Abstract
Answer questions and earn CME/CNE
Recent decades have seen an unprecedented rise in obesity, and the health impact thereof is increasingly evident. In 2014, worldwide, more than 1.9 billion adults were overweight (body mass index [BMI], 25‐29.9 kg/m2), and of these, over 600 million were obese (BMI ≥30 kg/m2). Although the association between obesity and the risk of diabetes and coronary artery disease is widely known, the impact of obesity on cancer incidence, morbidity, and mortality is not fully appreciated. Obesity is associated both with a higher risk of developing breast cancer, particularly in postmenopausal women, and with worse disease outcome for women of all ages. The first part of this review summarizes the relationships between obesity and breast cancer development and outcomes in premenopausal and postmenopausal women and in those with hormone receptor‐positive and ‐negative disease. The second part of this review addresses hypothesized molecular mechanistic insights that may underlie the effects of obesity to increase local and circulating proinflammatory cytokines, promote tumor angiogenesis and stimulate the most malignant cancer stem cell population to drive cancer growth, invasion, and metastasis. Finally, a review of observational studies demonstrates that increased physical activity is associated with lower breast cancer risk and better outcomes. The effects of recent lifestyle interventions to decrease sex steroids, insulin/insulin‐like growth factor‐1 pathway activation, and inflammatory biomarkers associated with worse breast cancer outcomes in obesity also are discussed. Although many observational studies indicate that exercise with weight loss is associated with improved breast cancer outcome, further prospective studies are needed to determine whether weight reduction will lead to improved patient outcomes. It is hoped that several ongoing lifestyle intervention trials, which are reviewed herein, will support the systematic incorporation of weight loss intervention strategies into care for patients with breast cancer. CA Cancer J Clin 2017;67:378–397. © 2017 The Authors. CA A Cancer Journal for Clinicians published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: breast cancer, diet and exercise, hormone receptor, immunity, inflammatory cytokines, nuclear factor kappa B (NF‐κB), obesity, postmenopausal and premenopausal, sex steroids, weight loss
Practical Implications for Continuing Education.
Obesity increases postmenopausal ER positive breast cancer risk and mortality.
Increased estrogens and inflammatory mediators contribute to the aggressive breast cancer phenotype in obesity.
Observational studies support ongoing trials to test whether exercise/weight loss interventions will decrease breast cancer mortality.
Introduction
Obesity, defined as a body mass index (BMI) ≥30 kg/m2, affects over 600 million adults worldwide, or 13% of the world population.1 Obesity is a major health problem of particular importance in developed countries, such as the United States, where obesity affects more than 36% of adults.2 Whereas the impact of obesity on diabetes and heart disease is well known,3, 4 our understanding of the impact of obesity on cancer is only beginning to change clinical practice. Although it has been known for several decades that obesity is associated with higher cancer mortality,5, 6 studies that may establish a causal link are still ongoing, and efforts to intervene effectively with weight‐reduction strategies in the cancer population have not yet entered routine clinical practice. Recent studies have demonstrated that overweight and obesity are associated with higher risks of adenocarcinoma of the esophagus, gastric cardia, thyroid, pancreas, colon, rectum, endometrium, prostate, gallbladder, ovary, and breast, in addition to multiple myeloma.7 Adipose tissue of obese individuals produces inflammatory cytokines and mediators, creating an environment that promotes cancer invasion and metastasis.8, 9, 10 Because breast cancer is the most common cancer and the second leading cause of cancer death among women in developed countries,11 understanding how obesity impacts this disease has important public health implications. Here, we review how obesity is associated with breast cancer incidence and mortality. We also briefly review the molecular mechanisms in obese adipose tissue that increase inflammation, down‐regulate antitumor immunity, and promote tumor angiogenesis, growth, and metastasis. Finally, we also review compelling evidence from observational studies indicating that exercise and weight loss are associated with lower disease risk and better survival, and ongoing studies designed to test whether weight loss intervention will ameliorate survival in patients with breast cancer.
Obesity and Breast Cancer Risk in Premenopausal Women
Overall Risk
Between 2011 and 2014, approximately 35% of premenopausal women ages 20 to 59 years in the United States were obese.2 Approximately 20% of breast cancers are diagnosed in women younger than 50 years. An inverse association between obesity and premenopausal breast cancer risk has been reported.12, 13, 14, 15 A pooled analysis of 7 studies, which included 337,819 women and 4385 invasive breast cancers, reported an inverse association between BMI and premenopausal breast cancer risk when comparing women who had a BMI >31 kg/m2 versus those who had a BMI ≤21 kg/m2 (risk ratio [RR], 0.54; 95% confidence interval [95% CI]. 0.34‐0.85).16 A 9‐study meta‐analysis also demonstrated an inverse correlation between premenopausal breast cancer risk and obesity (RR, 0.98; 95% CI, 0.97‐0.99) per unit increase in BMI.17 Another large meta‐analysis of 20 data sets, which included >2.5 million women and 7930 premenopausal breast cancers, demonstrated that premenopausal breast cancer risk is reduced by approximately 8% per 5 kg/m2 BMI increase (RR, 0.92; 95% CI, 0.88‐0.97 [P = .001]).18 That meta‐analysis was comprised of prospective cohort studies, which provide a stronger study design than case‐control studies.
The reduced premenopausal breast cancer risk with obesity is not observed in all studies. A case‐control study demonstrated a modestly positive association between obesity and premenopausal breast cancer risk.19 Moreover, the Breast Cancer Prevention P‐1 trial, which included 5864 premenopausal women, found that obesity was associated with higher premenopausal breast cancer risk (hazard ratio [HR], 1.70; 95% CI, 1.10‐2.63).20 Differences in the distribution of women with hormone receptor‐positive and negative breast cancer in the studies described above might explain these differing results. Furthermore, 2 meta‐analyses have reported differences between ethnicities, showing an inverse association between increased BMI and premenopausal breast cancer risk in most of groups, but a positive association in the Asian population.18, 21
Hormone Receptor‐Positive Premenopausal Breast Cancer
The effect of obesity on premenopausal breast cancer risk differs across disease subtypes. Most studies report that obesity is associated with lower estrogen receptor (ER)‐positive breast cancer risk before menopause,14, 22, 23, 24, 25 while others report no association.19, 26 Two meta‐analyses, which included 6106 and 2486 premenopausal women with ER‐positive breast cancer, respectively, demonstrated an inverse association between BMI and ER‐positive breast cancer before menopause.27, 28 These observations appear to apply to both the Hispanic and non‐Hispanic white populations in the United States.24 Interestingly, a smaller study found that this inverse association between BMI and ER‐positive or progesterone receptor (PR)‐positive premenopausal breast cancer risk was restricted to white women (n = 677; P = .02), and there was a null association in African American women (n = 884; P = .89).29 Figure 1A summarizes forest plots of these studies.14, 19, 22, 23, 24, 25, 26, 27, 28
Figure 1.
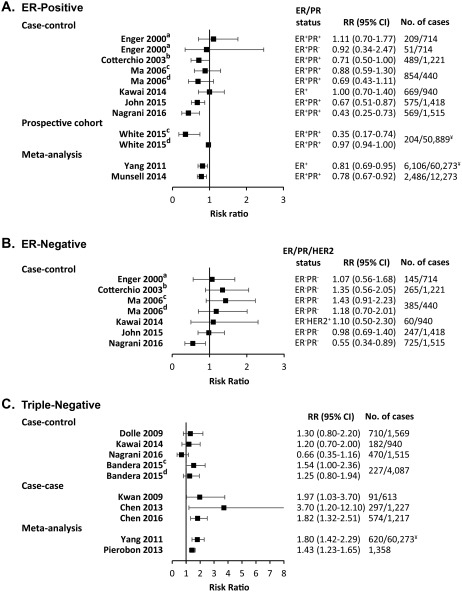
Forest Plots for the Association of Premenopausal Breast Cancer Risk and Body Mass Index. Body mass index (BMI) is compared between obese (BMI >30 kg/m2) and normal‐weight (BMI <25 kg/m2) women for (A) estrogen receptor‐positive (ER+) or/and progesterone receptor‐positive (PR+) breast cancer from case‐control studies (Kawai et al,19 Cotterchio et al,22 Ma et al,23 John et al,24 Nagrani et al,25 and Enger et al26), a prospective cohort study (White et al14), and meta‐analyses (Yang et al27 and Munsell et al28); (B) ER‐negative/PR‐negative (ER‐PR‐) and human epidermal growth factor 2‐positive (Her2+) or unknown breast cancer from case‐control studies (Kawai et al,19 Cotterchio et al,22 Ma et al,23 John et al,24 Nagrani et al,25 and Enger et al26); and (C) triple‐negative breast cancer from case‐control studies (Kawai,19 Nagrani,25 Dolle et al,31 and Bandera et al33), case‐case‐studies (Kwan et al,30 Chen et al,32 and Chen et al34), and meta‐analyses (Yang et al27 and Pierobon et al36). Risk ratio (RR) estimates include odds ratios, rate ratios, and hazard ratios. No. of cases indicates the number of premenopausal breast cancer cases/controls (for case‐control studies) or premenopausal breast cancer cases/total population (prospective cohort, case‐case studies, and meta‐analyses). ¥ indicates the total number of women (not only premenopausal). Superscript letters indicate studies that compared: awomen with a BMI ≥27.1 kg/m2 versus < 21.7 kg/m2; bwomen with a BMI >27 kg/m2 versus ≤25 kg/m2; cwomen with grade I obesity (30‐34.99 kg/m2) versus normal‐weight women; and dwomen with grade II and III obesity (>35 kg/m2) versus normal‐weight women. 95% CI indicates 95% confidence interval.
