Supplemental Digital Content is available in the text
Keywords: diabetes mellitus, dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 receptor, response, type 2
Abstract
Incretin hormone-based therapy in type 2 diabetes has been widely used, and dipepdityl peptidase-4 (DPP-4) inhibitors, which prevent incretin degradation, have become popular oral hypoglycemic agents. The efficacy of DPP-4 inhibitors varies from individuals, and factors determining responses to DPP-4 inhibitors have not been fully established. We aimed to investigate whether genetic variations in glucagon-like peptide (GLP-1) receptor are associated with responses to DPP-4 inhibitors in patients with type 2 diabetes.
Genetic variations of rs3765467 in GLP-1 receptor were explored in 246 patients with type 2 diabetes who received DPP-4 inhibitors treatment for 24 weeks in addition to previous medication. Patients with glycated hemoglobin (HbA1c) > 7% and who were naive to any DPP-4 inhibitors were enrolled. Responders were defined as those who showed a > 10% reduction in HbA1c after DPP-4 inhibitor treatment.
DPP-4 inhibitors improved glycemic parameters and lipid profiles. Compared to the major genotype (GG), a larger proportion of patients with the minor allele genotype (GA/AA) were responders (P = 0.018), and also showing greater HbA1c reductions (1.3 ± 1.1 vs 0.9 ± 1.2%; P = 0.022). This genetic effect remained significant even after adjustment for other confounding factors (OR = 2.00, 95% CI = 1.03–3.89).
Polymorphism in the GLP-1 receptor may influence DPP-4 inhibitor response. Further studies in larger population will help determine the association between genetic variation and interindividual differences in DPP-4 inhibitor therapy.
1. Introduction
Incretin hormones regulate glucose-modulated insulin secretion from pancreatic β cells.[1] The 2 incretin hormones, glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are inactivated by dipeptidyl peptidase-4 (DPP-4), the peptidase enzyme soon after they secreted from intestinal cells.[2] Incretins act on G protein-coupled receptors on the pancreatic β cell membranes to stimulate cyclic adenosine monophosphate (cAMP) formation, protein kinase A activation, and insulin secretion.[1] The process of stimulated insulin release is rapid, and DPP-4 inhibitors prevent degradation of endogenous incretin hormones, to improve the duration of GLP-1 and GIP activity. In fact, 6-month DPP-4 inhibitor treatment elevated insulin secretion and decreased postprandial glucagon-to-insulin ratio in a clinical study.[3] Additionally, DPP-4 inhibitors also involve a low risk of hypoglycemia.[4] Although safety concerns for acute pancreatitis and DPP-4 inhibitor use are controversial, a population-based case-control study showed that incretin-based therapy did not increase acute pancreatitis risk.[5]
A number of studies have reported on associations between specific genetic variations and glycemic responses obtained with antidiabetic medications.[6–9] Genetic mutation of the peroxisome proliferator-activated receptor (PPAR) γ2 was shown to affect responses to rosiglitazone treatment in patients with type 2 diabetes mellitus.[6] However, DPP-4 inhibitors, to which responses by T2DM patients vary, the genetic factors are not fully understood.[10] A 52-week treatment with DPP-4 inhibitor as add-on therapy in Korean population showed that DPP-4 inhibitor responders had higher basal fasting plasma glucose, with no effect of age on their response rate.[10] This was in contrast to another study that was mainly based on Caucasian population of DPP-4 inhibitor responders characterized older age with mild fasting hyperglycemia.[11] GLP-1 receptors are involved in the DPP-4 inhibitor action mechanism, and GLP-1 responses may help predict the efficacy DPP-4 inhibitors.[12] A recent study reported that DPP-4 inhibitors could activate incretin receptors and influence the gut-to-pancreas neural axis.[2] The efficacy of DPP-4 inhibitors might be affected by GLP-1 receptor, in that the altered structure or affinity of GLP-1 receptor could determine the difference in responses to DPP-4 inhibitors.
