Abstract
Background:
To evaluate the efficacy of fractional carbon dioxide (CO2) laser combined with luliconazole 1% cream for the treatment of onychomycosis and to compare it with that of fractional CO2 laser alone.
Methods:
This was a randomized, parallel group, 2-arm, positive-controlled, single-center, superiority trial with a 1:2 allocation ratio. Sixty patients with clinical and mycological diagnosis of onychomycosis were enrolled from the Dermatology Department of the First Affiliated Hospital of Nanjing Medical University in Nanjing, China from March 2015 to May 2015. Patients were randomized following simple randomization procedures (computerized random number generator) into 2 groups; L group only received 12 sessions of laser treatment at 2-week interval for 6 months, while L + D group received 12 sessions of laser treatment at 2-week interval combined with luliconazole 1% cream once daily for 6 months. This was not a blind trial. The main outcome measures were the clinical efficacy rate (CER) assessed from the percentage of fully and >60% normal-appearing nails and the mycological clearance rate (MCR) assessed from the percentage of nails with negative fungal microscopy. There were no changes to trial outcome measures after the trial commenced.
Results:
A total of 60 patients (N = 233 nails) completed treatments and follow-up, and were randomized and divided into 2 groups: L group (31 patients, N = 108 nails) and L + D group (29 patients, N = 115 nails). The CER and MCR of L + D group were 69.6% and 57.4%, respectively. L + D group showed significantly higher CER (69.6% vs 50.9%; χ2 = 8.1, P = 0.004) and MCR (57.4% vs 38.9%; χ2 = 7.6, P = 0.006) compared with those in L group. Some patients experienced mild pain during laser treatment, but there was no bleeding or oozing during or after treatment. There were no adverse effects reported during the observation period.
Conclusion:
Fractional CO2 laser treatment combined with 1% luliconazole cream for 6 months was an effective and safe method for the treatment of onychomycosis, and had a higher efficacy than fractional CO2 laser treatment alone.
Keywords: fractional CO2 laser, luliconazole, onychomycosis, topical treatment
1. Introduction
Onychomycosis, a fungal infection of the nail, is a refractory chronic infectious disease. It is the most common nail disorder in adults, accounting for up to 50% of all nail diseases.[1] The most common therapeutic options are oral and topical antifungal agents. However, oral medications have more adverse effects such as hepatotoxicity and potential drug interactions, and its clinical application is often limited, especially in patients with comorbidities.[2] Topical antifungal agents are often ineffective because of their limited ability to penetrate through the nail plate.[2] Therefore, many therapeutic trials have been conducted to find an alternative therapy that can increase the efficacy of onychomycosis treatment while avoiding its adverse effects.
In recent years, laser treatment of onychomycosis has garnered increasing attention. Currently used lasers for the treatment of onychomycosis are Nd:YAG laser, ultrapulse fractional carbon dioxide (CO2) laser, 870/930 nm dual wavelength diode laser, and photodynamic therapy.[3] Yang et al[4] reported fractional CO2 laser with a total of 8 sessions within 3 months showed a clinical efficacy of 52.11% in the treatment of onychomycosis. Furthermore, there were 2 studies reported that fractional CO2 laser combined with a topical antifungal agent showed a clinical efficacy of 71% and 73.32% in the treatment of onychomycosis, suggesting the combination therapy had a higher efficacy for treating onychomycosis than fractional CO2 laser alone.[5,6] It is generally believed that the mechanism by which fractional CO2 laser treats onychomycosis may mainly through vaporization and decomposition of target's local tissue and its bactericidal effect. Fungi are extremely sensitive to temperature above 55 °C; the photothermal effect of fractional CO2 laser can increase the temperature of local tissue, thus it plays a direct role in killing the fungi in the laser-treated affected nail.[7] Additionally, fractional CO2 laser makes the local tissue of affected nail vaporize and exfoliate, causing diffuse remodeling and at the same time destroys the fungal growth environment, thus contributing in fungal growth inhibition.[8] Furthermore, fractional CO2 laser can enhance the absorption of topical antifungal agents, thereby improving its efficacy.[9] However, the main drawback is the lack of control group in their clinical trials, therefore further study is needed.
Luliconazole is a newly developed imidazole antifungal drugs, it exerts a broad spectrum of antifungal effect by blocking ergosterol biosynthesis.[10] In this study, we used fractional CO2 laser combined with luliconazole 1% cream to treat onychomycosis. We aim to evaluate the efficacy of fractional CO2 laser combined with luliconazole 1% cream for treating onychomycosis and to compare it with that of fractional CO2 laser alone. To our knowledge, this is the first report on the efficacy and safety of fractional CO2 laser therapy combined with luliconazole 1% cream.
2. Methods
2.1. Trial design
This was a randomized, parallel group, 2-arm, positive-controlled, single-center, superiority trial with a 1:2 allocation ratio. There were no changes to methods after trial commencement.
2.2. Participants
A total of 60 patients were enrolled at the Dermatology Department of the First Affiliated Hospital of Nanjing Medical University in Nanjing, China. The study protocol was approved by the Local Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (trial registration, 2015-SRFA-032). All patients provided written informed consent. This study was conducted in accordance with CONSORT statement.[11]
The key inclusion criterion was a diagnosis of onychomycosis affecting fingernails and/or toenails by clinical nail morphology confirmed by positive fungal microscopy. Exclusion criteria were: patients with a history of keloid, localized bacterial infections, concomitant nail disease such as psoriasis, lichen planus or atopic dermatitis, or other skin diseases that may interfere with diagnosis and treatment; with allergy to drug used in the study; with heart, liver, kidney diseases, diabetes, or mental illness; had taken oral antifungal medication within the last 3 months or used topical antifungal medication within the last 2 weeks; had taken oral glucocorticoids or immunosuppressants within the last 3 months; with nail polish; and pregnant patients. Patients with negative fungal culture were not excluded if fungal microscopy was positive.
2.3. Randomization, interventions, and sample size
Patients were randomized following simple randomization procedures (computerized random number generator) into 2 groups: control group or L group (31 patients, N = 108 nails) only received laser treatment at 2-week intervals for 6 months; treatment group or L + D group (29 patients, N = 115 nails) received laser treatment at 2-week intervals combined with luliconazole 1% cream once daily for 6 months. This was not a blind trial.