Hormone Receptor‐Negative Premenopausal Breast Cancer
Triple‐negative breast cancers (TNBCs) lack expression of ER, PR, and human epidermal growth factor receptor 2 (HER2) and have a very aggressive disease course. In contrast to ER‐positive breast cancers, obesity is associated with a higher risk of premenopausal ER‐negative breast cancer19, 22, 23, 24, 25, 26 and TNBC in most studies.19, 30, 31, 32, 33, 34 The Cancer and Steroid Hormone (CASH) population‐based, case‐control study, with 3432 breast cancers, reported a strong positive association between BMI and premenopausal TNBC risk.35 In contrast, an Indian case‐control study failed to associate premenopausal ER‐negative/PR‐negative breast cancer and TNBC with obesity but showed that greater waist circumference and waist‐hip ratio was associated with an elevated risk of premenopausal TNBC (P < .001).25 Two meta‐analyses of 620 women27 and 1358 women36 with TNBC reported an 80% and 43% higher risk of developing TNBC in obese premenopausal women, respectively. Figures 1B and 1C summarize forest plots of these studies.19, 22, 23, 25, 26, 27, 30, 31, 32, 33, 34, 36
Inflammatory Premenopausal Breast Cancer
Inflammatory breast cancer (IBC) has a rapid, aggressive disease course.37 A recent case‐control study from the Breast Cancer Surveillance Consortium database (1994‐2009) showed that obesity is associated with higher premenopausal IBC risk (RR, 3.62; 95% CI, 1.30‐10.04) for all cases and for those with ER‐positive (RR, 3.53; 95% CI, 1.20‐10.39) and ER‐negative (RR, 4.67; 95% CI, 1.45‐15.02) IBC.38 An older case‐comparison study that included 68 IBCs also reported that a BMI > 26.65 kg/m2 was associated with an up to 4‐fold higher risk of premenopausal IBC.39
Obesity and Breast Cancer Risk in Postmenopausal Women
Overall Risk
The prevalence of obesity among US women ages 60 years and older between 2011 and 2014 was approximately 39%.2 Metabolic syndrome has increased with the rise of obesity40 and is significantly associated with a higher postmenopausal breast cancer risk.41 Obesity consistently associates with higher postmenopausal breast cancer risk in many studies.14, 16, 22, 42, 43, 44, 45 The Million Women Study followed 1.2 million UK women ages 50 to 64 years for a mean of 5.4 years, including 45,037 with breast cancer, and identified a nearly 30% higher risk of developing postmenopausal breast cancer with obesity (RR, 1.29; 95% CI, 1.22‐1.36).46 Similarly, a meta‐analysis of 34 studies comprising >2.5 million women, including 23,909 postmenopausal breast cancers, indicated that postmenopausal breast cancer risk was positively associated with each 5‐kg/m2 increase in BMI (RR, 1.12; 95% CI, 1.08‐1.16 [P < .0001]).18 The association of obesity with a higher risk of postmenopausal breast cancer is greater in, and may be limited to, women who have not used menopausal hormone therapy (HT).47, 48, 49
Hormone Receptor‐Positive Postmenopausal Breast Cancer
The association of postmenopausal breast cancer risk with obesity may be limited to ER‐positive breast cancers. Obesity associates most strongly with postmenopausal hormone receptor‐positive breast cancer risk in many prospective cohort studies and case‐control studies.14, 22, 26, 42, 43, 48, 49, 50, 51, 52, 53, 54, 55 This association was stronger in women from Asia‐Pacific than in those from North America, Europe, and Australia.18 Furthermore, an increase in BMI after age 18 years is associated with an elevated risk of postmenopausal ER‐positive/PR‐positive breast cancer.26, 49, 54 Weight gain was associated with a higher risk of ER‐positive/PR‐positive breast cancer after menopause only in Hispanics who did not receive HT and who had a normal BMI as young adults.53 Notably, an Indian case‐control study reported that obesity was associated with an elevated risk of ER‐positive/PR‐positive breast cancer only 10 years after menopause.25 ER‐positive/PR‐positive postmenopausal breast cancer risk is also associated with higher waist circumference and waist‐to‐hip ratio.14, 25, 52, 54 The higher ER‐positive/PR‐positive breast cancer risk with obesity is most notable among women who never used HT.42, 49, 56 A meta‐analysis of 89 studies from 1980 to 2012, which included 59,185 women, demonstrated that the increment in postmenopausal ER‐positive/PR‐positive breast cancer risk with obesity (RR, 1.39; 95% CI, 1.14‐1.70) was greater among HT never users (RR, 1.42; 95% CI, 1.30‐1.55) than among HT users (RR, 1.18; 95% CI, 0.98‐1.42).28 In contrast to ER‐positive/PR‐positive cancers, several studies indicate that the risk of ER‐positive/PR‐negative cancer is not higher with obesity.26, 42, 43, 48, 49, 50, 51, 54 This subset of cancers has a more aggressive phenotype and more often has a luminal B than luminal A gene expression profile.57
In summary, a large body of evidence indicates that, although obesity is inversely associated with receptor‐positive breast cancer development before menopause, it is a risk factor for postmenopausal hormone receptor‐positive breast cancer. Figures 2A and 2B presents forest plots of these studies.14, 22, 23, 25, 26, 28, 42, 43, 48, 49, 50, 51, 52, 54, 55
Figure 2.
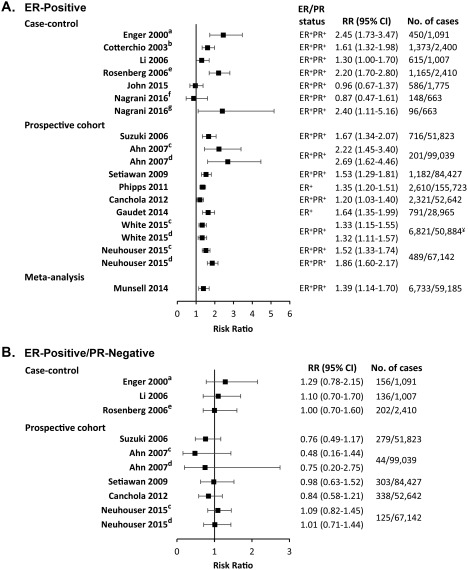
Forest Plots for the Association of Postmenopausal Breast Cancer Risk and Body Mass Index. Body mass index (BMI) is compared between obese (BMI > 30 kg/m2) and normal‐weight (BMI < 25 kg/m2) women for (A) estrogen receptor‐positive (ER+) and progesterone receptor‐positive (PR+) or unknown breast cancer from case‐control studies (Cotterchio et al,22 John et al,23 Nagrani et al,25 Enger et al,26 Rosenberg et al51, and Li et al42), prospective cohort studies (White et al,14 Neuhouser et al,43 Suzuki et al,48 Ahn et al,49 Setiawan et al,50 Phipps et al,52 Canchola et al,54 and Gaudet et al55), and a meta‐analysis (Munsell28); and (B) ER+ PR‐negative (PR‐) breast cancer from case‐control studies (Enger et al,26 Li et al,42 and Rosenberg et al51) and prospective cohort studies (Neuhouser et al,43 Suzuki et al,48 Ahn et al,49 Setiawan et al,50 and Canchola et al54). Risk ratio (RR) estimates included odds ratios, rate ratios, and hazard ratios. No. of cases indicates the number of postmenopausal breast cancer cases/control (case‐control studies) or postmenopausal breast cancer cases/total population (prospective cohort studies and meta‐analysis). ¥ indicates the total number of women (not only premenopausal). Superscript letters indicate studies that compared: awomen with a BMI ≥27.1 kg/m2 versus ≥21.7 kg/m2; bwomen with a BMI > 27 kg/m2 versus ≤25 kg/m2; cwomen with grade I obesity (30‐34.99 kg/m2) versus normal‐weight women; dwomen with grade II and III obesity (>35 kg/m2) versus normal‐weight women; ewomen with a BMI ≥28.3 kg/m2 versus <22.2 kg/m2; fwomen who were postmenopausal for less than 10 years; and gwomen who were postmenopausal for 10 years or more. 95% CI indicates 95% confidence interval.
Hormone Receptor‐Negative Postmenopausal Breast Cancer
The association of obesity with the risk of ER‐negative breast cancer and TNBC appears to differ before and after menopause. Although obesity is associated with higher risk before menopause, the risk of ER‐negative breast cancer and TNBC is minimally or inversely associated with obesity after menopause. A Swedish mammography cohort of 51,823 postmenopausal women found an inverse association between obesity and hormone receptor‐negative breast cancer,48 as did other studies.25, 33, 48, 49, 50, 53, 54, 55 In contrast, others reported a null or modest positive association,14, 25, 26, 42, 43, 52, 58 and few reports demonstrated a significant association between obesity and the risk of ER‐negative/PR‐negative breast cancer after menopause.22, 51 Despite these conflicting reports, 2 large meta‐analyses, including 2302 ER‐negative/PR‐negative breast cancers and 1883 TNBCs, respectively, both reported a null association between obesity and the risk of postmenopausal hormone receptor‐negative breast cancer.28, 36 Forest plots in Figures 3A and 3B summarize these studies.14, 22, 23, 25, 26, 28, 33, 36, 42, 43, 48, 49, 50, 51, 52, 54, 55, 58
Figure 3.
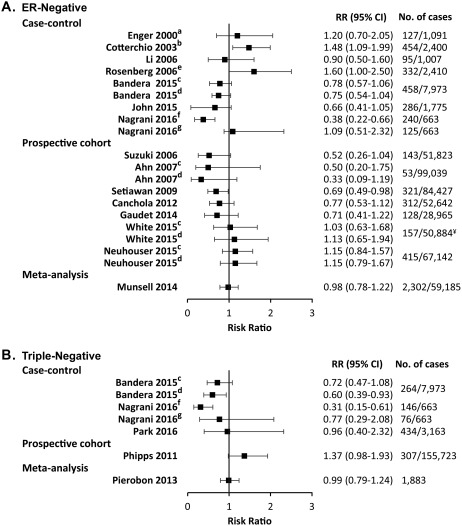
Forest Plots for the Association of Postmenopausal Breast Cancer Risk and Body Mass Index. Body mass index (BMI) is compared between obese (BMI > 30 kg/m2) and normal‐weight (BMI < 25 kg/m2) women for (A) estrogen receptor‐negative/progesterone receptor‐negative and human epidermal growth factor receptor 2‐unknown breast cancer from case‐control studies (Cotterchio et al,22 John et al,23 Nagrani et al,25 Enger et al,26 Rosenberg et al51, Bandera et al,33 and Li et al,42), prospective cohort studies (White et al,14 Neuhouser et al,43 Suzuki et al,48 Ahn et al,49 Setiawan et al,50 Canchola et al,54 and Gaudet et al55), and a meta‐analysis (Munsell et al28); and (B) triple‐negative breast cancer from case‐control studies (Nagrani et al,25 Bandera et al,33 and Park et al58), a prospective cohort study (Phipps et al52), and a meta‐analysis (Pierobon et al36). Risk ratio (RR) estimates included odds ratios, rate ratios, and hazard ratios. No. of cases indicates the number of postmenopausal breast cancer cases/control (case‐control studies) or postmenopausal breast cancer cases/total population (prospective cohort studies and meta‐analysis). ¥ indicates the total number of women, not only those who were premenopausal. Superscript letters indicate studies that compared: awomen with a BMI ≥ 27.1 kg/m2 versus <21.7 kg/m2; bwomen with a BMI > 27 kg/m2 versus ≤25 kg/m2; cwomen with grade I obesity (30‐34.99 kg/m2) versus normal‐weight women; dwomen with grade II and III obesity (>35 kg/m2) versus normal‐weight women; ewomen with a BMI ≥ 28.3 kg/m2 versus <22.2 kg/m2; fwomen who were postmenopausal for less than 10 years; and gwomen who were postmenopausal for 10 years or more. 95% CI indicates 95% confidence interval.
