Summary
Gene expression noise (heterogeneity) leads to phenotypic diversity among isogenic individual cells. Our current understanding of gene expression noise is mostly limited to transcription, as separating translational noise from transcriptional noise has been challenging. It also remains unclear how translational heterogeneity originates. Using a transcription-normalized reporter system, we discovered that stop codon readthrough is heterogeneous among single cells, and individual cells with higher UGA readthrough grow faster from stationary phase. Our work also revealed that individual cells with lower protein synthesis levels exhibited higher UGA readthrough, which was confirmed with ribosome-targeting antibiotics (e.g., chloramphenicol). Further experiments and mathematical modeling suggest that varied competition between ternary complexes and release factors perturbs the UGA readthrough level. Our results indicate that fluctuations in the concentrations of translational components lead to UGA readthrough heterogeneity among single cells, which enhances phenotypic diversity of the genetically-identical population and facilitates its adaptation to changing environments.
eTOC Blurb
Protein synthesis accuracy is important for cell physiology and has been mostly studied at the population level. Fan et al. develop a dual-reporter system to quantitate protein synthesis errors in single bacterial cells, and show that the slowing protein synthesis increases some translational errors, namely readthrough of stop codons.
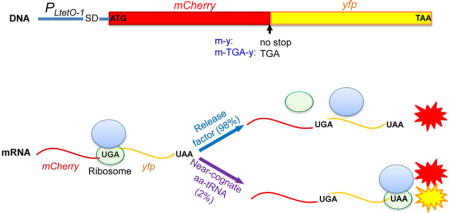
INTRODUCTION
Protein synthesis is a fundamental and essential process in all three domains of life. Accurate protein synthesis requires correct matching of amino acids and transfer RNAs (tRNAs) by aminoacyl-tRNA synthetases, proofreading of aminoacyl-tRNAs (aa-tRNAs) by trans-editing factors, precise decoding of mRNA codons by proper aa-tRNAs, and accurate translocation of mRNA by the ribosome (Fredrick and Noller, 2002; Ling et al., 2009; Liu et al., 2015; Mascarenhas et al., 2008; Zaher and Green, 2009). High translational errors cause growth defects and cell death in bacteria (Bacher et al., 2005; Davis, 1987; Karkhanis et al., 2007), mitochondrial dysfunction in yeast (Reynolds et al., 2010), shortened life span in flies (Lu et al., 2014), and neurodegeneration and cardioproteinopathy in mammals (Lee et al., 2006; Liu et al., 2014). Surprisingly, naturally isolated Escherichia coli strains display a wide range of ribosomal fidelity, suggesting that high translational errors may be favored under some natural habitats (Mikkola and Kurland, 1992). Recent evidence suggests that increased translational errors paradoxically provides benefits to microorganisms under certain stress conditions (Bezerra et al., 2013; Ling et al., 2015; Pan, 2013; Pouplana et al., 2014). For example, amino acid misincorporation in the β subunit of RNA polymerase increases resistance of mycobacteria to rifampicin (Javid et al., 2014; Su et al., 2016), and translational errors improve bacterial tolerance to oxidative stress by activating the general stress response (Fan et al., 2015; Fredriksson et al., 2007). Interestingly, in such cases only subpopulations of genetically-identical cells survive severe stresses (Fan et al., 2015; Su et al., 2016), suggesting that stress response activated by translational errors may be heterogeneous (noisy) in individual cells. However, the noise levels of translational errors have not been determined, and how such heterogeneity originates remains unknown.
Gene expression has been shown to be stochastic and noisy (Balazsi et al., 2011; Kaern et al., 2005; Levine and Hwa, 2007; Levine et al., 2013; Newman et al., 2006; Sanchez and Golding, 2013; Taniguchi et al., 2010; Young et al., 2013). The first experimental evidence came from pioneering work by Elowitz et al., showing that transcription is intrinsically noisy (Elowitz et al., 2002). Later studies revealed that transcription is bursty and noncontinuous (Blake et al., 2003; Levine et al., 2013; Sanchez and Golding, 2013), and the promoter architecture regulates transcriptional noise (Sanchez et al., 2013). Noise in gene expression can be harmful by disrupting regulatory networks, but increasing evidence shows that such noise can also be beneficial by generating phenotypic heterogeneity that helps the population to quickly adapt to environmental changes through bet-hedging (Ackermann, 2015; Blake et al., 2006; Sanchez and Golding, 2013). Compared with transcriptional noise, translational noise is extremely poorly understood. Earlier studies used a single fluorescent reporter to determine the noise levels when translation initiation (Ozbudak et al., 2002) or the codon context (Blake et al., 2003) is varied. It was suggested that in Bacillus subtilis, increasing the efficiency of translation initiation enhances translational noise (Ozbudak et al., 2002). However, it remains a significant challenge to separate translational noise from transcriptional noise (Paulsson, 2004). Here we have developed transcription-normalized dual-fluorescence reporters to quantitate the noise levels of stop codon readthrough and investigate the sources of such noise. Our work suggests that reduced translation promotes UGA readthrough by altering competition between the ternary complex (TC) and release factors, and provides a model for the heterogeneity of UGA readthrough among single cells. We also show that increased UGA readthrough promotes growth of E. coli cells from stationary phase, indicating that UGA readthrough heterogeneity provides an advantage to the population during environmental shifts.
RESULTS
Dual-fluorescence Reporters to Quantitate Translational Errors
To separate the noise of translational errors from transcriptional noise, we have constructed a series of mCherry-YFP (red and yellow fluorescent proteins) fusion reporters that measure the errors of stop-codon readthrough and frameshifting (Figure 1). The mCherry and yfp genes are transcribed as a single mRNA and translated from the same start codon to yield a single polypeptide (Figure 1A), therefore minimizing the influence of the noise from transcription and translation initiation in single-cell analysis. Additionally, both mCherry and YFP are stable in E. coli (Figures S1A and S1B), and degradation of the reporter proteins should produce a minimal level of noise. Expression of the dual-fluorescence reporter on a low-copy number plasmid does not affect bacterial growth (Figure S1C). With these reporters, 0.2–3% error rates were detected in wild-type (WT) E. coli MG1655 grown in Luria-Bertani broth (LB) at 37 °C using fluorescenc e spectrometry (Figures 1B and S1D). Such error rates were orders of magnitude higher than DNA mutational and transcriptional error rates (Gordon et al., 2015; Rosenberg et al., 2012), suggesting that the observed errors directly result from translation. In support of this notion, a ribosomal ambiguity mutation (rpsD I199N or rpsD*), which affects the ribosomal decoding center to reduce translational fidelity (Bjorkman et al., 1999; Fan et al., 2015; Kramer and Farabaugh, 2007), increased all four types of errors that we tested (Figure 1B).
Figure 1. Dual-fluorescent Reporters for Translational Errors.
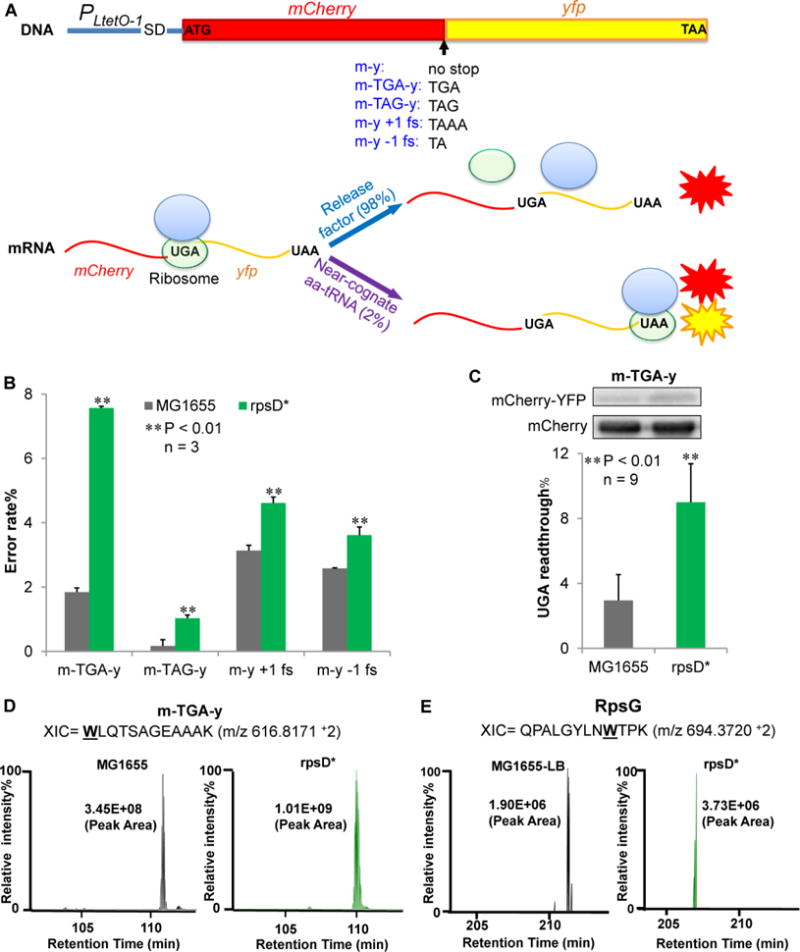
(A) Dual-fluorescence reporters that measure translational errors. MCherry (m) and yfp (y) genes are fused under the control of a constitutive promoter PLtetO-1. At the end of mCherry is a stop codon, a frameshifting (fs) codon, or no stop codon. Readthrough of the stop codon or frameshifting produces a single mCherry-YFP fusion protein and yields YFP signal. (B) The reporters on a low-copy number plasmid were expressed for 24 hours in Luria-Bertani broth (LB) at 37 °C in wild-type (MG1655) and ribosomal error-prone (rpsD*) E. coli strains. Fluorescence was quantified by spectrometry on a plate reader. The error rate was calculated as the YFP/mCherry ratio of the error reporter normalized by the YFP/mCherry ratio of the m-y control. (C) Western blotting showing that UGA readthrough of the m-TGA-y reporter. Anti-mCherry antibody was used to detect both mCherry-YFP fusion and mCherry. Data are represented as mean ± standard deviation. (D, E) Label-free quantitative mass spectrometry analysis of UGA readthrough in the dual-fluorescence reporter protein (m-TGA-y) and native protein RpsG. Extracted ion chromatograms (XIC) for the m-TGA-y reporter peptide WLQTSAGEAAAK (W, or tryptophan, marks the UGA readthrough site in the peptide) and the RpsG peptide QPALGYLNWTPK are shown from two different strains. XIC peak area indicates the relative abundance of the detected peptide. Relative peak intensities were fixed at 100% individually for comparative purposes.
