Summary
The significance of serum lactate dehydrogenase (LDH) in light chain (AL) amyloidosis has not been previously explored. We studied 1019 newly diagnosed patients and correlated the elevation of LDH above the upper limit of normal (ULN) with disease characteristics and outcome. Four hundred and nine patients had an LDH above ULN, representing 40% of the study population. Patients with an elevated LDH were older, were less likely to be male and had more extensive organ involvement compared to patients with a normal LDH. Patients with high LDH had greater cardiac and renal dysfunction. Elevated LDH was an independent prognostic marker for overall survival and for death within 6 month of diagnosis, but this was restricted to patients not eligible for stem cell transplant. Serum LDH may act as a marker for organ damage and should be explored as a potential marker for tissue healing and organ recovery.
Keywords: LDH, tissue, damage, prognosis, early death
Introduction
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme found in all tissues. Elevation of serum LDH is associated with various causes of cell damage,(Glick 1969) such as myocardial infarction,(Danese and Montagnana 2016) acute necrotizing pancreatitis,(Waldemar, et al 1995) acute ischaemic renal tubular necrosis(Green, et al 2016) and haemolysis(Tabbara 1992). Elevated serum LDH is associated with various malignancies, potentially as an indicator of tumour metabolic activity, proliferation and invasiveness.(Gallo, et al 2015) Serum LDH is elevated in both solid and haematological cancers (Petrelli, et al 2015, Walenta and Mueller-Klieser 2004, Wulaningsih, et al 2015) and is used for prognostic purposes in several types of cancer.(Miles, et al 2012, Palumbo, et al 2015, Petrelli, et al 2015, Winter 2007)
Immunoglobulin light chain (AL) amyloidosis is a unique syndrome due to a monoclonal expansion of plasma cells, in which nearly half of patients reach the threshold of multiple myeloma, with more than 10% clonal plasma cells in the bone marrow.(Kourelis, et al 2013) However, amyloidosis is associated with misfolded protein deposition and tissue damage. We hypothesized that elevation of serum LDH in AL amyloidosis may be a marker for tissue damage rather than a measure of tumour mass in this low proliferative clonal process.
Methods
One thousand and nineteen patients (n=1019) with biopsy-proven systemic AL amyloidosis who were seen in our institution within 90 days of diagnosis between 1 January 2000 and 31 August 2015 and had serum LDH measurement before treatment were included in this study. Patients were excluded if they had been previously treated for AL amyloidosis or multiple myeloma (MM), had AL amyloidosis due to a lymphoproliferative disorder or had incidental amyloid deposits without demonstrable visceral involvement.
Data were extracted from prospectively maintained databases and patient records were reviewed for data accuracy and completeness. The study was approved by the Institutional Review Board (IRB). All patients gave written informed consent for medical record review according to the IRB and Minnesota state law.
Serum LDH was measured using a commercially available reagent (Roche diagnostics, Indianapolis, IN, USA). Patients with serum LDH activity greater than the upper limit of normal (ULN) were designated as “high serum LDH” group, while those with serum LDH below ULN were categorized in the “normal serum LDH” group.
Assessment of organ involvement, haematological response and organ response were determined by consensus criteria.(Comenzo, et al 2012, Gertz, et al 2005) Bone marrow plasma cells (BMPCs) were considered as the highest estimate of the bone marrow aspiration and biopsy. Immunoparesis was assessed using a quantitative method, where significant immunoparesis was defined when the average relative difference of the uninvolved immunoglobulins from their respective lower limit of normal was below zero, as previously described.(Muchtar, et al 2017a) Treatment was categorized at autologous stem cell transplant (ASCT) and non-ASCT regimens. Current eligibility for ASCT in our centre has been published.(Muchtar, et al 2016a)
The Pearson χ2 test and the Kruskal-Wallis test were used to ascertain differences between nominal and continuous variables, respectively. Survival analysis was performed using the Kaplan-Meier method. Cox proportional hazards regression models were built to identify independent prognostic factors for survival. One model included the whole study cohort and another model was restricted to patients who received standard therapy without ASCT. In addition, a binary logistic regression model was built to identify independent predictors of death within the first 6 months of diagnosis. All variables in the univariate analysis were entered into the multivariate analysis, regardless of significance reached in the univariate analysis. P values less than 0.05 were considered significant. Statistical analysis was performed using JMP software (SAS, Cary, NC).
Results
Of the 1019 patients included, 610 (60%) had serum LDH within the normal range and 409 patients (40%) had serum LDH level above the ULN.
