Abstract
Whole-body positron emission tomography/computed tomography with the glucose analog 2-[18F]fluoro-2-deoxy-d-glucose (FDG-PET/CT) has been extensively used to screen for underlying malignancies in asymptomatic individuals. We were able to survey a cohort of hospital employees using FDG-PET/CT and to report the results herein.
A total of 116 hospital employees older than 55 years old were offered whole-body FDG-PET in our hospital. Ninety-seven employees (83.6%) completed the assessment from February 2014 to August 2014 in our PET center. The final confirmation of cancer was based on pathologic examination and follow-up after more than 1 year.
Among the 97 participants, 92 were asymptomatic and 5 presented with previously diagnosed cancers. Six of the 92 asymptomatic participants (6.6%) with significant nodular lesions were referred for histological or cytological evaluation of the possibility of malignancy, and 1 case was considered clinically important and required surgical resection. The cancer discovery rate was 3.3% (3/92) with positive predictive value of 50% (3/6). In the 5 participants with previously identified cancers, no recurrence or metastasis was detected.
The offer of whole-body FDG-PET for cancer screening was welcomed with enthusiasm by most of the hospital employees. PET/CT combines the merits of PET and CT and can be administered to and provide benefits to a select group of hospital employees.
Keywords: cancer screening, FDG, nuclear medicine, oncologic imaging, PET/CT
1. Introduction
Cancer is a major public health problem worldwide and is the first or second leading cause of death in developed countries.[1–3] Early detection of cancer is crucial for initiating treatment, prolonging survival, reducing mortality, and decreasing economic burden. To achieve early detection, various modalities for cancer screening have been developed, studied, and debated.[3,4] The National Cancer Institute in the United States estimated that 3% to 35% of premature deaths could be avoided through cancer screening.[5] Similar to various tumor markers, conventional cancer screening is organ-specific. In other words, conventional screening is unable to detect cancers outside of the target organ or to determine disease severity. Ideally, cancer screening should be a noninvasive and painless procedure that can reliably detect various cancers at a potentially curable stage regardless of location.[3]
Positron emission tomography with the glucose analog 2-[18F]fluoro-2-deoxy-d-glucose (FDG-PET) has increasingly been recognized as a powerful tool with which to evaluate various malignant tumors. FDG is an analog of glucose, which is obtained by and trapped in cells and is labeled with a positron-emitting isotope. Cancer cells can trap more FDG than normal cells, subsequently causing more radiation to be detected by PET. FDG-PET is an imaging method based on the increased rate of glucose metabolism in malignant tumors, which can be detected before anatomic changes. FDG-PET also provides improved differentiation of tumor malignancy. FDG-PET has high sensitivity for tumor detection. However, there is physiologic FDG uptake in the brain, ocular muscles, nasopharynx, tonsils, salivary glands, intrinsic laryngeal muscles, heart, great vessels, breasts, liver, spleen, pancreas, stomach, intestines, kidneys, ureters, urinary bladder, genital organs, bone marrow, and muscles. A number of benign processes, particularly inflammation, also show higher uptake of FDG than background tissues. Recognition of the physiological uptake of FDG and these benign processes is important to avoid misinterpretation of PET. In a nationwide questionnaire survey of cancer screening in Japan, the cancer discovery rate of 1.35% associated with FDG-PET was much higher than the 0.1% rate in conventional cancer screening.[3] The discovered rate of cancer varied in different age groups and increased by 0.4%, 0.76%, 1.07%, 1.49%, 2.08%, and 2.56% in the ≤fourth, fifth, sixth, seventh, eighth, and ≥ninth decades of life, respectively.[6]
A multicenter study in the United States found that nearly all of the patients surveyed believed that full-body computed tomography (FBCT) would provide personal reassurance about their health and that it could enable the identification of cancers that were still curable.[7,8] Many people are also interested in cancer screening in Asian countries. Whole-body FDG-PET is currently widely used in cancer screening. Combined PET and computed tomography (PET/CT) systems have emerged as promising imaging modalities, gradually replacing PET and becoming more routinely applied in clinical situations. With the ability of CT to provide anatomic mapping images and attenuation correction data, FDG-PET/CT could decrease the false-positive rate and improve the specificity of FDG-PET.[3–5]
In a health promotion proposal project supported by our institution, hospital employees between 55 and 65 years old were provided the opportunity to receive a free whole-body FDG-PET/CT scan. This selected population exhibits a relatively higher incidence of cancer and is in the last decade before retirement, according to the regulations of our government. The aim of this study was to survey compliance in undergoing this examination and the preliminary results of cancer screening in a cohort of hospital employees.
2. Methods
2.1. Patients
A total of 116 hospital employees, 55 to 65 years of age, were offered whole-body FDG-PET in our hospital, including doctors (n = 44), nurses (n = 8), pharmacologists (n = 7), technicians (n = 3), and administrators (n = 54). Ninety-seven participants (83.6%) completed whole-body FDG-PET from February 2014 to August 2014 in our PET center. Eleven doctors, 3 nurses, 1 technician, and 4 administrators refused the examination. Among the 97 examinees, 5 presented with previously diagnosed cancers, including 1 participant with prostate cancer, 1 with endometrial cancer, 1 with nasopharyngeal cancer, and 2 with breast cancer. After completion of the PET/CT examination, an experienced attending physician provided a detailed explanation of the scan results to allay doubts of the subjects while providing information. A concluding report was provided to each participant within 1 week. If any subject was suspected of having cancer, he or she was provided with a clinic appointment and was introduced to an authoritative doctor to provide appropriate treatment. The final confirmation of cancer was based on a pathologic report and follow-up of more than 1 year. This study was approved by the Kaohsiung Veterans General Hospital institutional review board. Written or verbal informed consent to participate in this study was waived by the hospital ethics committee because of the retrospective nature of the study.
