Supplemental Digital Content is available in the text
Keywords: breast cancer, cyclophosphamide, doxorubicin, genetic polymorphism, neutropenia
Abstract
Chemotherapy-induced neutropenia (CIN) is one of the major adverse events that necessitate chemotherapy dose reduction. This study aimed to evaluate the association between grade 4 neutropenia and genetic polymorphisms in breast cancer patients. In this genetic polymorphism association study, peripheral blood samples from 100 consecutive breast cancer outpatients, between August 2012 and September 2014, treated with doxorubicin and cyclophosphamide (AC) combination chemotherapy were genotyped for polymorphisms in adenosine triphosphate-binding cassette subfamily B member 1 (ABCB1), cytochrome P450 (CYP) enzyme-coding genes (CYP2B6 and CYP3A5), glutathione S-transferase (GST), and excision repair cross-complementing 1 (ERCC1). Associations between grade 4 neutropenia and genotypes as well as risk factors were examined using multivariate logistic regression. From 100 patients, 32.0% had grade 4 neutropenia. Multivariate logistic regression analysis revealed that ERCC1 118C > T (odds ratio [OR], 3.43; 95% confidence interval [CI], 1.22–9.69; P = 0.020), CYP2B6∗6 (OR, 4.51; 95% CI, 1.21–16.95; P = 0.025), body mass index (BMI) (OR, 6.94; 95% CI, 1.15–41.67; P = 0.035), and baseline white blood cell (WBC) count (OR, 2.99; 95% CI, 1.06–8.40; P = 0.038) were significant predictors of grade 4 neutropenia. ERCC1 and CYP2B6 gene polymorphisms were associated with the extent of grade 4 neutropenia in patients receiving AC chemotherapy. In addition to previously known risk factors, BMI and WBC counts, ERCC1 and CYP2B6 gene polymorphisms were also identified as independent strong predictors of grade 4 neutropenia.
1. Introduction
Chemotherapy-induced neutropenia (CIN) is a major dose-limiting toxicity of systemic cancer chemotherapy and frequently necessitates the reduction of the initial chemotherapeutic dose. The degree and duration of neutropenia determine the risk of life-threatening infections.[1] CIN is directly associated with concomitant morbidity and mortality rates and health care costs.[2,3] Therefore, it is critical to identify risk factors for CIN, and an adequate management of CIN in cancer patients is essential. Several risk factors, such as female gender, older age, and lower pretreatment blood cell counts, for CIN are well known and were previously reported. In addition, pharmacogenetic factors, as potential risk factors for CIN, garnered increased interest in recent years.
Anthracycline-based chemotherapy containing doxorubicin and cyclophosphamide (AC) is a standard regimen commonly used worldwide to treat early-stage breast cancer.[4,5] Doxorubicin is subject to transport by organic anion transporters belonging to the amphiphilic solute transporter family 22 and is mainly exported by adenosine triphosphate-binding cassette subfamily B member 1 (ABCB1) transporter.[6,7]ABCB1 polymorphisms significantly impact the levels of doxorubicin in breast cancer patients, resulting in significantly increased serum levels and reduced clearance in patients with ABCB1 variant alleles.[8] Conversely, cyclophosphamide is administered as a prodrug that undergoes phase I activation metabolism by the cytochrome P450 (CYP) enzymes CYP2B6, CYP3A4, and CYP3A5 and phase II inactivation primarily through conjugation with a thiol or sulfate via glutathione S-transferases (GSTs).[9–12] The active metabolite, 4-hydroxy-cyclophosphamide diffuses into cancer cells and is responsible for the alkylating ability of cyclophosphamide. Functional genes related to drug transport and metabolism and DNA repair impact the cytotoxic effects associated with chemotherapy. Nevertheless, little is known regarding pharmacogenomics in breast cancer treatment.
Importantly, 1 study found that severe neutropenia during breast cancer treatment with standard-dose adjuvant AC combination chemotherapy was observed more frequently in Asian patients than in Caucasian patients.[13] Interethnic differences and pharmacoethnicity are increasingly recognized as underlying interindividual variations in drug responsiveness and anticancer drug responsiveness, respectively. One potential reason for this effect is genetic differences in the functions of drug metabolizing enzymes and/or transporters. Therefore, it is critical to determine the role of pharmacogenetic polymorphisms in each ethnic group to improve treatment outcomes and reduce adverse events.
In this study, we assessed the association between grade 4 neutropenia and genetic polymorphisms in breast cancer patients receiving AC combination chemotherapy to examine potential effects of gene polymorphisms on severe CIN. In addition, we assessed potential risk factors predisposing patients receiving adjuvant AC combination chemotherapy to severe neutropenia.