Previously, it was reported that the rs3765467 variant of the GLP-1 receptor gene (GLP1R) was associated with insulin secretory response to exogenous GLP-1 in nondiabetic subjects.[13] Therefore, we investigated whether this same variation in GLP1R could affect T2DM patients’ responses to DPP-4 inhibitors.
2. Methods
2.1. Subjects inclusion criteria and study preparation
As the current study was exploratory, proportions of responders for GA genotype and GG/AA genotype were estimated at 50% and 70%, respectively, which was modified from a previous study.[14] For an allocation rate of each genotype of 1:1 (α error = 0.05, β error = 0.20), the total sample size required was calculated as 186, with 80% statistical power. A total of 246 Korean patients with T2DM were recruited from outpatient clinics at Yonsei University Severance Hospital, and Seoul National University Hospital, both located in Seoul, Korea. Diagnosis of T2DM was defined according to the American Diabetes Association's criteria.[15] Study inclusion criteria were (1) ≥ 20 years-old age; (2) DPP-4 inhibitor naive at baseline; (3) received DPP-4 inhibitor treatment ≥ 24 weeks; (4) baseline glycated hemoglobin (HbA1c) > 7%; (5) no hypoglycemic medication change in the last 6 months. Exclusion criteria were patients with type 1 diabetes mellitus, pregnant or lactating women, and patients with missing data on baseline clinical or biochemical parameters. We used a modified definition of glycemic response according to a previous study.[14] Responders were defined as those exhibiting a ≥ 10% reduction in HbA1c values after 24 weeks of DPP-4 inhibitor treatment. The Institutional Review Board of the Yonsei University College of Medicine (4-2011-0912, 4-2001-0039), and Seoul National University Hospital approved this study (0412-138-017, 1205-130-411). All subjects provided written informed consent.
2.2. Metabolic and clinical parameters
Fasting blood glucose level, HbA1c, and lipid profiles were assessed at both baseline and 24 weeks after treatment. Fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were measured after overnight (8-hour) fasting. Clinical parameters of age, sex, height, weight, duration of diabetes, and type of DPP-4 inhibitors were collected. The body mass index (BMI) was calculated using the following formula: BMI = body weight (kg) / height m2. Obesity was defined as BMI ≥ 25 kg/m2.[16]
2.3. DNA extraction and resequencing of GLP-1 receptor gene
Genomic DNA was isolated from peripheral blood lymphocytes in 246 patients. The polymerase chain reaction (PCR) was performed to amplify exon 4 on chromosome 6 (forward primer GCGTATATGTCAGGGGAGGA, reverse primer TTTGTCCAGAAAGCATGGTG) and an automated genetic analyzer (Model 3730xl, Applied Biosystems, Foster City, CA) was used to sequence the sample. All DNA was stored at 4’C in 96-well DNA storage boxes.
2.4. Statistical analysis
Differences between genotype, allele, and responder groups were tested by the chi-square test for categorical variables or Student's t-test for continuous variables. The paired t-test was used to analyze the changes in glycemic and lipid parameters before and after treatment. Fisher's exact test was used if expected cell frequencies were <5. For an additive model, analysis of variance (ANOVA) was used to compare the means, followed by post hoc analysis. Proportions in additive model were analyzed in the chi-square test. Genotype frequencies were tested for Hardy–Weinberg equilibrium using the chi-square test. Multivariate logistic regression analyses were conducted to assess independent associations between patient's responses to DPP-4 inhibitors (dependent variable) and rs3765467 (independent variable), including covariates. Results were expressed as odds ratio (OR) and 95% confidence interval (CI). Given that triglyceride, LDL cholesterol, HDL cholesterol, aspartate aminotransferase (AST), and alanine transaminase (ALT) values were not normally distributed, statistical analyses of these values were conducted on log-transformed data. All statistical analyses were conducted using IBM SPSS (Version 20.0, IBM Corp. Armonk, NY). A P value < 0.05 was considered statistically significant. Statistical power and sample size were calculated using the G∗power program (Version 3.1.9.2, Erdfelder, Faul, & Buchner).[17]
3. Results
3.1. Characteristics of study population
Supplementary Table 1 shows the allele and genotype distributions of rs3765467 in the study population. G was the major allele, whereas A was the minor allele in this group. The genotype distribution did not deviated from Hardy–Weinberg equilibrium (P = 0.939). Table 1 shows clinical characteristics of the study participants at baseline. The mean duration of diabetes was 9.3 years, and the HbA1c level was ∼8.2%. After 24 weeks of DPP-4 inhibitor add-on treatment, fasting blood glucose levels and HbA1c values significantly decreased (from 155.5 ± 44.8 mg/dL to 133.7 ± 34.7, P < 0.001 for fasting blood glucose, and 8.2 ± 1.1% to 7.2 ± 0.9%, P < 0.001 for HbA1c). In addition, total cholesterol and triglyceride levels also decreased significantly (all Ps < 0.001).