We estimated the sample size based on the binomial categorical variables. With an estimated efficacy rate in the control group of 50%, an estimated efficacy rate in the treatment group of 70%, an alpha of 0.05, and an expected error value of 0.10, about 110 nails should be included into each group. If each patient has 3 to 4 affected nails, then each group should consist of approximately 30 persons for the study.
2.4. Fungal examination
Fungal microscopy and culture were done at the beginning of treatment. Only fungal microscopy was done at 3 months after the last treatment to determine mycological clearance.
Infected nails were cleaned with 75% alcohol. Subungual debris or nail plate debris from the involved nail plate was obtained by using a sterile scalpel or by nail clipping, then direct microscopy using potassium hydroxide preparation showing septate hyphae or pseudohyphae confirmed the fungal infection.
The specimen was cultured on Sabouraud dextrose agar medium with and without cycloheximide, both media contained chloramphenicol and peptone. The cultures were incubated at 25 °C and observed every other day for up to 4 weeks. No growth after 4 weeks confirmed a negative fungal culture.
2.5. Laser treatment
All patients were treated with 1 single pass of fractional CO2 laser (AcuPulse; Lumenis Ltd., Santa Clara, CA) in Deep mode at an energy of 10 to 15 mJ, a pulse duration of 0.5 to 1.0 seconds, a spot diameter of 4.0 to 10.0 mm, and a density of 10% over the affected area including 2 to 3 mm normal-appearing areas close to them. Laser treatment consisted of 12 sessions at 2-week interval within 6 months.
2.6. Topical antifungal treatment
Patients in L + D group were instructed to use topical luliconazole 1% cream (Hainan Hai Ling Pharmaceutical Co., Ltd., Hainan, China) on the affected areas, cover them with a plastic wrap once every night for 8 to 12 hours, and remove it in the next morning. Topical antifungal treatment was done everyday for 6 months.
2.7. Outcome assessment
The main outcome measures were the clinical efficacy rate (CER) assessed from the percentage of fully and >60% normal-appearing nails and the mycological clearance rate (MCR) assessed from the percentage of nails with negative fungal microscopy. There were no changes to trial outcome measures after the trial commenced.
2.8. Clinical cure
The nails were analyzed and classified into 4 grades, modified from Lim et al,[5] as follows: “complete response” (fully normal-appearing nail measured from the proximal nail fold to involved nail), “significant response” (>60% normal-appearing nail compared with the area of the initially infected nail), “moderate response” (20%–60% normal-appearing nail), and “no response” (<20% normal-appearing nail). The CER was defined as the total percentage of nails with complete response and significant response, and assessed at 3 and 6 months after the last treatment. Standardized photographs were obtained at the beginning of treatment and at 3, 6, and 9 months after the start of treatment using a digital single-lens camera (PowerShot G12, Canon, Tokyo, Japan).
2.9. Mycological cure
Fungal microscopy was performed for the mycological examination of all patients before treatment and at 3 months after the last treatment. MCR was defined as the total percentage of nails with a negative fungal microscopy showing either complete clinical cure or other clinical responses.
2.10. Influencing factors
Baseline data, such as age, sex, duration of disease, involved nails (thumb/big toenails or other finger/toenails; finger or toenails), clinical type (fully or partly damaged), affected nail's initial thickness, species of fungus, and Onychomycosis Severity Index (OSI) score, were collected before treatment. At 3 months after the last treatment, the influence of the above factors on treatment outcome was assessed.
Clinical severity was determined using OSI by evaluating the percentage of nail plate involvement (0–5 points), proximity of infection to the matrix (1–5 points), and degree of subungual hyperkeratosis or presence of dermatophytoma (10 points if nail's thickness ≥2 mm or dermatophytoma is present, otherwise no point is added).[12] A total score of 5 or less is classified as mild, 6 to 15 as moderate, and 16 to 35 as severe.[12]
2.11. Patient satisfaction
At the end of the study, the patients documented their degree of satisfaction as “very satisfied,” “satisfied,” “slightly satisfied,” or “not satisfied.”
2.12. Statistical analysis
We conducted all statistical analyses with the SPSS software (version 20.0; SPSS Inc, Chicago, IL). We used Chi-square test for analyzing categorical variables and t test for analyzing quantitative data. Multivariate logistic regression analysis was performed to identify independent factors. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Participants
Figure 1 shows the participant flow diagram. A total of 60 patients were assessed for eligibility and enrolled into the study from March 2015 to May 2015. None was excluded. They were randomly assigned into 2 groups: 31 patients in L group and 29 patients in L + D group, received intended treatment, and were analyzed for the outcome.
Figure 1.
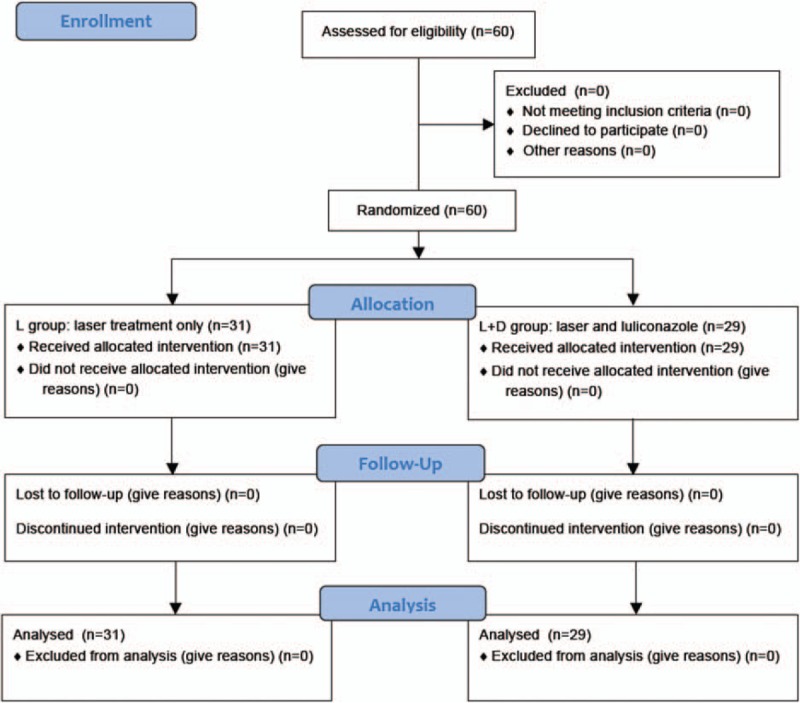
Participant flow diagram; 60 patients met eligibility criteria of a diagnosis of onychomycosis by clinical toenail morphology confirmed by positive fungal microscopy.