Baseline characteristic of the entire cohort and by serum LDH groups can be viewed in Table I. The two serum LDH groups differed by age (older population in the high serum LDH group, median 63 vs 61 years; P=0.01) and by sex (44% females in high LDH group vs 34% in the normal LDH group; P=0.001). Patients with an elevated LDH were more likely to present with a systolic blood pressure below 100 mmHg (26% vs 20%; P=0.02). Of note, patients presented with a lower frequency of extensive organ involvement (>2 organs) in the most recent period (2008–2015) compared to the earlier period (2000–2007) (22% vs 31%; P=0.002) as well as a lower proportion of cardiac involvement (66% vs 77%; P<0.001) and hepatic involvement (14% vs 26%; P<0.001), but not renal involvement (64% vs 64%; P=0.99). There was no difference in the proportion of high LDH between periods (42% vs 40%; P=0.57).
Table I.
Baseline characteristics of the entire cohort and serum LDH groups
| Characteristic | Entire cohort (n=1019) |
Normal serum LDH (n=610) |
High serum LDH (n=409) |
P |
|---|---|---|---|---|
|
| ||||
| Age, years; Median (IQR) | 62 (55–69) | 61 (54–68) | 63 (55–70) | 0.02 |
|
| ||||
| Male sex, N (%) | 633 (62%) | 403 (66%) | 230 (56%) | 0.001 |
|
| ||||
| Systolic blood pressure <100mmHg | 226 (22%) | 121 (20%) | 105 (26%) | 0.02 |
|
| ||||
| Organ involved, N (%) | ||||
| >2 organs | 283 (28%) | 151 (25%) | 132 (32%) | 0.008 |
| Cardiac | 744 (73%) | 405 (66%) | 339 (83%) | <0.001 |
| Renal | 645 (63%) | 348 (57%) | 297 (73%) | <0.001 |
| Hepatic | 217 (21%) | 114 (19%) | 103 (25%) | 0.01 |
| Nerve | 194 (19%) | 137 (22%) | 57 (14%) | <0.001 |
| GI | 168 (16%) | 108 (18%) | 60 (15%) | 0.19 |
|
| ||||
| Lambda restricted, N (%) | 755 (74%) | 480 (79%) | 275 (67%) | <0.001 |
|
| ||||
| 2004 Mayo AL amyloidosis stage, % (n=729) | ||||
| I / II / III | 23 / 37 / 40 | 32/ 44 / 24 | 11 / 29 / 60 | <0.001 |
|
| ||||
| 2012 Mayo AL amyloidosis stage, % (n=787) | ||||
| I / II / III / IV | 25 / 24 / 22 / 29 | 30 / 31 / 21 / 18 | 17 / 15 / 25 / 43 | <0.001 |
|
| ||||
| First line treatment, N (%) | ||||
| ASCT | 406 (40%) | 289 (47%) | 118 (29%) | |
| MDex | 272 (27%) | 120 (20%) | 152 (37%) | |
| MP | 104 (10%) | 69 (11%) | 35 (9%) | |
| Bortezomib-based | 59 (6%) | 31 (5%) | 28 (7%) | <0.001 |
| IMiD-based | 45 (4%) | 30 (5%) | 15 (4%) | |
| Dexamethasone alone | 32 (3%) | 20 (3%) | 12 (3%) | |
| No treatment | 71 (7%) | 18 (3%) | 6 (1%) | |
| Unknown | 24 (2%) | 30 (5%) | 41 (10%) | |
| Other | 5 (<1%) | 3 (<1%) | 2 (<1%) | |
Abbreviations: ASCT, Autologous stem cell transplantation, GI, Gastrointestinal; IMiD, Immunomodulatory drug; IQR, interquartile range; LDH, Lactate dehydrogenase; MDex, melphalan-dexamethasone; MP, melphalan-prednisone
Correlation between LDH and organ involvement pattern
Patients in the high serum LDH group were more likely to have extensive organ involvement (>2 organs) compared to patients in the normal serum LDH group (32% vs 25%; P=0.008). Also, elevated serum LDH patients were more likely to have cardiac (83% vs 66%; P<0.001), renal (73% vs 57%; P<0.001) and hepatic involvement (25% vs 19%; P=0.01) compared to their counterparts. The high LDH group had a lower proportion of nerve involvement (14% vs 22%; P<0.001). In an attempt to better characterize the source of LDH elevation, we analysed the frequency of LDH elevation among patients with single-organ involvement. Three hundred and forty-six patients (n=346) had predominantly single organ involvement. Among patients with cardiac involvement alone (n=150), 36% had a LDH above the ULN. In comparison, 29% of patients with only renal involvement (n=150) had an elevated LDH (P=0.17). Numbers of patients were lower in other organs (n=12 for nerve and n=6 for liver involvement as a single organ), with 17% of patients in each of these organ groups having an elevated LDH.