2. Patients and methods
2.1. Study design and patients
In this genetic polymorphism association study, a total of 100 consecutive Japanese breast cancer outpatients receiving 4 cycles of AC combination therapy between August 2012 and September 2014 at the Department of Medical Oncology at Seirei Hamamatsu General Hospital were analyzed. Patients were eligible for this pharmacogenetic study if they had histologically confirmed breast cancer and planned to receive 4 cycles of the standard AC regimen (60-mg/m2 doxorubicin and 600-mg/m2 cyclophosphamide on day 1 of a 21-day cycle) as neoadjuvant or adjuvant chemotherapy, were ≥20 years of age at diagnosis, had an Eastern Cooperative Oncology Group performance status of 0 to 1, and adequate baseline hematologic, hepatic, and renal functions (white blood cell [WBC] count ≥3 × 109/L; platelet count ≥100 × 109/L; aspartate aminotransferase [AST] < 100 IU/L; alanine aminotransferase [ALT] < 100 IU/L; total bilirubin <2 mg/dL; serum creatinine <1.5 mg/dL).
Written informed consent was obtained from all patients. This study was approved by the Institutional Review Board of the Seirei Hamamatsu General Hospital and complied with the provisions of the Declaration of Helsinki. The STROBE checklist is available as Supplementary Material (see Checklist 1, Supplementary Material).
2.2. Assessment
Hematologic toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 4.0. As absolute neutrophil count (ANC) is the most important indicator and most readily quantifiable parameter of myelotoxicity, clinically significant and severe myelotoxicity induced by chemotherapy was defined as the presence of grade 4 neutropenia (ANC < 0.5 × 109/L). The main evaluation parameter was the percentage of patients with grade 4 neutropenia. The neutropenia grade was based on the lowest recorded neutrophil count during the first cycle of AC chemotherapy.
Several baseline variables, including age, body mass index (BMI), clinical stage, and baseline laboratory data of all patients that could potentially influence severe CIN were included in this study. The cutoff values for laboratory data were based on the medians in this study. Patients with BMI > 25 kg/m2 were considered overweight; this cutoff was chosen in accordance with the universal BMI criteria developed by the World Health Organization.[14]
2.3. Pharmacogenetic analysis
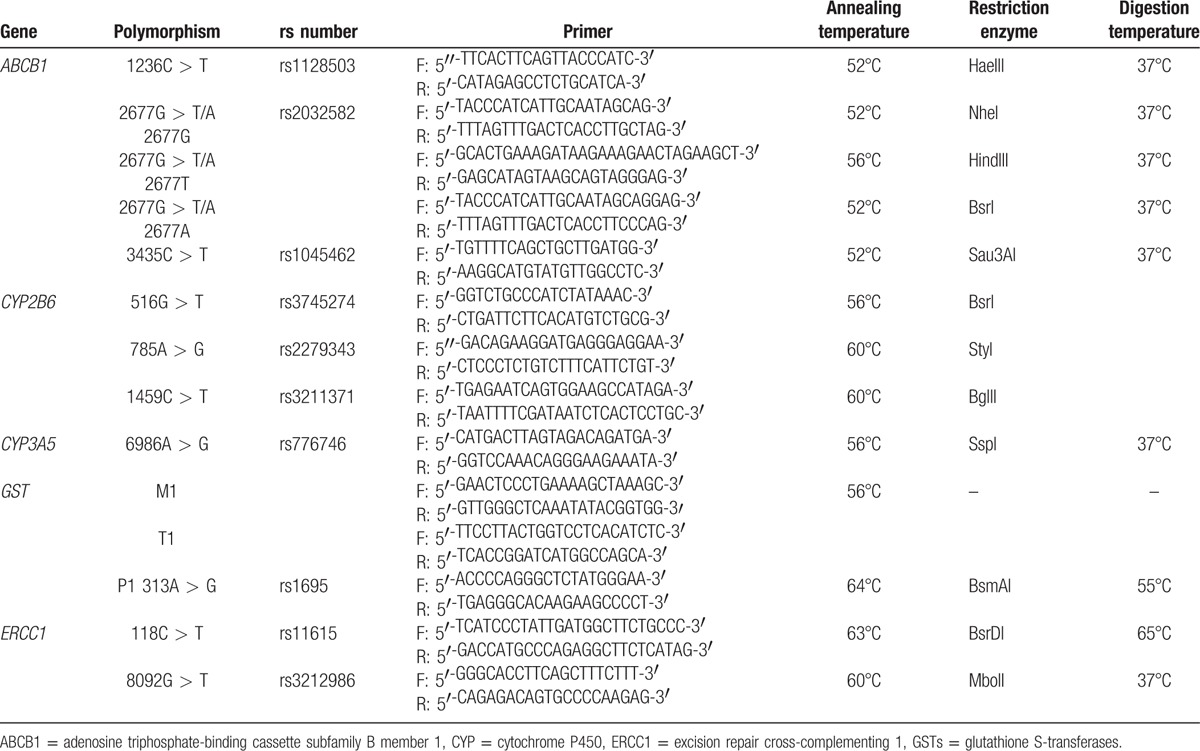
Five milliliters of whole venous blood samples from all patients were collected into EDTA-2Na Venoject II tubes (Terumo, Tokyo, Japan) and stored at −25°C before DNA extraction. Leukocyte genomic DNA was extracted directly from the blood specimen using a QIAamp DNA Blood Maxi Kit (Qiagen N.V., Limburg, the Netherlands). We selected the following polymorphisms in 5 genes that were associated with functional effects on gene expression, level, or activity: ABCB1 1236C > T, ABCB1 2677G > T/A, ABCB1 3435C > T, CYP2B6∗4 (785A > G), CYP2B6∗5 (1459C > T), CYP2B6∗6 (516G > T and 785A > G), CYP2B6∗9 (516G > T), CYP3A5 6986A > G, GSTM1/T1 null, GSTP1 313A > G, excision repair cross-complementing 1 (ERCC1) 118C > T, and ERCC1 8092G > T. Genotyping for all gene polymorphisms was performed with polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis. PCR conditions, primer, and restriction enzymes used for all reactions are shown in Table 1. All patients were classified into 2 groups: homozygous wild-type or carriers of 1 or more variant alleles.