3.2. Baseline characteristics
The 60 patients consisted of 22 males and 38 females with an average age of 37.4 years (age range, 19–76 years). There were a total of 223 nails with 3.7 affected nails per patient. The mean initial thickness of the nail plate was 2.27 mm (range, 1.1–4.4 mm). The mean duration of the disease was 6.4 years (range, 3 months–18 years).
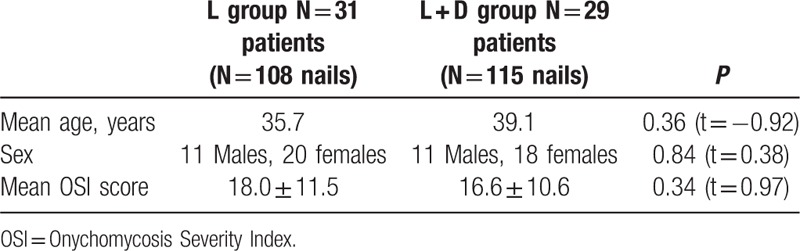
Table 1 describes the baseline characteristics of each group. A total of 31 patients in L group (N = 108 nails) consisted of 11 males and 20 females with an average age of 35.7 years; a total of 29 patients in L + D group (N = 115 nails) consisted of 11 males and 18 females with an average age of 39.1 years. Before treatment, the OSI score of all nails were assessed; the mean OSI score of nails in L group was 18.0 ± 11.5 and those in L + D group was 16.6 ± 10.6. There were no significant differences in age (t = −0.92, P = 0.36), gender (t = 0.38, P = 0.84), and OSI score (t = 0.97, P = 0.34) between L group and L + D groups before treatment.
Table 1.
Baseline characteristics.
3.3. Clinical efficacy
At 3 and 6 months after the last treatment, significant clinical improvement was observed and the CER was assessed. The appearance of most of the nails in L + D group improved significantly compared with that at baseline (Figs. 2 and 3). Figures 4 and 5 show improvement of the onychomycotic nails in the L group. The CER was significantly higher in L + D group than that in L group at 3 months (69.6% vs 50.9%; P = 0.004) and 6 months (73.0% vs 52.8%; P = 0.002) after the last treatment (Table 2). Figure 6 shows the comparison of clinical response between those in L group and L + D group. In all patients showing a complete response, there was no clinical or mycological recurrence at 3 months after the last treatment.
Figure 2.
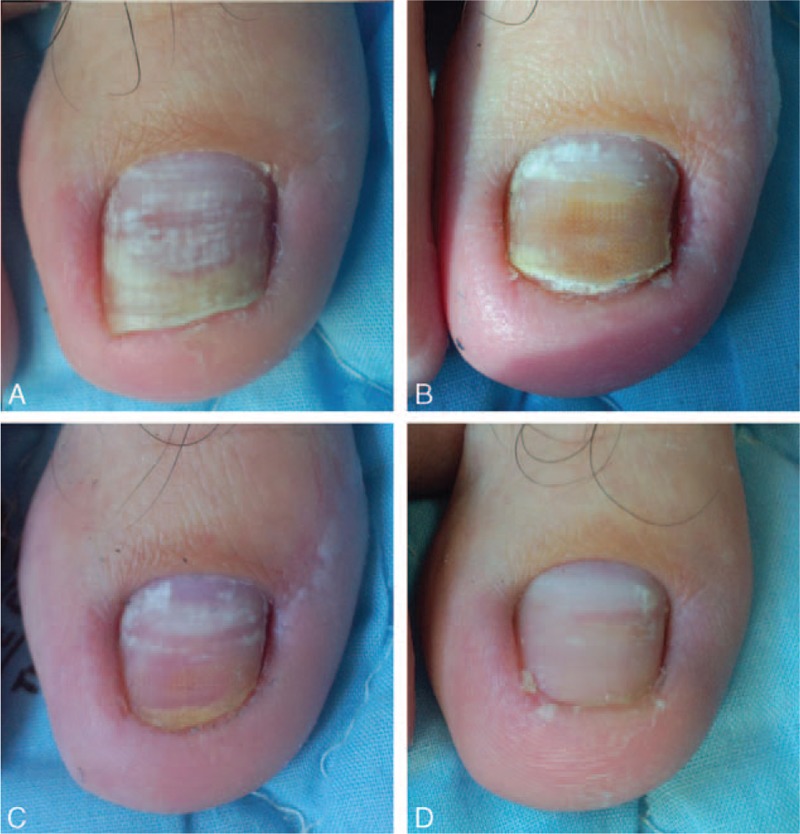
Photographs of right 1st toe with onychomycosis treated with fractional CO2 laser treatment combined with luliconazole 1% cream (L + D group) at baseline before intervention (A), 3 months (B), 6 months (C), and 9 months (D) from baseline.
Figure 3.