Table 1.
Baseline clinical characteristics of study population.

3.2. Association between GLP1R genetic variants and DPP-4 inhibitor efficacy
No significant differences in baseline fasting blood glucose and HbA1c were found between patients with GA/AA genotype and those with GG genotype. However, patients with GA/AA genotype were associated with a significantly greater reduction of HbA1c levels after DPP-4 inhibitor treatment (variation: 1.3 ± 1.1% vs 0.9 ± 1.2%; P = 0.022) (Table 2).
Table 2.
Clinical and biochemical characteristics of patients according to rs3765467 genotype.
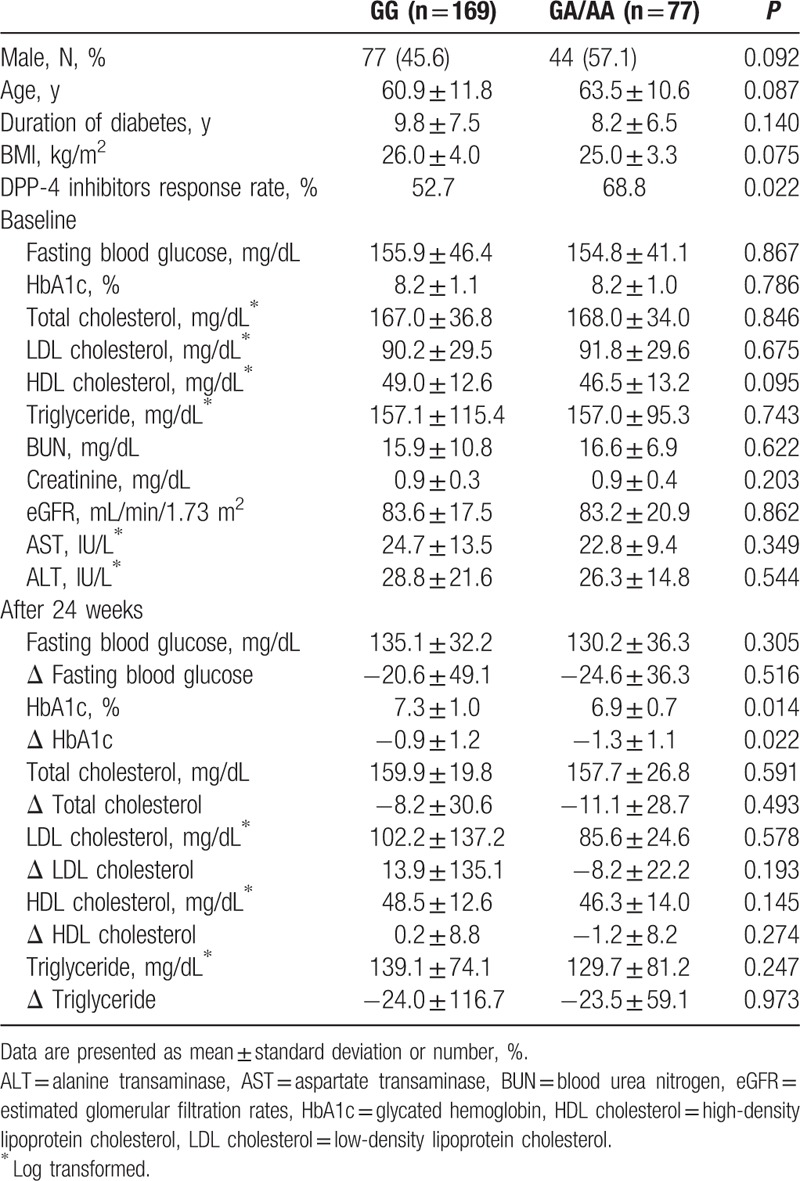
The proportion of DPP-4 inhibitor responders with GG genotype (52.7%) was significantly lower than that of responders with GA/AA genotype (68.8%, P = 0.018). Regarding the subjects’ responses to DPP-4 inhibitors, the responder group had shorter diabetes duration (8.1 ± 6.4 vs 10.8 ± 8.0 years, P = 0.008) with higher baseline fasting blood glucose level (162.4 ± 50.2 vs 146.2 ± 34.5 mg/dL, P = 0.008) and HbA1c (8.5 ± 1.2% vs 7.8 ± 0.6% P < 0.001). The same types of DPP-4 inhibitor were used in the 2 groups. When further analyzed with the additive model, only HbA1c levels following treatment reached statistical significance. The amount of HbA1c reduction was marginally different between the genotypes. However, the proportion of patients with AA genotype in the study population was only 