Abstract
Lymphocyte-to-monocyte ratio (LMR) was associated with survival benefit in some types of cancer. The relationship between LMR and rectal cancer has not been investigated. We conducted a retrospective cohort study to assess the prognostic significance of LMR in patients with nonmetastatic rectal cancer. Patients with rectal cancer who underwent potentially curative resection between January 2009 and December 2013 were enrolled. The LMR was calculated from preoperative blood test by dividing the absolute lymphocyte counts by the absolute monocyte counts. The optimal cut-off value for LMR was calculated as the median value. On the basis of the cut-off value, patients were divided into 2 groups: low group and high group. A total of 543 patients with rectal cancer were eligible for this study. The median follow-up time for all patients was 55 months (range 6–85 months). The cut-off value of LMR was 5.13 and patients were divided into 2 groups: low group (LMR < 5.13) and high group (LMR ≥ 5.13). In the univariate and multivariate analysis, the LMR was not significantly associated with overall survival (OS) [hazard ratio (HR): 1.034, 95% confidence intervals (CIs): 0.682–1.566, P = 0.876]. When disease-free survival (DFS) was compared, univariate and multivariate analysis also indicated that the LMR was not significantly associated with DFS (HR: 0.988, 95% CI: 0.671–1.453, P = 0.950). In addition, in the subgroup analysis by tumor-node-metastasis stage, there existed no significance between LMR and OS and DFS. Although as an easy access and highly efficient laboratorial inflammatory marker, LMR cannot predict the prognosis of nonmetastatic rectal cancer patients.
Keywords: disease-free survival, lymphocyte-to-monocyte ratio, overall survival, prognosis, rectal cancer
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and fourth leading cause of cancer-related deaths worldwide.[1] Although treatment strategies for CRC have been developed, surgery is still the main option for nonmetastatic disease. However, the outcomes for patients who underwent curative surgery were not satisfied because about 40% of those patients died of it.[2] Generally, outcomes following surgery are mainly determined by pathological tumor characteristics, such as tumor-node-metastasis (TNM) stage system. Patients with same TNM stage, however, may have different oncologic outcomes.[3] It is increasingly recognized that not only TNM stage but also neo-adjuvant therapy, adjuvant therapy, and systemic inflammatory response is connected with CRC outcomes.
Since Virchow[4] in 1863 first reported the link between inflammation and tumorigenesis, many other studies have shown that systemic inflammatory response played a critical role in the pathogenesis and progression of cancer, including CRC.[5–8] Approximately 20% of tumor occurrence was associated with chronic inflammation, and even about 15% of cancer-related deaths were closely associated with chronic inflammation or unresolved infection.[6,9] As a marker of systemic inflammatory response, recent studies have proved that the elevated pretreatment lymphocyte-to-monocyte ratio (LMR) was associated with favorable survival in hematologic malignancies.[10–12] In addition, it has been proved for the first time that preoperative LMR was a good prognostic marker in patients with colon cancer by Stotz et al in 2014.[13] Since then, several other studies also showed that LMR was a prognostic factor for patients with metastatic CRC.[14–18] Besides, Xiao et al[19] focused on rectal cancer patients with pT3N0 and suggested that elevated LMR can predict a favorable prognosis. However, to the best of our knowledge, there is no report that investigated LMR and nonmetastatic rectal cancer with a large sample size. Thus, we conducted this retrospective study to investigate the prognostic significance of LMR in patients with nonmetastatic rectal cancer with a large sample size.
2. Methods
2.1. Patients
Patients with rectal cancer underwent potentially curative resection between January 2009 and December 2013 at the Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, and were enrolled in this retrospective study. The inclusion criteria were histologically confirmed rectal carcinoma and checked blood test in 2 weeks before surgery and LMR could be calculated. Exclusion criteria were as follows: Neoadjuvant therapy, Presence of metastatic disease, Presence of infection, Beyond 85 years old, and Died within 1 month after surgery. Ethical approval was not necessary because this study was a retrospective study.