Correlation of serum LDH and laboratory studies
In an attempt to characterize the source of serum LDH, we correlated laboratory findings with the two study groups. The results are in given in Table II. Parameters associated with the plasma cell compartment (BMPCs, difference between involved and uninvolved light chains [dFLC], labelling index and immunoparesis state) did not demonstrate a correlation with LDH. A correlation with haemoglobin and bilirubin was not found, making haemolysis the cause of LDH elevation unlikely. Given that organ involvement distribution was unbalanced between the LDH groups, we analysed organ function in an organ-stratified fashion. Among patients with cardiac involvement, the cardiac biomarkers N-terminal pro b-type natriuretic peptide (NT-proBNP) and troponin T were significantly higher among patients with high LDH than those with LDH below the ULN (median NT-proBNP 6164 vs 2385 pg/ml; median troponin T 0.08 vs 0.02 ng/ml; P<0.001 for both). Among patients with renal involvement, the high LDH group had a lower estimated glomerular filtration rate (median 53 vs 66 ml/min/1.73 m2; P<0.001) and a higher proteinuria level (median 5.3 vs 4.1 g/24 h collection; P=0.008) compared with their counterparts. Among patients with hepatic amyloidosis, we found no association between LDH groups and serum alkaline phosphatase or total bilirubin levels.
Table II.
Laboratory evaluation in the whole cohort and by serum LDH groups
| Entire cohort (n=1019) |
Normal serum LDH (n=610) |
High serum LDH (n=409) |
P | |
|---|---|---|---|---|
| Plasma cell compartment evaluation | ||||
| BMPCs, median (IQR) | 9 (5–15) | 8 (5–14) | 9 (5–15) | 0.52 |
| dFLC, mg/dl, median (IQR) (n=888) | 23 (9–57) | 22 (8–57) | 24 (10–60) | 0.26 |
| Labelling index (n=668) | 0 (0–0.4) | 0 (0–0.3) | 0(0–0.4) | 0.42 |
| Significant immunoparesis, N (%) (n=928) | 335 (36%) | 212 (38%) | 123 (33%) | 0.11 |
| Organ function evaluation | ||||
| Haemoglobin, g/l median (IQR) | 131 (119–144) | 132 (119–144) | 131 (118–146) | 0.94 |
| Serum albumin, g/l, median (IQR) | 29 (23–33) | 30 (24–34) | 26 (19–32) | <0.001 |
| Thyroid stimulating hormone, u/ml, median (IQR) | 2.8 (1.8–4.5) | 2.6 (1.7–4.2) | 3.1 (2.1–4.7) | <0.001 |
| Cardiac amyloidosis population | ||||
| NT-proBNP, pg/ml, median (IQR) | 3766 (1333–8934) | 2385 (999–5128) | 6164 (2166–12691) | <0.001 |
| Troponin T, ng/ml, median (IQR) | 0.04 (0.01–0.1) | 0.02 (<0.01–0.05) | 0.08 (0.03–0.15) | <0.001 |
| Renal amyloidosis population | ||||
| eGFR, ml/min/1.73 m2, median (IQR) | 60 (41–78) | 66 (49–81) | 53 (30–70) | <0.001 |
| Proteinuria, g/24 h, median (IQR) | 4.8 (1.8–8.2) | 4.1 (1.8–7.4) | 5.3 (2.0–9.4) | 0.008 |
| Hepatic amyloidosis population | ||||
| Alkaline phosphatase, xUNL, median (IQR) | 2.4 (1.7–4.5) | 2.4 (1.6–4.2) | 2.6 (1.7–4.8) | 0.14 |
| Total bilirubin, µmol/l, median (IQR) | 13.7 (8.6–25.7) | 13.7 (8.6–23.9) | 13.7 (8.6–29.1) | 0.71 |
Abbreviations: BMPCs, bone marrow plasma cells; dFLC, difference between involved and uninvolved light chains; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDH, Lactate dehydrogenase; NT-proBNP, N-terminal pro b-type natriuretic peptide; ULN, upper limit of normal;.