2.8% (7/246). Compared to patients with G allele, those with an A allele showed greater reduction in HbA1c levels after 24 weeks (1.3 ± 1.1% vs 1.0 ± 1.2%, P = 0.044). In addition, proportion of responders was higher in patients with an A allele than those with a G allele (69.0% vs 55.4%, P = 0.021).
3.3. Effect of GLP1R genetic variation according to the baseline blood glucose level
As baseline HbA1c could influence the response to DPP-4 inhibitors, we stratified the subjects according to the median value of basal HbA1c (8.0%) and rs3765467 genotype. Proportion of DPP-4 inhibitor responders increased stepwise among the 4 groups, with the highest response rate shown in subjects with higher baseline HbA1c and GA/AA genotype (91.2%, P < 0.001 for trend, Fig. 1A), as well as A allele (89.2%, P < 0.001 for trend, Fig. 1B). Moreover, response rates to DPP-4 inhibitor in patients with higher baseline HbA1c (> 8%) were determined by rs3765467 variation (P = 0.005 for GA/AA genotype and 0.017 for A allele).
Figure 1.
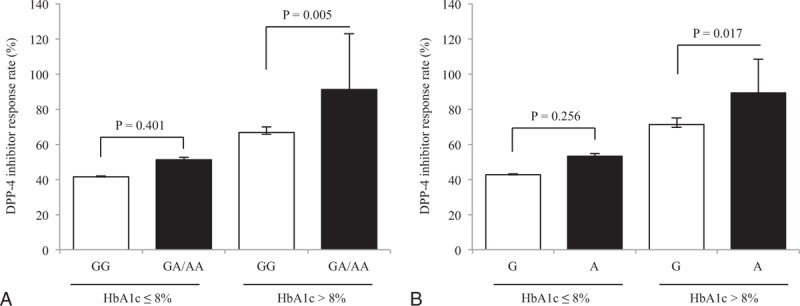
Differences in the response rates to DPP-4 inhibitors according to baseline HbA1c and rs3765467. (A) The proportion of responders according to HbA1c and rs3765467 genotype, (B) the proportion of responders according to HbA1c and rs3765467 allele. Error bars represent 95% confidence intervals. DPP-4 = dipepdityl peptidase-4, HbA1c = glycated hemoglobin.