Table 1.
Primer sequences, annealing temperatures, restriction enzymes, and digestion temperatures used for genetic polymorphisms analysis in this study.
2.4. Statistical analysis
The optimal sample size was not estimated a priori because this is an exploratory study. Continuous variables were summarized as means ± standard deviation, and categorical variables were presented as frequencies and proportions. Categorical variables were compared using the chi-square test. If assumptions of the chi-square test were not met, that is, >20% of the cells in the contingency table had expected values of <5 or the minimum expected frequency was <1, Fisher's exact test was used. The genotypic distribution of each polymorphism was also examined for deviation from Hardy–Weinberg equilibrium using the chi-square test. Risk factors associated with grade 4 neutropenia were examined using the chi-square test or Fisher's exact test in univariate analysis and multivariate logistic regression analysis. Risk factors with P values of <0.15 by univariate analysis were included in multivariate analysis. Results of multivariate analysis were reported as odds ratios (ORs) with 95% confidence intervals (CIs) and P values. All statistical tests were 2-sided, and P values of <0.05 were considered statistically significant. All statistical analyses were performed using PASW Statistics for Windows, version 22.0 (SPSS, Chicago, IL).
3. Results
3.1. Patient characteristics
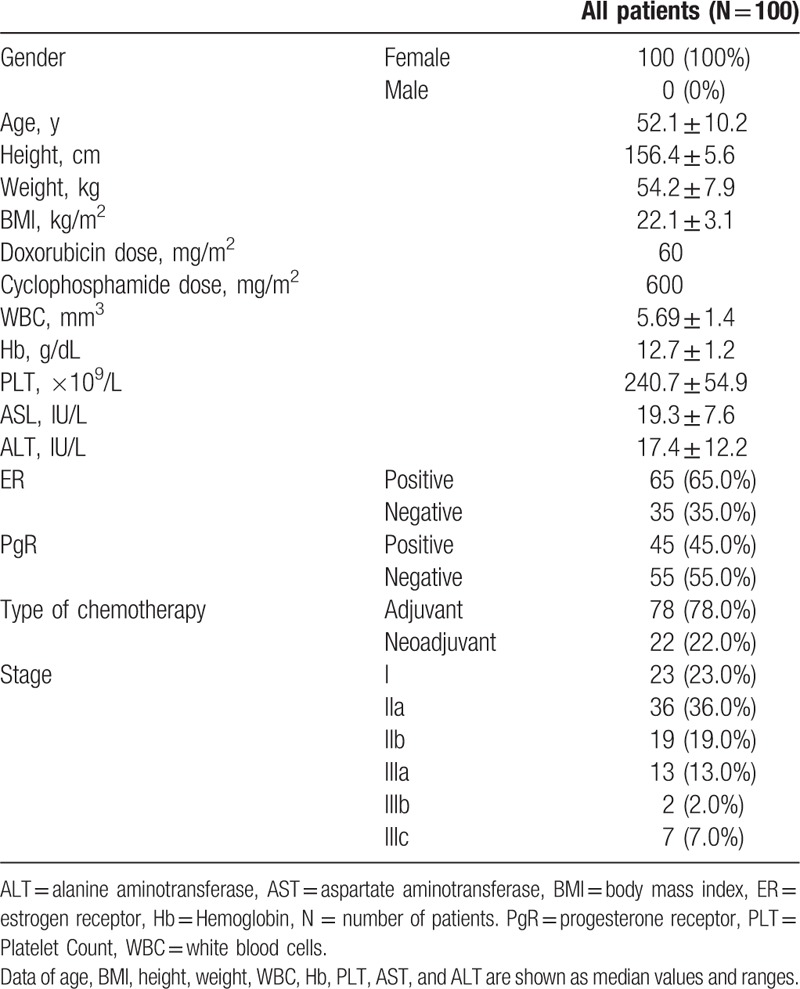
In this genetic polymorphism association study, a total of 100 patients with breast cancer were included. The baseline demographic and clinical characteristics are shown in Table 2. The mean age of study participants was 52 years. All patients were female and received a combination of 60-mg/m2 doxorubicin and 600-mg/m2 cyclophosphamide as adjuvant or neoadjuvant treatment.