Correlation to response to first line treatment
LDH elevation did not correlate with the haematological response to first line treatment. Partial response or better was similar between groups (82% in the normal LDH group and 80% in the high LDH group; P=0.64). There was also no association with depth of response (very good partial response or better 59% vs 61%, respectively; P=0.58). Correspondingly, the LDH groups were not associated with depth of response whether stem cell transplantation was used as primary therapy (P=0.48) or not (P=0.73). Similarly, no difference in organ response rate was seen between the LDH groups (51% vs 49%; P=0.56).
Survival analysis
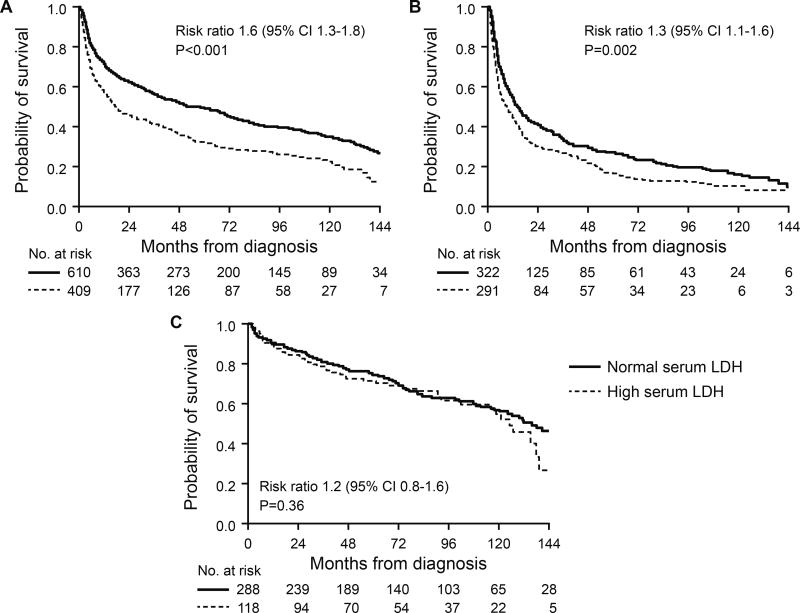
Patients with an elevated LDH had a shorter OS compared to patients with LDH within the normal range (median 18 vs 59 months; P<0.001) (Figure 1). This survival difference was seen among non-ASCT patients (median OS 9 vs 14 months; P=0.002), but not among patients who underwent ASCT as their primary therapy (median 125 vs 135 months; P=0.36). A survival difference was also seen in subgroup analysis by number of involved organs. In patients with single organ involvement, the high serum LDH group had a shorter OS compared to those with normal LDH (median 47 vs 102 months; P<0.001). Similarly, among patients with 2 or more involved organs, survival was shorter in the high LDH group (median 14 vs 31 months; P<0.001). In a multivariate analysis, high serum LDH did not demonstrate an independent impact on OS (P=0.08) (Table III, model A). Since an OS difference was restricted to the non-ASCT cohort, we performed a multivariate analysis limited to non-ASCT patients. In this patient group, high serum LDH was associated with an independent effect on OS with a hazard ratio of 1.3 (95% confidence interval [CI] 1.02–1.7; P=0.03) (Table III, model B). When multivariate analysis of model B was performed using the variables in their continuous form (when applicable), LDH retained its independent association with survival (hazard ratio 1.6 per one unit change; 95% CI 1.2–2.2; P=0.003).
Figure 1.
Overall survival Kaplan-Meier curves by serum LDH groups. A. Whole cohort. B. Non-ASCT cohort C. ASCT cohort.
ASCT, autologous stem cell transplant; CI, confidence interval; LDH, Lactate dehydrogenase;
Table III.