2.2. FDG PET/CT imaging
Patients fasted for at least 6 hours before whole-body FDG PET imaging. An intravenous catheter was placed for radiopharmaceutical administration, and the patient's blood glucose levels were measured before injecting the tracer. All of the patients exhibited blood glucose levels of <150 mg/dL at the time of injection. Each patient received 370 to 555 MBq of F-18 FDG, according to his or her body weight (7.03 MBq/kg). After the tracer injection, the patients rested for 1 hour on a comfortable bed in a dark room. Whole-body FDG-PET imaging (Discovery ST-16; GE Healthcare, Milwaukee, WI) was performed from the head to the upper thigh with the patients in a supine position. A delayed image, with or without the use of diuretics, was obtained when necessary. CT scanning was performed before PET imaging. The following parameters were used: 0.6 seconds per rotation: 120 kV, 100 mA, and 3.75-mm thick slices. After CT scanning, PET images of the same regions were acquired in 2-dimensional (2D) mode, and 4 minutes of data were collected per bed position. Attenuation-corrected PET images were reconstructed using an ordered subset expectation maximization iterative reconstructed algorithm. The 3.75-mm thick transaxial CT images were reconstructed at 3.27-mm intervals for fusion with the PET images. PET, CT, and fused PET/CT images were generated on a Xeleris image display and processing platform (GE Healthcare) for review on a computer workstation.
2.3. Image analysis
The PET, CT, and fused PET/CT images were interpreted by 2 qualified nuclear medicine physicians who were allowed to manipulate the image contrast, image intensity, and 3-dimensional images on a computer screen. The final diagnoses were made by consensus. The physicians were not blinded to the medical history or outcomes at the times of image analysis. Prior imaging, especially prior PET/CT and contrast-enhanced CT scans, was available at the time of review to enable the fullest analysis. Both physicians reviewed the data independently before reaching a consensus. Any increase in FDG uptake was compared to the corresponding anatomical findings on the CT image. For areas with abnormal FDG uptake, the physicians outlined the region of interest (ROI), which indicated the area with the greatest amount of uptake. The standardized uptake value (SUV), a marker of tumor glucose metabolism, was determined semiautomatically using the SUV tools available in the Xeleris software package (SUV = activity in the ROI [Bq/g]/[injected dose (Bq)/body weight (g)]). The 2D ROI was drawn around the tumor on each transaxial slice that contained tumor tissue. A single-pixel maximum SUV (SUVmax) was determined for each region, and the slice with the highest SUV was considered to be the SUVmax for the entire tumor.
2.4. Statistical analysis
The results were analyzed on a pathological basis (when histological sampling was possible), via other imaging modalities, and/or with clinical follow-up evaluations. The discovery of cancer was defined as the detection of a malignant lesion within 12 months of the whole-body FDG-PET scan. The chi-square test was used to examine differences between groups. Differences were considered to be significant at P < 0.05. All of the calculations were performed using SPSS software, version 12.0 (SPSS, Chicago, IL).
3. Results
3.1. Identification of patients
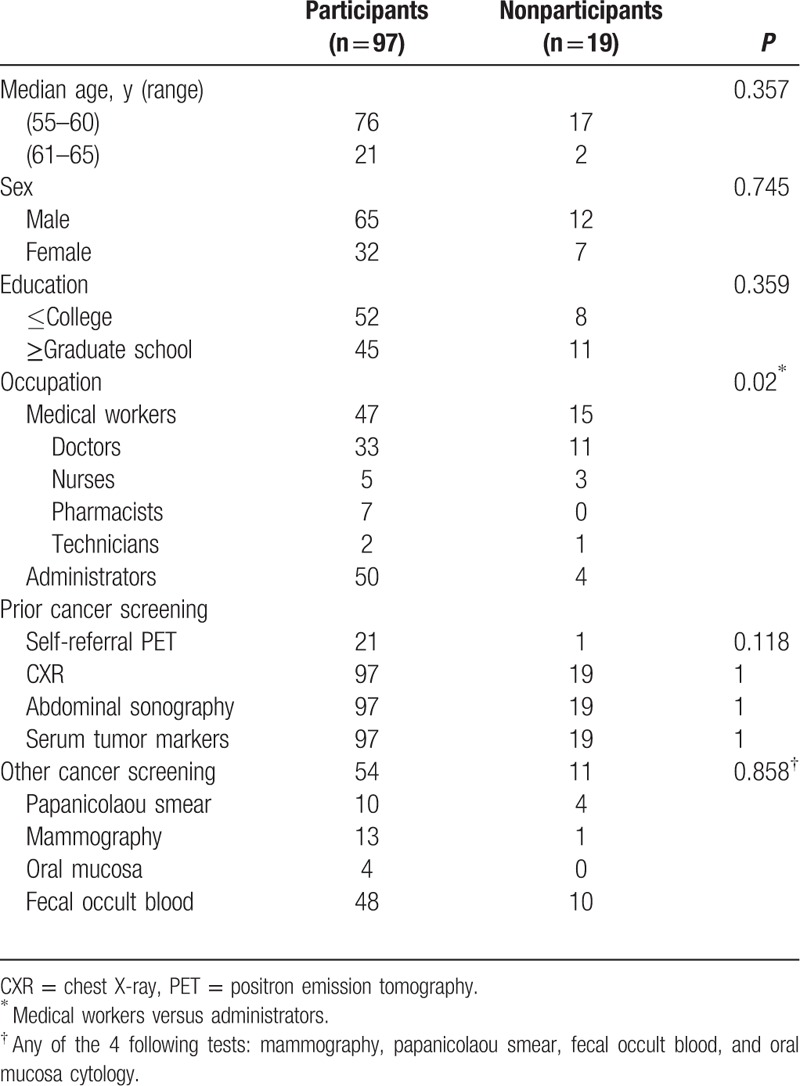
Characteristics of the 116 hospital employees in the present study are listed in Table 1. Ninety-seven employees (83.6%) underwent whole-body FDG-PET from February 2014 to August 2014 in our PET center. There were no significant differences in age, sex, education level, or prior cancer screening between the 97 examinees and the 19 hospital employees who opted out of the test. Medical employees (doctors, nurses, and technicians) were significantly less willing to undergo this examination compared to administrators (15/62 vs 4/54, P = 0.02). The reasons for opting out of the whole-body FDG-PET in the 19 hospital employees included the following: too busy to spend the time for the test (n = 7), concern about the harm of radiation (n = 5), worry about receiving bad news (n = 3), feeling healthy without the need for further testing (n = 3), and recent FDG-PET testing (n = 1). Twenty-one of the 97 examinees and only 1 of the 19 hospital employees who opted out of the test had received a prior self-referral PET in our center (21/97 vs 1/19, P = 0.118).