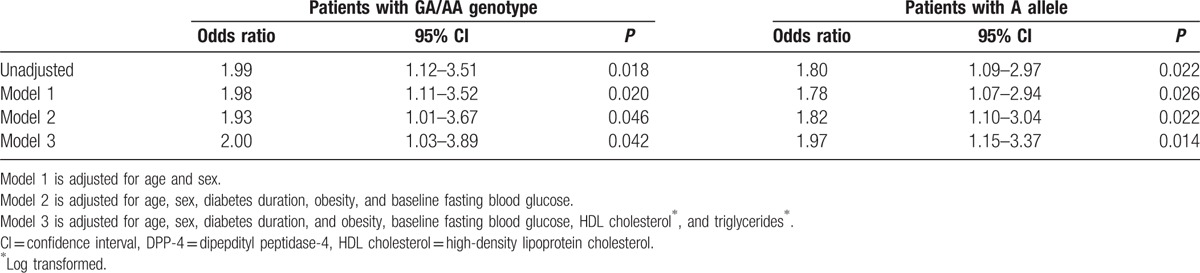
3.4. GLP1R genetic effect on DPP-4 inhibitor efficacy remained significantly in multivariate logistic analysis
Next, we performed multivariate analyses to evaluate whether this genetic effect remained significant, even after adjustment for other confounding factors. GLP1R variation remained a significant factor affecting the efficacy of DPP-4 inhibitors. Patients with an A allele (GA/AA) showed better responses to DPP-4 inhibitors than those without (GG). Compared with GG genotype, GA/AA genotype increased the likelihood of a response by 200% (Table 3). Furthermore, patients with an A allele tended to respond better to DPP-4 inhibitors (Table 3).
Table 3.
Logistic regression analysis for predicting DPP-4 inhibitor treatment response.
4. Discussion and conclusions
In the current study, we found that variation in GLP1R (rs3765426) is associated with DPP-4 inhibitor response efficacy. A larger number of patients with an A allele showed a reduction in HbA1c, with DPP-4 inhibitor treatment than those without. This genetic effect remained significant after adjustment for other confounding factors. In addition, as previously reported, responders to DPP-4 inhibitors had shorter duration of diabetes, higher fasting blood glucose, and HbA1c at baseline.[10]
Interestingly, in this study, the proportion of DPP-4 inhibitor responders increased in relation to GLP1R genotype and higher baseline HbA1c. However, the association between DPP-4 inhibitor efficacy and baseline HbA1c remains controversial. One cohort study reported that lower baseline HbA1c was linked to a good response in DPP-4 inhibitor treatment,[18] whereas others have shown that a higher baseline HbA1c could predict a greater DPP-4 inhibitor response.[10,19] In a meta-analysis that compared HbA1c changes according to baseline HbA1c value, the amount of reduction increased to 0.83%, with a baseline HbA1c of 8.5%.[19] In the current study, a greater reduction of HbA1c (1.3%) was observed in GA/AA genotype population with similar baseline HbA1c (8.2%), suggesting a role for GLP1R variation in the effect of DPP-4 inhibitors.
Many investigators have found the concepts of genetic variation and antidiabetic medication efficacy to be quite attractive. For PPAR agonists, polymorphisms of PPAR, adipose tissue, adiponectin, and cholesterol synthesis have all been shown to have an impact on drug potency.[6–8,20,21] Genetic variants in transcription factors have also been shown to influence pharmacokinetics and pharmacodynamics of metformin.[22] Although the correlation between DPP-4 inhibitors and genetic variation is controversial, our study would support such a correlation with the A allele of rs3765467.
Previously, we reported no significant association between genetic variations in DPP4 itself, and patient responses to DPP-4 inhibitors.[23] On the other hand, an association between interleukin-6 polymorphism and DPP-4 inhibitor response has been reported.[24] Recent preclinical studies have shown association between DPP-4 inhibitors and incretin receptors.[25,26] Since administration of DPP-4 inhibitor to incretin receptor knockout mice did not improve glycemic parameters, these findings suggest the importance of incretin receptor in glucoregulatory actions of DPP-4 inhibitors.