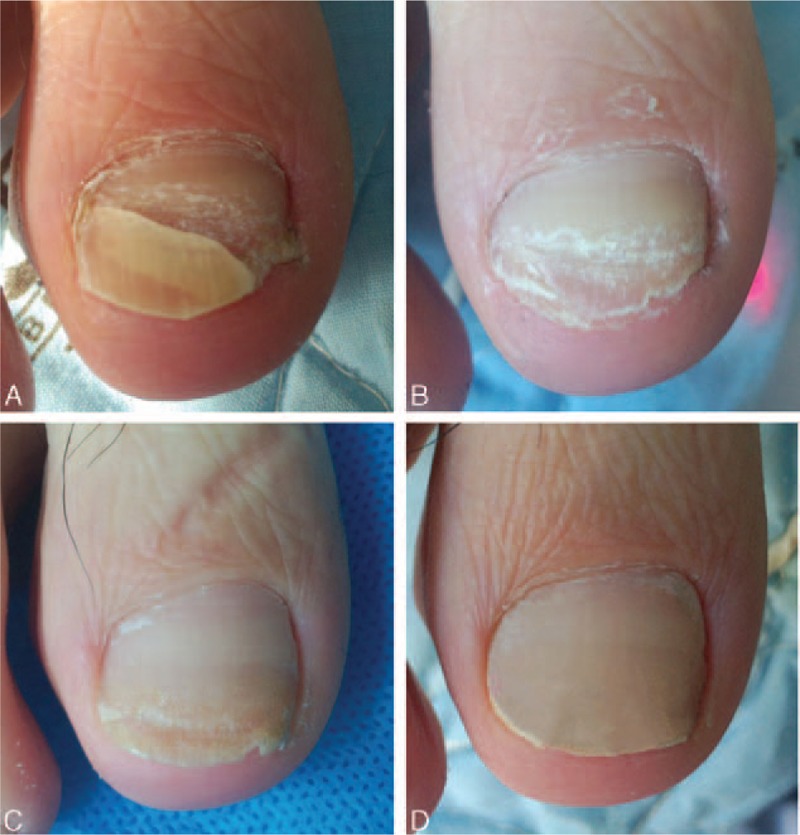
Photographs of right 1st toe with onychomycosis treated with fractional CO2 laser treatment combined with luliconazole 1% cream (L + D group) at baseline before intervention (A), 3 months (B), 6 months (C), and 9 months (D) from baseline.
Figure 4.
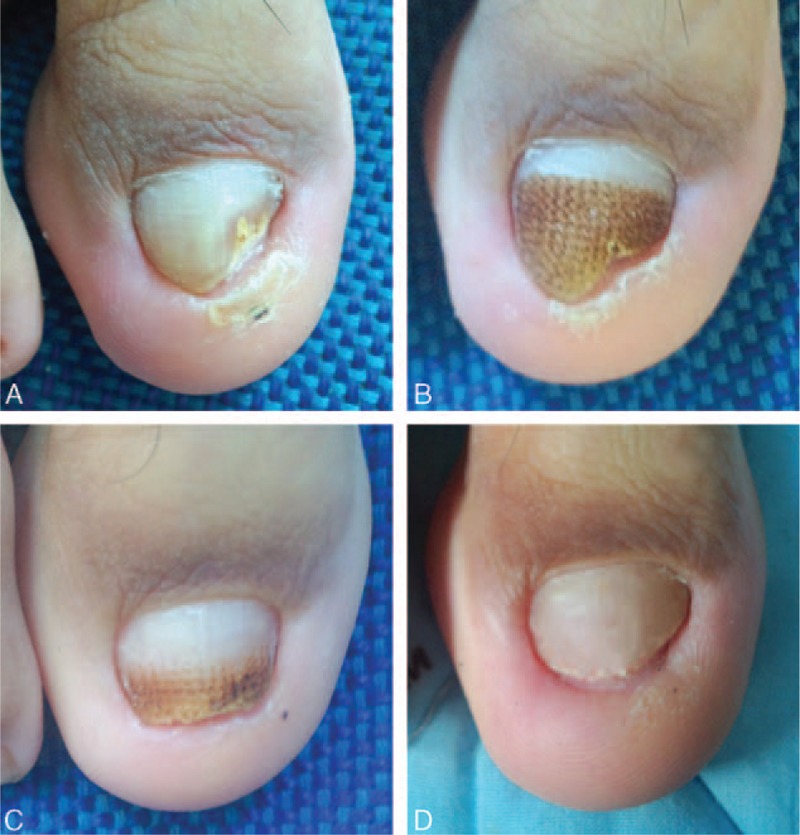
Photographs of right 1st toe with onychomycosis treated with only fractional CO2 laser treatment (L group) at baseline before intervention (A), 3 months (B), 6 months (C), and 9 months (D) from baseline.
Figure 5.
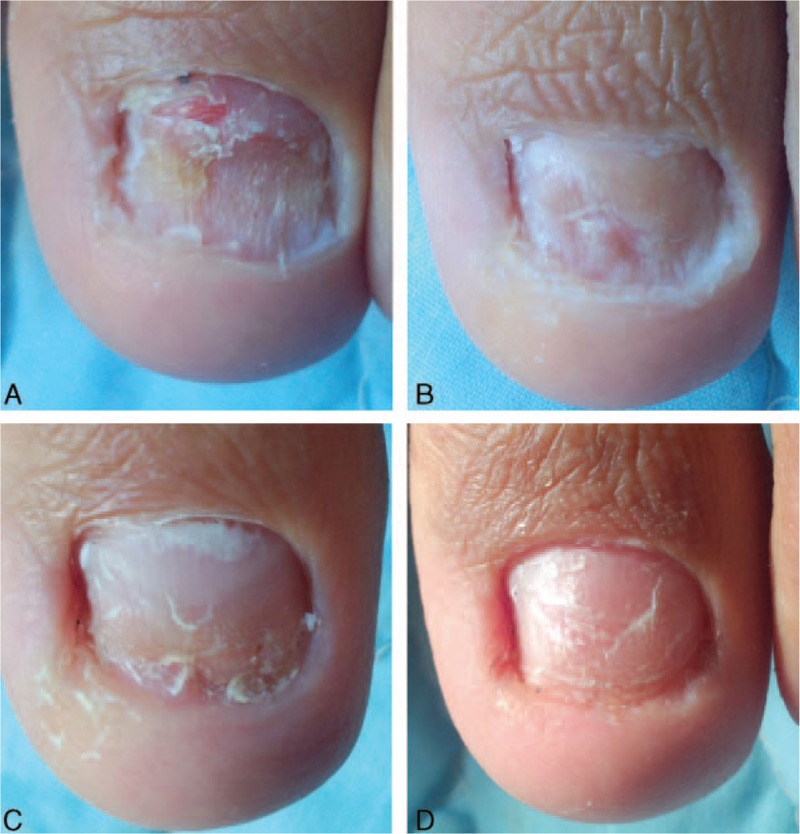
Photographs of left 1st toe with onychomycosis treated with only fractional CO2 laser treatment (L group) at baseline before intervention (A), 3 months (B), 6 months (C), and 9 months (D) from baseline.