Table 2.
Baseline demographics and clinical characteristics of the study patients.
Gene polymorphisms were evaluated using genomic DNA extracted from peripheral leukocytes in all patients included in this study. The observed genotype distributions were in line with Hardy–Weinberg equilibrium (P > 0.05 for all variants).
The genotype frequencies of gene polymorphisms included in this study were as follows: for ABCB1 1236C > T, the genotype frequency for CC was 14.0% and that for CT and TT was 86.0%; for ABCB1 2677G > T/A, the genotype frequency for GG was 22.0% and that for GT, GA, TT, TA, and AA was 78.0%; for ABCB1 3435C > T, the genotype frequency for CC was 35.0% and that for CT and TT was 65.0%; for CYP2B6∗4, the frequencies of genotypes with and without ∗4 mutations were 78.0% and 22.0%, respectively; for CYP2B6∗5, the frequencies of genotypes with and without ∗5 mutations were 95.0% and 5.0%, respectively; for CYP2B6∗6, the frequencies of genotypes with and without ∗6 mutations were 72.0% and 28.0%, respectively; for CYP2B6∗9, the frequencies of genotypes with and without ∗9 mutations were 99.0% and 1.0%, respectively; for CYP3A5 6986A > G, the genotype frequency for AA was 2.0% and that for AG and GG was 98.0%; for GSTM/T1, the frequencies of null/null and other genotypes were 24.0% and 76.0%, respectively; for GSTP1 313A > G, the genotype frequency for AA was 68.0% and that for AG and GG was 32.0%; for ERCC1 118C > T, the genotype frequency for CC was 58.0% and that for CT and TT was 42.0%; and for ERCC1 8092G > T, the genotype frequency for GG was 56.0% and that for GT and TT was 44.0%. The observed genotype frequencies were in line with an expected Hardy–Weinberg equilibrium (P > 0.05 for all variants).
3.2. Association between grade 4 neutropenia incidence and genotypes
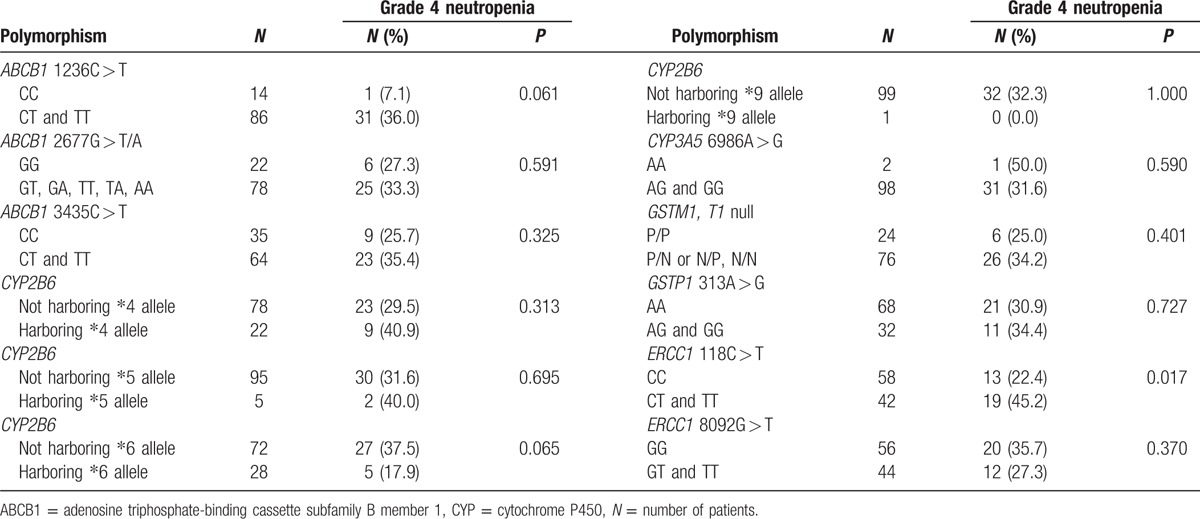
Of a total of 100 patients, the proportion of patients with grade 4 neutropenia was 32.0% (n = 32); the genotypes of these patients were classified into 2 groups (Table 3). Patients with the ERCC1 118 CT and TT genotypes had a significantly higher rate of grade 4 neutropenia than those with the CC genotype (45.2% vs 22.4%, P = 0.017). The rate of grade 4 neutropenia was higher in patients not harboring CYP2B6∗6 allele than in those harboring CYP2B6∗6 allele, which trended toward significance (37.5% vs 17.9%, P = 0.065). Similarly, the rate of grade 4 neutropenia was higher in patients harboring ABCB1 1236 CT and TT genotyes than in those harboring the CC genotype, which trended toward significance (36.0% vs 7.1%, P = 0.061).