Two possible functional mechanisms of genetic effects have been suggested:[27] (1) GLP-1 receptor enhances cAMP signaling, and (2) GLP-1 receptor alters pancreatic β cell apoptosis or proliferation. Several papers reported that variation in GLP1R could regulate intracellular postreceptor signaling.[28–31] Moreover, GLP1R variant is reported to be able to alter receptor binding affinity.[31,32] Rs3765467 is a nonsynonymous mutation in which amino acid arginine is replaced by glutamine at Exon 5 position 131 (R131Q) of the GLP-1 receptor, and it was also reported to be linked with cAMP signaling in oxynotomodulin.[32] A recent human study showed that rs3765467 had a very profound insulinotropic effect in response to infused GLP-1 agonist.[13] Given this point, a GLP1R variation, especially rs3765467, could be a candidate for determining the efficacy of DPP-4 inhibitors. Meanwhile, the degree of DPP-4 inhibitor efficacy could be explained by ethnicity.[33] Compared to non-Asians, Asians exhibit a higher capacity to respond to DPP-4 inhibitor treatment. In a meta-analysis study, lower BMI in Asians, which could be related to insulin sensitivity, was referred to as a primary factor.[33] According to an international database, minor allele frequency (MAF) for rs3765467 is reported to occur in up to 36% of Asians, which is relatively high compared to other ethnicities.[34] In the current study, the MAF was 17.1%. The relatively high A allele frequency in Asians may account for the better response in these populations.
A recent Taiwanese study showed that GLP-1 analog response was not affected by GLP1R variation.[35] The study enrolled 36 patients with poorly controlled hyperglycemia and treated them with a 6-day continuous subcutaneous insulin infusion and exenatide, a GLP-1 analogue. Patients with rs3765467 minor allele genotype showed a significant reduction in the plasma glucose level before adjustment. The small sample size might account for the statistical insignificance after adjustment. In contrast, our study showed response rates for GA and GG/GA genotypes of 52.7% and 68.8%, which were comparable to our estimation. In addition, the statistical power reached 83%, which slightly exceeded the 80% we had predicted.
Meanwhile, there are some limitations to our study. First, we could not assess insulin sensitivity or pancreatic beta cell function due to a lack of information on C peptides and insulin levels. However, a previous study showed that the minor allele of rs3765467 is associated with a 2-fold increase in insulin secretion in response to exogenous GLP-1 infusion.[13] Second, types and dose of DPP-4 inhibitors were not controlled in the study population. Despite uncontrolled medication, difference in the types of DPP-4 inhibitor between responder and nonresponder was insignificant. Third, physical activity and dietary changes that could improve hyperglycemia were not evaluated in the current study. Nevertheless, our data suggest for the first time that GLP1R variation can affect patient response to DPP-4 inhibitors. Finally, since the current study was an exploratory and observation study, we could not confirm the actual or specific mechanism of GLP1R and DPP-4 inhibitor response.
In conclusion, the present study shows that DPP-4 inhibitor treatment reduces blood glucose levels during a 24-week period, and that a greater number of DPP-4 inhibitor responders carry either the GA/AA genotype or an A allele. In multivariate regression analysis, both GA/AA genotype and an A allele for GLP1R were consistently associated with DPP-4 inhibitor response. This suggests that variation in GLP1R (rs3765467) can influence DPP-4 inhibitor efficacy. Further studies in a large population are warranted to determine the mechanisms of DPP-4 inhibitor response in GLP1R. Moreover, it would be interesting to identify the genetic differences related to DPP-4 inhibitor response among other ethnic groups. This finding could be clinically relevant in that one could potentially anticipate DPP-4 inhibitor responses before administering thereof.
Acknowledgments
The authors thank to all participants in this study.
Supplementary Material
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, CI = confidence interval, DPP-4 = dipepdityl peptidase-4, GLP-1 = glucagon-like peptide, GLP1R = GLP-1 receptor gene, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein, LDL = low-density lipoprotein, OR = odds ratio, T2DM = type 2 diabetes mellitus.