Inflammatory Postmenopausal Breast Cancer
In premenopausal women, IBC risk after menopause is 3‐fold to 5‐fold higher with obesity.39 A recent case‐control study including 435 patients with IBC and 72,096 controls demonstrated that obesity was associated with a higher risk of IBC after menopause (RR, 3.75; 95% CI, 1.92‐7.34).38 Another case‐control study reported that overweight and obesity were associated with higher IBC risk for premenopausal and postmenopausal women (odds ratio [OR], 3.77; 95% CI, 2.00‐7.08).59
Weight Loss and Breast Cancer Risk
Weight loss in adulthood has been associated with a lower breast cancer risk. Prospective cohort studies show that weight loss after age 18 years60 or after menopause61 are both associated with a reduction in the risk of postmenopausal breast cancer, with stronger associations in women who never used postmenopausal HT.60 Two prospective cohort studies that included 4047 and 16,038 obese patients, respectively, reported that bariatric surgery resulted in a weight reduction and was associated with reduced all‐cancer incidence in obese women but had no significant effects in men.62, 63 Similarly, a prospective cohort study that included 1035 patients who underwent bariatric surgery and 5746 controls reported a reduced 5‐year incidence of breast cancer after bariatric surgery64
Weight Gain After Breast Cancer
From 50% up to 96% of women with breast cancer gain weight during treatment, and even those who do not usually gain weight over the next 3 years.65, 66 Weight gain is greater in premenopausal women, those treated with chemotherapy, and women who are overweight at diagnosis.67 Most large observational studies show that weight gain postdiagnosis is inversely associates with disease‐free survival.65, 66, 67, 68, 69 Weight gain >5.9 kg after diagnosis is associated with a 1.6‐fold higher risk of death.68 Nichols et al reported that each 5‐pound weight gain postdiagnosis was associated with a 13% increase in breast cancer‐specific mortality and a 12% increase in all‐cause mortality.67 Weight gain after diagnosis is also associated with greater incidence and severity of complications after primary and reconstructive surgery, increased fatigue, more arthralgias, and up to twice the rate and severity of hot flashes.66
Increased Breast Cancer Mortality in Obese Patients
Obesity is associated with a shorter time to disease recurrence and greater mortality for both premenopausal and postmenopausal breast cancer. The American Cancer Society's Cancer Prevention Study II followed 495,477 women from 1982 to 1998 and reported a positive association between BMI and breast cancer mortality (P < .001): Women who had a BMI >40 kg/m2 had a greater than 2‐fold higher risk of mortality compared with those who had a BMI from 18 to 24.9 kg/m2 (RR, 2.12; 95% CI, 1.41‐3.19).6 Other studies showed that obesity was associated with larger tumors, positive lymph node status, shorter distant disease‐free interval and overall survival,70, 71, 72 and triple‐negative tumor subtype.71 A recent meta‐analysis of 82 studies that included 213,075 breast cancer survivors confirmed that obesity was associated with greater breast cancer mortality (RR, 1.41; 95% CI, 1.29‐1.53) in both premenopausal (RR, 1.75; 95% CI, 1.26‐2.41) and postmenopausal (RR, 1.34; 95% CI, 1.18‐1.53) women.73 In the Multiethnic Cohort Study, obesity was associated with higher all‐cause and breast cancer‐specific mortality irrespective of ethnicity in American women older than 50 years.74 In contrast, the Contraceptive and Reproductive Experiences population‐based, case‐control study demonstrated that obesity was associated with increased breast cancer‐specific mortality in white women, but not in African American women.75
Outcome of Hormone Receptor‐Positive Breast Cancer
Several studies have evaluated obesity and receptor‐positive breast cancer outcome. Analyses of data from 3385 women with hormone receptor‐positive breast cancers from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B‐14 Protocol indicated that obese women had greater all‐cause mortality (HR, 1.31; 95% CI, 1.12‐1.54).76 Among 6885 women from 3 US clinical trials, a BMI ≥30 kg/m2 was associated with decreased disease‐free survival (P = .008) and overall survival (P = .002) in patients with hormone receptor‐positive breast cancer.77 A meta‐analysis of 13 studies indicated that the adverse effects of obesity did not differ by hormone receptor or menopausal status. In that study, obesity was associated with increased breast cancer‐specific mortality among women who had hormone receptor‐positive breast cancer (HR, 1.36; 95% CI, 1.20‐1.54), including both premenopausal (HR, 1.23; 95% CI, 1.07‐1.42) and postmenopausal (HR, 1.15; 95% CI, 1.06‐1.26) women.78
Outcome of Hormone Receptor‐Negative Breast Cancer
The 10% to 20% of women who have breast cancers of the TNBC subtype have a shorter survival than most other breast cancer subtypes.36 Although several studies have demonstrated that the risk of TNBC is increased with obesity, particularly before menopause, the association of obesity with outcomes in women with TNBC is more controversial. Among patients with TNBC, obesity is associated with greater size (P = .02), stage (P = .001), and grade (P = .01).79 In a retrospective study of 418 US patients with TNBC, 164 of whom were obese, no effect of obesity on disease‐free or overall survival was observed.80 In contrast, another study that included 107 patients with TNBC reported shorter disease‐free survival (P = .006) and overall survival (P = .015) among obese patients.81 Given the small size of these studies, and because TNBC itself is associated with a shorter time to recurrence and death, larger studies or meta‐analyses will be needed to determine whether obesity is associated with poorer survival in women with TNBC.
Outcome of IBC
IBC accounts for 2.5% of breast cancers and is characterized by rapid local and distant metastasis, younger age, and <5% long‐term survival.37 A study of 706 patients who had stage III locally advanced breast cancers, including 111 with IBC and 595 without IBC, showed that a BMI >25 kg/m2 was associated with worse disease‐free and overall survival (P = .001), but the difference did not reach significance in the IBC subset (P = .45).82 In contrast, another study of 177 patients with IBC showed that obesity was associated with worse survival only in the postmenopausal subgroup (HR, 1.86; 95% CI, 1.02‐3.4).83
The Influence of Obesity on Breast Cancer Response to Chemotherapy and Radiation Therapies
Chemotherapy and radiation doses in obese patients have been a matter of controversy. Comorbidities in obese patients with breast cancer have historically led to reduced chemotherapy dosing. Many centers routinely limit body surface area to 2 m2 for calculations of chemotherapy dosing to reduce toxicity,84 but decreased dose intensity of adjuvant chemotherapy has been associated with poorer outcomes.85 A community oncology practice survey revealed consistent underdosing of obese patients for chemotherapy, and dose reductions in radiation therapy also limit treatment efficacy and outcome.85 Notably, dosing chemotherapy in obese patients based on actual body weight does not increase adverse effects. A retrospective analysis identified no excess toxicity in obese patients with breast cancer who received full‐dose adjuvant chemotherapy compared with nonobese patients. Obese women who received reduced chemotherapy doses had worse survival.86 The American Society of Clinical Oncology guidelines recommend using actual body weight when calculating chemotherapy dosing regardless of BMI.87
Obese Adipose Tissue
Hypoxia, Adipokines, and Inflammation in Obese Adipose Tissue
The adipose tissue is an endocrine organ that releases bioactive adipokines, including >50 different cytokines, chemokines, and hormone‐like factors.8 In lean adipose tissue, mature adipocytes secrete mainly the antimitogenic hormone, adiponectin, and low levels of proangiogenic and promitogenic leptin.88, 89 In obesity, preadipocyte to adipocyte maturation is decreased, yielding more preadipocytes,90 which secrete high levels of leptin.88, 89 As adipose tissue expands in obesity, oxygen demand exceeds supply, and hypoxia induces adipocyte gene expression changes, including those of inflammation‐related adipokines.91 Hypoxia‐inducible factor‐1 (HIF‐1) acts as a molecular oxygen sensor to alter gene expression in hypoxia.91 HIF‐1 directly upregulates expression of leptin and vascular endothelial growth factor (VEGF) and inhibits adiponectin expression (Fig. 4).91, 92, 93
Figure 4.
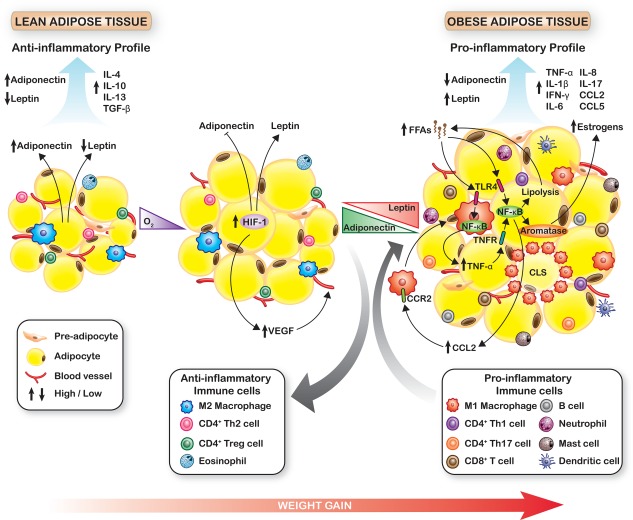
Changes in Adipose Tissue During Weight Gain. During obese adipose tissue expansion, preadipocyte differentiation is impaired, and hypoxia activates hypoxia‐inducible factor 1 (HIF‐1) to decrease adiponectin expression and upregulate leptin. HIF‐1 also promotes angiogenesis by turning on vascular endothelial growth factor (VEGF). The altered leptin:adiponectin ratio promotes proinflammatory immune cell infiltration and the formation of crown‐like structures (CLS). Dying adipocytes release free fatty acids (FFAs), which bind toll‐like receptor 4 (TLR4) on macrophages and adipocytes to activate nuclear factor kappa B (NF‐κB) and upregulate secretion of inflammatory cytokines, including tumor necrosis factor α (TNF‐α), interleukin‐6 (IL‐6), IL‐8, chemokine (C‐C motif) ligand 5 (CCL5), and CCL2. These cytokines promote lipolysis and FFA release to further activate the NF‐κB pathway, which also increases aromatase expression and estrogen synthesis. CCL2 and other cytokines serve as chemoattractants to recruit monocytes/macrophages. These feed‐forward loops establish a chronic inflammatory milieu in obese adipose tissue. IFN‐γ indicates interferon gamma; TGF‐β, transforming growth factor beta; TNFR, tumor necrosis factor receptor; Th1, T‐helper 1 cells; Th17, T‐helper 17 cells; Treg, T‐regulatory cells.