Table 3.
Association between grade 4 neutropenia incidence and genotypes by univariate analysis.
3.3. Association between grade 4 neutropenia incidence and patient characteristics
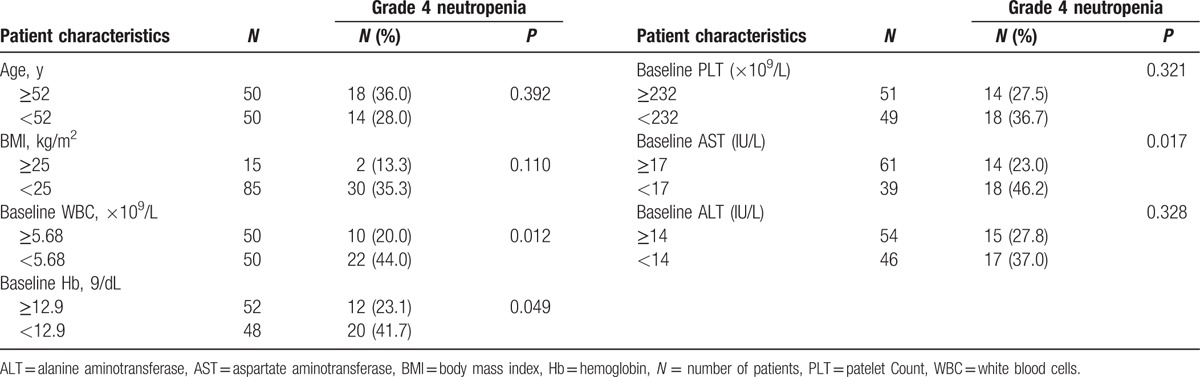
We performed univariate analysis to examine the relationship of patient characteristics with grade 4 neutropenia (Table 4). The following factors exhibited either a significant correlation or a trend toward significant correlation in univariate analysis: baseline WBC count (P = 0.012), baseline AST (P = 0.017), baseline hemoglobin level (P = 0.049), and BMI (P = 0.110). No association was found between clinical stage and grade 4 neutropenia. The cutoff values for all parameters were determined based on the median value for continuous variables.
Table 4.
Association between grade 4 neutropenia incidence and patient characteristics by univariate analysis.
3.4. Risk factors for grade 4 neutropenia
To explore genetic polymorphisms as potential predictors of grade 4 neutropenia, we performed univariate analysis. The following 7 factors exhibited either a significant correlation or a trend toward a significant correlation in univariate analysis and were included in the multivariate logistic regression analysis: baseline WBC count, baseline AST, baseline hemoglobin level, baseline BIM, and genotypes ERCC1 118C > T, CYP2B6 ∗6, and ABCB1 1236C > T.
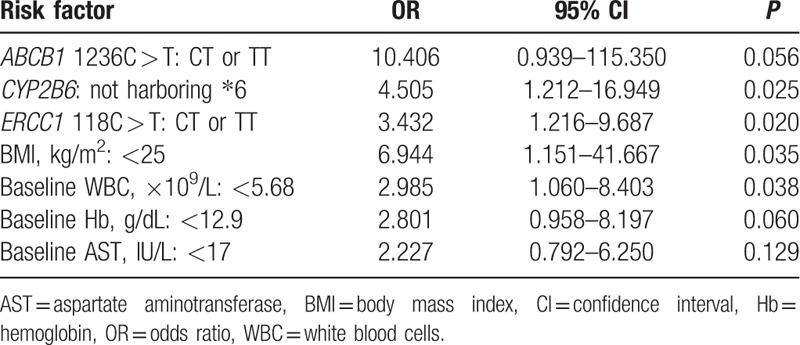
The multivariate analysis adjusted for these risk factors showed that ERCC1 118C > T: non-CC (OR, 3.432; 95% CI, 1.16–9.687; P = 0.020), non-∗6 CYP2B6 allele (OR, 4.505; 95% CI, 1.212–16.949; P = 0.025), body mass index (BMI) < 25 kg/m2 (OR, 6.944; 95% CI, 1.151–41.667; P = 0.035), and baseline WBC count <5.68 × 109/L (OR, 2.985; 95% CI, 1.060–8.403; P = 0.038) were significant determinants of grade 4 neutropenia in patients receiving AC combination chemotherapy (Table 5).