Table 1.
Characteristics of the 116 hospital employees in the present study.
3.2. Cancer screening
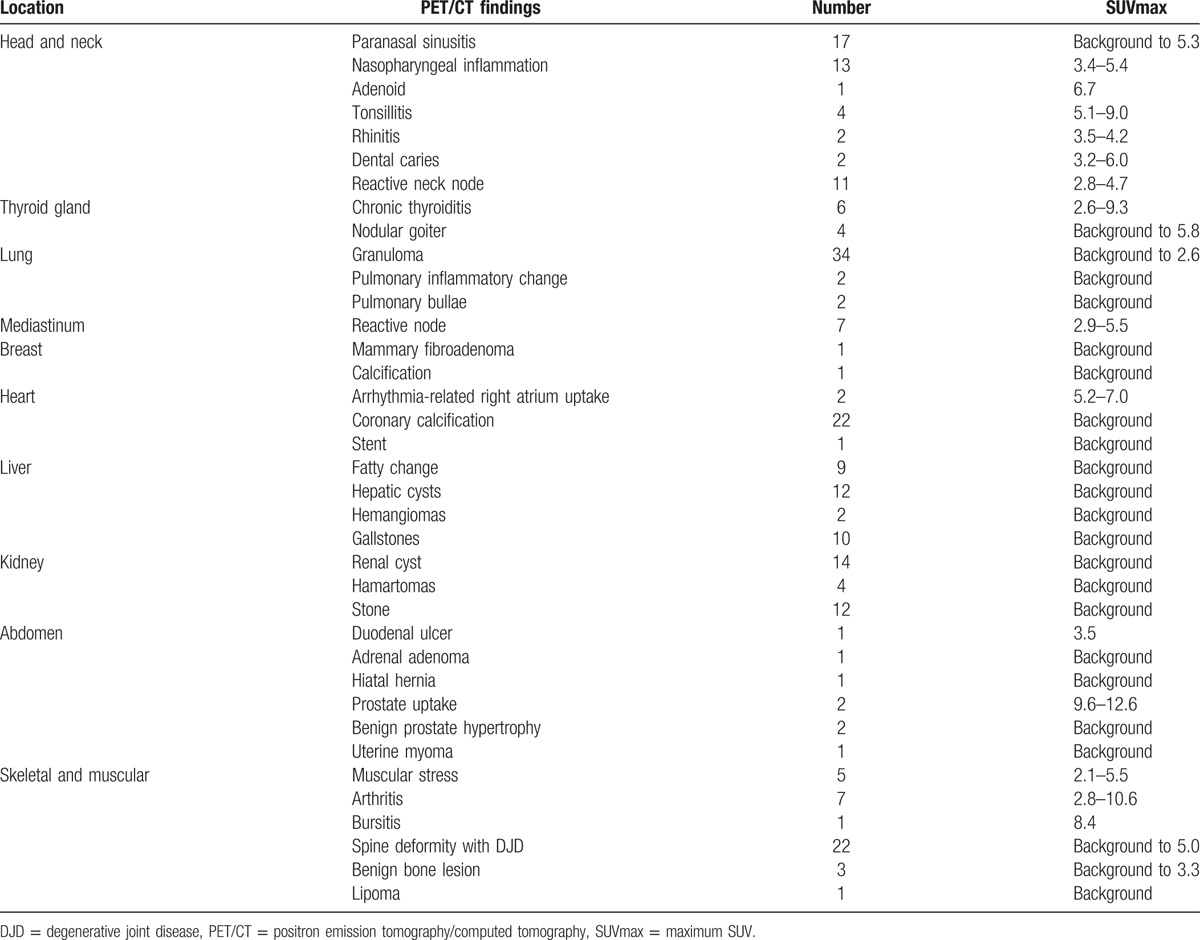
Among the 97 examinees, 92 were asymptomatic and 5 presented with previously diagnosed cancers. Seven of the 92 asymptomatic examinees (7.6%) were referred to the clinic for further examination due to significant nodular lesions (Table 2). Six of them received surgical interventions; pathological findings demonstrated malignancy in 3 participants and benign lesions in 3 participants. Cytology of the 1 thyroid aspiration revealed only a few macrophages. All of the confirmed malignant lesions were papillary thyroid cancers (Fig. 1). They were classified as stage I, II, or III, and the patients subsequently underwent total thyroidectomy and I-131 ablation. All of the patients are now under regular follow-up. Benign lesions included meningioma (Fig. 2), Castleman disease (Fig. 3), follicular adenoma, and nodular goiter. Meningioma was considered clinically relevant and required surgical resection. Other patients underwent surgical intervention to exclude the possibility of malignancy in lesions. The true positive rate for subjects with suggested possible cancer (positive predictive value, PPV) by FDG-PET/CT was 50% (3/6).
Table 2.
Positive PET/CT findings with pathologic or cytological verification in 92 asymptomatic participants.