CSK and ESK contributed equally to this work.
Funding: This study is financially supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2016R1A2B4013029 and 2015M3A9B6029138) and the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI14C0060) and the Faculty Fund from Yonsei University College of Medicine (No. 6-2011-0085).
Authorship: Study concept and design—EH, SHK, and ESK; analysis and interpretation of data—EH, HSP, CSK, and ESK,
Drafting of the manuscript—EH and ESK; critical revision of the manuscript for important intellectual content: OK, EYC, HJW, Y-hL, S-HL, CHK, L-KK, SHK, and KSP; statistical analysis—EH and HSP; administrative, technical, or material support—Y-hL, S-HL, CHK, L-KK, SHK, CSK, and KSP.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165. [DOI] [PubMed] [Google Scholar]
- 2.Waget A, Cabou C, Masseboeuf M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology 2011; 152:3018–3029. [DOI] [PubMed] [Google Scholar]
- 3.Yang HK, Kang B, Lee SH, et al. Effects of 6-month sitagliptin treatment on insulin and glucagon responses in Korean patients with type 2 diabetes mellitus. Diabetes Metab J 2015; 39:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HM, Lim JS, Lee BW, et al. Optimal candidates for the switch from glimepiride to sitagliptin to reduce hypoglycemia in patients with type 2 diabetes mellitus. Endocrinol Metab 2015; 30:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomsen RW, Pedersen L, Moller N, et al. Incretin-based therapy and risk of acute pancreatitis: a nationwide population-based case-control study. Diabetes Care 2015; 38:1089–1098. [DOI] [PubMed] [Google Scholar]
- 6.Kang ES, Park SY, Kim HJ, et al. Effects of Pro12Ala polymorphism of peroxisome proliferator-activated receptor gamma2 gene on rosiglitazone response in type 2 diabetes. Clin Pharmacol Ther 2005; 78:202–208. [DOI] [PubMed] [Google Scholar]
- 7.Kang ES, Park SY, Kim HJ, et al. The influence of adiponectin gene polymorphism on the rosiglitazone response in patients with type 2 diabetes. Diabetes Care 2005; 28:1139–1144. [DOI] [PubMed] [Google Scholar]
- 8.Kang ES, Park SE, Han SJ, et al. LPIN1 genetic variation is associated with rosiglitazone response in type 2 diabetic patients. Mol Genet Metab 2008; 95:96–100. [DOI] [PubMed] [Google Scholar]
- 9.Min SH, Kwak SH, Cho YM, et al. Clinical characteristics of subjects with sulfonylurea-dependent type 2 diabetes. Endocrinol Metab 2015; 30:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YA, Yoo WS, Hong ES, et al. Clinical characteristics and metabolic predictors of rapid responders to dipeptidyl peptidase-4 inhibitor as an add-on therapy to sulfonylurea and metformin. Diabetes Metab J 2015; 39:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monami M, Cremasco F, Lamanna C, et al. Predictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trials. Diabetes/Metab Res Rev 2011; 27:362–372. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705. [DOI] [PubMed] [Google Scholar]
- 13.Sathananthan A, Man CD, Micheletto F, et al. Common genetic variation in GLP1R and insulin secretion in response to exogenous GLP-1 in nondiabetic subjects: a pilot study. Diabetes Care 2010; 33:2074–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bluher M, Lubben G, Paschke R. Analysis of the relationship between the Pro12Ala variant in the PPAR-gamma2 gene and the response rate to therapy with pioglitazone in patients with type 2 diabetes. Diabetes Care 2003; 26:825–831. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015; 38 suppl:S8–S16. [DOI] [PubMed] [Google Scholar]
- 16.Kim CS, Ko SH, Kwon HS, et al. Prevalence, awareness, and management of obesity in Korea: data from the Korea national health and nutrition examination survey (1998–2011). Diabetes Metab J 2014; 38:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faul F, Erdfelder E, Lang AG, et al. G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39:175–191. [DOI] [PubMed] [Google Scholar]