Table 5.
Multivariate analysis of associations between grade 4 neutropenia and clinical variables.
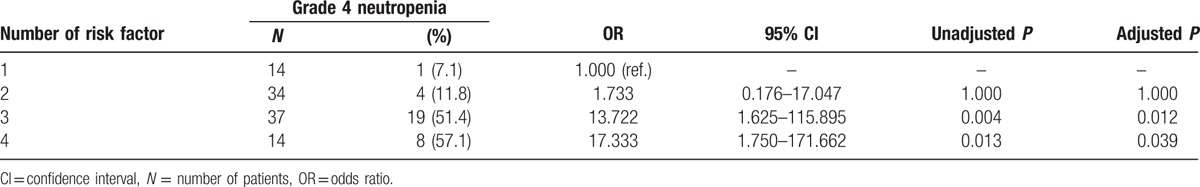
3.5. Association between the number of identified risk factors and grade 4 neutropenia development
Based on multivariate analysis results, 4 patient-related risk factors, including BMI, baseline WBC, ERCC1 118C > T, and CYP2B6∗6, were determined as significantly associated with grade 4 neutropenia development. The number of risk factors was significantly associated with grade 4 neutropenia development and their associations are summarized in Table 6. All patients but 1 had more than 1 risk factor. The frequency of grade 4 neutropenia was observed in 7.1% of patients with 1 risk factor, 11.8% with 2 risk factors, 51.4% with 3 risk factors, and 57.1% with all 4 risk factors. Compared with those carrying 1 risk factor, OR for grade 4 neutropenia development in patients carrying 2, 3, and 4 risk factors were 1.733 (95% CI, 0.176–17.047; P = 1.000), 13.722 (95% CI, 1.625–115.895; P = 0.0129), and 17.333 (95% CI, 1.750–171.662; P = 0.039), respectively.
Table 6.
Number of risk factors and grade 4 neutropenia development.
4. Discussion
This study aimed to determine whether doxorubicin/cyclophosphamide-related genetic polymorphisms were associated with grade 4 neutropenia development and to assess risk factors for grade 4 neutropenia including genetic polymorphisms in Japanese patients receiving AC combination chemotherapy.
Several factors, such as female gender,[15–17] younger age,[18–20] lower BMI,[21,22] and lower pretreatment blood cell counts,[17,23,24] and hemoglobin levels,[19,25–27] were identified as risk factors for severe CIN and/or febrile neutropenia. However, a pharmacogenetic approach based solely on polymorphisms involved in antitumor agent activity appears to be insufficient. A multifaceted approach, including genetic factors, is required to address CIN. Thus, germline polymorphisms are attractive candidates that can be easily assessed in available samples.
In this study, we evaluated the effect of genetic polymorphisms on grade 4 neutropenia induced by AC combination chemotherapy in breast cancer patients. The multivariate logistic regression analysis revealed 4 factors, CYP2B6, ERCC1, BMI, and baseline WBC count, to be the independent and significant risk factors. Our findings showing low BMI and low baseline WBC count as risk factors for grade 4 neutropenia was consistent with those of previous reports. We showed a strong association between CYP2B6 and ERCC1 genotypes and grade 4 neutropenia development. OR for patients with grade 4 neutropenia and possessing 1 or more CYP2B6∗6 and ERCC1 118C > T variant alleles were 4.505 (95% CI, 1.212–16.949) and 3.432 (95%CI, 1.216–9.687), respectively. Therefore, in addition to previously reported risk factors, we found that CYP2B6 and ERCC1 polymorphisms were good predictors of grade 4 neutropenia.
In this analysis, the frequency of grade 4 neutropenia was lower in patients harboring CYP2B6∗6 allele than in those not harboring CYP2B6∗6 allele. CYP2B6 is the primary enzyme that substantially metabolizes cyclophosphamide to its active metabolite, 4-hydroxycyclophosphamide. Several studies showed that CYP2B6 polymorphisms altered the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide.[28–30] As CYP2B6∗6 allele is associated with its reduced protein and mRNA expression,[31] it is likely that the lower levels of 4-hydroxycyclophosphamide led to the lower frequency of grade 4 neutropenia in patients with CYP2B6∗6 allele.