Patient demographics and clinicopathological characteristics were collected, including gender, age, pretreatment carcinoembryonie antigen (CEA), tumor location, tumor size, differentiation, TNM stage, vascular invasion, perineural invasion, adjuvant therapy, and lymphocyte and monocyte counts. TNM stage was assessed according to the American Joint Committee on Cancer TNM staging standard, 7th edition. The LMR was calculated from blood test by dividing the absolute lymphocyte counts by the absolute monocyte counts.
2.2. Follow-up
Follow-up was performed every 3 months intervals for the first 2 years, every 6 months intervals in the next 3 years, and every 12 months intervals after 5 years after surgery. The examinations included physical examination, blood test, CEA levels, computed tomography (CT) of chest, and CT or magnetic resonance imaging (MRI) of abdominal and pelvic (every 6 months within the first 2 years and every 12 months after 2 years after surgery) and colonoscopy (every 2 years). Local recurrence was defined as the recurrent disease in the pelvis or at the incision, while the distant recurrence was defined as the recurrence beyond the above parts. Both of them were confirmed by biopsy, CT, or MRI. Follow-up data of all enrolled patients were available.
2.3. Statistical analysis
The primary endpoint and the secondary endpoint of this study were overall survival (OS) and disease-free survival (DFS), respectively. OS was the time of surgery to the date of death from any causes or the date of follow-up, while DFS was calculated from the surgery to the recurrence or end of follow-up.
The optimal cut-off value for LMR was calculated as the median value. The Chi-square test or Fisher exact test was used to analyze the association between clinicopathologic characteristics and LMR. The OS and DFS were analyzed and compared by using the Kaplan–Meier method and the log-rank test. Multivariate analysis was performed using Cox proportional hazards regression. Data analyses were all carried out using SPSS software (version 22.0; SPSS Inc., Chicago, IL). A P value <0.05 was recognized as statistical significance.
3. Results
3.1. Patient characteristics
A total of 543 patients with rectal cancer who underwent potentially curative resection were eligible for this study. All patients were followed up until January 31, 2016. And the median follow-up time was 55 months (range 6–85 months). The cut-off value of LMR, based on the median value, was 5.13. According to the cut-off value, patients were divided into 2 groups: low group (LMR <5.13) and high group (LMR ≥5.13).
3.2. Correlations between LMR and clinicopathological factors
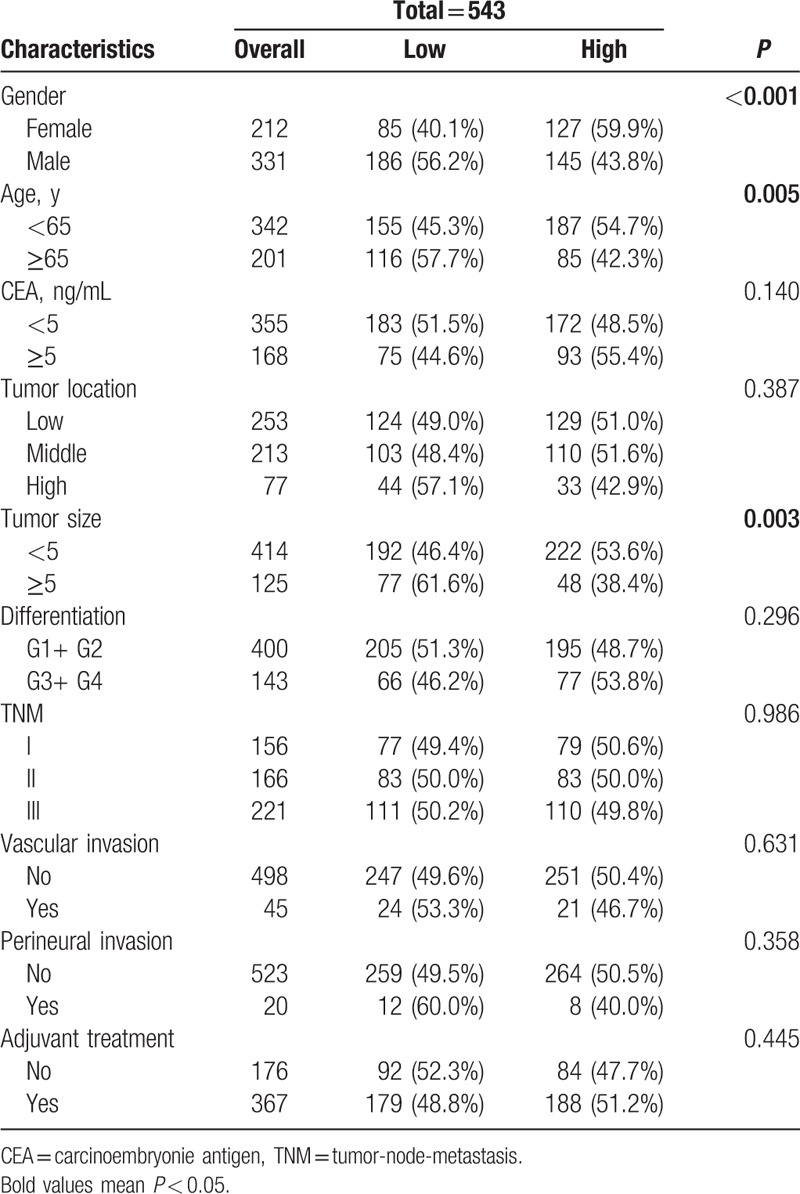