In lean adipose tissue, the anti‐inflammatory cytokines interleukin 4 (IL‐4), IL‐10, IL‐13, and transforming growth factor beta 1 (TGF‐β1) are produced by immune cells, including M2 macrophages, eosinophils, CD4‐positive T helper 2 (Th2) cells, and regulatory T (Treg) cells (Fig. 4).94, 95, 96 Adipokines in obese adipose tissue, particularly leptin, alter the immune environment.94, 97 Adipose tissue macrophages are increased and shift from the anti‐inflammatory M2 subtype to proinflammatory M1 macrophages, which secrete tumor necrosis factor alpha (TNF‐α), IL‐1β, IL‐6, IL‐8, chemokine (C‐C motif) ligand 2 (CCL2), and VEGF.94, 95 T‐lymphocyte populations change in obesity. Proinflammatory CD8‐positive T cells and CD4‐positive Th1 and Th17 cells, which produce interferon gamma (IFN‐γ) and IL‐17A, are increased, whereas anti‐inflammatory CD4‐positive Th2 and Treg cells are reduced.94, 95, 96 Infiltration of neutrophils, mast cells, mature B cells, and immature dendritic cells (DCs), which produce proinflammatory TNF‐α, IL‐6, and IL‐8, increases, and eosinophils that release anti‐inflammatory IL‐4 and IL‐13 are reduced (Fig. 4).94, 95, 96
Obesity, Inflammation, and the NF‐κB Pathway
Inflammation in obese adipose tissue is activated and maintained by the nuclear factor kappa B (NF‐κB) pathway.98 The increased inflammatory cytokines in obese fat induce lipolysis, releasing free fatty acids (FFAs), which stimulate toll‐like receptors (TLRs)99 on adipocytes100 and macrophages101 to turn on the NF‐κB pathway.100 NF‐κB activates expression of genes encoding inflammatory cytokines, including TNF‐α, IL‐1β, IL‐6, IL‐8 and CCL2,8, 98 which, in turn, feed‐forward to further activate NF‐κB.88, 98, 102 Circulating monocytes infiltrate adipose tissue, attracted by CCL2 via their surface receptor CCR2, and differentiate into macrophages (Fig. 4).103
Estrogen Synthesis in Obese Adipose Tissue
Estrogen biosynthesis after menopause is catalyzed largely in adipose tissue, through the conversion of adrenal androgens into estrogens by aromatase.104 Several cellular and molecular changes in obese adipose tissue alter estrogen biosynthesis and metabolism. NF‐κB pathway activation leads to an increase in aromatase expression in breast adipocytes and hence to greater estrogen synthesis (Fig. 4).8, 105 Similarly, several cytokines that are upregulated in obese adipose tissue, such as TNF‐α and IL‐6, stimulate aromatase activity.106 In obese mammary fat, microscopic foci of dying adipocytes surrounded by macrophages, called crown‐like structures, exhibit increased aromatase activity.107 In postmenopausal women, BMI correlates positively with serum estrone and estradiol levels and negatively with sex hormone‐binding globulin levels, leading to an increase in total bioavailable estrogen.104, 108 Compared with women who have a BMI <22.5 kg/m2, obese women have an 86% increase in circulating estradiol, a 60% increase in estrone, and a 20% increase in testosterone.108
Obese Adipose Tissue Creates a Pro‐Oncogenic Environment
Obesity, Insulin, Insulin‐Like Growth Factor‐1, and the Leptin Adiponectin Reversal
Patients with the metabolic syndrome have increased levels of circulating insulin and insulin‐like growth factor‐1 (IGF‐1) and a greater risk of breast cancer.109 Elevated fasting insulin is associated with poor breast cancer outcome.110 Hyperinsulinemia reduces sex hormone‐binding globulin levels and increases estrogen bioavailability, thereby increasing breast cancer risk.111 These factors have been associated with poorer outcomes in patients with breast cancer.109, 112 The high levels of TNF‐α and IL‐6 in obese fat impair insulin receptor β subunit activation and decrease glucose transport and fatty acid metabolism,113, 114 mediating insulin resistance and upregulating insulin and IGF‐1 levels. Because IGF‐1 actions are mediated by IGF‐1 receptor (IGF‐1R), which is frequently overexpressed in breast cancers,115 and IGF‐1 is a potent breast cancer mitogen, it has been posited that the increased levels of circulating insulin and IGF‐1 in obesity make an important contribution to the increased breast cancer risk and mortality in obese individuals.116
In obese adipose tissue, the adiponectin:leptin ratio is decreased. A recent case‐control study showed that the highest serum leptin quartile was associated with a higher postmenopausal breast cancer risk relative to the lowest quartile (HR, 1.94; 95% CI, 1.37‐2.17).117 This may relate to the effects of leptin to upregulate both the estrogen and insulin‐signaling pathways.118, 119 Leptin and leptin receptors are frequently increased in breast cancers and both are associated with poor outcome.120, 121, 122 High serum and intratumor leptin levels are also associated with poor breast cancer outcome.122 In contrast, adiponectin is reduced in obesity, and this also constitutes a risk factor for postmenopausal breast cancer.123, 124
Inflammation and Cancer
Chronic NF‐κB activation in obese adipose tissue not only drives obesity‐mediated inflammation but also stimulates antiapoptotic genes and breast cancer proliferation, invasion, angiogenesis, and metastasis.125 Recent work suggests that NF‐κB mediates tumor progression through proinflammatory cytokines. Several proinflammatory/proangiogenic cytokines, including IL‐6, IL‐8, CCL2, CCL5, and VEGF, that are elevated in obese fat8, 126 are associated with a poor prognosis when overexpressed in primary breast cancers and, with greater stage and grade, and with poor outcomes.127, 128, 129 Notably, IL‐6 levels in peritumoral fat are higher than in all other breast quadrants106 and increase with increasing tumor size and lymph node involvement.10 Recent work has shown that contact between breast cancer cells and adipocytes induces both cell types to secrete more IL‐6, IL‐8, CCL2, and CCL5 and the interaction of cancer cells with cancer‐associated adipocytes promotes tumor invasion and metastasis.9, 10 Adipose tissue contributes up to 35% of circulating IL‐6,130 and the increase in serum IL‐6 after menopause131, 132 may contribute to increased breast cancer risk and tumor progression. Elevated IL‐8 from cancer cells, surrounding adipocytes, endothelial cells, infiltrating neutrophils, and tumor‐associated macrophages (TAMs) promotes angiogenesis, tumor growth, metastasis, and chemotherapy resistance (Fig. 5).8, 9, 128 CCL2 and CCL5 also mediate tumor‐promoting cross‐talk between cancer cells and the microenvironment.127, 129 Both chemokines are also expressed by tumor‐invading mesenchymal stem cells, cancer‐associated fibroblasts, and surrounding adipocytes to drive breast cancer cell motility and metastasis.8, 9, 133, 134, 135, 136 In the inflammatory tumor microenvironment of obesity, TNF‐α and IL‐1 also enhance breast cancer growth and migration (Fig. 5).126, 137
Figure 5.
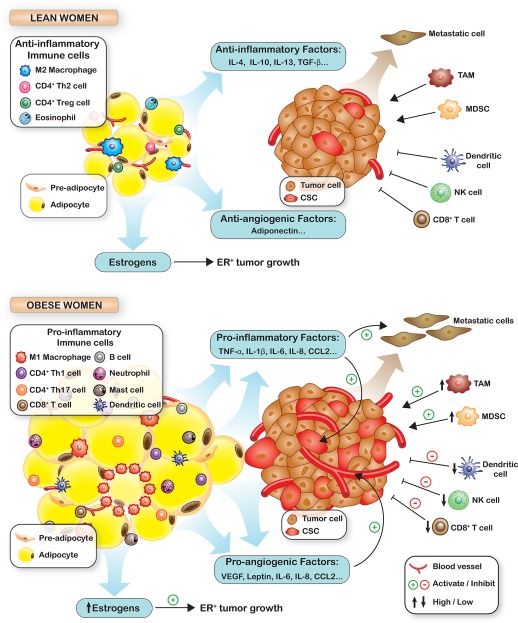
The Role of Obesity in Tumorigenesis. With obesity, secreted cytokines shift from an anti‐inflammatory profile to a proinflammatory/proangiogenic profile. Proinflammatory and proangiogenic cytokine secretion also increases upon adipocyte:breast cancer cell contact to increase angiogenesis, cancer stem cell expansion, invasion, and metastasis. In obese adipose tissue, mediators of antitumor immunity, such as CD8‐positive (CD8+) T cells, natural killer (NK) cells, and dendritic cells, decrease and myeloid‐derived suppressor cells (MDSCs) and tumor‐associated macrophages (TAMs) that suppress antitumor immunity accumulate. High aromatase levels drive higher estrogen synthesis to support estrogen receptor‐positive (ER+) breast cancer growth. CCL indicates chemokine (C‐C motif) ligand; IL, interleukin; TGF‐β, transforming growth factor β; TNF‐α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Interaction of Adipocytes With Invading Breast Cancer Cells Upregulates Cancer Stem Cells
Most solid tumors appear to be generated and maintained by a stem‐like population that can self‐renew, give rise to heterogeneous tumor growth, and mediate drug resistance, tumor recurrence, and metastasis.138, 139, 140 TLR4 is expressed not only on adipocytes and immune cells, but many breast cancers also express this receptor.141 Free fatty acids (FFAs) produced by obese fat lipolysis stimulate TLR4 on the breast cancer cell surface to activate NF‐κB.141 This continuous NF‐κB activation leads to an increase in cancer stem cells (CSCs).142 Moreover, cytokines synergistically upregulated by contact between breast cancer cells and adipocytes, as occurs early during breast cancer invasion, increase the abundance of stem‐like cancer cells.9 Exposure of breast cancer cells to adipocytes, or to the cytokines they upregulate, led to evidence of CSC enrichment, including increased aldehyde dehydrogenase‐1 (ALDH1) activity and mammosphere formation in vitro, and increased tumor‐initiating cells and metastasis in mouse models.9 Adipocyte contact and proinflammatory cytokines CCL2, CCL5, IFN‐γ‐induced protein 10 (IP‐10), IL‐6, and IL‐8 upregulated embryonic stem cell transcription factors, including c‐Myc, sex‐determining region Y box 2 (Sox2), and Nanog, to mediate CSC expansion9 (Fig. 5).
Others have also demonstrated that each of these cytokines individually promotes CSC expansion. IL‐8 increased ALDH1 activity and mammosphere and breast cancer xenograft formation, and IL‐8 blockade improved response to chemotherapy in mouse models.143 IL‐6,144 CCL2,145 and CCL5146 have all been shown to stimulate CSCs. Because all of these cytokines are overexpressed in obese adipose tissue and are upregulated upon contact with invading breast cancer cells, breast CSC enrichment may underlie the worse prognosis of breast cancer in obese women (Fig. 5).