Multivariate analysis for survival for the whole study population and for the non-ASCT cohort
| Model A (Whole cohort) | Model B (non-ASCT cohort) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Prognostic factor | HR | 95% CI | P | HR | 95% CI | P |
|
| ||||||
| Age >65 years | 1.4 | 1.1–1.7 | 0.003 | 1.4 | 1.1–1.8 | 0.002 |
|
| ||||||
| Male sex | 1.3 | 1.1–1.7 | 0.006 | 1.2 | 0.95–1.6 | 0.11 |
|
| ||||||
| >2 involved organs | 1.1 | 0.8–1.3 | 0.66 | 0.9 | 0.7–1.1 | 0.31 |
|
| ||||||
| dFLC ≥18 mg/dl | 1.3 | 1.03–1.6 | 0.02 | 1.2 | 0.95–1.6 | 0.11 |
|
| ||||||
| BMPCs ≥10% | 1.1 | 0.9–1.3 | 0.46 | 1.0 | 0.8–1.3 | 0.98 |
|
| ||||||
| Serum LDH above ULN | 1.2 | 0.97–1.5 | 0.08 | 1.3 | 1.02–1.7 | 0.03 |
|
| ||||||
| eGFR <30 ml/min/1.73 m2 | 0.8 | 0.6–1.1 | 0.25 | 0.8 | 0.5–1.04 | 0.08 |
|
| ||||||
| Proteinuria >2 g/24 h | 0.8 | 0.6–0.98 | 0.03 | 0.8 | 0.6–1.01 | 0.06 |
|
| ||||||
| Troponin T ≥0.025 ng/ml | 1.4 | 1.1–1.9 | 0.008 | 1.6 | 1.2–2.2 | 0.003 |
|
| ||||||
| NT-proBNP ≥1800 pg/ml | 1.9 | 1.4–2.5 | <0.001 | 1.9 | 1.4–2.6 | <0.001 |
|
| ||||||
| Systolic blood pressure <100 mmHg | 1.5 | 1.2–1.9 | <0.001 | 1.4 | 1.1–1.8 | 0.01 |
|
| ||||||
| ASCT as primary therapy (vs non-ASCT regimen) | 0.4 | 0.3–0.5 | <0.001 | Not included | ||
|
| ||||||
| Treatment category | Not included | |||||
| MDex | Reference | |||||
| Bortezomib-based | 0.7 | 0.5–1.1 | 0.12 | |||
| IMiD-based | 1.3 | 0.8–1.9 | 0.27 | |||
| MP/Dex alone | 1.4 | 0.9–2.1 | 0.14 | |||
| No treatment | 5.4 | 3.6–7.9 | <0.001 | |||
Abbreviations: ASCT, autologous stem cell transplant; BMPCs, bone marrow plasma cells; CI, confidence interval; dFLC, difference between involved and uninvolved light chains; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IMiD, Immunomodulatory drug; LDH, Lactate dehydrogenase; MDex, melphalan-dexamethasone; MP, melphalan-prednisone; NT-proBNP, N-terminal pro b-type natriuretic peptide; SBP, systolic blood pressure; ULN, upper limit of normal.
Effect on early death (≤6 months of diagnosis)
Finally, the impact of LDH on the risk of early death (within 6 month of diagnosis) was assessed. Patients with elevated LDH had a greater likelihood of early death compared to patients with normal LDH (32% vs 20%; P<0.001). Sub-analysis by the primary treatment showed this difference to be seen among the non-ASCT cohort (43% vs 32%; P=0.005), but not among ASCT patients (4% vs 7%; P=0.34). A binary logistic regression analysis was performed to identify independent predictors of early death in the whole study cohort and in the non-ASCT cohort. The results of this analysis can be viewed in Table IV. Serum LDH above ULN was associated with an independent increase in early death (odds ratio [OR] 1.9, 95% CI 1.1–3.1; P=0.01). Other predictors for early death in this model were dFLC ≥18 mg/dl (OR=1.7); systolic blood pressure (SBP) <100 mmHg (OR=1.7); NT-proBNP ≥1800 pg/ml (OR=5.1) and troponin T ≥0.025 ng/ml (OR=2.1).
Table IV.
Univariate and multivariate analysis for predictor of early death (≤6 months) in the non-ASCT cohort
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age ≥65 years | 0.8 (0.6–1.1) | 0.11 | 0.9 (0.6–1.4) | 0.65 |
| >2 organs involved | 1.9 (1.4–2.7) | <0.001 | 1.4 (0.9–2.3) | 0.14 |
| SBP <100 mmHg | 3.1 (2.1–4.5) | <0.001 | 1.6 (0.9–2.7) | 0.08 |
| dFLC ≥18 mg/dl | 2.3 (1.5–3.5) | <0.001 | 1.7 (1.0–2.9) | 0.05 |
| NT-proBNP ≥1800 pg/ml | 8.5 (4.4–18.6) | <0.001 | 5.8 (2.6–14.9) | <0.001 |
| Troponin T ≥0.025 ng/ml | 5.3 (3.3–8.8) | <0.001 | 2.0 (1.03–4.0) | 0.03 |
| LDH above ULN | 1.6 (1.2–2.2) | 0.005 | 1.8 (1.1–3.0) | 0.02 |
Abbreviations: ASCT, autologous stem cell transplant; CI, confidence interval; dFLC, difference between involved and uninvolved light chains; eGFR, estimated glomerular filtration rate; LDH, Lactate dehydrogenase; NT-proBNP, N-terminal pro b-type natriuretic peptide; OR, odd ratio; SBP, Systolic blood pressure; ULN, upper limit of normal.