A comparison of the basic clinicopathological characteristics of those 2 groups is summarized in Table 1. The CEA, tumor location, difference, TNM stage, vascular invasion, perineural invasion, and adjuvant treatment were not statistically different between the 2 groups, while the gender (P = <0.001), age (P = 0.005), and tumor size (P = 0.003) existed statistically different between the 2 groups.
Table 1.
Patient clinicopathological characteristics.
3.3. Survival outcomes
As summarized in Tables 2, in the univariate and multivariate analysis, the LMR was not significantly associated with OS [hazard ratio (HR): 1.034, 95% confidence intervals (CIs): 0.682–1.566, P = 0.876; Fig. 1A], while age, CEA, tumor location, difference, and TNM stage were associated with OS. When DFS was compared in Table 3, univariate and multivariate analysis indicated that the LMR was also not significantly associated with DFS (HR: 0.988, 95% CI: 0.671–1.453, P = 0.950; Fig. 1B) (Table 2). In addition, in the subgroup analysis by TNM stage, there existed no significance between LMR and OS and DFS (Fig. 2).
Table 2.
Univariate and multivariate analyses of LMR for OS in patients with nonmetastatic rectal cancer.
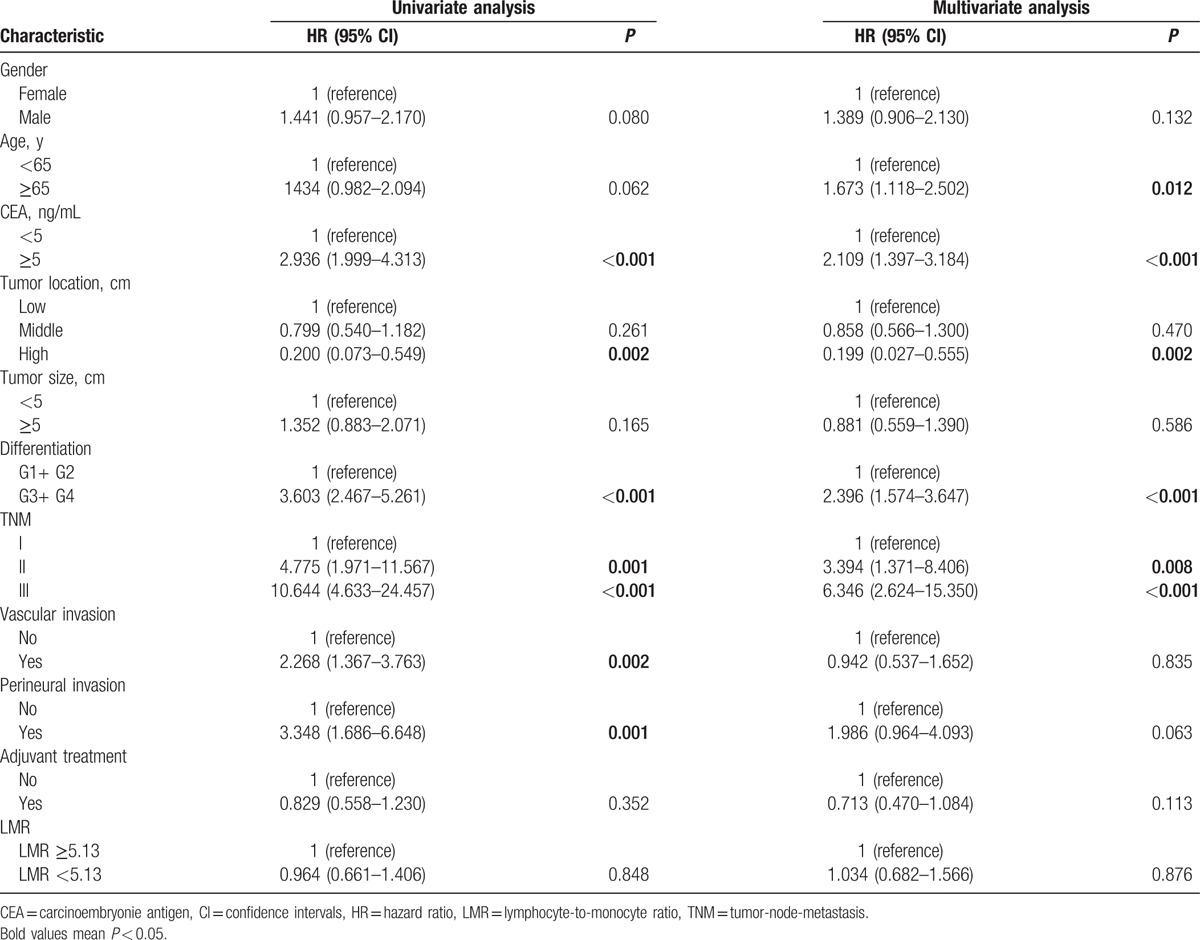
Figure 1.
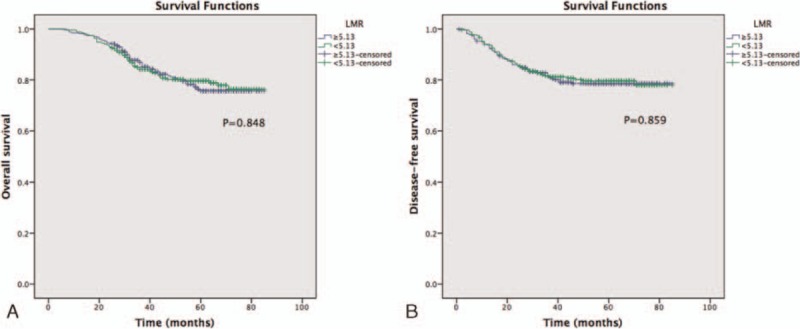
Preoperative LMR and OS and DFS: (A) OS, (B) DFS.
Table 3.
Univariate and multivariate analyses of LMR for DFS in patients with nonmetastatic rectal cancer.