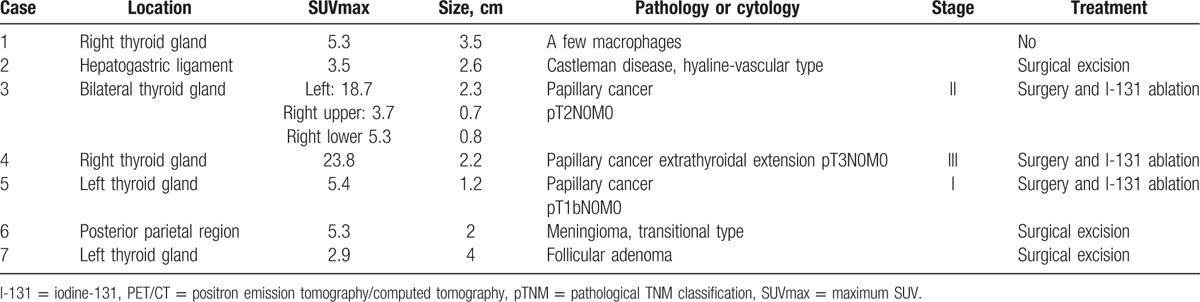
Figure 1.
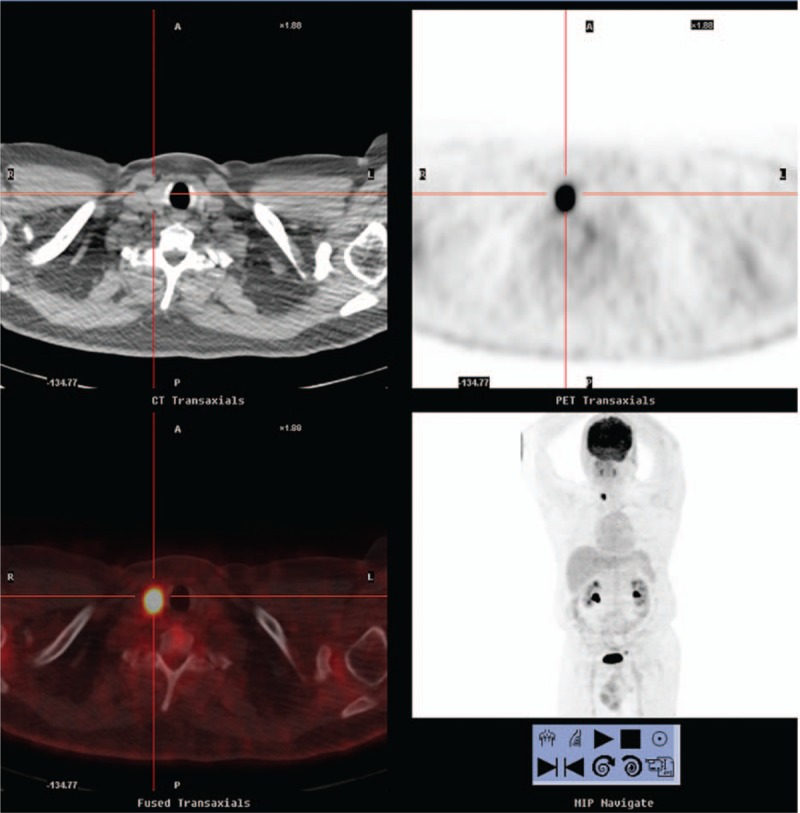
A 56-year-old asymptomatic man with no indication of systemic disease. Positron emission tomography/computed tomography demonstrated a 2.2-cm 2-[18F]fluoro-2-deoxy-d-glucose–avid lesion in the right thyroid lobe (maximum standard uptake value : 23.8, cross cursor). Pathology revealed papillary carcinoma with focal minimal extension into the perithyroid soft tissue, pT3N0M0, stage III. The patient received total thyroidectomy and I-131 ablation. He is currently under clinical follow-up.
Figure 2.
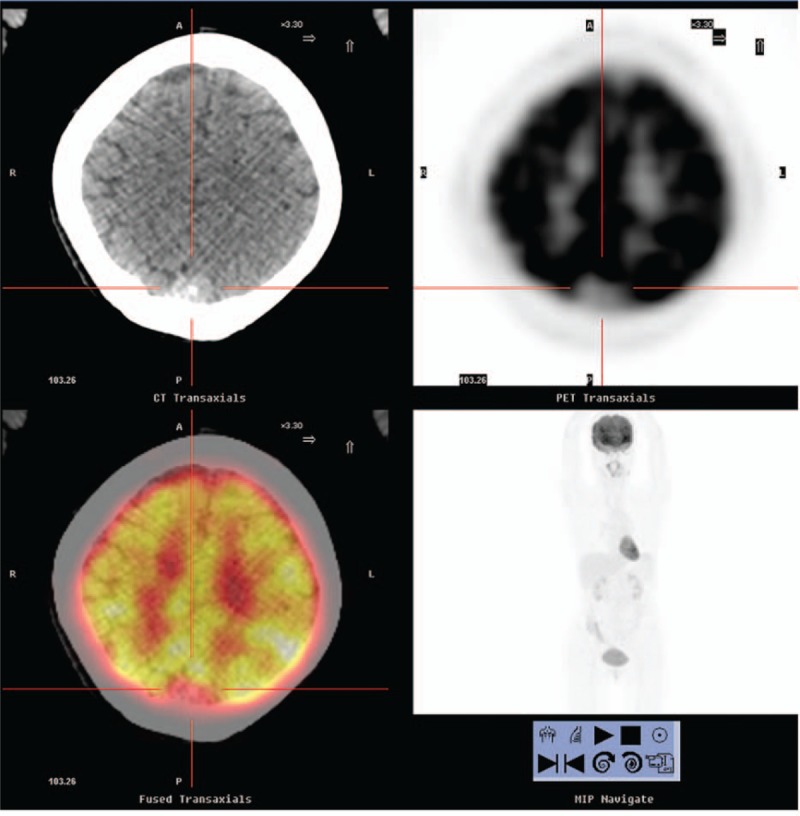
A 57-year-old woman with no indication of systemic disease. Positron emission tomography/computed tomography demonstrated a non-2-[18F]fluoro-2-deoxy-d-glucose–avid calcified dura-based nodule in the posterior sagittal region approximately 2 cm in size (maximum standard uptake value: lower than cerebral background, cross cursor), indicating the presence of a meningioma over the parasagittal occipito–parietal region. The patient underwent surgery, and pathology revealed a transitional type meningioma.