To validate that the fluorescence of the reporters reflects the actual protein level, we used Western blotting to detect and quantitate mCherry and mCherry-YFP fusion protein (Figure 1C). In WT cells expressing the m-TGA-y reporter, the mCherry-YFP fusion accounted for 2.5% of total mCherry proteins produced. This was in good agreement with the 2% UGA readthrough level determined by fluorescence spectrometry (Figure 1B) and consistent with previous reports (Devaraj et al., 2009; Eggertsson and Söll, 1988). The fraction of mCherry-YFP in the rpsD* strain increased to 8%, which was again consistent with the UGA readthrough level determined by fluorescence (Figure 1B).
To identify the amino acid(s) incorporated at UGA sites as well as to detect UGA readthrough events in the native proteome, we further applied quantitative mass spectrometry of WT and rpsD* cells with and without the m-TGA-y reporter (Table S1, Figures 1D, 1E and S2). UGA readthrough in the m-TGA-y reporter and native proteins were indeed detected with liquid chromatography tandem mass spectrometry (LC MS/MS). Our unbiased MS/MS search shows that tryptophan (W) is the predominant amino acid that suppresses UGA codons in E. coli. The level of the peptide resulting from UGA readthrough in the m-TGA-y reporter was increased three-fold in the rpsD* strain compared to the WT (Figure 1D), in line with the fluorescence and Western blotting results (Figures 1B and 1C). These results demonstrate that our dual-fluorescence reporter system is robust for quantitation of translational errors.
UGA Readthrough Is Heterogeneous among Single Cells
We next applied our dual-fluorescence reporters to determine the heterogeneity of stop codon readthrough among genetically-identical single cells grown under the same condition. Using fluorescence microscopy, we visualized mCherry and YFP signals in single cells (Figures 2 and S1E). Whereas the YFP signal of the m-TGA-y reporter was substantially higher than background, cells without reporters showed no detectable fluorescence signal (Figure S1E), suggesting that the influence of auto fluorescence on signal quantification is negligible under our experimental setting. Our results showed that the control m-y reporter exhibited tight linear correlation between the YFP and mCherry signals, and the YFP/mCherry ratio was mostly homogeneous (Figures 2A, 2C and S3A). In contrast, the YFP/mCherry ratio of the m-TGA-y reporter (indicating UGA readthrough level) was more dispersed (Figures 2B, 2D and S3B), suggesting that the concentration of UGA readthrough products was heterogeneous among single cells.
Figure 2. UGA Readthrough Is Heterogeneous among Single Cells.
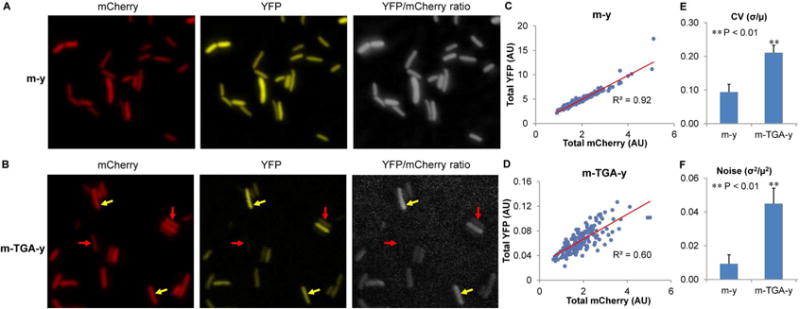
(A, B) YFP and mCherry fluorescence in MG1655 cells carrying either the m-y or m-TGA-y reporter. Cells were grown in LB for 24 h. The YFP/mCherry ratio is the relative YFP signal normalized by the mCherry signal. Cells with high and low UGA readthrough are indicated by yellow and red arrows, respectively. (C, D) In the scatter plots, each dot represents a single cell. The fluorescence is background-subtracted and arbitrary units are shown. (E, F) Heterogeneity of UGA readthrough among single cells is indicated by CV and noise of the YFP/mCherry ratio. μ, the mean of the YFP/mCherry ratio; σ, standard deviation. Data are represented as mean ± standard deviation. AU, arbitrary units.
Heterogeneity of gene expression is quantified with coefficient of variation (CV), calculated as the ratio of the standard deviation (σ) over the mean (μ), or noise (σ2/μ2) (Blake et al., 2003; Elowitz et al., 2002; Taniguchi et al., 2010). Our results revealed that the CV of YFP/mCherry ratio in cells with the control m-y reporter was approximately 0.1 (Figure 2E). Such a low level of heterogeneity may be caused by noise from fluorophore maturation, partial degradation of mRNA and protein, ribosomal drop-off during translation, missense errors, and the imaging system. The CV of YFP/mCherry ratio in cells with the m-TGA-y reporter increased to 0.2, corresponding to a four-fold increase in noise (Figures 2E and 2F). It has been suggested that when the copy number of protein molecules is low (<1,000/cell), partitioning errors and finite-number effect would contribute to the overall protein noise, whereby reducing the fluorescence mean would increase the CV and noise (Huh and Paulsson, 2011; Kaern et al., 2005). However, the estimated concentration of mCherry-YFP fusion protein in cells with the m-TGA-y reporter is about 6,000 molecules per cell (see methods). Therefore, partitioning noise and finite-number effect is expected to have little contribution to the observed CV of the YFP/mCherry ratio. In support of this argument, we found that reducing the mean of mCherry by decreasing the promoter strength of the m-TGA-y reporter did not further increase the CV (Figure S3C). We thus reason that the higher CV and noise of the YFP/mCherry ratio in m-TGA-y compared to m-y is largely due to the heterogeneity of UGA readthrough events among individual cells.
Reduced Protein Synthesis Increases UGA Readthrough
To understand how the heterogeneity of UGA readthrough arises, we analyzed the correlation between UGA readthrough (indicated by the YFP/mCherry ratio) and various cell parameters (Figures 3 and S3). We found that cells with lower mCherry expression levels exhibited higher UGA readthrough (Figures 3A and 3B). To test whether high UGA readthrough reduces mCherry expression or reduced protein expression increases UGA readthrough, we first used a UGA suppressor tRNASer variant with a UCA anticodon ( ) (Figures S3G and S3H). The suppressor tRNA substantially increased UGA readthrough, but did not affect the mCherry protein level. We next tested whether reducing protein expression would enhance UGA readthrough. A low mCherry level may result from reduced transcription or translation. We demonstrated that reducing transcription with rifampicin (Rif), which inhibits RNA polymerase, did not significantly affect UGA readthrough (Figure S3I). In contrast, treating E. coli with low concentrations of chloramphenicol (Chl), which binds to the peptidyl transferase center (PTC) of the ribosome and impedes binding of aa-tRNAs and release factors (Wilson, 2014), reduced protein synthesis and increased UGA readthrough, as determined by fluorescence spectrometry and Western blotting (Figures 3C and 3D). To verify that the observed increase in UGA readthrough by Chl was not an artifact due to the sequence context of the dual-fluorescence reporter, we tested UGA readthrough at a native chromosomal site of the cspC gene (Figure 3E), and confirmed that Chl also significantly enhanced readthrough of the native UGA codon (Figures 3E and 3F). To determine whether increased UGA readthrough is a specific effect of Chl, we further tested other ribosomal inhibitors tetracycline (Tet, inhibiting aa-tRNA delivery to the ribosome), spectinomycin (Spc, inhibiting translocation), and erythromycin (Ery, blocking the 50S exit tunnel). Tet, Spc and Ery also significantly enhanced UGA readthrough as Chl (Figure S4A).
Figure 3. Reducing Protein Synthesis Increases the Level of UGA Readthrough.