Changes in Antitumor Immunity Associated with Obesity
Obesity not only affects the profile of immune cells within adipose tissue but also modifies circulating and tumor‐infiltrating immune cells and their functional activities. For example, obese individuals have decreased peripheral blood CD8‐positive T cells, reduced lymphocyte proliferation in response to mitogens, and dysregulated cytokine expression.147 Natural killer (NK) cells play an important role in the innate immune response against cancer. In obesity, both NK cell numbers and their cytotoxic activity are diminished.147, 148, 149 Dendritic cells (DCs) are antigen‐presenting cells that activate the T cells essential for antitumor immunity.150 Studies in diet‐induced obese mice indicate that obesity impairs the efficacy of DC‐dependent antitumor immunotherapies (Fig. 5).151
Tumor‐associated macrophages (TAMs) actively promote all aspects of tumor initiation, growth, and development.152 TAMs derive from circulating monocytes in response to the chemokines produced by stromal and tumor cells, particularly CCL2. In obesity, circulating CCL2 is elevated, and high CCL2 levels are associated with increased TAMs and a poor prognosis in breast cancer.152, 153 Myeloid‐derived suppressor cells (MDSCs) home to human cancers and play a role in inhibiting antitumor responses and promoting tumor expansion and metastasis.154, 155 Increased intratumor MDSC infiltration is associated with a poor prognosis (Fig. 5).94, 156 In mice, obesity increases MDSC infiltration into the tumor microenvironment.94
Obesity and Angiogenesis in Breast Cancer
Angiogenesis, which is the formation of new blood vessels from a preexisting vascular network, is required for tumor expansion and is an independent indicator of poor breast cancer prognosis.157 VEGF is a potent proangiogenic growth factor that is essential for continued tumor growth, and elevated intratumor VEGF is associated with breast cancer aggressiveness and a poor prognosis.157, 158 In obesity, angiogenesis is driven by hypoxia‐induced HIF‐1 activation and VEGF induction.159 Leptin160, 161, 162 and several cytokines that are increased in obese adipose tissue, including IL‐1β, IL‐6, IL‐8, and TNF‐α, upregulate VEGF to stimulate breast tumor angiogenesis.126, 163 TNF‐α and IL‐1β also activate leptin production through preadipocytes to further stimulate angiogenesis.90 IL‐8 increases the migration, proliferation, and survival of both endothelial cells and cancer cells.128 The obese mammary gland may promote angiogenesis through a novel CCL2/IL‐1β/CXCL12 pathway that bypasses VEGF and its receptor. Infiltrating immune cells, cancer‐associated adipocytes, and TAMs in the peritumoral microenvironment of obese fat promote tumor angiogenesis by upregulating the proangiogenic cytokines VEGF, IL‐6, and IL‐8, which support vessel growth in breast tumors (Fig. 5).164
Obesity, Estrogens, and Breast Cancer
The stronger association between obesity and ER‐positive, postmenopausal breast cancer risk, compared to that of ER‐negative breast cancer after menopause, points to the importance of the estrogenic milieu of obesity.28, 165, 166 High postmenopausal BMI and recent or current postmenopausal hormone use, especially estrogen plus progestin, are associated with higher postmenopausal breast cancer risk.167 In contrast, both tamoxifen and aromatase‐inhibitor drugs, which block ER and prevent estrogen formation, respectively, decrease the risk of breast cancer.167 Postmenopausal breast cancer risk in obesity is associated with elevated circulating free and total estradiol, estrone, and testosterone and decreased sex hormone‐binding globulin (which increases free estrogens).108, 168, 169, 170, 171, 172, 173, 174 Relative to women with the lowest quintile of circulating sex steroid levels, breast cancer risk is approximately double for those in the highest quintile, with an HR of 2.15 (95% CI, 1.87‐2.46) for estradiol, 1.81 (95% CI, 1.5‐2.10) for estrone, and 2.04 (95% CI, 1.76‐2.37) for testosterone.108 Elevated serum sex steroid levels account for a large component of the excess risk of postmenopausal breast cancer among obese women.174 Estrogen levels are higher in the breast than in circulation and are higher in cancerous than benign breast tissue.175 A recent study of paired serum and postmenopausal breast cancer tissue hormone levels concluded that tissue estradiol is associated with ER‐positive breast cancer size and that BMI affects tissue levels of estradiol and its precursors.176 The proestrogenic milieu in obese women is linked to inflammation in obese adipose tissue. Elevated IL‐6 stimulates aromatase expression.106 The resulting increased aromatization in breast cancer cells, cancer‐associated fibroblasts, and cancer‐associated adipocytes would increase local and circulating estrogens, driving an obesity‐inflammation‐aromatase axis to promote local breast cancer development and growth.
Physical Activity and Breast Cancer Risk and Outcome
Physical Activity Associated With Reduced Breast Cancer Risk
In the Women's Health Initiative study of 155,723 women who were followed over a median of 7.3 years, the risk of breast cancer was lower by 15% to 23% in the tertile with the highest recreational physical activity (PA) relative to the lowest tertile.52 Similarly, among 25,624 Norwegian women, over 4 hours of leisure time PA per week was associated with a 37% lower risk (HR, 0.63; 95% CI, 0.42‐0.95 [P = .04]), with an inverse dose response between PA and risk.177 Two case‐control studies also associated higher recreational PA versus none with a 30% to 60% lower breast cancer risk (most of borderline significance), with a significant decrease in premenopausal, receptor‐negative cancers (HR, 0.46; 95% CI, 0.24‐0.92).26
Breast Cancer Mortality Is Inversely Associated With PA Before and After Breast Cancer Diagnosis
Many large, observational, prospective cohort studies have associated PA before and after a diagnosis of invasive breast cancer with better outcome. Recent meta‐analyses indicate that, relative to sedentary women with breast cancer, those in the highest categories of activity before diagnosis have a 16% to 27% greater breast cancer‐specific survival rate, and those in the highest PA category after diagnosis have 28% to 41% greater disease‐specific survival.178, 179 A meta‐analysis of 22 prospective cohort studies that included 123,574 survivors of breast cancer who were followed for a mean of 4.3 to 12.7 years179 showed that the highest categories of lifetime prediagnosis PA were associated with nearly 30% lower breast cancer‐specific mortality (HR, 0.73; 95% CI, 0.54‐0.98), and the HR for the highest recent prediagnosis PA was 0.84 (95% CI, 0.73‐0.97) relative to the lowest category. The highest postdiagnosis PA category versus sedentary had an HR of 0.59 (95% CI, 0.45‐0.78) for breast cancer‐specific mortality, and those who met the recommended PA guideline of 8 or more metabolic equivalent of task (MET)‐hours per week (approximately 2.5 hours of moderate activity per week) postdiagnosis had a 33% reduction in breast cancer mortality.179 A pooled analysis of 4 studies that included 13,302 survivors associated PA of 10 MET‐hours per week postdiagnosis with 27% lower all‐cause mortality and 25% lower breast cancer‐specific mortality.180 It appears that all subgroups benefit regardless of age, BMI, race, stage, hormone receptor status, or menopausal status,178, 179, 181 with greater benefit seen in those who have higher initial BMI.179 Most studies of PA postdiagnosis show a dose‐response effect.180, 182, 183, 184, 185, 186 Notably, the Nurses' Health Study (NHS) showed a dose response for PA between 0 and 9 to 14.9 MET‐hours per week, with an HR for breast cancer mortality of 0.50 (95% CI, 0.31‐0.82).184 Greater energy expenditure was not associated with greater risk reduction. Those spending 9 MET‐hours per week (equivalent to walking 3‐5 hours at average pace) in PA after diagnosis showed absolute, unadjusted mortality risk reductions of 4% at 5 years and 6% at 10 years versus women who had PA less than 3 MET‐hours per week.184 These risk reductions are similar to those derived from adjuvant chemotherapy.187
Observational studies identify associations between factors and disease outcomes, but they do not establish causal links. To date, only a single large, randomized controlled trial (RCT) has reported an effect of diet and/or PA on breast cancer disease‐free survival,188 and none have yet reported a change in breast cancer‐specific or all‐cause mortality. Completed and ongoing lifestyle intervention trials of diet and/or exercise in breast cancer survivors are discussed below.
Ongoing Weight Loss Intervention Trials Will Test Effects on Breast Cancer Outcome
Several relatively small randomized and nonrandomized trials (n = 10‐103 women) in breast cancer survivors have established that weight loss of up to 5% or more over 2 to 18 months is feasible.189 In the prospective, randomized Women's Intervention Nutrition Study (WINS), a low‐fat diet intervention yielded a mean 3.7% weight loss (mean, 2.3 kg at 1 year and 2.7 kg at 5 years; P < .005) and decreased the risk of recurrence (HR, 0.76; 95% CI, 0.60‐0.98).188 In contrast, in the Women's Healthy Eating and Living (WHEL) Study of a high‐fiber, fruits and vegetables diet, women had a stable weight and showed no improvement in overall survival.190
Ongoing, large, randomized trials aim to determine whether diet and exercise can improve breast cancer outcomes. The Canadian Life Style Intervention in Adjuvant Treatment of Early Breast Cancer (LISA) trial accrued 338 women with a BMI from 24 to 40 kg/m2 to a 2‐year, telephone‐based, structured intervention aiming at a 500 to 100 kilocalorie per day deficit through diet and exercise. This yielded a weight loss of 5.3% at 6 months and 3.6% at 24 months versus 0.7% and 0.4% at these respective time points in controls (P < .001) over all BMI categories.191 Similarly, the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial192 randomized participants with BMI from 25 to 40 kg/m2 to group‐based interventions across 2 years with similar energy deficit goals and achieved a 6% mean weight loss at 12 months in the intervention group versus 1.5% in controls, with 26% of participants showing 10% or greater weight loss. Weight loss was lower in younger patients and in those receiving chemotherapy.192 The ongoing German Docetaxel‐Based Anthracycline‐Free Adjuvant Treatment Evaluation as Well as Life Style Intervention (SUCCESS‐C) trial will randomize 3547 women with lymph node‐positive or high‐risk, lymph node‐negative cancers to 1 of 2 chemotherapy arms. A second randomization will compare disease‐free survival in participants with a BMI from 24 to 40 kg/m2 assigned to observation versus a telephone‐based lifestyle intervention with diet and 150 to 200 minutes per week of moderate exercise. The Italian Diet and Androgens‐5 (DIANA‐5) trial accrued 1208 survivors of breast cancer between 2008 and 2010 to test the effects of an intensive group diet and exercise intervention over 5 years on patient outcome.193 The ongoing Breast Cancer Weight Loss Study (BWEL) of the Alliance of Clinical Trials in Oncology will randomize more than 3000 overweight or obese, premenopausal and postmenopausal women with breast cancer to a health education program with or without weight loss intervention within 12 months of diagnosis and will follow effects on insulin resistance, biomarkers, and disease‐free and overall survival over 10 years (clinicaltrials.gov identifier NCT02750826). Data on outcome benefits from these trials are not yet available.
Effects of Lifestyle Interventions on Biomarkers of Obesity
Several RCTs have indicated that exercise and diet interventions not only change biomarkers associated with poor outcome in obesity, including leptin,194, 195 insulin,191 and estrogens,194, 195, 196 but that these effects are driven by weight loss.197 Notably, in the absence of weight loss, diet and/or exercise appear to have little effect on these biomarkers. Because all of these factors are associated with poor breast cancer prognosis, it is speculated that they play causal roles in the adverse effect of obesity on outcome and that their downregulation through diet and PA predict a benefit on disease outcome.