Discussion
AL amyloidosis can be viewed as a two-compartment disease. The first compartment is the clonal plasma cell disorder that resides in the bone marrow and produces the amyloidogenic protein depositing in this disease. The biological characteristics of the plasma cells, such as their percentage in the bone marrow,(Kourelis, et al 2013) their proliferation rate(Gertz, et al 1989, Kumar, et al 2012) and their cytogenetic content(Warsame, et al 2015) have been shown to be prognostic. Cytogenetics also correlates with response to various regimens.(Bochtler, et al 2015, Muchtar, et al 2016b) The second disease compartment in AL amyloidosis is the organ dysfunction caused by the amyloid deposits. These deposits accumulate in organs and tissues throughout the body, leading to damage at both the cellular level and tissue architecture, which lead to organ dysfunction.
No data is available on LDH elevation in AL amyloidosis. In MM, serum LDH is elevated in approximately 12% of patients, is prognostic and used to define high-risk disease.(Gkotzamanidou, et al 2011, Palumbo, et al 2015) This study demonstrated that elevation of LDH above normal occurs in 40% of newly diagnosed AL amyloidosis patients, higher than observed in MM. This prevalence is unexpected in light of the fact that AL amyloidosis is a plasma cell disorder, often with a low clonal cell burden (median 7–10%). The source of LDH does not appear to be from the plasma cell compartment. Our analysis did not show any correlation between serum LDH and various measures of the clonal plasma cell burden, including the percentage of bone marrow plasma cells, the light chain level, the proliferation rate and the presence of significant immunoparesis. In contrast, we have demonstrated that high levels of serum LDH are associated with more extensive disease (>2 involved organs) as well as higher levels of dysfunction of the heart and kidneys. High LDH was also more likely in patients with kappa light chain. While light chain restriction is a feature of the plasma cells, kappa light chain has a tissue tropism for liver and thyroid gland involvement.(Huang, et al 2015, Muchtar, et al 2017b) Serum LDH was not associated with response to therapy. This also suggests the plasma cell compartment is not the source of serum LDH.
The exact source of the elevated LDH in AL amyloidosis cannot be determined from our data and may be widespread given the abundance of LDH in various body tissues. In patients with single organ involvement, a similar rate of elevated LDH was seen between those with heart involvement and those with renal involvement. Therefore, our data do not suggest any predilection of either heart or kidneys. The high LDH group was more likely to have SBP below 100 mmHg, which may contribute to tissue hypoxemia and cellular damage, with no organ specificity. Amyloid deposits, soluble amyloid aggregates and the precursor amyloidogenic proteins, the free light chains, have been implicated as the mediators for tissue injury.(Merlini, et al 2011) Assessment of LDH level in other forms of amyloidosis will enable a better understanding of tissue injury by amyloidosis subtype.
We have shown that LDH was an independent prognostic marker (overall survival and early death alike), but only in the non-ASCT cohort. Non-ASCT patients tend to be more affected from the extent of organ deposition rather than the plasma cell compartment. The non-ASCT patient cohort is usually older, has a poorer performance status and more affected organs and the degree of organ dysfunction is greater.(Muchtar, et al 2017c) Therefore, organ repair may not occur once a haematological response is achieved. It is not uncommon to have a deep haematological response, without impact on organ damage. Therefore, LDH may act as a potential marker for tissue damage and amyloid burden and is more likely to have an impact on patients with advanced organ dysfunction.
This study has several limitations. It is a retrospective single-centre study, which impacts the generalizability of its findings. However, given the rarity of AL amyloidosis, this study provides the first dataset on LDH in AL amyloidosis. We lacked data for several variables in 10–30% of patients, owing to the study period extending back to 2000, a time in which cardiac biomarkers and dFLC were not routinely performed. Disease presentation and management have changed considerably over the years, and this requires caution when interpreting the data. Nevertheless, the multivariate analysis included variables associated with severity of disease as well as type of treatment employed. Finally, our data interpretation and conclusions are based on inference rather than causality. However, we believe that our analysis justifies the conclusions.