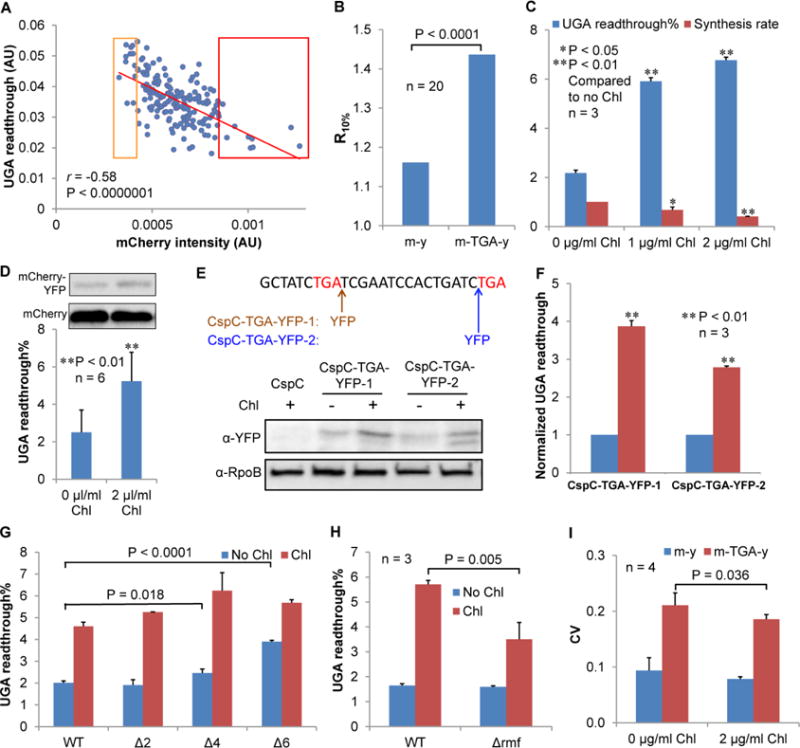
(A) In single MG1655 cells, higher UGA readthrough correlates (Spearman’s Rank correlation) with lower mCherry protein level. The mCherry intensity is the mCherry signal normalized by cell volume with arbitrary units. Relative UGA readthrough is calculated from the YFP/mCherry ratio. Experimental conditions were the same as in Figure 2. (B) The Y-axis (R10%) is the ratio of the average UGA readthrough in the bottom 10% of cells divided by the average UGA readthrough in the top 10% of cells ranked by mCherry intensity from panel A. (C) MG1655 cells were grown in LB with and without Chl for 24 h, and UGA readthrough and protein synthesis rates were determined by fluorescence spectrometry. Treating MG1655 with low doses of Chl decreases protein synthesis rate and enhances UGA readthrough. (D) Western blotting confirms that Chl increases UGA readthrough of the m-TGA-y reporter. (E, F) UGA readthrough of chromosomally-encoded CspC increases in the presence of chloramphenicol. The nucleotide sequence of the 3′-end of the chromosomal constitutively expressed cspC gene is shown. Red letters indicate the first and second stop codons. An in-frame YFP tag is inserted immediately after the first TGA codon of the native cspC gene (CspC-TGA-YFP-1) or before the second stop codon (CspC-TGA-YFP-2) in MG1655. Western blotting result shows that readthrough of the cspC UGA codon is significantly increased by addition of Chl. (G) Reducing ribosome copy number increases UGA readthrough. WT MG1655 and its ribosomal operon deletion mutants (Δ2, ΔrrnEG; Δ4, ΔrrnGBAD; Δ6, ΔrrnGADBHC) were grown in LB with and without Chl for 24 h, and UGA readthrough levels were determined with fluorescence spectrometry. (H) Deleting rmf partially suppresses the effect of Chl to increase UGA readthrough. (I) Chl treatment decreases the CV of UGA readthrough. Data are represented as mean ± standard deviation. AU, arbitrary units.
The efficiency of stop codon readthrough is a direct result of competition between TC (composed of elongation factor Tu (EF-Tu), aa-tRNA and GTP) and release factors. UGA stop codons are suppressed by Trp-tRNATrp (Table S1, Figures 1D, 1E and S2) and terminated by release factor 2 (RF2) (Engelberg-Kulka, 1981; Youngman et al., 2007). It has been reported that in E. coli K-12 strains (including MG1655), RF2 contains an A246T mutation that decreases termination efficiency (Dincbas-Renqvist et al., 2000; Mora et al., 2007). To test whether reduced protein synthesis specifically increases UGA readthrough in K-12 strains, we examined E. coli BL21 and Salmonella enterica Serovar Typhimurium LT2 strains that both carry wild-type RF2. Like MG1655, both BL21 and S. Typhimurium strains exhibited strong negative correlation between UGA readthrough and mCherry intensity, and increased UGA readthrough upon Chl treatment (Figure S5), demonstrating that reduced protein synthesis enhances UGA readthrough independent of the A246T mutation.
The next question is how reduced translation may increase UGA readthrough errors, which is surprising given previous kinetic studies showing that there is a trade-off between translational speed and accuracy (Johansson et al., 2008). Using acidic gel northern blotting, we show that the overall Trp-tRNATrp level increases in the presence of Chl due to an increase in total tRNATrp (Figure 4A). Further time-course analysis shows that tRNATrp is stable both in the presence and absence of Chl (Figure 4B), indicating that the increased level of tRNATrp with Chl is not caused by increased tRNA stability, but rather by enhanced transcription. We next performed mathematical modeling to understand how reduced translation may increase UGA readthrough (Figures S4B, S4C and Table S2). Our modeling suggests that increasing TC concentration enhances UGA readthrough rate. Based on these experimental and modeling results, we reason that reduced translation increases the effective concentration of EF-Tu:Trp-tRNATrp:GTP complex to more efficiently compete against RF2, thereby promoting UGA readthrough.
Figure 4. Chloramphenicol Increases Trp-tRNATrp Level.
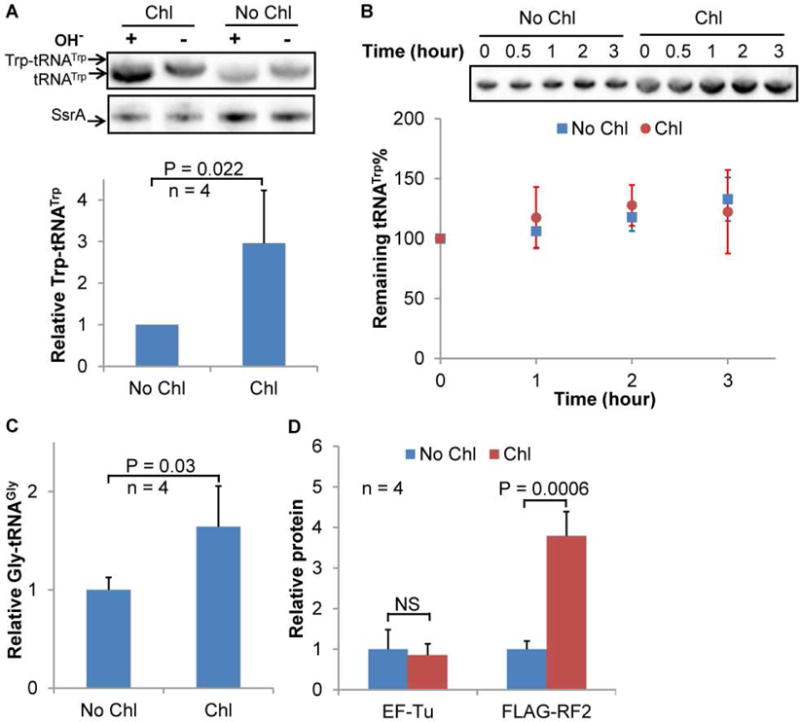
(A) Chl treatment increases the level of Trp-tRNATrp shown by acidic northern blotting. Total RNA was isolated from MG1655 cells grown in LB with or without 2 μg/ml Chl under acidic conditions, and treated with or without alkaline (OH−) before acid gel electrophoresis and northern blotting. Alkaline treatment causes deacylation of aminoacyl-tRNAs. Almost 100% of tRNATrp was aminoacylated without alkaline treatment. SsrA was used as an internal standard to calculate the relative concentration of Trp-tRNATrp. (B) Stability of tRNATrp with and without Chl. MG1655 cells grown in LB in the presence absence of Chl were treated with a high concentration of rifampicin to stop transcription. RNA samples were prepared at indicated time points following addition of Rif and subjected to northern blotting analysis. Chl increases the overall level of tRNATrp, but not the stability. (C) The level of Gly-tRNAGly was determined using acidic gel northern blotting as in Figure 4. (D) The protein levels of EF-Tu and FLAG-RF2 with and without Chl revealed by Western blotting. A FLAG tag is fused to the C-terminus of RF2 at the native chromosomal site. Data are represented as mean ± standard deviation.
A recent study shows that Chl and Tet decrease the active fraction of ribosomes (Dai et al., 2016). To test the effects of active ribosomes on UGA readthrough, we used mutant strains lacking several copies of the rrn operon that encodes ribosomal RNAs (Quan et al., 2015). Deleting 6 out of 7 copies of the rrn operon significantly increased UGA readthrough (Figure 3G), suggesting that reducing the cellular ribosome concentration promotes UGA readthrough. We also tested a strain that lacks RMF (ribosome modulation factor), which promotes 70S ribosomes to form inactive dimers during stationary phase and stress conditions (Polikanov et al., 2012; Yoshida and Wada, 2014). Deleting rmf is expected to increase the concentrations of active ribosomes. Indeed, deleting rmf mitigates the effect of Chl to enhance UGA readthrough (Figure 3H), supporting that Chl increases UGA readthrough by reducing the fraction of active ribosomes. It has been suggested that low codon adaptation of highly-transcribed genes (e.g., our m-TGA-y reporter, expressed at over 300,000 protein molecules per cell) may also sequester ribosomes and reduce the active fraction (Kudla et al., 2009). In contrast, reduced translation initiation of the reporter is not expected to affect the active ribosome concentration. We therefore reduced expression of the reporter proteins by either introducing non-optimal codons to mCherry or decreasing translation initiation efficiency. Non-optimal codons enhanced UGA readthrough as Chl (Figure S4D), but reducing translation initiation efficiency by changing the Shine-Dalgarno sequence did not alter the UGA readthrough level (Figure S4E). These results are consistent with the notion that reducing the concentration of active ribosomes promotes UGA readthrough.
Next, we investigated how reduced translation affects the heterogeneity of UGA readthrough. Both Chl and codon non-optimization decreases the CV of YFP/mCherry ratio (Figures 3I and S4F), suggesting that UGA readthrough heterogeneity is reduced when translation is attenuated across the population. Collectively, these results suggest that reduced translation enhances the level of UGA readthrough, and various levels of protein synthesis among single cells contribute to heterogeneous UGA readthrough in a bacterial population.