Increased ERCC1 mRNA levels are directly associated with platinum resistance in various cancers. Furthermore, ERCC1 gene polymorphisms were already reported to associate with the efficacy and cytotoxicity of platinum agents.[32–35] Given that ERCC1 118C > T polymorphisms were associated with differential mRNA levels and lower enzyme levels [36] and that the T allele was associated with reduced translation and presumably reduced DNA repair capability, lower DNA repair capacity resulting from non-CC ERCC1 118C > T alleles might lead to decreased ability to repair the damage done by platinum agents in normal tissue to precipitate increased toxicity. The findings of this study showed that possessing at least 1 ERCC1 118C > T variant allele was associated with a 3.4-fold increase in the risk for developing grade 4 neutropenia. One study reported that ERCC1 gene products were crucial for the repair of 4-hydroxycyclophosphamide-induced DNA damage and that ERCC1 mutants might exhibit hypersensitivity to DNA crosslinking agents.[37] Therefore, our findings suggested that ERCC1 118C > T polymorphisms might affect DNA repair of normal cells damaged by cyclophosphamide.
In this study, the incidence of grade 4 neutropenia was 32.0%. Furthermore, all patients but one developed grade 4 neutropenia during the first treatment cycle. In a study among breast cancer patients treated with standard-dose adjuvant AC combination chemotherapy, Ma et al[13] reported that the incidence of grade 4 neutropenia was higher in Chinese patients (25%) than in Caucasian patients (0.3%), demonstrating significant interethnic differences. This study might have also encountered patients with different pharmacoethnicity. Granulocyte colony-stimulating factor (G-CSF) was proven to effectively reduce the incidence of febrile neutropenia when prophylactically given 24 to 72 hours after chemotherapy. However, due to high cost, G-CSF is not routinely prescribed to all patients. Because Asian patients have higher frequency of severe neutropenia, those with 1 or more risk factors might be considered for prophylactic G-CSF administration.
To the best of our knowledge, this is the first clinical report showing an association between CYP2B6 and ERCC1 polymorphisms and severe neutropenia in patients treated with AC combination chemotherapy. However, the study has 2 major limitations. First, pharmacokinetic data for doxorubicin and cyclophosphamide were not available in this study as blood samples were collected before the first cycle of chemotherapy. Therefore, we could not elucidate the mechanisms underlying CYP2B6 polymorphisms and cyclophosphamide toxicity. The second limitation was the relatively small sample size used to evaluate the association.
In conclusion, we observed a significant association of grade 4 neutropenia with genetic polymorphisms in CYP2B6 and ERCC1. Our analysis also demonstrated its association with BMI and WBC, 2 previously identified risk factors for CIN. Our findings from clinical data might contribute to future clinical practice. However, our study included a relatively small sample size; thus, future, large-scale studies are critical to evaluate the utility of CYP2B6 and ERCC1 polymorphisms as predictors of grade 4 neutropenia.
Acknowledgments
The authors wish to thank all the patients and medical staff who participated in this study.
Supplementary Material
Footnotes
Abbreviations: ABCB1 = adenosine triphosphate-binding cassette subfamily B member 1, AC = doxorubicin and cyclophosphamide, ALT = alanine aminotransferase, ANC = absolute neutrophil count, AST = aspartate aminotransferase, BMI = body mass index, CI = confidence interval, CIN = chemotherapy-induced neutropenia, CYP = cytochrome P450, ERCC1 = excision repair cross-complementing 1, G-CSF = granulocyte colony-stimulating factor, GSTs = glutathione S-transferases, OR = odds ratio, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, WBC = white blood cell.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 1966; 64:328–340. [DOI] [PubMed] [Google Scholar]
- 2.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34: 730–651. [DOI] [PubMed] [Google Scholar]
- 3.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006; 106:2258–2266. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 5.Gianni L, Norton L, Wolmark N, et al. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol 2009; 27:4798–4808. [DOI] [PubMed] [Google Scholar]
- 6.Fairchild CR, Ivy SP, Kao-Shan CS, et al. Isolation of amplified and overexpressed DNA sequences from adriamycin-resistant human breast cancer cells. Cancer Res 1987; 47:5141–5148. [PubMed] [Google Scholar]
- 7.Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood 1999; 94:1086–1099. [PubMed] [Google Scholar]
- 8.Lal S, Wong ZW, Sandanaraj E, et al. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci 2008; 99:816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang TK, Weber GF, Crespi CL, et al. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 1993; 53:5629–5637. [PubMed] [Google Scholar]
- 10.Roy P, Yu LJ, Crespi CL, et al. Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 1999; 27:655–666. [PubMed] [Google Scholar]