In summary, we have demonstrated that serum LDH is elevated in 40% of newly diagnosed AL amyloidosis. The source of elevation is probably tissue damage caused by the amyloid deposits, with no clear organ source. Elevated serum LDH above the normal reference values is an independent prognostic marker in the non-ASCT population, predicting both overall survival and early death. The clinical significance of elevated LDH should be further investigated as a marker for tissue healing and organ recovery. Measuring LDH in non-ASCT candidates is an easy way to assess for a poor prognosis. ASCT may be able to overcome the poor prognosis associated with LDH due to the higher associated haematological responses seen with stem cell transplantation.
Acknowledgments
The study was supported by the Jabbs foundation (Birmingham, UK), the Henry J. Predolin Foundation (USA) and the Specialized Programs of Research Excellence (SPORE), grant CA186781 from the National Cancer Institute, National Institutes of Health, USA.
Footnotes
Disclosure of Conflicts of Interest
Eli Muchtar: None; Angela Dispenzieri received research funding from Celgene, Millennium, Pfizer and Janssen, and travel grant from Pfizer; Martha Q. Lacy received research funding from Celgene; Francis K. Buadi: None; Prashant Kapoor received research funding from Takeda, Celgene and Amgen; Suzanne R. Hayman: None; Wilson Gonsalves: none; Rahma Warsame: None; Taxiarchis V. Kourelis: None; Stephen Russel: None; John A. Lust: None; Yi Lin: None; Ronald S. Go: None; Steven Zeldenrust: None; David Dingli received research funding from Karyopharm Therapeutics, Amgen and Millenium Pharmaceuticals; Nelson Leung: None; S. Vincent Rajkumar: None; Robert A. Kyle: None; Shaji Kumar received consultancy from Celgene, Millennium, Onyx, Janssen and BMS and research funding from Celgene, Millennium, Novartis, Onyx AbbVie, Janssen and BMS; Morie A. Gertz received consultancy from Millenium and honoraria from Celgene, Millenium, Onyx, Novartis, Smith Kline, Prothena, Ionis and Amgen.
Authorship Contributions
E.M. designed the study, collected and analysed the data, wrote the first draft and approved the final version of the manuscript; A.D., M.Q.L., F.K.B., P.K., S.R.H., W.G., R.W., T.V.K., S.R., J.A.L., Y.L., R.S.G., S.Z., D.D., N.L., S.V.R., S.K.K. performed patient management, participated in final data analysis, revised the manuscript critically and approved of the final version of the manuscript. R.C. analysed data, provided critical review of the manuscript and approved the last version of the manuscript; R.A.K. Performed patients’ follow-up, participated in final data analysis, revised the manuscript critically and approved of the final version of the manuscript; M.A.G. performed patient management, designed the study, analysed the data, wrote the first draft and approved the final version of the manuscript.
References
- Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, Kimmich C, Goldschmidt H, Ho AD, Hose D, Jauch A, Schonland SO. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1371–1378. doi: 10.1200/JCO.2014.57.4947. [DOI] [PubMed] [Google Scholar]
- Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, Falk R, Wells K, Solomon A, Wechalekar A, Zonder J, Dispenzieri A, Gertz M, Streicher H, Skinner M, Kyle RA, Merlini G. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26:2317–2325. doi: 10.1038/leu.2012.100. [DOI] [PubMed] [Google Scholar]
- Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Annals of translational medicine. 2016;4:194. doi: 10.21037/atm.2016.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M, Sapio L, Spina A, Naviglio D, Calogero A, Naviglio S. Lactic dehydrogenase and cancer: an overview. Frontiers in bioscience. 2015;20:1234–1249. doi: 10.2741/4368. [DOI] [PubMed] [Google Scholar]
- Gertz MA, Kyle RA, Greipp PR. The plasma cell labeling index: a valuable tool in primary systemic amyloidosis. Blood. 1989;74:1108–1111. [PubMed] [Google Scholar]
- Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. American journal of hematology. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- Gkotzamanidou M, Kastritis E, Gavriatopoulou MR, Nikitas N, Gika D, Mparmparousi D, Matsouka C, Terpos E, Dimopoulos MA. Increased serum lactate dehydrongenase should be included among the variables that define very-high-risk multiple myeloma. Clinical lymphoma, myeloma & leukemia. 2011;11:409–413. doi: 10.1016/j.clml.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Glick JH., Jr Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase values in health and disease, and clinical evaluation of these tests by means of discriminant analysis. American journal of clinical pathology. 1969;52:320–328. doi: 10.1093/ajcp/52.3.320. [DOI] [PubMed] [Google Scholar]