Figure 3.
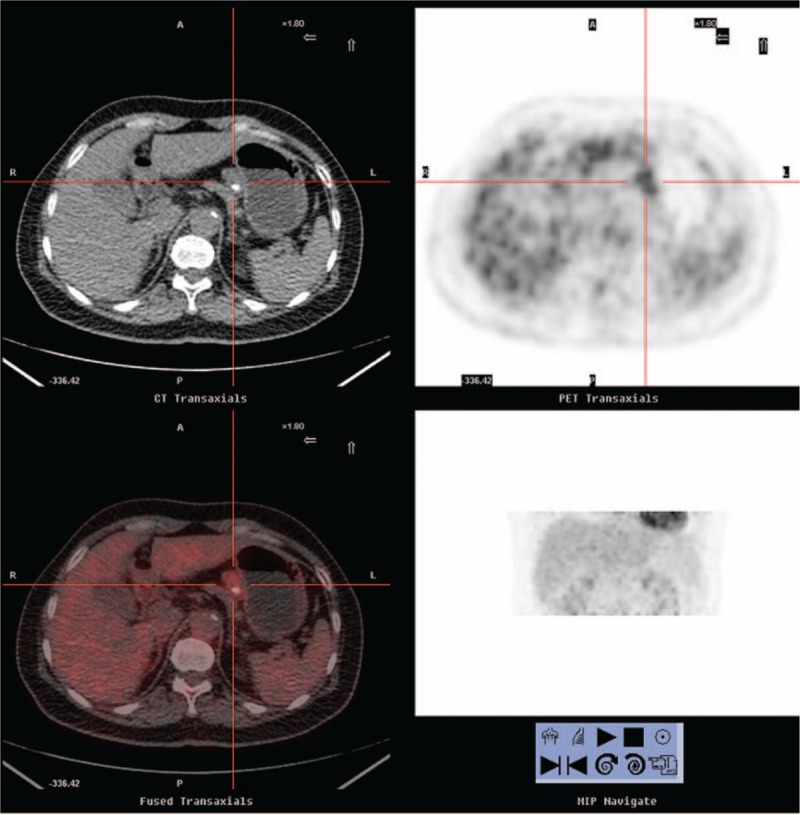
A 61-year-old woman with no indication of systemic disease. Positron emission tomography/computed tomography demonstrated a lesion with moderate 2-[18F]fluoro-2-deoxy-d-glucose uptake in the hepatogastric ligament (maximum standard uptake value: 3.5, cross cursor), approximately 2.6 cm in size with a calcified spot inside it. The patient received surgery, and pathological examination revealed Castleman disease of the hyaline-vascular type.
Other findings addressed in the reports of noncancerous lesions are listed in Table 3. All tumors were rechecked by physical examination or medical history, or the patients were referred to a specialist for further confirmation or follow-up for at least 1 year. Two participants with right atrial uptake exhibited a medical history of arrhythmia with right bundle branch block.[9] One participant with a 1.6-cm non-FDG-avid low-CT-attenuation nodule in the right adrenal gland was diagnosed with a benign adenoma. Two participants exhibited focal FDG uptake in the prostate, which demonstrated decreased activity on delayed imaging. These participants were under clinical follow-up with negative serum prostate-specific antigen (PSA). Six participants with diffuse uptake in the thyroid gland were offered serum thyroid surveillance. Three of the 6 were diagnosed with autoimmune thyroid disease, and 1 participant was receiving medical treatment for previously diagnosed hyperthyroidism. Two of the 6 patients with diffuse thyroid uptake tested negative during follow-up. One participant with unilateral elevated tonsil activity developed tonsillitis a few days after the scan. One patient with a strong signal in the left iliopsoas bursa was diagnosed with iliopsoas bursitis on follow-up MRI.
Table 3.
Other findings of PET/CT in the 97 participants.
For the 5 participants with previous cancer diagnoses, none exhibited a positive PET for recurrence or metastasis. All of the participants with negative PET/CT findings remained asymptomatic during more than 1 year of follow-up. The sensitivity of FDG-PET/CT was 100% in this study.
3.3. Psychological effects after screening
A psychological survey was performed 1 year after screening. Eighty-one participants (81/97, 83.5%) responded to the questionnaire. Some of the participants were unavailable due to retirement. All of the respondents (81/81, 100%) reported that they were satisfied with the examination procedures. Seventy-two (72/81, 88.9%) stated that they were happy to have enrolled in this project, and 75 (75/81, 92.6%) were willing to recommend this examination to family and friends. Because a detailed interpretation of the PET/CT results was provided, most of the participants (77/81, 95.1%) reported no experience of worry, anxiety, depression, or psychological distress after the PET/CT examination.