- 11.Oliveira AL, Rodrigues FF, Santos RE, et al. GSTT1, GSTM1, and GSTP1 polymorphisms and chemotherapy response in locally advanced breast cancer. Genet Mol Res 2010; 9:1045–1053. [DOI] [PubMed] [Google Scholar]
- 12.Gor PP, Su HI, Gray RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res 2010; 12:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma B, Yeo W, Hui P, et al. Acute toxicity of adjuvant doxorubicin and cyclophosphamide for early breast cancer—a retrospective review of Chinese patients and comparison with an historic Western series. Radiother Oncol 2002; 62:185–189. [DOI] [PubMed] [Google Scholar]
- 14.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J Clin Oncol 2004; 22:4302–4311. [DOI] [PubMed] [Google Scholar]
- 16.Crawford J, Glaspy JA, Stoller RG, et al. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther 2005; 3:36–46. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji D, Kamezato M, Daimon T, et al. Retrospective analysis of severe neutropenia in patients receiving concomitant administration of docetaxel and clarithromycin. Chemotherapy 2013; 59:407–413. [DOI] [PubMed] [Google Scholar]
- 18.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 2003; 21:4524–4531. [DOI] [PubMed] [Google Scholar]
- 19.Lyman GH, Morrison VA, Dale DC, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 2003; 44:2069–2076. [DOI] [PubMed] [Google Scholar]
- 20.Morrison VA, Picozzi V, Scott S, et al. The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin's lymphoma receiving initial CHOP chemotherapy: a risk factor analysis. Clin Lymphoma 2001; 2:47–56. [DOI] [PubMed] [Google Scholar]
- 21.Chan A, Chen C, Chiang J, et al. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 2012; 20:1525–1532. [DOI] [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003; 98:484–495. [DOI] [PubMed] [Google Scholar]
- 23.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J Clin Oncol 2004; 22:4302–4311. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins P, Scaife J, Freeman S. Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann Oncol 2012; 23:1766–1771. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Brogan K, Panageas KS, et al. Change in cycle 1 to cycle 2 haematological counts predicts toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Drugs Aging 2005; 22:709–715. [DOI] [PubMed] [Google Scholar]
- 26.Salar A, Haioun C, Rossi FG, et al. The need for improved neutropenia risk assessment in DLBCL patients receiving R-CHOP-21: findings from clinical practice. Leuk Res 2012; 36:548–553. [DOI] [PubMed] [Google Scholar]
- 27.Moreau M, Klastersky J, Schwarzbold A, et al. A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann Oncol 2009; 20:513–519. [DOI] [PubMed] [Google Scholar]
- 28.Joy MS, La M, Wang J, et al. Cyclophosphamide and 4-hydroxycyclophosphamide pharmacokinetics in patients with glomerulonephritis secondary to lupus and small vessel vasculitis. Br J Clin Pharmacol 2012; 74:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima M, Komagata S, Fujiki Y, et al. Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genomics 2007; 17:431–445. [DOI] [PubMed] [Google Scholar]
- 30.Ekhart C1, Doodeman VD, Rodenhuis S, et al. Influence of polymorphisms of drug metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide. Pharmacogenet Genomics 2008; 18:515–523. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann MH, Blievernicht JK, Klein K, et al. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6∗6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther 2008; 325:284–292. [DOI] [PubMed] [Google Scholar]
- 32.Kamikozuru H, Kuramochi H, Hayashi K, et al. ERCC1 codon 118 polymorphism is a useful prognostic marker in patients with pancreatic cancer treated with platinum-based chemotherapy. Int J Oncol 2008; 32:1091–1096. [PubMed] [Google Scholar]
- 33.Kang S, Ju W, Kim JW, et al. Association between excision repair cross-complementation group 1 polymorphism and clinical outcome of platinum-based chemotherapy in patients with epithelial ovarian cancer. Exp Mol Med 2006; 38:320–324. [DOI] [PubMed] [Google Scholar]
- 34.Viguier J, Boige V, Miquel C, et al. ERCC1 codon 118 polymorphism is a predictive factor for the tumor response to oxaliplatin/5-fluorouracil combination chemotherapy in patients with advanced colorectal cancer. Clin Cancer Res 2005; 11:6212–6217. [DOI] [PubMed] [Google Scholar]
- 35.Inada M, Sato M, Morita S, et al. Associations between oxaliplatin-induced peripheral neuropathy and polymorphisms of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol Ther 2010; 48:729–734. [DOI] [PubMed] [Google Scholar]
- 36.Yu JJ, Lee KB, Mu C, et al. Comparison of two human ovarian carcinoma cell lines (A2780/CP70 and MCAS) that are equally resistant to platinum, but differ at codon 118 of the ERCC1 gene. Int J Oncol 2000; 16:555–560. [DOI] [PubMed] [Google Scholar]
- 37.Andersson BS, Sadeghi T, Siciliano MJ, et al. Nucleotide excision repair genes as determinants of cellular sensitivity to cyclophosphamide analogs. Cancer Chemother Pharmacol 1996; 38:406–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