Table 2.
The comparison of clinical and mycological results between L group and L + D group.
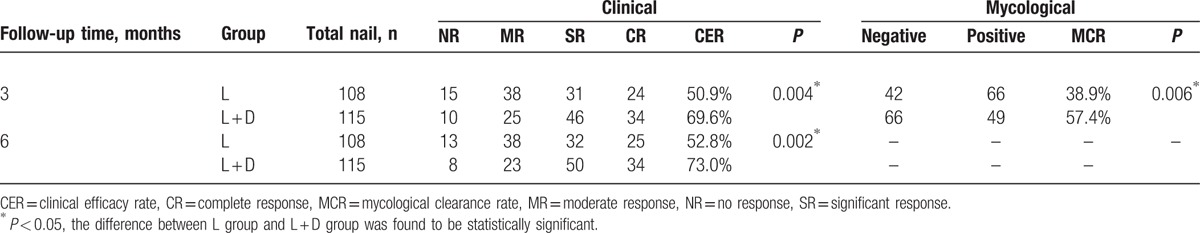
Figure 6.
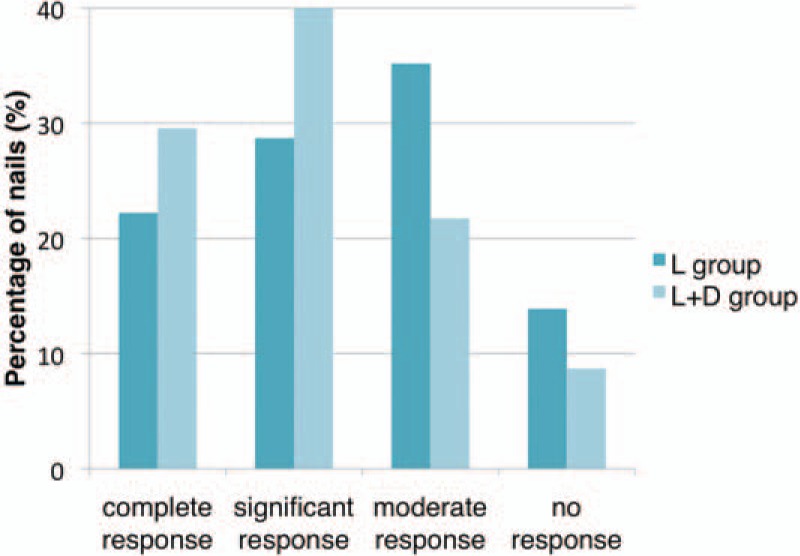
The comparison of clinical response at 3 months after last treatment between those in L group and L + D group.
3.4. Mycological clearance
At 3 months after the last treatment, mycological examination using fungal microscopy showed the MCR in L group was 38.9% (42 nails), while that in L + D group was 57.4% (66 nails) (Table 2). The MCR was significantly higher in L + D group than that in L group (χ2 = 7.6, P = 0.006).
3.5. Influencing factors
Factors that might influence treatment outcome were assessed (Table 3). The OSI score, nail's thickness, and involved nail were the 3 factors that significantly influenced the treatment outcome in the L + D group; a significantly higher CER was observed in nails with OSI score of less than 16 (P = 0.04), in nails of less than 2 mm thick (P = 0.006), and in fingernails (P = 0.02). On the other hand, there were 4 factors that significantly influenced the treatment outcome within the L group: clinical type, nail's thickness, OSI score, and involved nail; there were significantly higher CER observed in partly damaged nails (P = 0.01), nails of less than 2 mm thick (P = 0.04), nails with OSI score of less than 16 (P = 0.007), and fingernails (P = 0.01).
Table 3.
The clinical efficacy according to influencing factors.
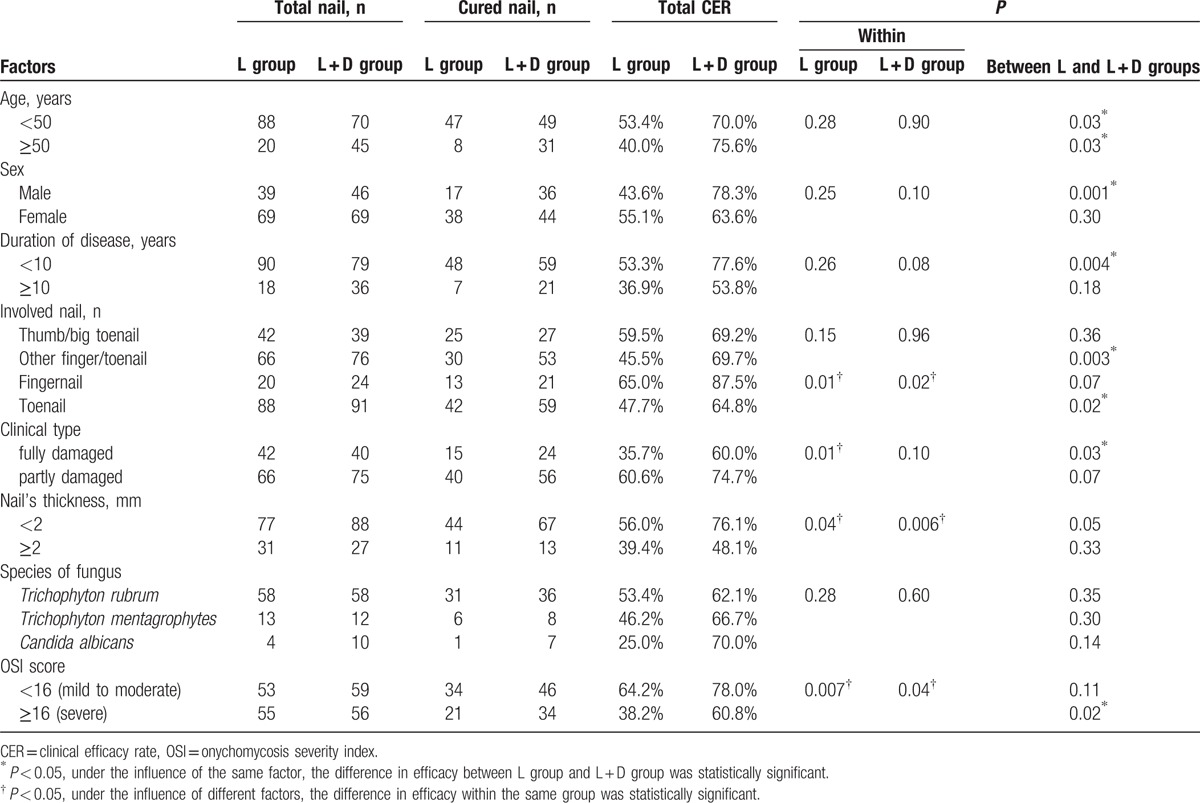
L + D group showed a significantly higher CER compared with that of L group in patients with all ages (P = 0.03 in <50 years old, P = 0.03 in ≥50 years old), in males (P = 0.001), nails with a duration of disease <10 years (P = 0.004), in finger/toenails other than thumb/big toenails (P = 0.003), in toenails (P = 0.02), in fully damaged nails (P = 0.03), and nails with OSI score ≥16 (P = 0.02).
