Supplemental Digital Content is available in the text
Keywords: C-reactive protein, meta-analysis, peanut, tree nut
Abstract
Background:
The effects of different types of tree nut, peanut, and soy nut consumption on serum C - reactive protein (CRP) are not well established. we aimed to undertake a systematic review and meta-analysis of prospective studies to determine the effect of nut consumption (tree nuts, peanuts, and soy nuts) on serum CRP.
Method:
PubMed-Medline, Web of Science, Cochrane Database, and Google Scholar databases were searched (up until April 20 2016) to identify prospective studies evaluating the impact of tree nut, peanut, and soy nut consumption on serum CRP. Random effects models meta-analysis was used for quantitative data synthesis. Sensitivity analysis was conducted using the leave-one-out method. Heterogeneity was quantitatively assessed using the I2 index. Systematic review registration: CRD42016038044.
Results:
From a total of 844 entries identified via searches, 20 studies were included in the final selection. The meta-analysis indicated a nonsignificant increase in serum CRP concentrations following nut consumption (weighted mean difference [WMD] 0.17 mg/L, (95% CI –0.67 to 0.33, I2 52.1%). The WMDs for IL6 was –0.06(ng/dL), (95% CI –0.69 to 0.56, I2 9.6%), –0.71(mg/dL), (95% CI –1.11 to –0.30, I2 6.3%), for leptin, and -0.60(mg/dL), (95% CI –1.88 to 0.68, I2 5.6%) for adiponectin, and −0.18(mg/dL), (95% CI –1.24 to 0.88, I2 9.3%) for IL10 and –0.37 (pg/mL), (95% CI –0.90 to 0.16, I2 7.9%) for TNF-α. These findings were robust in sensitivity analyses.
Conclusions:
This meta-analysis suggests that nut consumption significantly decrease leptin while have no significant effect on CRP, IL6, adiponectin, IL10, and TNF-α.
1. Introduction
Inflammation play a crucial role in the progress of cardiovascular disease (CVD) and type 2 diabetes (T2D).[1,2] Inflammatory and endothelial markers such as serum C-reactive protein (CRP), Interleukin-6 (IL-6), fibrinogen, vascular cell adhesion molecule-1 (VCAM-1) and Intracellular adhesion molecule-1 (ICAM-1) have been recognized as independent predictors of CVD or (T2D).[3] Serum CRP is a indicator of general inflammation and is raised in the existence of chronic situation such as CVD,[4] obesity,[5] (T2D),[6] and components of metabolic syndrome[7]; hypertension,[8] increased waist circumference,[9] fasting hyperglycemia,[10] low serum high-density lipoprotein cholesterol, and hypertriglyceridemia.[11] Recently, mostly the focuses of dietary components were on interaction between diet and inflammation. Clinical and epidemiologic surveys proposed that dietary factors includingn-3 polyunsaturated fatty acids (PUFA), antioxidant vitamins, dietary fiber, and L-arginine might play an curial role in regulating inflammation.[12–14] Nuts are full of unsaturated fatty acids and have nonlipid components including antioxidant vitamins (especially vitamin E), dietary fiber, magnesium, plant protein have a lot of arginine and numerous phytochemicals.[15] Earlier experiments reported that the cardio protective properties of nuts consumption might be accredited to the amended insulin sensitivity, endothelial function, or anti-inflammatory role of nuts.[3,16] Studies suggested that common nuts consumption is related with improvements stages of inflammatory factors including CRP, IL-6, and fibrinogen, even after adjustment for covariates.[17] Though the action of nuts which are high in monounsaturated fat, including almonds, has not been formerly explained in association with inflammation, some of the constituents of nuts including arginine, magnesium, fiber, and vitamin E have confirmed an anti-inflammatory effect.[14,18,19] A randomized clinical trial verified that a filtered palmitoleic acid (16-1; omega-7) can make a weighty decreases in serum CRP following administration for a month.[20] In this regard, Jiang et al[17] reported the reverse association between nut consumption and the inflammatory factors such as CRP, fibrinogen, and IL-6. In same line, it has been reported that the administration of almonds made a substantial decrease in serum CRP.[21]
As of yet, no systematic review and meta-analysis is available on the impact of different types of tree nut, peanut, and soy nut consumption serum CRP. Consequently, in this survey, we aimed to perform a systematic review and meta-analysis of published randomized control trials (RCTs) to review the data on impacts of nuts (pistachios, cashews, hazelnuts, almonds, walnuts, pecans, macadamia nuts, peanuts, and soy nuts) on serum CRP. Via meta-analysis we computed the impact of before mentioned nuts. Moreover, we have reported our results based on different type of nuts.