Defective Translation Termination Increases UGA Readthrough Heterogeneity
We next tested how RF2 fluctuations affect UGA readthrough. In E. coli, the coding region of the prfB gene (encoding RF2) contains a TGAC frameshifting codon, which autoregulates the production of RF2 (Curran, 1993; Curran and Yarus, 1988). A high level of RF2 decreases the frameshifting efficiency at the TGAC site by promoting early termination, therefore reducing RF2 translation. To test how RF2 autoregulates UGA readthrough heterogeneity, we used a genome engineering tool (Wang et al., 2009) to change the chromosomal site of TGAC in prfB to TAGC. RF2 does not terminate translation at UAG codons. Therefore, the TAGC mutation in prfB is expected to abolish autoregulation of RF2 production and increase fluctuations of RF2 protein levels among single cells. Surprisingly, the TAGC mutation did not significantly alter the level or CV of UGA readthrough (Figure S6), suggesting that the RF2 activity was possibly close to saturation for translation termination at UGA codons in the WT cells. In line with this, overexpressing RF2 in WT cells did not further decrease the level of UGA readthrough (Figures 5A and 5B). However, when prfC (encoding RF3 that facilitates RF2 during termination) was deleted, overexpressing RF2 decreased the level of UGA readthrough, suggesting that the RF2 activity was no longer saturating in the absence of RF3. Consequently, deleting prfC increased both the level and heterogeneity of UGA readthrough (Figures 5C and 5D). These results suggest that RF2 fluctuations do not significantly contribute to UGA readthrough heterogeneity in WT E. coli cells, but defects in translation termination enhance the sensitivity of UGA readthrough to RF2 fluctuations among single cells, thereby increasing readthrough heterogeneity.
Figure 5. Defective Termination Increases UGA Readthrough Heterogeneity.
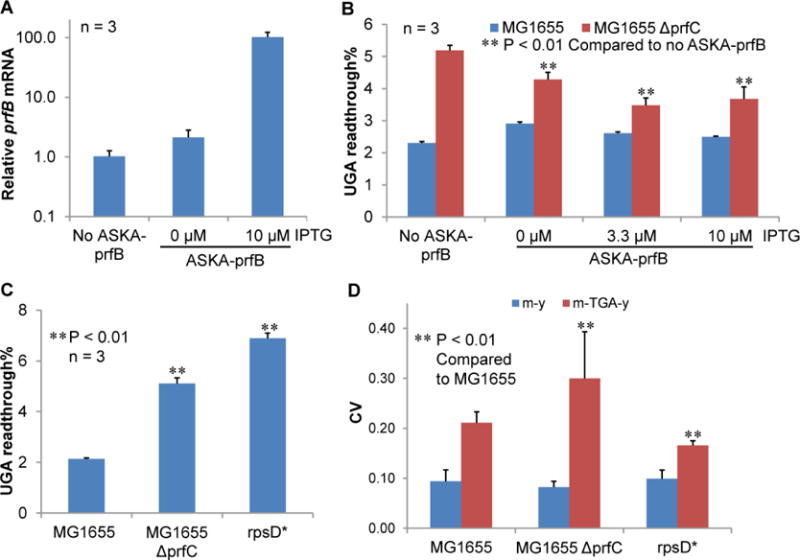
(A) ASKA-prfB (Kitagawa et al., 2005) leads to overproduction of prfB mRNA as determined with quantitative reverse transcriptase PCR. In the absence of isopropyl β-D-1-thiogalactopyranoside (IPTG), the promoter of the ASKA plasmid is leaky (Kitagawa et al., 2005). (B) The m-TGA-y reporter was tested in strains with and without the RF2 overexpression plasmid. In MG1655, increasing the RF2 level does not further decrease UGA readthrough, suggesting that the RF2 activity is close to saturation. In contrast, excess levels of RF2 decrease UGA readthrough in the RF3 (encoded by prfC) deleting strain, indicating increased sensitivity of UGA readthrough to RF2 fluctuations when the release factor activity is compromised. The ΔprfC result also suggests the RF2 protein is successfully overproduced from the plasmid. (C) Deleting RF3 and introducing the rpsD* mutation both increases UGA readthrough of the m-TGA-y reporter. (D) RF3 deletion increases the CV of UGA readthrough. In contrast, the rpsD* mutation decreases the CV. In addition to recycling release factors, RF3 also maintains fidelity during translation elongation (Zaher and Green, 2011). The rpsD* result suggests that increased UGA readthrough heterogeneity upon RF3 deletion is caused by defective termination rather than reduced fidelity during elongation. Data are represented as mean ± standard deviation.
UGA Readthrough Promotes Cell Growth from Stationary Phase
Altering translational fidelity results in various phenotypic changes (Drummond and Wilke, 2009; Pan, 2013). To provide insights into how UGA readthrough heterogeneity affects bacterial physiology, we used time-lapse fluorescence microscopy to monitor division of individual cells with various levels of UGA readthrough. Stationary-phase WT cells carrying the m-TGA-y reporter were tested on agar pad with minimal glucose medium in the chamber of an automated fluorescence microscope. We found that cells with high levels of UGA readthrough required a shorter time to reach the first division than cells with low UGA readthrough levels (Figure 6A). To test whether increased UGA readthrough is sufficient to cause faster growth from stationary phase, we expressed the WT and suppressor tRNASer in MG1655. The suppressor tRNA indeed improved regrowth of stationary-phase cells in minimal media with various carbon source (Figure 6B, 6C and S7A). However, growth in rich media LB was not affected by the suppressor tRNA (Figure S7A), suggesting that the growth advantage provided by UGA readthrough is manifested under poor-nutrient conditions. In addition, regrowth of log-phase cells was not much affected by the suppressor tRNA even in minimal media (Figure S7B). Collectively, these results suggest that heterogeneous UGA readthrough allows a subpopulation of cells to grow faster from the stationary phase, thereby improving the overall fitness of the bacterial population.
Figure 6. UGA Readthrough Promotes Cell Growth from Stationary Phase.
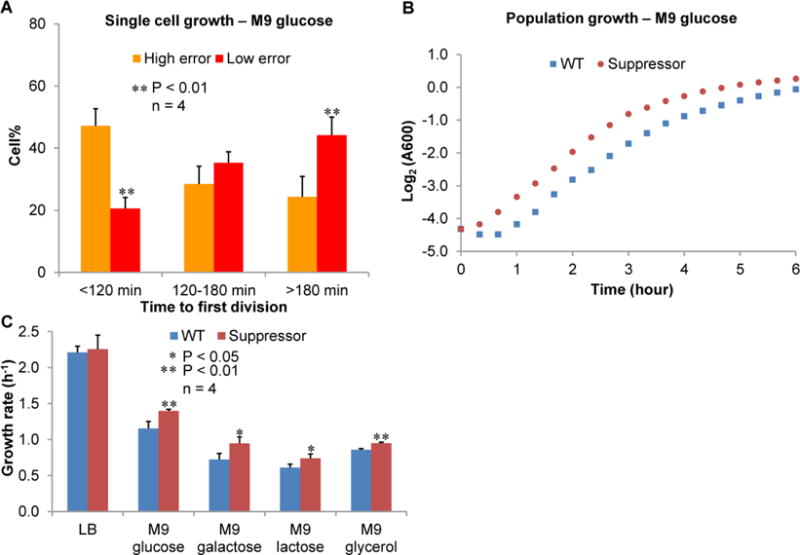
(A) Percentage of cells with high and low error levels that require different time periods to reach the first cell division. Stationary-phase MG1655 cells were placed on M9 glucose agar pad and monitored for fluorescence and growth. High and low error cells are the top and bottom quartiles of individual cells ranked by the YFP/mCherry ratio of the m-TGA-y reporter, respectively. A significantly higher percentage of cells with high UGA readthrough levels divide within 120 min compared with cells with low UGA readthrough levels. (B) Stationary-phase cultures of MG1655 carrying the WT or UGA suppressor tRNASer were diluted 50-fold, and growth was monitored over time. (C) UGA suppressor tRNASer improves growth in minimal media with various carbon sources (1% each), but not in LB. Data are represented as mean ± standard deviation.
DISCUSSION
Noise in gene expression has been extensively studied at the transcriptional level, but the levels and sources of noise during translation are poorly understood. Here we have developed a dual-fluorescence reporter system to determine the noise of stop codon readthrough with minimal influence from noise produced during transcription, translation initiation, and protein degradation. Such reporters will be broadly useful to determine the levels and heterogeneity of translational errors in single cells in their native environments, e.g., within biofilms and during host-microbe interactions. We have further provided in-depth analyses of the regulation of UGA readthrough noise. The sources of gene expression noise include intrinsic sources that result from stochastic transcription and translation of individual genes, and extrinsic sources caused by fluctuations of global resources among single cells (Ackermann, 2015; Blake et al., 2003; Elowitz et al., 2002). The heterogeneity of UGA readthrough over long periods of growth under our experimental conditions is likely dominated by extrinsic sources of noise, such as fluctuations of the concentrations of nutrients and translational components among individual cells. For example, amino acid levels may reduce or deplete in some cells after 24 h of growth in LB. UGA readthrough results from occasional recognition of the stop codon by the EF-Tu:Trp-tRNATrp:GTP ternary complex, which competes with RF2 that releases the elongating peptide (Figure 7). Fluctuations of TC and RF2 among single cells would lead to heterogeneity of UGA readthrough and increase its noise. We have shown that reduced translation increases the level of UGA readthrough and Trp-tRNATrp (Figures 3, 4, S4 and S5). Because tRNATrp is stable (Figure 4B), the increase in tRNATrp in the presence of Chl is likely due to enhanced transcription. In addition to tRNATrp, the level of tRNAGly is also increased by Chl (Figure 4C). It is possible that some unknown protein factor is involved in repressing the tRNA promoters, and Chl reduces synthesis of the repressor to upregulate tRNA transcription. We also show that the protein level of EF-Tu is not significantly affected by Chl (Figure 4D). Given that EF-Tu is very abundant and in 6-fold excess relative to the ribosome (Dai et al., 2016), an increase in Trp-tRNATrp is expected to drive the formation of TC. Another factor that may affect UGA readthrough is the RF2 protein level. We show that the FLAG-tagged RF2 level increases in the presence of Chl (Figure 4D), which is not expected to enhance UGA readthrough. Our data thus suggest that reduced protein synthesis promotes UGA readthrough by increasing the effective concentration of TC.