The OSI score, nail's thickness, and involved nail were the 3 factors that significantly influenced the CER in both L group and L + D group. Thus, both L group and L + D group showed a higher CER in nails with OSI score of less than 16, in nails of less than 2 mm thick, and in fingernails.
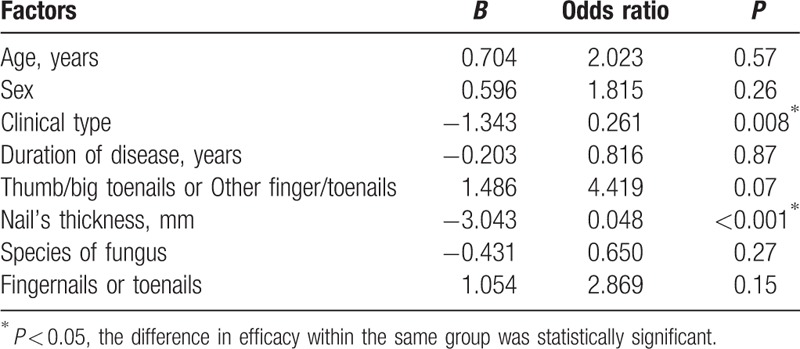
The results of multivariate analysis for significant factors affecting the CER in L and L + D groups are listed in Tables 4 and 5, respectively. Clinical type and nail's thickness were found to be the 2 factors that may independently predict a higher CER in both L and L + D groups after controlling for potential confounders. The CER in partly damaged nails and in nails <2 mm thick were significantly higher in both L and L + D groups. The CER was significantly higher in the fingernails compared with that in the toenails of both L + D group (P = 0.02) and L group (P = 0.01). Remarkably, L + D group showed a significantly higher CER compared with that of L group in toenails (P = 0.02). However, the results of multivariate analysis showed that the involved nail (fingernails or toenails) was a factor that may independently predict a higher CER in L group, but not in L + D group.
Table 4.
Multivariate regression model on fractional CO2 laser-treated group (L group).
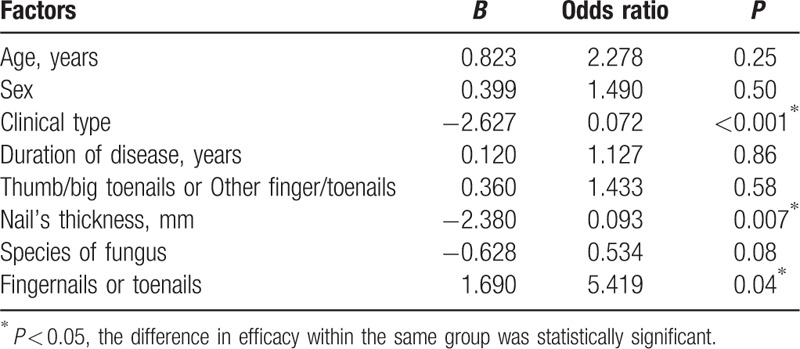
Table 5.
Multivariate regression model on fractional CO2 laser combined with 1% luliconazole cream group (L + D group).
Fungal cultures before treatment revealed there were 155 cases with positive results: 116 cases of Trichophyton rubrum, 25 cases of Trichophyton mentagrophytes, and 14 cases of Candida albicans. At 3 months after the last treatment, the CER in the cases of T rubrum, T mentagrophytes, and C albicans treated with fractional CO2 laser and luliconazole 1% cream were 62.1%, 66.7%, and 70.0%, respectively; the CER in the cases of T rubrum, T mentagrophytes, and C albicans treated with fractional CO2 laser alone were 53.4%, 46.2%, and 25.0%, respectively. There were no significant differences in the above results when compared within the same group or between the 2 groups, suggesting the species of fungus was not a factor that could influence the treatment outcome.
3.6. Patient satisfaction
The evaluation of patient satisfaction in L + D group showed higher satisfaction than that of L group. More patients were “very satisfied” in L + D group (12; 41.4%) compared with those in L group (5; 16.1%). Figure 7 shows the comparison of patient satisfaction between those in L group and L + D group.
Figure 7.