Sex steroids
Circulating sex steroid levels increase with increasing BMI, and women with the highest quintiles of circulating estrogen, estrone, and testosterone have up to 2‐fold higher breast cancer risk.108 Elevated sex steroids account for most of the excess risk of postmenopausal breast cancer with obesity.174 RCTs have shown that PA effectively reduces serum sex steroid levels in obese postmenopausal women without194, 196 and with breast cancer.195, 198 In postmenopausal women with a BMI >24 kg/m2, a 3‐month RCT of PA decreased estrone by 3.8% and estradiol by 7.7%. Estradiol decreased by 30% in those with a 10% weight loss.196 Combining PA and diet yielded greater weight loss (−11.9%) than either diet (−10%) or PA (−3.3%) alone and even more striking decreases in estrone, estradiol, and testosterone, indicating that weight loss is the driver linking PA to decreased sex steroids in postmenopausal women with BMI >25 kg/m2.194 Postmenopausal breast cancer survivors randomized in the Survivor's Health and Physical Exercise (SHAPE) RCT to increased PA and decreased energy intake showed a weight loss 5% or greater at 6 months, with decreased estradiol (P = .002), decreased estrone (P = .02), and increased sex hormone‐binding globulin (P < .01).195
Leptin
As noted previously, obese individuals have higher serum leptin levels,119 which are associated with higher postmenopausal breast cancer risk117 and a poor outcome.122 Aerobic exercise decreases leptin levels in association with weight loss.122, 197 Invasive breast cancer survivors with the highest quartile of PA had lower serum leptin levels than sedentary patients (P = .001).199 A PA intervention decreased serum leptin levels in postmenopausal women with a BMI >25 kg/m2, and the decrease was most pronounced in the diet and exercise arm, which showed the greatest weight loss compared with either diet or exercise alone.194 A 3‐month PA intervention decreased leptin levels (P = .031) in patients with breast cancer200; and, in the SHAPE study of postmenopausal survivors of breast cancer, an intervention that yielded a mean weight loss of 5% or more (or 8.7 kg) decreased serum leptin levels (P < .0001).195
Insulin, IGF‐1, and glucose tolerance
Obesity is often associated with insulin resistance, leading to elevated levels of insulin and IGF‐1, both of which are potent breast cancer growth factors.116 A decrease in fasting insulin levels is among the most consistent results of PA.119 The decrease in fasting glucose levels produced in PA intervention studies required weight loss.201 In the DIANA RCT, a 4.06‐kg mean weight loss in postmenopausal survivors of breast cancer decreased fasting glucose levels and improved glucose tolerance.202 RCTs of PA in postmenopausal survivors of breast cancer have shown decreased insulin levels by 4% to 10.3% with little change in controls.186, 203 Interestingly, although PA and weight loss interventions consistently improve glucose tolerance and insulin levels, reductions in serum IGF‐1 and IGF‐binding protein levels are variable and small.119, 197, 203
Inflammatory biomarkers
Obese adipose tissue is a site of chronic inflammation. Numerous studies have shown that exercise with weight loss decreases inflammatory markers, including C‐reactive protein, in postmenopausal women with or without breast cancer.197 TNF‐α decreases of 4% to 26% accompany weight loss of 6% to 26%, and reductions in IL‐6 levels are decreased by 6% to 50%.197 Several earlier small, short‐term RCTs of exercise with minimal to no weight loss in survivors of breast cancer failed to show decreases in IL‐6.203 Chronic long‐term PA is needed to significantly decrease IL‐6.197, 204 An intervention study in overweight and obese postmenopausal women who achieved an average of 7.3‐kg weight loss showed that initial serum IL‐6 levels strongly correlated with BMI and that IL‐6 was decrease by diet and exercise.205 Similarly, a PA intervention RCT yielding a mean 5.7‐kg weight loss in postmenopausal survivors of breast cancer showed a significant decrease in IL‐6 levels, whereas the levels in sedentary controls were unchanged.206 The effect of weight loss to decrease IL‐6 is greatest in obese individuals.203 Thus, the vicious cycle of inflammation, insulin resistance, and high estrogen levels, which are associated with and may be causally linked to the poor outcome of obese patients with breast cancer, can be interrupted by diet and exercise weight loss interventions.
Conclusions
The effects of obesity on the risk of breast cancer in premenopausal and postmenopausal women differ based on ER status. In premenopausal women, obesity is associated with a lower risk of ER‐positive breast cancer and a higher risk of TNBC. However, in postmenopausal women, obesity is associated with a markedly higher risk of ER‐positive breast cancer, particularly in nonusers of HT, but with only a modest or no effect on the risk of ER‐negative breast cancer. In contrast, obesity increases the risk of death in both premenopausal and postmenopausal patients with breast cancer. Both biologic factors and undertreatment of obese patients appear to contribute to poor breast cancer outcomes in obesity.
The molecular mechanisms underlying the higher risk and poor breast cancer outcome with obesity are complex. Local hypoxia in obese adipose tissue upregulates the secretion of leptin, and VEGF and decreases adiponectin. High levels of certain cytokines and leptins secreted by the obese adipose tissue mediate infiltration of proinflammatory immune cells that increase preadipocyte numbers, increase the release of FFAs, and activate the NF‐κB pathway, in both adipocytes and immune cells, to sustain a chronic inflammatory milieu. Expansion of the preadipocyte population produces high levels of IL‐6, IL‐8, CCL2, CCL5, and VEGF to feed‐forward and further upregulate NF‐κB and cytokine production.90, 114 Furthermore, contact between adipocytes and invading cancer cells would synergistically upregulate cytokine secretion.9 TNF‐α and IL‐6 impair insulin receptor activation113, 114 to mediate insulin resistance and the high levels of insulin and IFG‐1 that drive breast cancer growth. The NF‐κB pathway, TNF‐α, and IL‐6 also stimulate aromatase expression in breast stromal fibroblasts and adipocytes106 to upregulate estrogen production in both cancer and stroma. These changes in the leptin:adiponectin ratio and the secretion of proinflammatory cytokines, IGF‐1, and estrogen create a local microenvironment that is propitious for breast cancer development. Furthermore, proinflammatory and proangiogenic adipokines secreted by obese adipose tissue, together with impaired antitumor immunity in obesity, favor tumor progression, CSC expansion, and metastasis, leading to the poorer outcomes of obese patients with breast cancer.
A large body of observational studies has demonstrated that obesity, decreased PA, and weight gain all are associated with poor survival in patients with breast cancer. Exercise and weight loss decrease the inflammatory microenvironment in obese patients, improve antitumor immunity, decrease estrogen levels, and are associated with reduced breast cancer risk and better outcomes. Lifestyle intervention trials suggest that weight loss is feasible, and observational studies strongly suggest that it may indeed improve breast cancer survival. Long‐term lifestyle changes, beyond a single year, may be required for sustained weight loss. Moreover, biomarker data suggest that there are plausible molecular mechanisms underlying these effects. Most patients with breast cancer are either overweight or obese at diagnosis.65, 66 Practicing oncologists know that most women coping with new breast cancer therapy fail to lose weight on their own, and weight gain is very frequent.65, 66 We have yet to fully realize the consequences of the recent rise of obesity prevalence on cancer incidence and outcomes. The time has come for systematic weight loss intervention through standardized diet and exercise programs during or after adjuvant therapy. One could argue, based on compelling data from observational studies, that the current state of knowledge supports the routine incorporation of weight loss intervention as part of management for patients with breast cancer. Ongoing lifestyle intervention trials should help to clarify this issue.
DISCLOSURES: The authors report no conflicts of interest.
This work was supported by a Susan G. Komen Foundation grant PDF16380958 to Manuel Picon‐Ruiz and by funding from the Breast Cancer Research Foundation and from an NIH grant R01‐CA210440‐01A1 to Joyce Slingerland.
The first 2 authors contributed equally to this work.
A correction to the author list was added after online publication on August 3, 2017.
References
- 1. World Health Organization . Obesity and Overweight. Fact Sheet 311 (Reviewed May 2014). Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011‐2014. NCHS Data Brief, no. 201. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 3. Gonzalez N, Moreno‐Villegas Z, Gonzalez‐Bris A, Egido J, Lorenzo O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes [serial online]. Cardiovasc Diabetol. 2017;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011;96:1654‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Agency for Research on Cancer/World Health Organization . Weight Control and Physical Activity. IARC Handbook of Cancer Prevention. Vol 6 Lyon, France: IARC Publications; 2002. [Google Scholar]
- 6. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625‐1638. [DOI] [PubMed] [Google Scholar]
- 7. World Cancer Research Fund (WCRF) . Continuous Update Project: 2016. London, UK: WCRF International; 2016. [Google Scholar]
- 8. Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45‐57. [DOI] [PubMed] [Google Scholar]
- 9. Picon‐Ruiz M, Pan C, Drews‐Elger K, et al. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR‐302b mediated malignant progression. Cancer Res. 2016;76:491‐504. [DOI] [PubMed] [Google Scholar]
- 10. Dirat B, Bochet L, Dabek M, et al. Cancer‐associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455‐2465. [DOI] [PubMed] [Google Scholar]
- 11. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 12. Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166:2395‐2402. [DOI] [PubMed] [Google Scholar]
- 13. Berstad P, Coates RJ, Bernstein L, et al. A case‐control study of body mass index and breast cancer risk in white and African‐American women. Cancer Epidemiol Biomarkers Prev. 2010;19:1532‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121:3700‐3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris HR, Willett WC, Terry KL, Michels KB. Body fat distribution and risk of premenopausal breast cancer in the Nurses' Health Study II. J Natl Cancer Inst. 2011;103:273‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514‐527. [DOI] [PubMed] [Google Scholar]
- 17. Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421‐430. [DOI] [PubMed] [Google Scholar]
- 18. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet. 2008;371:569‐578. [DOI] [PubMed] [Google Scholar]
- 19. Kawai M, Malone KE, Tang MT, Li CI. Height, body mass index (BMI), BMI change, and the risk of estrogen receptor‐positive, HER2‐positive, and triple‐negative breast cancer among women ages 20 to 44 years. Cancer. 2014;120:1548‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high‐risk women in NSABP P‐1 and STAR breast cancer prevention trials. Cancer Prev Res (Phila). 2012;5:583‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose‐response meta‐analysis. Obes Rev. 2013;14:665‐678. [DOI] [PubMed] [Google Scholar]
- 22. Cotterchio M, Kreiger N, Theis B, Sloan M, Bahl S. Hormonal factors and the risk of breast cancer according to estrogen‐ and progesterone‐receptor subgroup. Cancer Epidemiol Biomarkers Prev. 2003;12:1053‐1060. [PubMed] [Google Scholar]
- 23. Ma H, Bernstein L, Ross RK, Ursin G. Hormone‐related risk factors for breast cancer in women under age 50 years by estrogen and progesterone receptor status: results from a case‐control and a case‐case comparison [serial online]. Breast Cancer Res. 2006;8:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. John EM, Sangaramoorthy M, Hines LM, et al. Overall and abdominal adiposity and premenopausal breast cancer risk among Hispanic women: the Breast Cancer Health Disparities study. Cancer Epidemiol Biomarkers Prev. 2015;24:138‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagrani R, Mhatre S, Rajaraman P, et al. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian ethnicity. Eur J Cancer. 2016;66:153‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Enger SM, Ross RK, Paganini‐Hill A, Carpenter CL, Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: results from two case‐control studies. Cancer Epidemiol Biomarkers Prev. 2000;9:681‐687. [PubMed] [Google Scholar]