4. Discussion
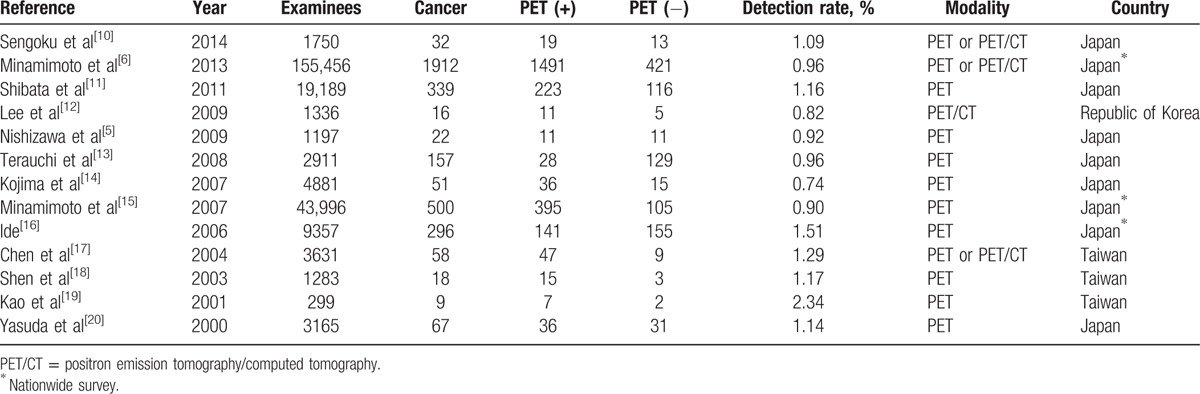
Whole-body FDG-PET has been used for decades to screen underlying malignancies in asymptomatic individuals. Cancer screening focuses on early detection to reveal curable cancers that would be fatal if left untreated. FDG-PET has the potential to detect a wide variety of cancers at a potentially curable stage.[3,4] In this study, we utilized FDG-PET/CT in a cohort of hospital employees ranging in age from 55 to 65 years old, and we uncovered malignant tumors in 3 of 92 (3.3%) asymptomatic participants with a PPV of 50%. This discovery rate was much higher than previously reported.[5,6,10–20] However, the PPV in prior articles has varied from 3.3% to 70%.[18,19,21] Some authors have indicated that the interpretation of the cancer discovery rate and PPV should be considered in the context of disease prevalence, and disease prevalence changes with age. A few studies have examined prevalence and divided subjects by age range.[6,12] Minamimoto et al[6] demonstrated increased cancer discovery rates of 0.4%, 0.76%, 1.07%, 1.49%, 2.08%, and 2.56%, and PPV of 16.5%, 22.9%, 24.1%, 27.4%, 33.1%, and 40.9% in the fourth, fifth, sixth, seventh, eighth, and ≥ninth decades, respectively. Lee et al[12] reported that cancer detection rates were 1.0%, 0.9%, 1.5%, 1.2%, and 0.9% in the ≤fourth, fifth, sixth, seventh, and ≥eighth decades, respectively. The discovery rate of cancer and PPV in our study were closer to the rates reported in older patients.
FDG-PET was first used for cancer screening in Japan.[22] Additional reports of the use of FDG-PET in cancer screening have emerged, mostly from Asian countries (Table 4). The cancer detection rate with FDG-PET in asymptomatic individuals varies from 0.74% to 2.34% with follow-up periods of 6 months to 2 years. In a nationwide questionnaire survey of cancer screening in Japan, the discovery rate of cancers was 0.9% (358/39,785) by FDG-PET alone and 1.35% (526/39,785) by combined methods of detection. The most commonly detected malignancies were thyroid, lung, colon, and breast cancers.[3] In our study, the 3 confirmed cancers were all papillary thyroid tumors at potentially curable stages. This phenomenon might have been due to the increased incidence of thyroid cancer in recent years, primarily in patients aged 60 to 70 years old.[23,24] Most of the hospital employees in our study received additional tests for cancer screening (Table 1). In our hospital, standard tests including physical examination, blood, urine, and stool tests, serum tumor markers (CA-125, CA-153, carcinoembryonic antigen [CEA], alpha-fetoprotein [AFP] and CA-199 for women and PSA, CEA, AFP and CA-199 for men), as well as chest X-ray and abdominal sonography, are performed every 2 to 3 years. Furthermore, 4 conventional cancer screenings including mammography, Papanicolaou smear, fecal occult blood, and oral mucosa cytology are provided free to a selected population in Taiwan. One-fifth of the hospital employees in our study had received a prior self-referral FDG-PET. Therefore, some cancers could have been previously screened out. However, FDG-PET/CT served as a complementary test to conventional cancer screening, and did discover 3 unexpected thyroid cancers. In summary, focal FDG uptake in the thyroid gland required further evaluation due to a significant risk of malignancy and an increasing incidence of thyroid cancer in recent years. In addition to the high cost of FDG-PET/CT, we suggested that a survey of the thyroid gland, especially with the high sensitivity and specificity of ultrasonography for detecting thyroid lesions, should be included in the standard tests for cancer screening.
Table 4.
Review summary of cancer screenings by PET or PET/CT in asymptomatic participants.