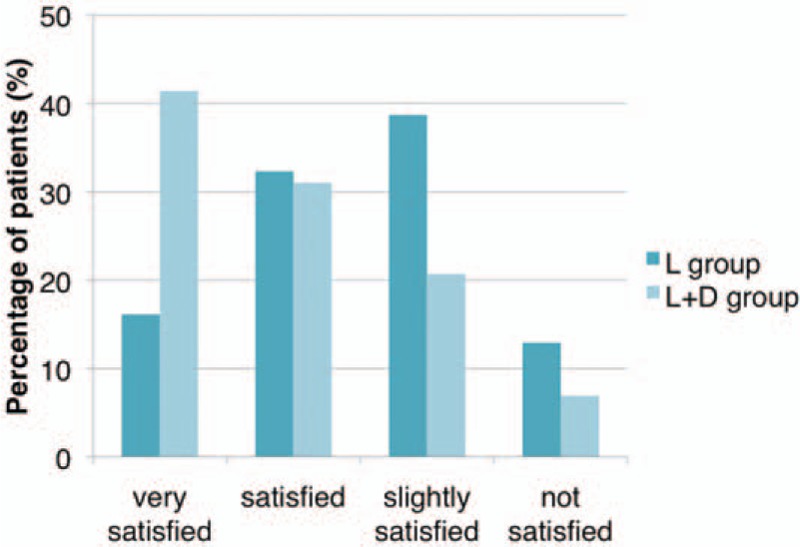
The comparison of patient satisfaction at 3 months after last treatment between those in L group and L + D group.
3.7. Adverse effects
Some patients experienced mild pain during laser treatment, but there was no bleeding or oozing during or after treatment. There were no adverse reactions reported during the observation period.
4. Discussion
We performed a randomized controlled trial of fractional CO2 laser treatment combined with luliconazole 1% for onychomycosis. To our knowledge, there were only 2 uncontrolled trials using fractional CO2 laser combined with a topical antifungal agent to treat onychomycosis. Lim et al[5] were known to be the first to report the efficacy of fractional CO2 laser combined with a topical antifungal agent to treat onychomycosis. They treated 24 patients (N = 119 affected toenails) with a total of 3 sessions of fractional CO2 laser treatment at 4-week interval combined with amorolfine cream once daily within 3 months. At 3 months after the last treatment, they reported 71% of patients (17 patients) had fully or >60% normal-appearing nails. They only did fungal microscopy on patients with fully normal-appearing nails and all of them had a negative fungal microscopic result (50%, 12 patients).[5] Another trial was done by Bhatta et al[6] using a total of 3 sessions of fractional CO2 laser at 4-week interval combined with once-daily application of terbinafine cream within 3 months to treat 75 patients (N = 356 affected nails). The percentage of patients with fully or >60% normal-appearing nails at 3 months after the last treatment in their study was 73.32% (55 patients) and there were 94.66% of patients (71 patients) with a negative fungal microscopic result.[6] Similar to their studies, we found fractional CO2 laser combined with luliconazole 1% cream to be effective for treating onychomycosis with 69.6% of nails (80 nails) with fully or >60% normal appearance and 57.4% of nails (66 nails) with a negative fungal microscopic result. The treatment outcome (CER) was maintained at least 6 months posttreatment, and there was no obvious recurrence.
Although we used the same method as that used by Lim et al[5] and Bhatta et al[6] to evaluate the clinical outcome, the clinical efficacy in our study was assessed from the percentage of nails with fully or >60% normal-appearance. Mycological cure in our study was also assessed from the percentage of nails with a negative fungal microscopic result, while Lim et al and Bhatta et al evaluated their results from the percentage of patients whose nails obtained a negative fungal microscopic result. Therefore, it makes comparison with the previous studies of Lim et al and Bhatta et al difficult. Nevertheless, the percentage of nails, instead of the percentage of patients, can better indicate the clinical efficacy and MCRs since the number of affected nails in each patient can vary.
In our study, the combined therapy was given within a period of 6 months, which was twice longer than those in the study of Lim et al and Bhatta et al.[5,6] Our team, Xu et al,[13] treated 16 patients (N = 29 affected nails) with onychomycosis using a long-pulsed 1064-nm Nd:YAG laser weekly and oral terbinafine daily for up to 6 months. Mycological and clinical examinations of each patient were performed at 2, 4, 8, 12, 16, and 24 weeks of treatment. The results showed the MCR (100%; 29 nails) and CER (96.55%; 28 nails) at 24 weeks of treatment were higher than the MCR (93.10%; 27 nails) and CER (86.21%; 25 nails) at 12 weeks of treatment, indicating that extended duration of treatment increased the efficacy of the treatment of onychomycosis. Therefore, we also extended the treatment duration in our study to achieve better efficacy.
To understand the patient's benefits of fractional CO2 laser therapy combined with luliconazole 1% cream in the treatment of onychomycosis, we investigated the factors that influenced the therapeutic clinical efficacy. Our analysis showed that an initial nail's thickness of less than 2 mm and OSI score of less than 16 were the best predictors for therapeutic efficacy. Compared to fractional CO2 laser alone, fractional CO2 laser treatment combined with luliconazole 1% cream showed higher efficacy in patients of all ages, male patients, nails with OSI score of more than 16, fully damaged nails, in finger/toenails other than thumb/big toenails, in toenails, and nails with a duration of disease <10 years.
Dermatophytes such as T rubrum and T mentagrophytes account for 80% to 90% of all cases, and C albicans accounts for approximately 70% of onychomycosis caused by yeasts.[14] Our study showed fractional CO2 laser combined with luliconazole 1% cream was effective to treat nails infected with T rubrum (62.1%; 36 nails), T mentagrophytes (66.7%; 8 nails), and C albicans (70%; 7 nails). However, there were no significant differences in the clinical efficacy rates in the groups treated with fractional CO2 laser and luliconazole 1% cream compared with the fractional CO2 laser group.
Studies evaluating monotherapy with topical antifungal agents for treating onychomycosis have not been satisfying, except in a very mild case.[15] Lim et al also reported that their patients were treated by topical monotherapy before laser treatment.[5] All patients had failed to improve with topical therapy for their onychomycosis, before undergoing combined therapy with fractional CO2 laser and topical amorolfine. Luliconazole is a newly developed imidazole antifungal drugs, they inhibit the activity of the enzyme lanosterol 14α-demethylase, thereby blocking the conversion of lanosterol to ergosterol, a necessary constituent of the fungal cell wall.[10] Studies have shown that luliconazole has a good antifungal activity, such as against dermatophytes, Malassezia and Candida.[16] However, we had treated another group of patients with only luliconazole 1% cream, but the results were of little value. On the other hand, Yang et al conducted a trial using only fractional CO2 laser to treat onychomycosis and the result showed a CER of 52.11%. Therefore, we designated fractional CO2 laser treatment as the control and did not include topical antifungal monotherapy in our study.