- 18.Monami M, Ragghianti B, Zannoni S, et al. Identification of predictors of response to basal insulin and DPP4 inhibitors in patients with type 2 diabetes failing to other therapies. Acta Diabetol 2015; 53:35–40.epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Chiodini P, Capuano A, et al. Baseline glycemic parameters predict the hemoglobin A1c response to DPP-4 inhibitors: meta-regression analysis of 78 randomized controlled trials with 20,053 patients. Endocrine 2014; 46:43–51. [DOI] [PubMed] [Google Scholar]
- 20.Park SE, Kang ES, Kim DH, et al. Effect of ABCA1 variant on atherogenic dyslipidaemia in patients with Type 2 diabetes treated with rosiglitazone. Diabetic Med 2009; 26:577–581. [DOI] [PubMed] [Google Scholar]
- 21.Kang ES, Cha BS, Kim HJ, et al. The 11482G >A polymorphism in the perilipin gene is associated with weight gain with rosiglitazone treatment in type 2 diabetes. Diabetes Care 2006; 29:1320–1324. [DOI] [PubMed] [Google Scholar]
- 22.Goswami S, Yee SW, Stocker S, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther 2014; 96:370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon O, Choe EY, Choi Y, et al. Discovery of dipeptidyl peptidase-4 gene variants and the associations with efficacy of vildagliptin in patients with type 2 diabetes—a pilot study. J Diabetes Metab 2013; 4:S13–006. [Google Scholar]
- 24.Matsui M, Takahashi Y, Takebe N, et al. Response to the dipeptidyl peptidase-4 inhibitors in Japanese patients with type 2 diabetes might be associated with a diplotype of two single nucleotide polymorphisms on the interleukin-6 promoter region under a certain level of physical activity. J Diabetes Invest 2015; 6:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansotia T, Baggio LL, Delmeire D, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 2004; 53:1326–1335. [DOI] [PubMed] [Google Scholar]
- 26.Flock G, Baggio LL, Longuet C, et al. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 2007; 56:3006–3013. [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA, Vardarli I. Genetic determinants predicting efficacy of glucose-lowering drugs? a long way to go. Diabetes Care 2010; 33:2123–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heller RS, Kieffer TJ, Habener JF. Point mutations in the first and third intracellular loops of the glucagon-like peptide-1 receptor alter intracellular signaling. Biochem Biophys Res Commun 1996; 223:624–632. [DOI] [PubMed] [Google Scholar]
- 29.Salapatek AMF, MacDonald PE, Gaisano HY, et al. Mutations to the third cytoplasmic domain of the glucagon-like peptide 1 (GLP-1) receptor can functionally uncouple GLP-1-stimulated insulin secretion in HIT-T15 cells. Mol Endocrinol 1999; 13:1305–1317. [DOI] [PubMed] [Google Scholar]
- 30.Tokuyama Y, Matsui K, Egashira T, et al. Five missense mutations in glucagon-like peptide 1 receptor gene in Japanese population. Diabetes Res Clin Pr 2004; 66:63–69. [DOI] [PubMed] [Google Scholar]
- 31.Beinborn M, Worrall CI, McBride EW, et al. A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Peptides 2005; 130:1–6. [DOI] [PubMed] [Google Scholar]
- 32.Koole C, Wootten D, Simms J, et al. Polymorphism and ligand dependent changes in human glucagon-like peptide-1 receptor (GLP-1R) function: allosteric rescue of loss of function mutation. Mol Pharmacol 2011; 80:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia 2013; 56:696–708. [DOI] [PubMed] [Google Scholar]
- 34.International HapMap 1000 Genomes Project. Available at: https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/. [Google Scholar]
- 35.Lin CH, Lee YS, Huang YY, et al. Polymorphisms of GLP-1 receptor gene and response to GLP-1 analogue in patients with poorly controlled type 2 diabetes. J Diabetes Res 2015; 2015:176949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