2. Materials and methods
2.1. Literature search strategy
The current study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines.[22,23] Furthermore, this systematic reviewed meta-analysis protocol is registered in the International Prospective Register of Systematic Reviews (registration no: CRD42016038044). The primary exposure of interest was to evaluate the effect of tree nut, peanut, and soy nut consumption on serum CRP. We searched multiple databases including PUBMED/ Medline, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), Google scholar, and Web of Science; until April 2016 using a combination of search term shown in the supplementary Table 1. The wild-card term “∗” was used to surge the sensitivity of the search strategy .No language restriction was applied. This was completed by hand search of the reference list of eligible articles, and email correspondences to authors for additional data where relevant.
2.2. Selection criteria
We included all prospective studies which assessed the effect of nut consumption on our outcomes of interested. Eligible studies had to meet the following criteria: (1) being a controlled trial with either parallel or crossover design, (2) prospective studies of patients treated with nut consumption compared to control group (either no nut or placebo), (3) presentation of sufficient information on primary outcome at baseline and at the end of follow-up in each group or providing the net change values. Exclusion criteria were: (i) nonclinical studies; (ii) observational studies with case–control, cross-sectional or cohort design; and (iii) studies that did not provide mean (or median) serum or plasma concentrations of our interested outcomes at baseline and/or at the end of trial. Abstracts, narrative reviews, comments, opinion pieces, methodological, editorials, letters, or any other publications lacking primary data and/or explicit method explanations, were excluded. Study selection began with the removal of duplicates; next, 2 reviewers (MM and EK) excluded some papers based on titles and abstracts only. To avoid bias, they were blinded to the names, qualifications, or the institutional affiliations of the study authors. The agreement between the reviewers was excellent (Kappa index: 0.88; P < 0.001). Disagreements were fixed at a meeting between reviewers prior to selected articles being regained (a flowchart is available in Fig. 1).
Figure 1.
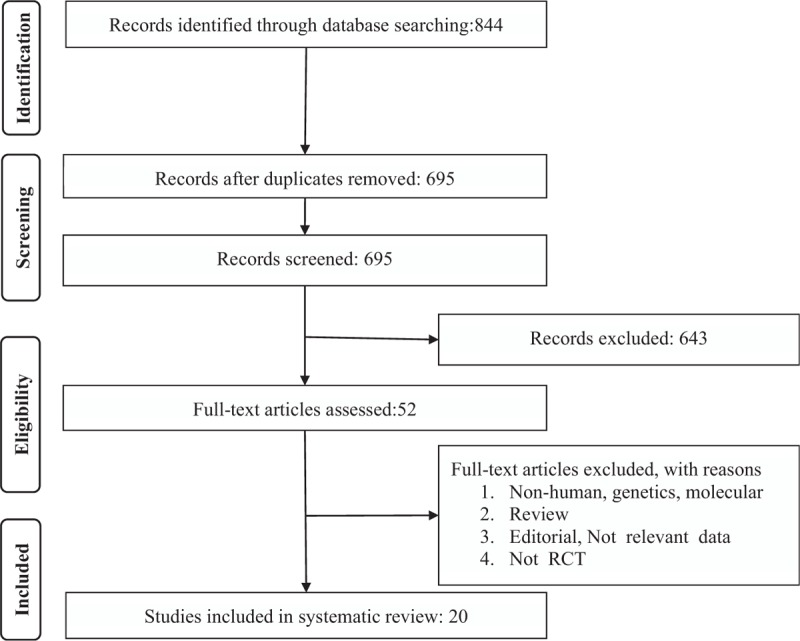
PRISMA flow chart for the studies selection. PRISMA = Systematic Reviews and Meta-Analyses.