Figure 7. Model for UGA Readthrough Heterogeneity.
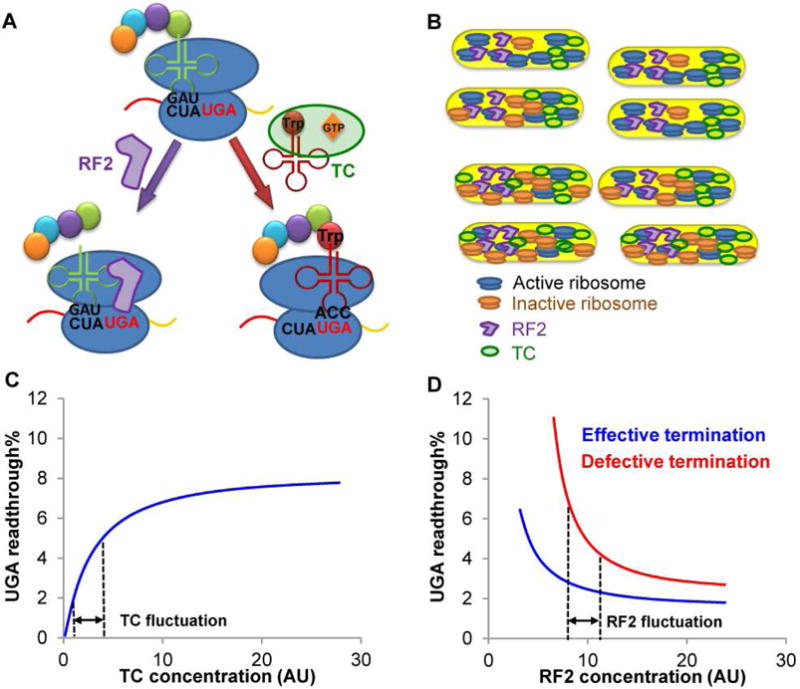
(A) The near-cognate Trp-tRNATrp forms a ternary complex (TC) with EF-Tu and GTP to compete with RF2 for the UGA stop codon. Recognition of UGA by RF2 leads to release of the growing peptide from the ribosome, whereas Trp-tRNATrp suppresses UGA by adding Trp to the growing peptide. The UGA readthrough level in a single cell is determined by the effective concentrations of TC and RF2. (B) The concentration of active ribosomes may vary among single cells due to fluctuations of global resources and ribosome maturation/inactivation. A low concentration of active ribosomes decreases global protein synthesis, which increases the effective concentration of TC due to its reduced usage during translation. (C) UGA readthrough is sensitive to TC fluctuations. An increase in effective TC leads to enhanced UGA readthrough. (D) WT cells with effective translation termination exhibit nearly saturated RF2 activity, and UGA readthrough is insensitive to RF2 fluctuations. Defects in RF2 activity, e.g., due to RF3 deletion, enhance the sensitivity of UGA readthrough to RF2 fluctuations and thus increase UGA readthrough heterogeneity. Dashed lines indicate arbitrary ranges of concentrations. AU, arbitrary units.
Recently, Hwa and coworkers have shown that some ribosome-targeting antibiotics, including Chl and Tet, reduce the active fraction of ribosomes rather than decreasing translation elongation rates (Dai et al., 2016). Our results suggest that reducing the concentration of active ribosomes promotes UGA readthrough (Figures 3, 4, and S5). Several lines of evidence indicate that protein synthesis is intrinsically heterogeneous: (a) the mCherry protein level is highly heterogeneous among single cells even with the chromosomal reporters (Figure S3A), indicating that overall protein production is heterogeneous; (b) the components of the protein synthesis machinery, including ribosomal proteins and elongation factors, vary among single cells at the protein level in E. coli (Taniguchi et al., 2010); (c) translation initiation has been shown to be noisy in bacteria (Ozbudak et al., 2002); and (d) ribosome hibernation alters gene expression heterogeneity (Guido et al., 2007). Collectively, such evidence suggests that the concentration of active ribosomes may vary among genetically-identical single cells due to heterogeneity during expression of ribosomal proteins and rRNAs, ribosome maturation and inactivation. This would exemplify fluctuations of TC and contribute to the heterogeneity of stop codon readthrough (Figure 7).
Our proteomic analysis identified Trp to be the major amino acid that suppresses UGA in E. coli, which is in agreement with previous studies (Engelberg-Kulka, 1981). E. coli tRNATrp contains a modification at A37 that facilitates C-A pairing at the wobble position (Vacher et al., 1984), which may explain why tRNATrp suppresses UGA better than other near-cognate tRNAs such as tRNACys. The suppression efficiency of UAG appears to be lower than UGA, presumably because of the stronger termination activity of RF1. Indeed, in an RF1 deletion strain, multiple amino acids have been detected to suppress UAG (Aerni et al., 2015).
In E. coli, UGA is used by approximately 30% of the protein-coding genes as the stop codon. Readthrough of stop codons may cause misfolded protein stress or production of protein isoforms with new functions (Dunn et al., 2013; True and Lindquist, 2000). For example, sequences following stop codon readthrough may contain novel localization signals (Dunn et al., 2013). In this study, we show that increased UGA readthrough in E. coli enhances growth under poor-nutrient conditions and promotes growth from stationary phase (Figures 6B, 6C, and S7A). The underlying mechanism remains to be elucidated. A pathway enrichment analysis suggests that genes with UGA as stop codons are most significantly enriched in ABC transporters (Table S3), which are involved in nutrient uptake (Hollenstein et al., 2007; Jones and George, 2004). It is possible that UGA readthrough may alter the function of such transporters and provide growth advantage when nutrients are limited. In line with this notion, UGA suppression does not enhance growth in rich media (Figure S7A). Such advantage of high UGA readthrough on cell growth is also observed in single cells (Figure 6A). Heterogeneous UGA readthrough among single cells may thus provide benefits to the microbial population by enhancing phenotypic diversity and facilitating adaptation to ever-changing environments.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to, and will be fulfilled by the lead contact Jiqiang Ling (Jiqiang.Ling@uth.tmc.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial Strains, Plasmids and Growth Conditions
All the bacterial strains and main plasmids used in this study are listed in the Key Resources Table. All the oligos for making the mutant strains and plasmids are listed in Table S4. The mutant strains are derivatives of E. coli K-12 MG1655 (WT). To construct MG1655 CspC-TGA-YFP-1, MG1655 CspC-TGA-YFP-2, MG1655 attB∷Ptet-m-y, MG1655 attB∷Ptet-m-TGA-y, and MG1655 prfB∷3×FLAG strains, a cassette (kan-ccdB) containing the toxin encoding gene ccdB under the control of araBAD promoter and a kanamycin resistance gene was amplified from template genomic DNA of CR201 strain (obtained from N. De Lay, UTHealth), and introduced into the specific sites on the chromosome of the parental strain harboring plasmid pSIM6 by λ red recombinase-mediated gene replacement (Datta et al., 2006). Strains containing pSIM6 were induced for Red expression by growth at 42 °C for 15, and electroporated with PCR fragments containing the kan-ccdB cassette. 1 ml LB was then added and cells were incubated at 32 °C for 2 h. The successful transformants were selected by kanamycin resistance. The kan-ccdB cassette was then replaced with respective DNA fragments. The positive clones were selected by growth on 0.5% arabinose LB plate, and verified by colony polymerase chain reaction (PCR). The MG1655 prfB TAGC strain was generated with a modified multiplex automated genome engineering (MAGE) method (Wang et al., 2009). Briefly, prfB-MAGE-TAGC oligo was electroporated into the MG1655 strain carrying plasmid pKD46, and cells were plated onto LB plates after 5 h incubation at 32 °C. Colony PCR was applied with primer pairs prfB-TAGC-MT-F and prfB-TAGC-R0 to select for the positive clones. All mutant strains were verified by sequencing.
To construct plasmids pZS-Ptet-m-y, pZS-Ptet-m-TGA-y, pZS-Ptet-m-TAG-y, pZS-Ptet-m-y +1 fs, and pZS-Ptet-m-y −1 fs, the pZS- Ptet-29AA-y plasmid was generated first by ligating a 29 amino acid (LQTSAGEAAAKEAAAKEAAAKEAAAKAAA) linker with plasmid pZS*11-yfp13 using In-Fusion HD Cloning according to manufacturer’s protocol. Fragments amplified from optimized mCherry gBlock by primer pZS-mCherry-29AA-IF paired with pZS-mCherry-29AA-IR, pZS-mCherryTGA-29AA-IR, pZS-mCherryTAA-29AA-IR, pZS-mCherryTAG-29AA-IR, pZS-mCherryTAAA-29AA-IR, and pZS-mCherryTA-29AA-IR, respectively, were ligated into the pZS- Ptet-29AA-Y plasmid through In-Fusion HD Cloning.
Unless otherwise noted, E. coli strains were grown in LB at 37 °C. For fluorescence microscopy analysis, overnight cultures were diluted 1: 1,000 and grown for 24 h. 100 μg/ml Chl was added to immediately stop protein synthesis. After 4 h incubation to allow full maturation of mCherry and YFP, samples were subjected to fluorescence microscopy analysis. The minimal medium contains 47.8 mM Na2PO4, 22.0 mM KH2PO4, 8.6 mM NaCl, 18.7 mM NH4Cl, 4 mM MgSO4, 0.2 mM CaCl2, 40 μg/ml each of the 20 amino acids, and 0.4–1% glucose or indicated sugar.