- Green H, Tobar A, Gafter-Gvili A, Leibovici L, Klein T, Rahamimov R, Mor E, Grossman A. Serum Lactate Dehydrogenase is Elevated in Ischemic Acute Tubular Necrosis but Not in Acute Rejection in Kidney Transplant Patients. Progress in transplantation. 2016;27:53–57. doi: 10.1177/1526924816664089. [DOI] [PubMed] [Google Scholar]
- Huang X, Wang Q, Jiang S, Chen W, Zeng C, Liu Z. The clinical features and outcomes of systemic AL amyloidosis: a cohort of 231 Chinese patients. Clinical kidney journal. 2015;8:120–126. doi: 10.1093/ckj/sfu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, Zeldenrust S, Leung N, Kyle RA, Russell S, Dingli D, Lust JA, Lin Y, Kapoor P, Rajkumar SV, McCurdy A, Dispenzieri A. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4319–4324. doi: 10.1200/JCO.2013.50.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1924–1933. doi: 10.1200/JCO.2010.32.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. British journal of haematology. 2012;156:730–743. doi: 10.1111/j.1365-2141.2011.09024.x. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Buadi FK, Dispenzieri A, Gertz MA. Immunoglobulin Light-Chain Amyloidosis: From Basics to New Developments in Diagnosis, Prognosis and Therapy. Acta Haematol. 2016a;135:172–190. doi: 10.1159/000443200. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Dispenzieri A, Kumar SK, Ketterling RP, Dingli D, Lacy MQ, Buadi FK, Hayman SR, Kapoor P, Leung N, Chakraborty R, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Gertz MA. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia. 2016b doi: 10.1038/leu.2016.369. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Dispenzieri A, Kumar SK, Buadi FK, Lacy MQ, Zeldenrust S, Hayman SR, Leung N, Kourelis TV, Gonsalves W, Chakraborty R, Russell S, Dingli D, Lust JA, Lin Y, Kapoor P, Go R, Kyle RA, Rajkumar SV, Gertz MA. Immunoparesis in newly diagnosed AL amyloidosis is a marker for response and survival. Leukemia. 2017a;31:92–99. doi: 10.1038/leu.2016.140. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Dean DS, Dispenzieri A, Dingli D, Buadi FK, Lacy MQ, Hayman SR, Kapoor P, Leung N, Russell S, Lust JA, Lin Y, Warsame R, Gonsalves W, Kourelis TV, Go RS, Chakraborty R, Zeldenrust S, Kyle RA, Rajkumar SV, Kumar SK, Gertz MA. Prevalence and predictors of thyroid functional abnormalities in newly diagnosed AL amyloidosis. J Intern Med. 2017b doi: 10.1111/joim.12617. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Dispenzieri A. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017c;129:2111–2119. doi: 10.1182/blood-2016-11-751628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F, Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S, Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BG, Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, Barni S. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta oncologica. 2015;54:961–970. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- Tabbara IA. Hemolytic anemias. Diagnosis and management. The Medical clinics of North America. 1992;76:649–668. doi: 10.1016/s0025-7125(16)30345-5. [DOI] [PubMed] [Google Scholar]
- Waldemar H, Buchler U, Buchler MW. Classification and severity staging of acute pancreatitis. Annali italiani di chirurgia. 1995;66:171–179. [PubMed] [Google Scholar]
- Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Seminars in radiation oncology. 2004;14:267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Warsame R, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, Leung N, Dingli D, Lust JA, Ketterling RP, Lin Y, Russell S, Hwa L, Kapoor P, Go RS, Zeldenrust SR, Kyle RA, Rajkumar SV, Dispenzieri A. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood cancer journal. 2015;5:e310. doi: 10.1038/bcj.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JN. Prognostic markers in diffuse large B-cell lymphoma: Keys to the underlying biology. Current hematologic malignancy reports. 2007;2:235–241. doi: 10.1007/s11899-007-0032-0. [DOI] [PubMed] [Google Scholar]
- Wulaningsih W, Holmberg L, Garmo H, Malmstrom H, Lambe M, Hammar N, Walldius G, Jungner I, Ng T, Van Hemelrijck M. Serum lactate dehydrogenase and survival following cancer diagnosis. British journal of cancer. 2015;113:1389–1396. doi: 10.1038/bjc.2015.361. [DOI] [PMC free article] [PubMed] [Google Scholar]