- 27. Yang XR, Chang‐Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham‐Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen‐progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in black women, the Carolina Breast Cancer Study, 1993‐2001. Cancer Causes Control. 2014;25:1101‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors [serial online]. Breast Cancer Res. 2009;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dolle JM, Daling JR, White E, et al. Risk factors for triple‐negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Cook LS, Tang MT, et al. Body mass index and risk of luminal, HER2‐overexpressing, and triple negative breast cancer. Breast Cancer Res Treat. 2016;157:545‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bandera EV, Chandran U, Hong CC, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen FY, Ou HY, Wang SM, Wu YH, Yan GJ, Tang LL. Associations between body mass index and molecular subtypes as well as other clinical characteristics of breast cancer in Chinese women. Ther Clin Risk Manag. 2013;9:131‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population‐based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130:587‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple‐negative breast cancers: a systematic review and meta‐analysis. Breast Cancer Res Treat. 2013;137:307‐314. [DOI] [PubMed] [Google Scholar]
- 37. Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351‐375. [DOI] [PubMed] [Google Scholar]
- 38. Schairer C, Li Y, Frawley P, et al. Risk factors for inflammatory breast cancer and other invasive breast cancers. J Natl Cancer Inst. 2013;105:1373‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16:3731‐3735. [DOI] [PubMed] [Google Scholar]
- 40. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629‐636. [DOI] [PubMed] [Google Scholar]
- 41. Agnoli C, Berrino F, Abagnato CA, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case‐control study. Nutr Metab Cardiovasc Dis. 2010;20:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li CI, Malone KE, Daling JR. Interactions between body mass index and hormone therapy and postmenopausal breast cancer risk (United States). Cancer Causes Control. 2006;17:695‐703. [DOI] [PubMed] [Google Scholar]
- 43. Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women's Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:611‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sebastiani F, Cortesi L, Sant M, et al. Increased incidence of breast cancer in postmenopausal women with high body mass index at the Modena Screening Program. J Breast Cancer. 2016;19:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhaskaran K, Douglas I, Forbes H, dos‐Santos‐Silva I, Leon DA, Smeeth L. Body‐mass index and risk of 22 specific cancers: a population‐based cohort study of 5.24 million UK adults. Lancet. 2014;384:755‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study [serial online]. BMJ. 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2004;111:762‐771. [DOI] [PubMed] [Google Scholar]
- 48. Suzuki R, Rylander‐Rudqvist T, Ye W, Saji S, Wolk A. Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer. 2006;119:1683‐1689. [DOI] [PubMed] [Google Scholar]
- 49. Ahn J, Schatzkin A, Lacey JV Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167:2091‐2102. [DOI] [PubMed] [Google Scholar]
- 50. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169:1251‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rosenberg LU, Einarsdottir K, Friman EI, et al. Risk factors for hormone receptor‐defined breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:2482‐2488. [DOI] [PubMed] [Google Scholar]
- 52. Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple‐negative and estrogen receptor‐positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:454‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. John EM, Sangaramoorthy M, Hines LM, et al. Body size throughout adult life influences postmenopausal breast cancer risk among Hispanic women: the Breast Cancer Health Disparities study. Cancer Epidemiol Biomarkers Prev. 2015;24:128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Canchola AJ, Anton‐Culver H, Bernstein L, et al. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control. 2012;23:473‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gaudet MM, Carter BD, Patel AV, Teras LR, Jacobs EJ, Gapstur SM. Waist circumference, body mass index, and postmenopausal breast cancer incidence in the Cancer Prevention Study‐II Nutrition Cohort. Cancer Causes Control. 2014;25:737‐745. [DOI] [PubMed] [Google Scholar]
- 56. Hvidtfeldt UA, Tjonneland A, Keiding N, et al. Risk of breast cancer in relation to combined effects of hormone therapy, body mass index, and alcohol use, by hormone‐receptor status. Epidemiology. 2015;26:353‐361. [DOI] [PubMed] [Google Scholar]
- 57. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 58. Park B, Choi JY, Sung HK, et al. Attribution to heterogeneous risk factors for breast cancer subtypes based on hormone receptor and human epidermal growth factor 2 receptor expression in Korea [serial online]. Medicine (Baltimore). 2016;95:e3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Atkinson RL, El‐Zein R, Valero V, et al. Epidemiological risk factors associated with inflammatory breast cancer subtypes. Cancer Causes Control. 2016;27:359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193‐201. [DOI] [PubMed] [Google Scholar]
- 61. Parker ED, Folsom AR. Intentional weight loss and incidence of obesity‐related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 2003;27:1447‐1452. [DOI] [PubMed] [Google Scholar]
- 62. Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653‐662. [DOI] [PubMed] [Google Scholar]
- 63. Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring). 2009;17:796‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691‐695. [DOI] [PubMed] [Google Scholar]
- 65. Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282‐294. [DOI] [PubMed] [Google Scholar]
- 66. Demark‐Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118:2277‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nichols HB, Trentham‐Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all‐cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128‐1143. [DOI] [PubMed] [Google Scholar]
- 69. Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370‐1378. [DOI] [PubMed] [Google Scholar]
- 70. Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686‐1691. [DOI] [PubMed] [Google Scholar]
- 71. Copson ER, Cutress RI, Maishman T, et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26:101‐112. [DOI] [PubMed] [Google Scholar]
- 72. Rosenberg L, Czene K, Hall P. Obesity and poor breast cancer prognosis: an illusion because of hormone replacement therapy? Br J Cancer. 2009;100:1486‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta‐analysis of 82 follow‐up studies. Ann Oncol. 2014;25:1901‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29:3358‐3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor‐positive early‐stage breast cancer. J Natl Cancer Inst. 2003;95:1467‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor‐positive operable breast cancer. Cancer. 2012;118:5937‐5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta‐analysis. Breast Cancer Res Treat. 2012;134:769‐781. [DOI] [PubMed] [Google Scholar]
- 79. Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple‐negative breast cancer? J Surg Res. 2013;184:253‐259. [DOI] [PubMed] [Google Scholar]
- 80. Ademuyiwa FO, Groman A, O'Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple‐negative breast cancer. Cancer. 2011;117:4132‐4140. [DOI] [PubMed] [Google Scholar]
- 81. Turkoz FP, Solak M, Petekkaya I, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18:335‐341. [PubMed] [Google Scholar]
- 82. Dawood S, Broglio K, Gonzalez‐Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14:1718‐1725. [DOI] [PubMed] [Google Scholar]
- 83. Chang S, Alderfer JR, Asmar L, Buzdar AU. Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat. 2000;64:157‐163. [DOI] [PubMed] [Google Scholar]
- 84. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lyman GH. Weight‐based chemotherapy dosing in obese patients with cancer: back to the future. J Oncol Pract. 2012;8:e62‐e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rosner GL, Hargis JB, Hollis DR, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from Cancer and Leukemia Group B Study 8541. J Clin Oncol. 1996;14:3000‐3008. [DOI] [PubMed] [Google Scholar]
- 87. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553‐1561. [DOI] [PubMed] [Google Scholar]
- 88. McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity‐induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies [serial online]. Front Endocrinol (Lausanne). 2013;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772‐783. [DOI] [PubMed] [Google Scholar]
- 90. Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L. Cytokine‐mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor‐alpha‐ and interleukin‐1beta‐treated human preadipocytes are potent leptin producers. Cytokine. 2005;32:94‐103. [DOI] [PubMed] [Google Scholar]
- 91. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1‐21. [DOI] [PubMed] [Google Scholar]
- 92. Chen B, Lam KS, Wang Y, et al. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor‐1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549‐556. [DOI] [PubMed] [Google Scholar]
- 93. Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol. 2008;198:127‐134. [DOI] [PubMed] [Google Scholar]
- 94. Apostolopoulos V, de Courten MP, Stojanovska L, Blatch GL, Tangalakis K, de Courten B. The complex immunological and inflammatory network of adipose tissue in obesity. Mol Nutr Food Res. 2016;60:43‐57. [DOI] [PubMed] [Google Scholar]
- 95. Catalan V, Gomez‐Ambrosi J, Rodriguez A, Fruhbeck G. Adipose tissue immunity and cancer. Front Physiol. 2013;4:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Naylor C, Petri WA Jr. Leptin regulation of immune responses. Trends Mol Med. 2016;22:88‐98. [DOI] [PubMed] [Google Scholar]
- 98. Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557‐566. [DOI] [PubMed] [Google Scholar]
- 99. Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010;31:228‐235. [DOI] [PubMed] [Google Scholar]
- 101. Castoldi A, de Naffah SC, Camara NO, Moraes‐Vieira PM. The macrophage switch in obesity development. Front Immunol. 2015;6:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vallabhapurapu S, Karin M. Regulation and function of NF‐kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693‐733. [DOI] [PubMed] [Google Scholar]
- 103. Panee J. Monocyte chemoattractant protein 1 (MCP‐1) in obesity and diabetes. Cytokine. 2012;60:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liedtke S, Schmidt ME, Vrieling A, et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring). 2012;20:1088‐1095. [DOI] [PubMed] [Google Scholar]
- 105. Simpson ER, Brown KA. Minireview: obesity and breast cancer: a tale of inflammation and dysregulated metabolism. Mol Endocrinol. 2013;27:715‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Purohit A, Reed MJ. Regulation of estrogen synthesis in postmenopausal women. Steroids. 2002;67:979‐983. [DOI] [PubMed] [Google Scholar]
- 107. Mullooly M, Yang HP, Falk RT, et al. Relationship between crown‐like structures and sex‐steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients [serial online]. Breast Cancer Res. 2017;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Key TJ, Appleby PN, Reeves GK, et al. Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanalysis of eighteen prospective studies. Steroids. 2015;99:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Arcidiacono B, Iiritano S, Nocera A, et al. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012:789174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early‐stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42‐51. [DOI] [PubMed] [Google Scholar]
- 111. Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf). 2013;78:321‐329. [DOI] [PubMed] [Google Scholar]
- 112. Voudouri K, Berdiaki A, Tzardi M, Tzanakakis GN, Nikitovic D. Insulin‐like growth factor and epidermal growth factor signaling in breast cancer cell growth: focus on endocrine resistant disease [serial online]. Anal Cell Pathol (Amst). 2015;2015:975495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin‐6 (IL‐6) treatment increased IL‐6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311:372‐379. [DOI] [PubMed] [Google Scholar]
- 114. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin‐6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745‐E751. [DOI] [PubMed] [Google Scholar]