Three of the 92 asymptomatic participants (3.3%) in our patient group exhibited histological benignancy, and another patient exhibited cytological benignancy. These benign lesions included meningioma, Castleman disease, follicular adenoma, and nodular goiter. Meningioma is the most common benign intracranial tumor, accounting for approximately 13% to 26% of all primary intracranial tumors.[25] FDG-PET allowed for the differentiation of histopathologic grade and the biological aggressiveness of meningiomas.[26–30] The meningioma identified in the present study belonged to a low-grade class, which was compatible with a final pathology of meningioma, transitional type. Castleman disease, alternatively referred to as giant lymph node hyperplasia or angiofollicular lymph node hyperplasia, is a rare lymphoproliferative disorder of uncertain etiology. Histopathology demonstrates 2 types of Castleman disease: a hyaline-vascular type and a plasma cell type. Although initially described as a benign localized lymphoproliferative disease, the systemic forms of Castleman disease have been associated with an increased risk for related neoplasms including Kaposi sarcoma; non-Hodgkin lymphoma; Hodgkin lymphoma; and polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome.[31] Most patients with follicular adenomas are clinically and biochemically euthyroid. Approximately 1% of follicular adenomas are “toxic adenomas”, which can lead to symptomatic hyperthyroidism. Approximately 20% of nonfunctioning follicular adenomas possess oncogene mutations that can predispose them to progressing to follicular carcinoma.[32,33] Although a higher mean SUVmax is observed in malignant nodules compared to benign nodules (6.9 vs 4.8, P < 0.001), an optimal cutoff value of SUVmax when detecting focal thyroid FDG uptake remains to be determined. Follow-up with ultrasound or fine needle aspiration is highly recommended.[34,35] Other significant findings without pathologic verification in this study included recent upper respiratory infection, adenoid hyperplasia, paranasal sinusitis, dental disease, tonsillitis, autoimmune thyroid disease, duodenal ulcer, arthritis, and iliopsoas bursitis. Most of these findings were correlated with participant medical history, physical examination, laboratory data, or follow-up imaging. Inherent false positive results for cancer screening exist, although patients can benefit from these findings. In the present study, false positives also resulted in the surgical resection of significant tumors. Furthermore, some significant infectious/inflammatory diseases were detected and appropriately treated or referred for follow-up. The early detection of these diseases could lead to more effective treatment options and could improve patient survival rates.
The proposal of whole-body FDG-PET for cancer screening was greeted with great enthusiasm by the hospital employees. Ninety-seven employees (83.6%) underwent whole-body FDG-PET in our PET center. Medical workers (doctors, nurse, and technicians) were significantly less willing to participate in this examination than administrators (15/62 vs 4/54, P = 0.02). Most of the medical workers explained that they were too busy for the test and/or were worried about the harm of radiation and/or the possibility of receiving bad news. In our hospital, there are many radiation workers, including radiologists, cardiologists, radiation oncologists, nuclear medicine physicians, radiology technicians, nuclear pharmacists, and others. They undergo radiation exposure at work, and their doses were limited according to ionizing radiation regulations. Therefore, they were more concerned about radiation damage. Most of the participants were satisfied with the procedure, happy to be enrolled in this project, and willing to refer this examination to their family and friends. A US national telephone survey of 500 adults addressing public enthusiasm for cancer screening revealed that 86% would undergo free total body imaging if offered; 85% preferred FBCT to receiving $1000 in cash, and 87% of respondents felt that “routine cancer screening tests for healthy persons are almost always a good idea”. Although nearly half of the respondents who had experienced a false-positive result expressed fear, all of them were glad to have undergone the screening test.[36] Most of the respondents (93.5%) agreed that the screening provided peace of mind and reduced worries about their health.[7] Most of the participants in the current study (77/81, 95.1%) reported no sense of worry, anxiety, depression, or psychological distress after the PET/CT examination. We believed that an immediate and detailed interpretation of the PET/CT results dispelled the doubts and fears of participants and encouraged them to accept the scan results.
Our study had some limitations. First, this study was retrospective, FDG-PET/CT was applied in a specific cohort of hospital employees, and 19 of 116 (16.4%) candidates did not complete the assessment. Second, only 7 participants received histopathologic conformation of suspected lesions; other patients were diagnosed via additional imaging modalities and/or clinical follow-up. Third, we actually limited whole-body imaging from the head to the upper thigh in most of the examinees because cancer below the thigh is rare in adults. Limited whole-body PET/CT has advantages over true whole-body PET/CT, primarily decreased scanning time, and decreased radiation.[37,38] Finally, the total number of participants in this study was small. Additional studies with a prospective trial design investigating a specific cohort of patients would be valuable.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CEA = carcinoembryonic antigen, FBCT = full-body computed tomography, FDG = 2-[18F]fluoro-2-deoxy-d-glucose, PET/CT = positron emission tomography/computed tomography, PPV = positive predictive value, PSA = prostate-specific antigen, ROI = region of interest, SUV = standard uptake value, SUVmax = maximum SUV.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jan J Clin Oncol 2015; 45:1–6. [DOI] [PubMed] [Google Scholar]
- 3.Ide M, Suzuki Y. Is whole-body FDG-PET valuable for health screening? For Eur J Nucl Med Mol Imaging 2005; 32:339–341. [DOI] [PubMed] [Google Scholar]
- 4.Schöder H, Gönen M. Screening for cancer with PET and PET/CT: potential and limitations. J Nucl Med 2007; 48 (suppl 1):4S–18S. [PubMed] [Google Scholar]