Although the efficacy of monotherapy with topical antifungal agent has not been satisfying, there were significantly higher clinical efficacy (P = 0.004) and MCRs (P = 0.006) in the group treated with both fractional CO2 laser and luliconazole 1% cream compared with those of fractional CO2 laser alone. Studies suggest that fractional CO2 laser therapy creates columns of destruction that enhance the penetration of topical antifungal agents into the nail bed or matrix, thereby improving the efficacy of onychomycosis treatment.[5,9] Our study confirmed that topical antifungal agent when combined with fractional CO2 laser had a significant efficacy.
Evaluation of patient satisfaction at the end of our study showed there were 41.4% of patients (21 patients) who were very satisfied with the results of fractional CO2 laser treatment combined with luliconazole 1% cream. There were a lower percentage of very satisfied patients in our study compared to that of Lim et al[5] (59%; 14 patients) and Bhatta et al[6] (66.67%; 50 patients), although the CER of our study was quite satisfying. This might be because of the long treatment duration and the inconvenience of repeated hospital visits. Moreover, we assessed the clinical and mycological cure based on the percentage of nails, instead of the percentage of patients, which was used in the previous studies; therefore, this may also explain the lack of consistency between the CER and the level of patient satisfaction in our study.
Some patients experienced mild pain during laser treatment, but there was no bleeding or oozing during or after treatment. There were no adverse reactions reported during the observation period. Fractional CO2 laser has the advantage of smaller diseased skin tissue damage, faster recovery, less pain treatment, can be used in patients with comorbid conditions, and no risk of developing resistance.[5,6,17,18]
There are a number of limitations to our study. First, there was no blinding. Thus, knowledge of group assignment may affect the patients’ behavior in the trial and their responses to subjective outcome measures. Second, our study had a limited number of cases. Third, we only evaluated the mycological cure from a fungal microscopy without a fungal culture examination. Further randomized controlled blinded trial with a larger sample size, more follow-ups during treatment, and a longer follow-up time is needed. Further study is also required to determine the optimal settings and duration of fractional CO2 laser treatment.
5. Conclusion
In conclusion, we found that the fractional CO2 laser combined with luliconazole 1% cream is a safe and effective treatment for the treatment of onychomycosis, and its efficacy is better than fractional CO2 laser treatment alone. Our findings cannot be generalized to other lasers with different fluences, wavelengths, or pulse rates.
Footnotes
Abbreviations: CER = clinical efficacy rate, CO2 = carbon dioxide, MCR = mycological clearance rate, OSI = Onychomycosis Severity Index.
Trial registration: 2015-SRFA-032
Funding/support: This work was supported by Grants from the China National Natural Science Foundation (81573072 and 81301384) and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (JX10231801), and Special Project Fund for Clinical Research of Luliconazole, Professional Committee of Dermatology and Venereology, and Chinese Association of Integrative Medicine.
The authors have no conflicts of interest to disclose.
References
- 1.Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol 2000; 43:641–648. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Paquet M, Simpson FC. Therapies for the treatment of onychomycosis. Clin Dermatol 2013; 31:544–554. [DOI] [PubMed] [Google Scholar]
- 3.Ledon JA, Savas J, Franca K, et al. Laser and light therapy for onychomycosis: a systematic review. Lasers Med Sci 2014; 29:823–829. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Liu H, Yang RY, et al. Efficacy of a superpulse-mode fractional carbon dioxide laser for the treatment of onychomycosis: a clinical observational study (fx1). Chinese J Dermatol 2015; 48:526–530. [Google Scholar]
- 5.Lim EH, Kim HR, Park YO, et al. Toenail onychomycosis treated with a fractional carbon-dioxide laser and topical antifungal cream. J Am Acad Dermatol 2014; 70:918–923. [DOI] [PubMed] [Google Scholar]
- 6.Bhatta AK, Keyal U, Huang X, et al. Fractional carbon-dioxide (CO2) laser-assisted topical therapy for the treatment of onychomycosis. J Am Acad Dermatol 2016; 74:916–923. [DOI] [PubMed] [Google Scholar]
- 7.Hira K, Yamada H, Takahashi Y, et al. Successful treatment of chromomycosis using carbon dioxide laser associated with topical heat applications. J Eur Acad Dermatol Venereol 2002; 16:273–275. [DOI] [PubMed] [Google Scholar]
- 8.Alam M, Pantanowitz L, Harton AM, et al. A prospective trial of fungal colonization after laser resurfacing of the face: correlation between culture positivity and symptoms of pruritus. Dermatol Surg 2003; 29:255–260. [DOI] [PubMed] [Google Scholar]
- 9.Borovoy M, Tracy M. Noninvasive CO 2 laser fenestration improves treatment of onychomycosis. Clin Laser Mon 1992; 10:123–124. [PubMed] [Google Scholar]
- 10.Zeichner JA. New topical therapeutic options in the management of superficial fungal infections. J Drugs Dermatol 2015; 14 (10 Suppl):s35–s41. [PubMed] [Google Scholar]
- 11.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010; 115:1063–1070. [DOI] [PubMed] [Google Scholar]
- 12.Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: onychomycosis severity index. Arch Dermatol 2011; 147:1277–1282. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Miao X, Zhou B, et al. Combined oral terbinafine and long-pulsed 1,064-nm Nd: YAG laser treatment is more effective for onychomycosis than either treatment alone. Dermatol Surg 2014; 40:1201–1207. [DOI] [PubMed] [Google Scholar]
- 14.Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther 2010; 35:497–519. [DOI] [PubMed] [Google Scholar]
- 15.Baran R, Kaoukhov A. Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J Eur Acad Dermatol Venereol 2005; 19:21–29. [DOI] [PubMed] [Google Scholar]
- 16.Koga H, Nanjoh Y, Makimura K, et al. In vitro antifungal activities of luliconazole, a new topical imidazole. Med Mycol 2009; 47:640–647. [DOI] [PubMed] [Google Scholar]
- 17.Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther 2014; 23:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majid I, Imran S. Fractional CO2 laser resurfacing as monotherapy in the treatment of atrophic facial acne scars. J Cutan Aesthet Surg 2014; 7:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