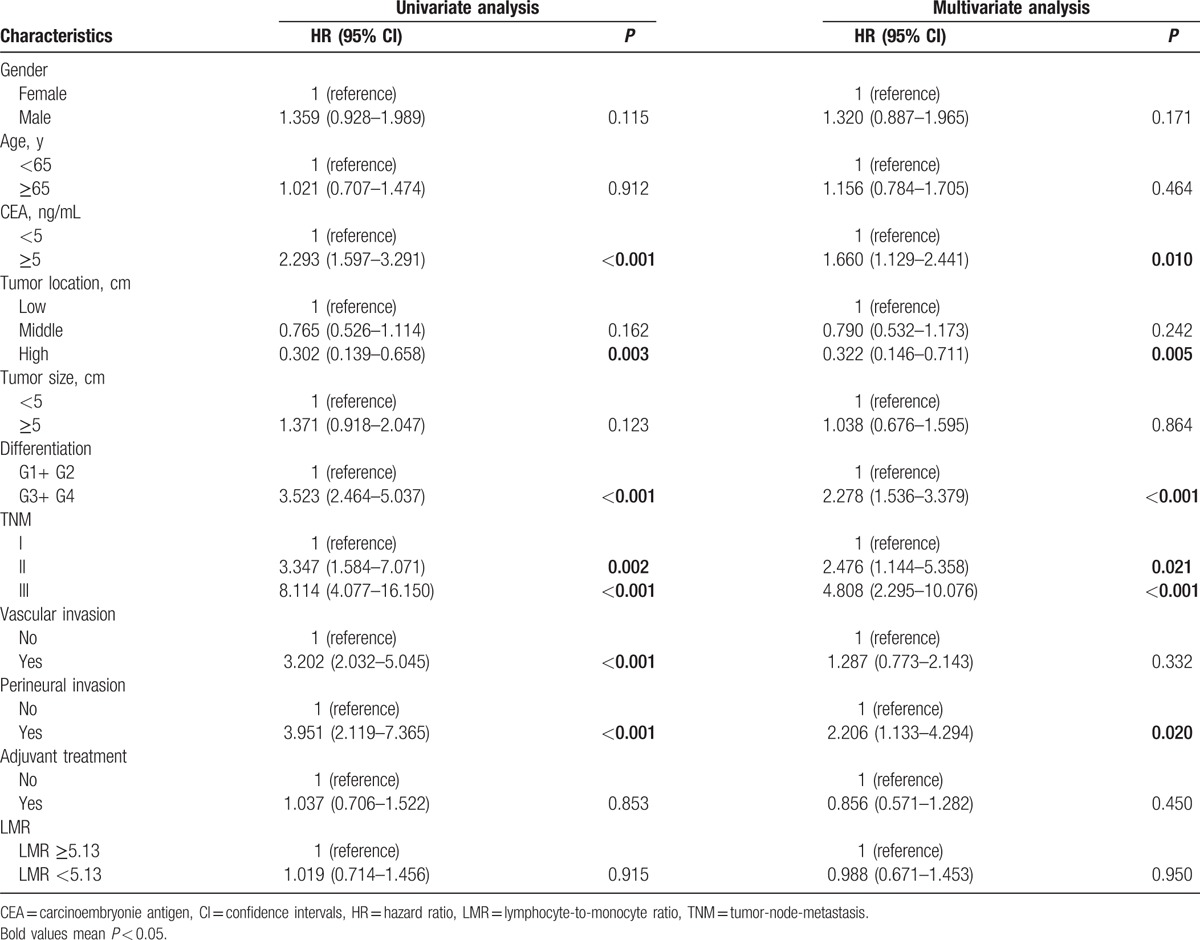
Figure 2.
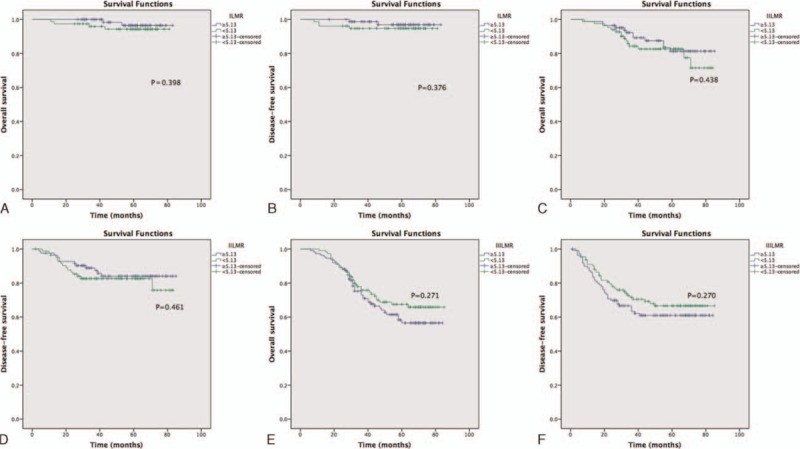
Preoperative LMR and OS and DFS in different TNM stages: (A) OS for stage I, (B) DFS for stage I, (C) OS for stage II, (D) DFS for stage II, (E) OS for stage III, (F) DFS for stage III. TNM = tumor-node-metastasis.