- 5.Nishizawa S, Kojima S, Teramukai S, et al. Prospective evaluation of whole-body cancer screening with multiple modalities including [18F]fluorodeoxyglucose positron emission tomography in a healthy population: a preliminary report. J Clin Oncol 2009; 27:1767–1773. [DOI] [PubMed] [Google Scholar]
- 6.Minamimoto R, Senda M, Jinnouchi S, et al. The current status of an FDG-PET cancer screening program in Japan, based on a 4-year (2006–2009) nationwide survey. Ann Nucl Med 2013; 27:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolber CT, Zipp G, Glendinning D, et al. Patient expectations of full-body CT screening. Am J Roentgenol 2007; 188:W297–W304. [DOI] [PubMed] [Google Scholar]
- 8.Kalish GM, Bhargavan M, Sunshine JH, et al. Self-referred whole-body imaging: where are we now? Radiology 2004; 233:353–358. [DOI] [PubMed] [Google Scholar]
- 9.Fujii H, Ide M, Yasuda S, et al. Increased FDG uptake in the wall of the right atrium in people who participated in a cancer screening program with whole-body PET. Ann Nucl Med 1999; 13:55–59. [DOI] [PubMed] [Google Scholar]
- 10.Sengoku T, Matsumura K, Usami M, et al. Diagnostic accuracy of FDG-PET cancer screening in asymptomatic individuals: use of record linkage from the Osaka Cancer Registry. Int J Clin Oncol 2014; 19:989–997. [DOI] [PubMed] [Google Scholar]
- 11.Shibata K, Arai M, Uno K, et al. Relationship of detection rate of PET cancer screening examinees and risk factors: analysis of background of examinees. Ann Nucl Med 2011; 25:261–267. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Kang KW, Paeng JC, et al. Cancer screening using F-18 FDG-PET/CT in Korea asymptomatic volunteers: a preliminary report. Ann Nucl Med 2009; 23:685–691. [DOI] [PubMed] [Google Scholar]
- 13.Terauchi T, Murano T, Daisaki H, et al. Evaluation of whole-body cancer screening using F-18 2-deoxy-2-fluoro-d-glucose positron emission tomography: a preliminary report. Ann Nucl Med 2008; 22:379–385. [DOI] [PubMed] [Google Scholar]
- 14.Kojima S, Zhou B, Teramukai S, et al. Cancer screening of healthy volunteers using whole-body 18F-FDG-PET scans: The Nishidai clinic study. Eur J Cancer 2007; 43:1842–1848. [DOI] [PubMed] [Google Scholar]
- 15.Minamimoto R, Senda M, Uno K, et al. Performance profile of FDG-PET and PET/CT for cancer screening on the basis of a Japanese Nationwide Survey. Ann Nucl Med 2007; 21:481–498. [DOI] [PubMed] [Google Scholar]
- 16.Ide M. Cancer screening with FDG-PET. Q J Nucl Med Mol Imaging 2006; 50:23–27. [PubMed] [Google Scholar]
- 17.Chen YK, Ding HJ, Su CT, et al. Application of PET and PET/CT imaging for cancer screening. Anticancer Res 2004; 24:4103–4108. [PubMed] [Google Scholar]
- 18.Shen YY, Su CT, Chen GJ, et al. The value of 18F-fluorodeoxyglucose positron emission tomography with the additional help of tumor markers in cancer screening. Neoplasma 2003; 50:217–221. [PubMed] [Google Scholar]
- 19.Kao CH, Kwan AS, Kwan JK, et al. The role of F-18 fluorodeoxyglucose positron emission tomography in cancer screening—a preliminary report. Oncology Reports 2001; 8:1145–1148. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda S, Ide M, Fujii H, et al. Application of positron emission tomography imaging to cancer screening. Br J Cancer 2000; 83:1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghotbi N, Iwanaga M, Ohtsuru A, et al. Cancer screening with whole-body PET/CT for healthy asymptomatic people in Japan: re-evaluation of its test validity and radiation exposure. Asian Pac J Cancer Prev 2007; 8:93–97. [PubMed] [Google Scholar]
- 22.Yasuda S, Ide M. PET and cancer screening. Ann Nucl Med 2005; 19:167–177. [DOI] [PubMed] [Google Scholar]
- 23.Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update, epidemiology and risk factors. J Can Epidemiol 2013; 2013:965212.article ID 965212, 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris LG, Silkora AG, Tosteson TD, et al. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 2013; 23:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marosi C, Hassler M, Roessler K, et al. Meningioma. Crit Rev Oncol Hematol 2008; 67:153–171. [DOI] [PubMed] [Google Scholar]
- 26.Cremerius U, Bares R, Weis J, et al. Fasting improves discrimination of grade 1 and atypical or malignant meningioma in FDG-PET. J Nucl Med 1997; 38:26–30. [PubMed] [Google Scholar]
- 27.Di Chiro G, Hatazawa J, Katz DA, et al. Glucose utilization by intracranial meningiomas as an index of tumor aggressivity and probability of recurrence: a PET study. Radiology 1987; 164:521–526. [DOI] [PubMed] [Google Scholar]
- 28.Lippitz B, Cremerius U, Mayfrank L, et al. PET-study of intracranial meningiomas: correlation with histopathology, cellularity and proliferation rate. Acta Neurochir Suppl 1996; 65:108–111. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Kang KW, Park SH, et al. 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging 2009; 36:1574–1582. [DOI] [PubMed] [Google Scholar]
- 30.Okuchi S, Okada T, Yamamoto A, et al. Grading meningioma: a comparative study of thallium-SPECT and FDG-PET. Medicine 2015; 94:e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan R, Chen JH, Trotman-Dickenson B, et al. The spectrum of Castleman's disease: mimics, radiologic pathologic correlation and role of imaging in patient management. Eur J Radiol 2012; 81:123–131. [DOI] [PubMed] [Google Scholar]
- 32.McHenry CR, Phitayakom R. Follicular adenoma and carcinoma of the thyroid gland. Oncologist 2011; 16:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 2006; 6:292–306. [DOI] [PubMed] [Google Scholar]
- 34.Ho TY, Liou MJ, Lin KJ, et al. Prevalence and significance of thyroid uptake detected by F-18 FDG-PET. Endocrine 2011; 40:297–302. [DOI] [PubMed] [Google Scholar]
- 35.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz LM, Woloshin S, Fowler FJ, et al. Enthusiasm for cancer screening in the United States. J Am Med Assoc 2004; 291:71–78. [DOI] [PubMed] [Google Scholar]
- 37.Sammer MBK, Shulkin BL, Alessio A, et al. Role of limited whole-body PET/CT in pediatric lymphoma. Am J Roentol 2011; 196:1047–1055. [DOI] [PubMed] [Google Scholar]
- 38.Sebro R, Mari-Aparici C, Hemandez-Pampaloni H. Value of true whole-body FDG-PET/CT scanning protocol in oncology: optimization of its use based on primary diagnosis. Acta Radiol 2013; 54:534–539. [DOI] [PubMed] [Google Scholar]