METHOD DETAILS
Fluorescence Microscopy
Samples were placed on a 2 μl agarose (1.5%) phosphate buffer pad on 15-well Multitest Slides (MP Biomedicals, LLC.). Images were obtained on an Olympus IX81-ZDC inverted microscope using Slidebook imaging software. Background fluorescence was subtracted from each image using ImageJ Background Subtraction with a 50.0 pixel rolling ball radius. Single-cell fluorescence quantitation was completed with MicrobeTracker (Sliusarenko et al., 2011), a MATLAB-based software package. Single-cell fluorescence data were exported from MATLAB and all further data analysis was completed in Microsoft Excel.
Time-lapse Microscopy
Overnight cultures were diluted 1:1,000 in LB and grown for 24 h at 37 °C. Cultures were placed on a 200 μl 1.5% agarose LB pad. Fluorescent images were taken at the initial time point for quantitation. Cells were followed for 150 min at room temperature with DIC images taken at regularly spaced intervals throughout the experiment. Image analysis and editing were performed using ImageJ.
Rate Determination
E. coli cultures were incubated at 37 °C in a microplate re ader (Synergy HT, BioTek) using 96-well black side plates (Corning). The signals of mCherry, YFP, and A600 were measured every 20 minutes with fluorescence spectrometry. To calculate translational error rates, the YFP/mCherry ratio of m-TGA-y, m-TAG-y, m-y +1 fs, and m-y −1 fs was normalized by the YFP/mCherry ratio of the control m-y reporter. Protein synthesis rates were calculated as described (Subramaniam et al., 2013) with the following formula: .
To calculate translational error rates by Western blotting, E. coli strains were grown in LB medium with and without addition of chloramphenicol at 37 °C for 24 h before 10 ml of culture was harvested. The cells were lysed by sonication, and whole protein samples were analyzed by Western blotting with a primary antibody against mCherry. The signals were qualified by Image Lab (Bio-Rad), and the error rates were calculated as the percentage of mCherry-YFP fusion protein.
Calculation of CV and Noise
CV was calculated as the standard deviation (σ) divided by the mean (μ) of the YFP/mCherry ratio of each cell in the same microscopic image frame. Noise was calculated as the ratio of the variance (σ2) over the square of the mean (μ2).
Detection of Native UGA Readthrough in cspC
The readthrough level of cspC UGA was determined by Western blotting. E. coli strains were grown in LB with and without addition of Chl (2 μg/ml) at 37 °C for 24 h. 10 ml of cell culture were harvested and lysed by sonication, and total proteins were separated by SDS-PAGE. Antibodies against GFP (Roche) and RpoB (loading control, Santa Cruz) were used in Western blotting analysis. The signals were qualified by Image Lab (Bio-Rad).
RNA Isolation
To prepare total RNA for qRT-PCR, cells in LB medium with or without 10 μM IPTG were grown to midlog phase (OD600~0.6–0.8). 800 μl of culture was used for total RNA extraction using hot phenol, and residual chromosomal DNA was removed.
To isolate total RNA for acidic gel northern blotting, overnight cultures were diluted 1: 1,000 in LB and grown for 14 h at 37 °C. 10 ml of cell culture was harvested at 4 °C and resuspend with TRIzol reagent immediately. Cells were lysed by the beads beater with 0.1 mm glass beads (RPI). RNA sample was prepared according to manufacturer’s protocol, and was stored in 10 mM sodium acetate buffer (pH 5.0) with 1 mM ethylenediaminetetraacetic acid (EDTA) at −80 °C to preserve aminoacyl-tRNAs. Deacylated samples were obtained by incubating RNA in 200 mM Tris pH 9.0 at 37 °C for 30 min.
qRT-PCR
1 μg of total DNA-free RNA was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad) according to manufacturer’s protocol. cDNA was amplified with the corresponding primers (see Table S4). qPCR was performed using Bio-Rad CFX96 and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) according to manufacturer’s suggestion. mreB transcript level was used for normalization. The ΔΔCt method was used to obtain the fold changes of target genes.
Acidic Gel Northern Blotting
Acid urea polyacrylamide gel electrophoresis was performed according to the procedures described in (Janssen et al., 2012). Briefly, 12% Acid urea polyacrylamide gel was prepared freshly before use. 5 μg of RNA sample was loaded onto the gel, and subjected the electrophoresis with sodium acetate running buffer (100 mM sodium acetate pH5.0, 1 mM EDTA) in cold room for 6 h. Gel-separated RNA was transferred to GT membrane (Bio-Rad) by semi-dry electro-blotting at 15 V for 40 min in 0.5 × TBE.
The membrane was cross-linked by UV and probed with 5′ end biotin labeled DNA oligonucleotide probes (see Table S4). Trp-tRNATrp, tRNATrp, Gly-tRNAGly and tRNAGly were detected by the northern blotting, respectively, with SsrA as the loading control. Quantitation was performed with Image Lab (Bio-Rad).
Protein Digestion and Proteomics
Protein extraction and digestion from E. coli was performed using cell pellets from 200 μL stationary-phase cultures. Lysis, reduction, alkylation, trypsin digest, and acid cleavage were performed as in (Lajoie et al., 2013). All resulting peptides were purified and desalted using a C18 MacroSpin column (The Nest Group), dried in a rotary vacuum centrifuge, and resuspended in 6 μL 70% formic acid and 16 μL 0.1% trifluoroacetic acid. Following A280 peptide quantification, samples were diluted to a concentration of 0.5 μg/μL in the same buffer, and 4 μL of sample (2 μg total) were injected onto an analytical column using ACQUITY UPLC M-Class (Waters) for mass spectrometry. Column specifications, solvent gradients, and mass spectrometry parameters for the Q Exactive Plus (Thermo) were performed as described in (Ferdaus et al., 2016).
Peptide Database Construction and Searches
The MG1655 genome and annotated open reading frames (ORFs) were downloaded from ncbi.nlm.nih.gov on September 16th, 2016. ORFs terminating in TGA were mapped to the reference genome and extended to the next in-frame stop codon (TAG, TAA or TGA) using an ad hoc Python script. Extended ORFs were translated and separate entries were included in the search database for every natural amino acid or an in-frame skipping event at the first TGA position. The normal MG1655 proteome and the extended frame protein sequences (sequences beginning at the first tryptic residue N-terminal to the TGA), including the m-TGA-y reporter sequences, were used concurrently for mass spectrometry data searches using MaxQuant v.1.5.1.2 (Cox and Mann, 2008). The copy number of mCherry was estimated using Intensity Based Absolute Quantitation as described (Soufi et al., 2015). The concentration of total mCherry was similar to that of EF-Tu corresponding to ~300,000 molecules per cell. The mCherry-YFP fusion protein was ~2% of total mCherry as observed by fluorescence spectrometry and Western blotting (~6,000 molecules per cell).
Mathematical Modeling
Theoretical modeling of the variation of stop codon (UGA) read-through error with antibiotic chloramphenicol (Chl) concentration are based on the network shown in Figure S4B and S4C. In the scheme, E represents the bare ribosome with UGA mRNA stop codon in A-site, ER represents the release factor (RF2)-bound state that undergoes a conformational transition to state ER* and PR is the state generated after RF2-induced pH-dependent hydrolysis of the peptide chain in the ribosome P-site (Indrisiunaite et al., 2015). Here, RF2 is the right substrate. For the wrong substrate, i.e., the ternary complex (TC) of Trp-tRNATrp, the initial bound state ET undergoes GTP-hydrolysis to ET*. From state ET*, the system can take two routes: (i) it can accommodate the aminoacyl-tRNA that leads to peptide chain elongation, i.e., readthrough of the stop codon (state PT) or (ii) it can reset by a proofreading dissociation of the aa-tRNA from ribosome (Cochella and Green, 2005). No such proofreading appears to be present for the RF2 pathway (Freistroffer et al., 2000).
A recent study shows that although Chl reduces the overall growth rate of E. coli, the elongation rate actually rises with Chl concentration (Dai et al., 2016). This feature is attributed to an increase in RNA/protein ratio that leads to a rise in effective TC concentration. Our experiment also confirms this fact with the Trp-tRNATrp levels going up with [Chl] (see Fig. 4). In contrast, the protein levels go down with [Chl] (Dai et al., 2016). In addition, binding of Chl to ribosome stalls the translation process and this leads to a fall in the completion probability of translation. We model these three effects of antibiotic addition with the following three schemes.
Model 1
Here, we take the TC concentration to be an increasing function of Chl concentration as
| (1) |
Here, [TC]0 denotes TC concentration in absence of Chl. We assume [TC] to be proportional to the Trp-tRNATrp level and fit the experimentally obtained fold change using Eq.(1). This gives, f = 20.2, a = 18.6 g/ml. Now, our experimentally measured growth rate in absence of Chl is ~1.9 h−1. With this information and following a recent analysis of TC concentration for various tRNA species under different growth conditions (Rudorf and Lipowsky, 2015), we set [TC]0 = 3.2 μM. We do not consider here any dependence of [RF2] on [Chl]. We take [RF2]0 = 18 μM following a report of RF2 level variation at various E. coli growth rates (Adamski et al., 1994) Also, in this model, we assume the completion probability to be 100%, i.e., all translation initiation events go to completion.
Model 2
Here, [TC] follows Eq.(1). The [RF2] dependence on [Chl] is taken as
| (2) |
The parameter K (~0.4 μM−1) (Dai et al., 2016) is the binding constant of Chl with ribosome. This corresponds to the assumption that decrease in the fraction of active ribosome leads to proportional decrease in translation rates and correspondingly in the protein concentrations. Again, we take the completion probability to be 100%.