4. Discussion
In the present study, we evaluated the prognostic significance of LMR in patients with nonmetastatic rectal cancer who underwent potentially curative resection. Our study cohort demonstrated that there was no association between LMR and OS (HR: 1.034, 95% CI: 0.682–1.566, P = 0.876) or DFS (HR: 0.988, 95% CI: 0.671–1.453, P = 0.950). There was no significance between LMR and OS or DFS in the subgroup analysis by TNM stage, either.
The association between inflammation and tumorigenesis was first reported by Virchow.[4] Since then, strong evidence suggests that inflammation plays a critical role in cancer onset, development, and therapeutic response.[6,7,20,21] As a marker of systemic inflammatory response, the LMR, which can be easily gained from peripheral blood test, is drawing increasing attention.
Although lymphopenia usually predicts disease severity[22] and can cause immune escape of tumor cells from tumor-infiltrating lymphocytes,[23] different subtypes of lymphocyte can have a different influence on tumor. Even the same type of lymphocyte may have different function. It has been already shown that elevated levels of tumor-infiltrating lymphocytes predicted a better survival in patients with CRC.[24] However, regulatory T cells, which are kind of lymphocytes, may play a positive,[25–27] negative,[28–30] or nonpredictive[31,32] role in combating CRC.
Similarly, monocytes can also have a different influence on tumor. Monocyte-associated macrophages are suggested to have a crucial role in host antitumor immunity suppression, tumor cell migration, and invasion.[33–35] Moreover, monocytes and their progeny can produce factors promoting the growth and survival of tumor cells.[36,37] Nevertheless, Forssell et al[38] showed that a dense macrophage infiltration at the tumor invasive margin was a good prognostic factor for colon cancer patients. From the above, the relationship between LMR and the prognosis of CRC is not clear.
Different to these aforementioned reports which indicated that elevated LMR predicted a significantly favorable OS and/or DFS in CRC patients,[13–19] our study suggested that LMR was not associated with either OS or DFS in rectal cancer patients, as well subgroup analysis by TNM stage. Although we did not measure the subtype cells of lymphocyte or monocyte, we thought that those subtype cells could have an influence on rectal cancer survival. This is the first study demonstrating that LMR is not associated with the survival of rectal cancer. In addition, a previous study focusing on CRC demonstrated that LMR could not be used as a potential diagnostic biomarker because its area under the curve of the cut-off value was <0.50, but the survival data were not presented.[39] Another 2 studies showed that LMR could be a prognostic factor for CRC.[17,40] However, both of them did not present the data of rectal cancer and colon cancer, separately. Besides, there is a report focused on pT3N0 rectal cancer, and the result was positive.[19] But it enrolled older patients, which might influence the result, because as our results, age could also influence the LMR. Besides, in our study, patients’ blood test was checked in 2 weeks before surgery, which was not mentioned in that report.
This is the first large-scale cohort study demonstrating that there is no association between LMR and either OS or DFS in rectal cancer. Unfortunately, this study was a retrospective study. Still, a prospective cohort study is needed to evaluate the association between LMR and OS and DFS in rectal cancer patients.
In conclusion, although as an easy access and highly efficient laboratorial inflammatory marker, LMR cannot predict the prognosis of nonmetastatic rectal cancer patients.
Footnotes
Abbreviations: AUC = area under curve, CI = confidence intervals, CRC = colorectal cancer, CT = computed tomography, DFS = disease-free survival, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, MRI = magnetic resonance imaging, OS = overall survival.
Both Q-BW and MW contributed equally to this work.
Funding/support: This work was supported by the Science and Technology Support Program of the Science & Technology Department of Sichuan Province (No. 2016SZ0043 and No. 2012FZ0005).