- 115. LeRoith D, Roberts CT Jr. The insulin‐like growth factor system and cancer. Cancer Lett. 2003;195:127‐137. [DOI] [PubMed] [Google Scholar]
- 116. Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484‐498. [DOI] [PubMed] [Google Scholar]
- 117. Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C‐reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila). 2013;6:188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Barone I, Giordano C, Bonofiglio D, Ando S, Catalano S. Leptin, obesity and breast cancer: progress to understanding the molecular connections. Curr Opin Pharmacol. 2016;31:83‐89. [DOI] [PubMed] [Google Scholar]
- 119. Schmidt S, Monk JM, Robinson LE, Mourtzakis M. The integrative role of leptin, oestrogen and the insulin family in obesity‐associated breast cancer: potential effects of exercise. Obes Rev. 2015;16:473‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Miyoshi Y, Funahashi T, Tanaka S, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414‐1419. [DOI] [PubMed] [Google Scholar]
- 121. Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB‐R) in human breast cancer. Clin Cancer Res. 2004;10:4325‐4331. [DOI] [PubMed] [Google Scholar]
- 122. Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: a meta‐analysis [serial online]. PLoS One. 2013;8:e67349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ye J, Jia J, Dong S, et al. Circulating adiponectin levels and the risk of breast cancer: a meta‐analysis. Eur J Cancer Prev. 2014;23:158‐165. [DOI] [PubMed] [Google Scholar]
- 124. Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Prasad S, Ravindran J, Aggarwal BB. NF‐kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336:25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Esquivel‐Velazquez M, Ostoa‐Saloma P, Palacios‐Arreola MI, Nava‐Castro KE, Castro JI, Morales‐Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325‐337. [DOI] [PubMed] [Google Scholar]
- 128. Waugh DJ, Wilson C. The interleukin‐8 pathway in cancer. Clin Cancer Res. 2008;14:6735‐6741. [DOI] [PubMed] [Google Scholar]
- 129. Soria G, Ben‐Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271‐285. [DOI] [PubMed] [Google Scholar]
- 130. Kim JH, Bachmann RA, Chen J. Interleukin‐6 and insulin resistance. Vitam Horm. 2009;80:613‐633. [DOI] [PubMed] [Google Scholar]
- 131. Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL‐6 in breast cancer. Breast Cancer Res Treat. 2013;138:657‐664. [DOI] [PubMed] [Google Scholar]
- 132. Morley JE, Baumgartner RN. Cytokine‐related aging process. J Gerontol A Biol Sci Med Sci. 2004;59:M924‐M929. [DOI] [PubMed] [Google Scholar]
- 133. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557‐563. [DOI] [PubMed] [Google Scholar]
- 134. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature. 2011;475:222‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Khalid A, Wolfram J, Ferrari I, et al. Recent advances in discovering the role of CCL5 in metastatic breast cancer. Mini Rev Med Chem. 2015;15:1063‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gwendal L, Paula YL. Recent discoveries concerning the tumor‐mesenchymal stem cell interactions. Biochim Biophys Acta. 2016;1866:290‐299. [DOI] [PubMed] [Google Scholar]
- 137. Perrier S, Caldefie‐Chezet F, Vasson MP. IL‐1 family in breast cancer: potential interplay with leptin and other adipocytokines. FEBS Lett. 2009;583:259‐265. [DOI] [PubMed] [Google Scholar]
- 138. Tirino V, Desiderio V, Paino F, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13‐24. [DOI] [PubMed] [Google Scholar]
- 139. O'Brien CA, Kreso A, Dick JE. Cancer stem cells in solid tumors: an overview. Semin Radiat Oncol. 2009;19:71‐77. [DOI] [PubMed] [Google Scholar]
- 140. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717‐728. [DOI] [PubMed] [Google Scholar]
- 141. Yang H, Wang B, Wang T, et al. Toll‐like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis [serial online]. PLoS One. 2014;9:e109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rinkenbaugh AL, Baldwin AS. The NF‐κB pathway and cancer stem cells [serial online]. Cells. 2016;5. pii: E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sansone P, Storci G, Tavolari S, et al. IL‐6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988‐4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tsuyada A, Chow A, Wu J, et al. CCL2 mediates crosstalk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768‐2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Velasco‐Velazquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72:3839‐3850. [DOI] [PubMed] [Google Scholar]
- 147. Bandaru P, Rajkumar H, Nappanveettil G. The impact of obesity on immune response to infection and vaccine: an insight into plausible mechanisms [serial online]. Endocrinol Metab Synd. 2013;2:113. [Google Scholar]
- 148. O'Shea D, Cawood TJ, O'Farrelly C, Lynch L. Natural killer cells in obesity: impaired function and increased susceptibility to the effects of cigarette smoke [serial online]. PLoS One. 2010;5:e8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Laue T, Wrann CD, Hoffmann‐Castendiek B, Pietsch D, Hubner L, Kielstein H. Altered NK cell function in obese healthy humans [serial online]. BMC Obes. 2015;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24‐34. [DOI] [PubMed] [Google Scholar]
- 151. James BR, Tomanek‐Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA. Diet‐induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189:1311‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wagner M, Samdal Steinskog ES, Wiig H. Adipose tissue macrophages: the inflammatory link between obesity and cancer? Expert Opin Ther Targets. 2015;19:527‐538. [DOI] [PubMed] [Google Scholar]
- 154. Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dushyanthen S, Beavis PA, Savas P, et al. Relevance of tumor‐infiltrating lymphocytes in breast cancer [serial online]. BMC Med. 2015;13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Aalders KC, Tryfonidis K, Senkus E, Cardoso F. Anti‐angiogenic treatment in breast cancer: facts, successes, failures and future perspectives. Cancer Treat Rev. 2017;53:98‐110. [DOI] [PubMed] [Google Scholar]
- 158. Adams J, Carder PJ, Downey S, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60:2898‐2905. [PubMed] [Google Scholar]
- 159. Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227‐235. [DOI] [PubMed] [Google Scholar]
- 160. Sierra‐Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683‐1686. [DOI] [PubMed] [Google Scholar]
- 161. Gonzalez‐Perez RR, Lanier V, Newman G. Leptin's pro‐angiogenic signature in breast cancer. Cancers (Basel). 2013;5:1140‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF‐2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390‐6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;13:2825‐2830. [DOI] [PubMed] [Google Scholar]
- 164. Lin EY, Pollard JW. Tumor‐associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064‐5066. [DOI] [PubMed] [Google Scholar]
- 165. Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status—a meta‐analysis. Int J Cancer. 2009;124:698‐712. [DOI] [PubMed] [Google Scholar]
- 166. Vrieling A, Buck K, Kaaks R, Chang‐Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta‐analysis. Breast Cancer Res Treat. 2010;123:641‐649. [DOI] [PubMed] [Google Scholar]
- 167. Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015;99:8‐10. [DOI] [PubMed] [Google Scholar]
- 168. Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071‐1082. [DOI] [PubMed] [Google Scholar]
- 169. Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin‐like growth factor‐I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sieri S, Krogh V, Bolelli G, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:169‐176. [DOI] [PubMed] [Google Scholar]
- 171. Woolcott CG, Shvetsov YB, Stanczyk FZ, et al. Plasma sex hormone concentrations and breast cancer risk in an ethnically diverse population of postmenopausal women: the Multiethnic Cohort Study. Endocr Relat Cancer. 2010;17:125‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Baglietto L, Severi G, English DR, et al. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:492‐502. [DOI] [PubMed] [Google Scholar]
- 173. Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow‐up. Breast Cancer Res Treat. 2013;137:883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218‐1226. [DOI] [PubMed] [Google Scholar]
- 175. Stanczyk FZ, Mathews BW, Sherman ME. Relationships of sex steroid hormone levels in benign and cancerous breast tissue and blood: a critical appraisal of current science. Steroids. 2015;99:91‐102. [DOI] [PubMed] [Google Scholar]
- 176. Kakugawa Y, Tada H, Kawai M, et al. Associations of obesity and physical activity with serum and intratumoral sex steroid hormone levels among postmenopausal women with breast cancer: analysis of paired serum and tumor tissue samples. Breast Cancer Res Treat. 2017;162:115‐125. [DOI] [PubMed] [Google Scholar]
- 177. Thune I, Brenn T, Lund E, Gaard M. Physical activity and the risk of breast cancer. N Engl J Med. 1997;336:1269‐1275. [DOI] [PubMed] [Google Scholar]
- 178. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta‐analysis. Ann Oncol. 2014;25:1293‐1311. [DOI] [PubMed] [Google Scholar]
- 179. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta‐analysis of epidemiological studies. Acta Oncol. 2015;54:635‐654. [DOI] [PubMed] [Google Scholar]
- 180. Beasley JM, Kwan ML, Chen WY, et al. Meeting the physical activity guidelines and survival after breast cancer: findings from the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;131:637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ballard‐Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Holick CN, Newcomb PA, Trentham‐Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379‐386. [DOI] [PubMed] [Google Scholar]
- 183. Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable‐fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345‐2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479‐2486. [DOI] [PubMed] [Google Scholar]
- 185. Irwin ML, McTiernan A, Manson JE, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the women's health initiative. Cancer Prev Res (Phila). 2011;4:522‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Chen X, Lu W, Zheng W, et al. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila). 2011;4:1409‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767‐1776. [DOI] [PubMed] [Google Scholar]
- 189. Reeves MM, Terranova CO, Eakin EG, Demark‐Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev. 2014;15:749‐768. [DOI] [PubMed] [Google Scholar]
- 190. Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone‐based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32:2231‐2239. [DOI] [PubMed] [Google Scholar]
- 192. Rock CL, Flatt SW, Byers TE, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33:3169‐3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Villarini A, Pasanisi P, Traina A, et al. Lifestyle and breast cancer recurrences: the DIANA‐5 trial. Tumori. 2012;98:1‐18. [DOI] [PubMed] [Google Scholar]
- 194. Campbell KL, Foster‐Schubert KE, Alfano CM, et al. Reduced‐calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30:2314‐2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rock CL, Pande C, Flatt SW, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13:188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12‐month randomized clinical trial. Cancer Res. 2004;64:2923‐2928. [DOI] [PubMed] [Google Scholar]
- 197. Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;13:1063‐1072. [DOI] [PubMed] [Google Scholar]
- 198. Neilson HK, Conroy SM, Friedenreich CM. The influence of energetic factors on biomarkers of postmenopausal breast cancer risk. Curr Nutr Rep. 2014;3:22‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with C‐peptide, leptin, and insulin‐like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881‐2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12:323‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2017;26:355‐365. [DOI] [PubMed] [Google Scholar]
- 202. Berrino F, Bellati C, Secreto G, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the Diet and Androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10:25‐33. [PubMed] [Google Scholar]
- 203. Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11‐27. [DOI] [PubMed] [Google Scholar]
- 204. Dethlefsen C, Pedersen KS, Hojman P. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res Treat. 2017;162:399‐408. [DOI] [PubMed] [Google Scholar]
- 205. You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739‐1746. [DOI] [PubMed] [Google Scholar]
- 206. Pakiz B, Flatt SW, Bardwell WA, Rock CL, Mills PJ. Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors. Int J Behav Med. 2011;18:333‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]