Model 3
In this case, the [TC] and [RF2] dependences on [Chl] are given by Eq.(1) and Eq.(2), respectively. However, we now note that not every read-through even will result in the completed (functional) YFP protein. Therefore, if the translating ribosome binds Chl before YFP is completed we assume that the translation will be aborted and the read-through won’t result in YFP fluorescence signal. The resulting functional dependence of completion probability on Chl is modeled as
| (3) |
Here, T□ is the completion time of YFP protein synthesis, indicator of stop codon readthrough error. It is defined as N/r where N=251 is the number of amino acids (aa) in YFP and r is the elongation rate (Dai et al., 2016). We take r=17 aa/s based on the values reported in (Dai et al., 2016). The effective binding constant kb ~ 2 × 104 M−1.s−1 is taken from a recent study on Chl inhibition (Xaplanteri et al., 2003) that indicates that initial binding of Chl is fast followed by a slower isomerization step.
We study the kinetics of the whole network using the standard tools of first-passage technique (Kampen, 2007). The probability to reach a given end-state (PR or PT) before the other, starting from state-E, is called the splitting probability (9). The UGA read-through error is defined as the ratio of the splitting probability of reaching the wrong end (PT) to that of reaching the right end (PR). The parameter set to generate the nature of error variation as a function of Chl concentration are given in Table S2.
QUANTITATION AND STATISTICAL ANALYSES
Statistical parameters for each experiment are reported in the corresponding figures. All data presented are the mean of at least three repeats, with error bars showing one standard deviation. The P values were calculated with unpaired t tests.
Supplementary Material
Table S1. Identified Peptides Resulting from UGA Readthrough by LC MS/MS, Related to Figure 1
Table S2. Parameter values to fit the antibiotic (Chl) dependence of UGA read-through error, Related to Figure 3
Table S3. Pathway Enrichment of Genes with UGA Stop Codons, Related to Figure 7
Table S4. Oligonucleotides Used, Related to STAR Methods
Figure S1. Dual-fluorescence Reporters, Related to Figure 1
Figure S2. MS/MS spectra of peptides resulting from UGA readthrough, Related to Figure 1
Figure S3. UGA Readthrough in E. coli, Related to Figure 3
Figure S4. Reducing Protein Synthesis Increases UGA Readthrough, Related to Figure 3
Figure S5. Reducing Protein Synthesis Increases UGA Readthrough in E. coli BL21 and Salmonella Typhimurium, Related to Figure 3
Figure S6. TGAC Frameshifting Codon of PrfB Does Not Determine the Noise of UGA Readthrough, Related to Figure 5
Figure S7. Growth of E. coli with and without Suppressor tRNA in Different Media, Related to Figure 6
Highlights.
Stop codon readthrough is heterogeneous among single cells
Increased UGA readthrough promotes bacterial growth
Reducing protein synthesis levels enhances UGA readthrough
Fluctuations of translational components lead to UGA readthrough heterogeneity
Acknowledgments
We thank Drs. Arvind R. Subramaniam and Philippe Cluzel (Harvard University) and the National BioResource Project (Japan) for plasmids, Dr. Nicholas De Lay (The University of Texas Health Science Center at Houston) for strains, Dr. Veronica Rowlett (The University of Texas Health Science Center at Houston) for technical assistance and Dr. Marc Lajoie (University of Washington) for help with bioinformatics. This work was funded by NIGMS R01GM115431 (J.L.), NIGMS R01GM61074 (W.M.), NIGMS R01GM117230 (J.R.), NSF DGE-1122492 (K.W.B.), and NSF PHY-1427654 (O.A.I).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Y.F., C.R.E., K.W.B., W.M., O.A.I., J.R., and J.L. designed experiments; Y.F., C.E., K.W.B., K.J.W., and J.L. performed experiments; Y.F., C.E., K.W.B., K.J.W., J.R., and J.L. analyzed experimental data; K.B. and O.A.I. performed mathematical modeling; Y.F., C.R.E., K.W.B., K.B., W.M., O.A.I., J.R., and J.L. wrote the manuscript.
The authors declare no finacial interest related to this work.
References
- Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508. doi: 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- Adamski FM, McCaughan KK, Jorgensen F, Kurland CG, Tate WP. The concentration of polypeptide chain release factors 1 and 2 at different growth rates of Escherichia coli. J Mol Biol. 1994;238:302–308. doi: 10.1006/jmbi.1994.1293. [DOI] [PubMed] [Google Scholar]
- Aerni HR, Shifman MA, Rogulina S, O’Donoghue P, Rinehart J. Revealing the amino acid composition of proteins within an expanded genetic code. Nucleic Acids Res. 2015;43:e8. doi: 10.1093/nar/gku1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006. doi: 10.1038/msb4100050. 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher JM, Crécy-Lagard V, Schimmel PR. Inhibited cell growth and protein functional changes from an editing-defective tRNA synthetase. Proc Natl Acad Sci USA. 2005;102:1697–1701. doi: 10.1073/pnas.0409064102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazsi G, van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. 2011;144:910–925. doi: 10.1016/j.cell.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra AR, Simoes J, Lee W, Rung J, Weil T, Gut IG, Gut M, Bayes M, Rizzetto L, Cavalieri D, et al. Reversion of a fungal genetic code alteration links proteome instability with genomic and phenotypic diversification. Proc Natl Acad Sci USA. 2013;110:11079–11084. doi: 10.1073/pnas.1302094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman J, Samuelsson P, Andersson DI, Hughes D. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella Typhimurium. Mol Microbiol. 1999;31:53–58. doi: 10.1046/j.1365-2958.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- Blake WJ, Balazsi G, Kohanski MA, Isaacs FJ, Murphy KF, Kuang Y, Cantor CR, Walt DR, Collins JJ. Phenotypic consequences of promoter-mediated transcriptional noise. Mol Cell. 2006;24:853–865. doi: 10.1016/j.molcel.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Blake WJ, M KA, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- Cochella L, Green R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science. 2005;308:1178–1180. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Curran JF. Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res. 1993;21:1837–1843. doi: 10.1093/nar/21.8.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JF, Yarus M. Use of tRNA suppressors to probe regulation of Escherichia coli release factor 2. J Mol Biol. 1988;203:75–83. doi: 10.1016/0022-2836(88)90092-7. [DOI] [PubMed] [Google Scholar]
- Dai X, Zhu M, Warren M, Balakrishnan R, Patsalo V, Okano H, Williamson JR, Fredrick K, Wang YP, Hwa T. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol. 2016;2:16231. doi: 10.1038/nmicrobiol.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Davis BD. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987;51:341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj A, Shoji S, Holbrook ED, Fredrick K. A role for the 30S subunit E site in maintenance of the translational reading frame. RNA. 2009;15:255–265. doi: 10.1261/rna.1320109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincbas-Renqvist V, Engstrom A, Mora L, Heurgue-Hamard V, Buckingham R, Ehrenberg M. A post-translational modification in the GGQ motif of RF2 from Escherichia coli stimulates termination of translation. EMBO J. 2000;19:6900–6907. doi: 10.1093/emboj/19.24.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DA, Wilke CO. The evolutionary consequences of erroneous protein synthesis. Nat Rev Genet. 2009;10:715–724. doi: 10.1038/nrg2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JG, Foo CK, Belletier NG, Gavis ER, Weissman JS. Ribosome profiling reveals pervasive and regulated stop codon readthrough in Drosophila melanogaster. eLife. 2013;2:e01179. doi: 10.7554/eLife.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G, Söll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol Rev. 1988;52:354–374. doi: 10.1128/mr.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H. UGA suppression by normal tRNATrp in Escherichia coli: codon context effects. Nucleic Acids Res. 1981;9:983–991. doi: 10.1093/nar/9.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Wu J, Ung MH, De Lay N, Cheng C, Ling J. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Res. 2015;43:1740–1748. doi: 10.1093/nar/gku1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdaus MZ, Barber KW, Lopez-Cayuqueo KI, Terker AS, Argaiz ER, Gassaway BM, Chambrey R, Gamba G, Rinehart J, McCormick JA. SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J Physiol. 2016;594:4945–4966. doi: 10.1113/JP272311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrick K, Noller HF. Accurate translocation of mRNA by the ribosome requires a peptidyl group or its analog on the tRNA moving into the 30S P site. Mol Cell. 2002;9:1125–1131. doi: 10.1016/s1097-2765(02)00523-3. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Ballesteros M, Peterson CN, Persson O, Silhavy TJ, Nystrom T. Decline in ribosomal fidelity contributes to the accumulation and stabilization of the master stress response regulator sigmaS upon carbon starvation. Genes Dev. 2007;21:862–874. doi: 10.1101/gad.409407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistroffer DV, Kwiatkowski M, Buckingham RH, Ehrenberg M. The accuracy of codon recognition by polypeptide release factors. Proc Natl Acad Sci USA. 2000;97:2046–2051. doi: 10.1073/pnas.030541097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Satory D, Halliday JA, Herman C. Lost in transcription: transient errors in information transfer. Curr Opin Microbiol. 2015;24:80–87. doi: 10.1016/j.mib.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido NJ, Lee P, Wang X, Elston TC, Collins JJ. A pathway and genetic factors contributing to elevated gene expression noise in stationary phase. Biophys J. 2007;93:L55–57. doi: 10.1529/biophysj.107.118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Huh D, Paulsson J. Non-genetic heterogeneity from stochastic partitioning at cell division. Nat Genet. 2011;43:95–100. doi: 10.1038/ng.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrisiunaite G, Pavlov MY, Heurgue-Hamard V, Ehrenberg M. On the pH dependence of class-1 RF-dependent termination of mRNA translation. J Mol Biol. 2015;427:1848–1860. doi: 10.1016/j.jmb.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Janssen BD, Diner EJ, Hayes CS. Analysis of aminoacyl-and peptidyl-tRNAs by gel electrophoresis. Bacterial Regulatory RNA: Methods and Protocols. 2012:291–309. doi: 10.1007/978-1-61779-949-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javid B, Sorrentino F, Toosky M, Zheng W, Pinkham JT, Jain N, Pan M, Deighan P, Rubin EJ. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc Natl Acad Sci USA. 2014;111:1132–1137. doi: 10.1073/pnas.1317580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Lovmar M, Ehrenberg M. Rate and accuracy of bacterial protein synthesis revisited. Curr Opin Microbiol. 2008;11:141–147. doi: 10.1016/j.mib.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaern M, Elston TC, Blake WJ, Collins JJ. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- Kampen NGv. Stochastic Processes in Physics and Chemistry (North Holland) 2007. [Google Scholar]