The authors report no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:359–386. [DOI] [PubMed] [Google Scholar]
- 2.Oliphant R, Nicholson GA, Horgan PG, et al. Contribution of surgical specialization to improved colorectal cancer survival. Br J Surg 2013; 100:1388–1395. [DOI] [PubMed] [Google Scholar]
- 3.Horgan PG, McMillan DC. Surgeons and selection of adjuvant therapy for node-negative colonic cancer. Br J Surg 2010; 97:1459–1460. [DOI] [PubMed] [Google Scholar]
- 4.Virchow R. An address on the value of pathological experiments. Br Med J 1881; 2:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol 2016; 17:230–240. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 9.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis 2011; 70:104–108. [DOI] [PubMed] [Google Scholar]
- 10.Porrata LF, Ristow K, Colgan JP, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin's lymphoma. Haematologica 2012; 97:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZM, Huang JJ, Xia Y, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One 2012; 7:41658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rambaldi A, Boschini C, Gritti G, et al. The lymphocyte to monocyte ratio improves the IPI-risk definition of diffuse large B-cell lymphoma when rituximab is added to chemotherapy. Am J Hematol 2013; 88:1062–1067. [DOI] [PubMed] [Google Scholar]
- 13.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer 2014; 110:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozawa T, Ishihara S, Kawai K, et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J Surg Res 2015; 199:386–392. [DOI] [PubMed] [Google Scholar]
- 15.Neofytou K, Smyth EC, Giakoustidis A, et al. The preoperative lymphocyte-to-monocyte ratio is prognostic of clinical outcomes for patients with liver-only colorectal metastases in the neoadjuvant setting. Ann Surg Oncol 2015; 22:4353–4362. [DOI] [PubMed] [Google Scholar]
- 16.Neal CP, Cairns V, Jones MJ, et al. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol 2015; 32:144. [DOI] [PubMed] [Google Scholar]
- 17.Kozak MM, von Eyben R, Pai JS, et al. The prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancer. Am J Clin Oncol 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol 2015; 21:9966–9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao WW, Zhang LN, You KY, et al. A low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in pathological T3N0 rectal cancer patients following total mesorectal excision. J Cancer 2015; 6:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature 2001; 411:380–384. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137–148. [DOI] [PubMed] [Google Scholar]
- 22.Calman KC. Tumour immunology and the gut. Gut 1975; 16:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol 2006; 12:7233–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997; 182:318–324. [DOI] [PubMed] [Google Scholar]
- 25.Correale P, Rotundo MS, Del Vecchio MT, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010; 33:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 2010; 126:2635–2643. [DOI] [PubMed] [Google Scholar]
- 27.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263–1271. [DOI] [PubMed] [Google Scholar]
- 28.Yaqub S, Henjum K, Mahic M, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother 2008; 57:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen HK, Donskov F, Nordsmark M, et al. Increased intratumoral FOXP3-positive regulatory immune cells during interleukin-2 treatment in metastatic renal cell carcinoma. Clin Cancer Res 2009; 15:1052–1058. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo C, Li Z, Xu Y, et al. Higher FOXP3-TSDR demethylation rates in adjacent normal tissues in patients with colon cancer were associated with worse survival. Mol Cancer 2014; 13:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Rego RL, Ansell SM, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009; 137:1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Chikazawa N, Tasaka T, et al. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 2010; 59:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124:263–266. [DOI] [PubMed] [Google Scholar]
- 34.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia 2002; 7:177–189. [DOI] [PubMed] [Google Scholar]
- 35.Dirkx AE, Oude Egbrink MG, Wagstaff J, et al. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 2006; 80:1183–1196. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood 2009; 114:2936–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 2005; 93:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forssell J, Oberg A, Henriksson ML, et al. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 2007; 13:1472–1479. [DOI] [PubMed] [Google Scholar]
- 39.Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol 2014; 31:305. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016; 139:220–231. [DOI] [PubMed] [Google Scholar]


