of Two Distinct Sites for Antagonist and Biased Agonist Binding to the Human Chemokine Receptor CXCR3. Angew Chem Int Ed Engl. 2016 doi: 10.1002/anie.201607831. [DOI] [PubMed] [Google Scholar]
- 206.Mancini AD, Bertrand G, Vivot K, Carpentier E, Tremblay C, Ghislain J, Bouvier M, Poitout V. beta-Arrestin Recruitment and Biased Agonism at Free Fatty Acid Receptor 1. J Biol Chem. 2015;290:21131–21140. doi: 10.1074/jbc.M115.644450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rosethorne EM, Charlton SJ. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits beta-arrestin without activating G proteins. Mol Pharmacol. 2011;79:749–757. doi: 10.1124/mol.110.068395. [DOI] [PubMed] [Google Scholar]
- 208.Emery AC, DiRaddo JO, Miller E, Hathaway HA, Pshenichkin S, Takoudjou GR, Grajkowska E, Yasuda RP, Wolfe BB, Wroblewski JT. Ligand bias at metabotropic glutamate 1a receptors: molecular determinants that distinguish beta-arrestin-mediated from G protein-mediated signaling. Mol Pharmacol. 2012;82:291–301. doi: 10.1124/mol.112.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Barak LS, Bai Y, Peterson S, Evron T, Urs NM, Peddibhotla S, Hedrick MP, Hershberger P, Maloney PR, Chung TD, Rodriguiz RM, Wetsel WC, Thomas JB, Hanson GR, Pinkerton AB, Caron MG. ML314: A Biased Neurotensin Receptor Ligand for Methamphetamine Abuse. ACS Chem Biol. 2016;11:1880–1890. doi: 10.1021/acschembio.6b00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peddibhotla S, Hedrick MP, Hershberger P, Maloney PR, Li Y, Milewski M, Gosalia P, Gray W, Mehta A, Sugarman E, Hood B, Suyama E, Nguyen K, Heynen-Genel S, Vasile S, Salaniwal S, Stonich D, Su Y, Mangravita-Novo A, Vicchiarelli M, Roth GP, Smith LH, Chung TD, Hanson GR, Thomas JB, Caron MG, Barak LS, Pinkerton AB. Discovery of ML314, a Brain Penetrant Non-Peptidic beta-Arrestin Biased Agonist of the Neurotensin NTR1 Receptor. ACS Med Chem Lett. 2013;4:846–851. doi: 10.1021/ml400176n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.White KL, Scopton AP, Rives ML, Bikbulatov RV, Polepally PR, Brown PJ, Kenakin T, Javitch JA, Zjawiony JK, Roth BL. Identification of novel functionally selective kappa-opioid receptor scaffolds. Mol Pharmacol. 2014;85:83–90. doi: 10.1124/mol.113.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]