- Karkhanis VA, Mascarenhas AP, Martinis SA. Amino acid toxicities of Escherichia coli that are prevented by leucyl-tRNA synthetase amino acid editing. J Bacteriol. 2007;189:8765–8768. doi: 10.1128/JB.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- Kramer EB, Farabaugh PJ. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA. 2007;13:87–96. doi: 10.1261/rna.294907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli. Science. 2009;324:255–258. doi: 10.1126/science.1170160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie MJ, Rovner AJ, Goodman DB, Aerni HR, Haimovich AD, Kuznetsov G, Mercer JA, Wang HH, Carr PA, Mosberg JA, et al. Genomically recoded organisms expand biological functions. Science. 2013;342:357–360. doi: 10.1126/science.1241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- Levine E, Hwa T. Stochastic fluctuations in metabolic pathways. Proc Natl Acad Sci USA. 2007;104:9224–9229. doi: 10.1073/pnas.0610987104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JH, Lin Y, Elowitz MB. Functional roles of pulsing in genetic circuits. Science. 2013;342:1193–1200. doi: 10.1126/science.1239999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, O’Donoghue P, Söll D. Genetic code flexibility in microorganisms: novel mechanisms and impact on physiology. Nat Rev Microbiol. 2015;13:707–721. doi: 10.1038/nrmicro3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Reynolds N, Ibba M. Aminoacyl-tRNA synthesis and translational quality control. Annu Rev Microbiol. 2009;63:61–78. doi: 10.1146/annurev.micro.091208.073210. [DOI] [PubMed] [Google Scholar]
- Liu Y, Satz JS, Vo MN, Nangle LA, Schimmel P, Ackerman SL. Deficiencies in tRNA synthetase editing activity cause cardioproteinopathy. Proc Natl Acad Sci USA. 2014;111:17570–17575. doi: 10.1073/pnas.1420196111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Vargas-Rodriguez O, Goto Y, Novoa EM, Ribas de Pouplana L, Suga H, Musier-Forsyth K. Homologous trans-editing factors with broad tRNA specificity prevent mistranslation caused by serine/threonine misactivation. Proc Natl Acad Sci USA. 2015;112:6027–6032. doi: 10.1073/pnas.1423664112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bergert M, Walther A, Suter B. Double-sieving-defective aminoacyl-tRNA synthetase causes protein mistranslation and affects cellular physiology and development. Nat Commun. 2014;5:5650. doi: 10.1038/ncomms6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas AP, An S, Rosen AE, Martinis SA, Musier-Forsyth K. Fidelity mechanisms of the aminoacyl-tRNA synthetases. In: RajBhandary UL, Köhrer C, editors. Protein Engineering. New York: Springer-Verlag; 2008. pp. 153–200. [Google Scholar]
- Mikkola R, Kurland CG. Selection of laboratory wild-type phenotype from natural isolates of Escherichia coli in chemostats. Mol Biol Evol. 1992;9:394–402. doi: 10.1093/oxfordjournals.molbev.a040731. [DOI] [PubMed] [Google Scholar]
- Mora L, Heurgue-Hamard V, de Zamaroczy M, Kervestin S, Buckingham RH. Methylation of bacterial release factors RF1 and RF2 is required for normal translation termination in vivo. J Biol Chem. 2007;282:35638–35645. doi: 10.1074/jbc.M706076200. [DOI] [PubMed] [Google Scholar]
- Newman JR, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- Ozbudak EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. Regulation of noise in the expression of a single gene. Nat Genet. 2002;31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- Pan T. Adaptive translation as a mechanism of stress response and adaptation. Annu Rev Genet. 2013;47:121–137. doi: 10.1146/annurev-genet-111212-133522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson J. Summing up the noise in gene networks. Nature. 2004;427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- Polikanov YS, Blaha GM, Steitz TA. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science. 2012;336:915–918. doi: 10.1126/science.1218538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouplana LR, Santos MA, Zhu JH, Farabaugh PJ, Javid B. Protein mistranslation: friend or foe? Trends Biochem Sci. 2014;39:355–362. doi: 10.1016/j.tibs.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Quan S, Skovgaard O, McLaughlin RE, Buurman ET, Squires CL. Markerless Escherichia coli rrn Deletion Strains for Genetic Determination of Ribosomal Binding Sites. G3 (Bethesda) 2015;5:2555–2557. doi: 10.1534/g3.115.022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NM, Ling J, Roy H, Banerjee R, Repasky SE, Hamel P, Ibba M. Cell-specific differences in the requirements for translation quality control. Proc Natl Acad Sci USA. 2010;107:4063–4068. doi: 10.1073/pnas.0909640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SM, Shee C, Frisch RL, Hastings PJ. Stress-induced mutation via DNA breaks in Escherichia coli: a molecular mechanism with implications for evolution and medicine. Bioessays. 2012;34:885–892. doi: 10.1002/bies.201200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudorf S, Lipowsky R. Protein synthesis in E. coli: dependence of codon-specific elongation on tRNA concentration and codon usage. PLoS One. 2015;10:e0134994. doi: 10.1371/journal.pone.0134994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Choubey S, Kondev J. Regulation of noise in gene expression. Annu Rev Biophys. 2013;42:469–491. doi: 10.1146/annurev-biophys-083012-130401. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Golding I. Genetic determinants and cellular constraints in noisy gene expression. Science. 2013;342:1188–1193. doi: 10.1126/science.1242975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliusarenko O, Heinritz J, Emonet T, Jacobs-Wagner C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol Microbiol. 2011;80:612–627. doi: 10.1111/j.1365-2958.2011.07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi B, Krug K, Harst A, Macek B. Characterization of the E. coli proteome and its modifications during growth and ethanol stress. Front Microbiol. 2015;6:103. doi: 10.3389/fmicb.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HW, Zhu JH, Li H, Cai RJ, Ealand C, Wang X, Chen YX, Kayani MU, Zhu TF, Moradigaravand D, et al. The essential mycobacterial amidotransferase GatCAB is a modulator of specific translational fidelity. Nat Microbiol. 2016;1:16147. doi: 10.1038/nmicrobiol.2016.147. [DOI] [PubMed] [Google Scholar]
- Subramaniam AR, Pan T, Cluzel P. Environmental perturbations lift the degeneracy of the genetic code to regulate protein levels in bacteria. Proc Natl Acad Sci USA. 2013;110:2419–2424. doi: 10.1073/pnas.1211077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True HL, Lindquist SL. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- Vacher J, Grosjean H, Houssier C, Buckingham RH. The effect of point mutations affecting Escherichia coli tryptophan tRNA on anticodon-anticodon interactions and on UGA suppression. J Mol Biol. 1984;177:329–342. doi: 10.1016/0022-2836(84)90460-1. [DOI] [PubMed] [Google Scholar]
- Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, Forest CR, Church GM. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol. 2014;12:35–48. doi: 10.1038/nrmicro3155. [DOI] [PubMed] [Google Scholar]
- Xaplanteri MA, Andreou A, Dinos GP, Kalpaxis DL. Effect of polyamines on the inhibition of peptidyltransferase by antibiotics: revisiting the mechanism of chloramphenicol action. Nucleic Acids Res. 2003;31:5074–5083. doi: 10.1093/nar/gkg686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Wada A. The 100S ribosome: ribosomal hibernation induced by stress. Wiley Interdiscip Rev RNA. 2014;5:723–732. doi: 10.1002/wrna.1242. [DOI] [PubMed] [Google Scholar]
- Young JW, Locke JC, Elowitz MB. Rate of environmental change determines stress response specificity. Proc Natl Acad Sci USA. 2013;110:4140–4145. doi: 10.1073/pnas.1213060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman EM, He SL, Nikstad LJ, Green R. Stop codon recognition by release factors induces structural rearrangement of the ribosomal decoding center that is productive for peptide release. Mol Cell. 2007;28:533–543. doi: 10.1016/j.molcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Zaher HS, Green R. Fidelity at the molecular level: lessons from protein synthesis. Cell. 2009;136:746–762. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher HS, Green R. A primary role for release factor 3 in quality control during translation elongation in Escherichia coli. Cell. 2011;147:396–408. doi: 10.1016/j.cell.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Identified Peptides Resulting from UGA Readthrough by LC MS/MS, Related to Figure 1
Table S2. Parameter values to fit the antibiotic (Chl) dependence of UGA read-through error, Related to Figure 3
Table S3. Pathway Enrichment of Genes with UGA Stop Codons, Related to Figure 7
Table S4. Oligonucleotides Used, Related to STAR Methods
Figure S1. Dual-fluorescence Reporters, Related to Figure 1
Figure S2. MS/MS spectra of peptides resulting from UGA readthrough, Related to Figure 1
Figure S3. UGA Readthrough in E. coli, Related to Figure 3
Figure S4. Reducing Protein Synthesis Increases UGA Readthrough, Related to Figure 3
Figure S5. Reducing Protein Synthesis Increases UGA Readthrough in E. coli BL21 and Salmonella Typhimurium, Related to Figure 3
Figure S6. TGAC Frameshifting Codon of PrfB Does Not Determine the Noise of UGA Readthrough, Related to Figure 5
Figure S7. Growth of E. coli with and without Suppressor tRNA in Different Media, Related to Figure 6