2.3. Data extraction and management
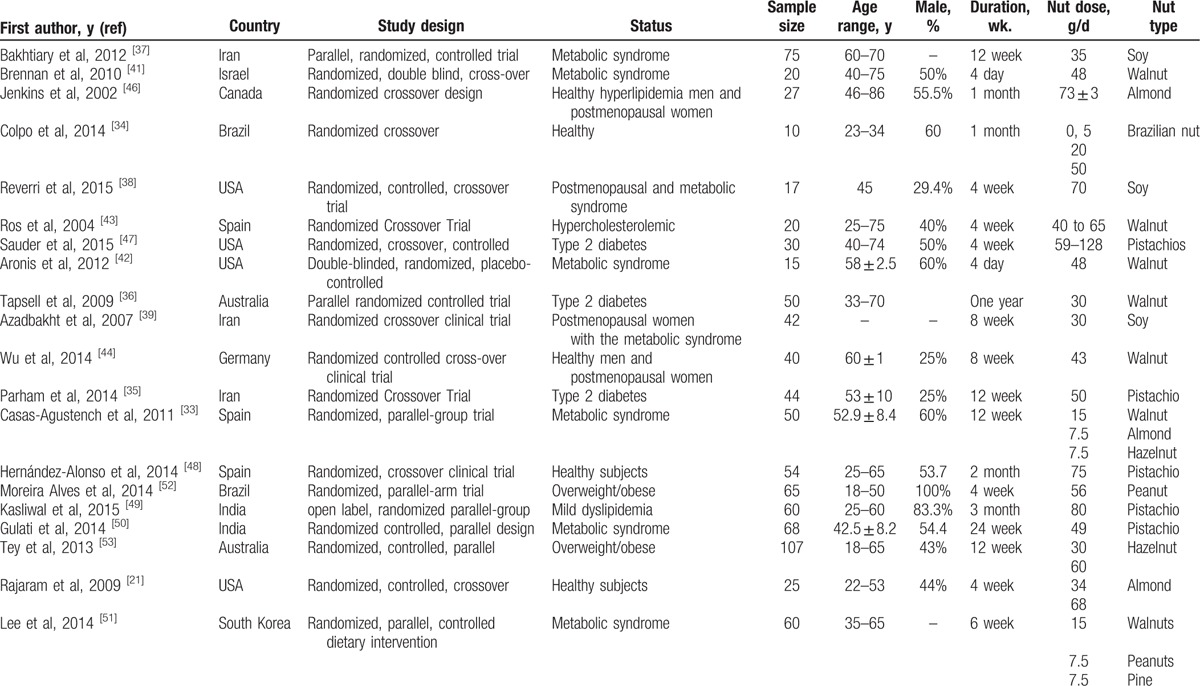
The full text of studies meeting inclusion criteria was recovered and screened to determine eligibility by 2 reviewers (MM, EK). Following assessment of methodological quality, the 2 reviewers extracted data onto a purpose-designed data extraction form and independently summaries what they consider to be the most important results from each study. Descriptive data extracted included the First author, year of publication, country, total sample size, study design; age range, male (%), follow-up duration (week), nut dose, and type of nut were summarized in Table 1. An independent reviewer confirmed all data entries.
Table 1.
General characteristics of the included studies.
2.4. Quality assessment
An assessment of bias in the included manuscript was performed by the Cochrane criteria.[24] The objects used for the assessment of manuscripts were including: acceptability of random sequence generation, distribution concealment, blinding of subjects, employees and consequence assessment, talking of drop-outs, selective outcome reporting, and additional possible causes of bias.[25]
2.5. Quantitative data synthesis
Based on recommendation of Cochrane Handbook[26] the mean change from baseline in the concentrations and SD of the variables of interest for both intervention and control groups were used to calculate the effect size. In brief, net changes in measurements (change scores) were calculated as follows: measure at end of follow-up − measure at baseline. Hozo et al[27] method was used in the case of the median and range (or 95% confidence interval [CL]). Moreover, standard deviation = standard error of the mean × square root (n), where n is the sample size.[25] Blood lipid and glucose levels were collated in mmol/L. A multiplication factor of 0.0259, 0.0113, or 0.0555 was used to convert cholesterol (total cholesterol, high-density lipoprotein (HDL)-C or low-density lipoprotein (LDL)-C), triglycerides and glucose levels respectively from mg/dL to mmol/L as appropriate.[25]
A random effects model (using the DerSimonian–Laird method) and the generic inverse variance method were used.[28] Heterogeneity was quantitatively assessed using the I2 index.[25] Effect sizes were stated as weighed mean difference (WMD) and 95% CL. Sensitivity analysis was performed using the leave-one-out method, that is, removing 1 study each time and repeating the analysis.[29,30]
2.6. Publication bias
Publication bias was discovered using visual inspection of Begg's funnel plot asymmetry, Begg's rank correlation, and Egger's weighted regression tests. Duval & Tweedie “trim and fill” and “fail-safe N” methods were used to adjust the analysis for the effects of publication bias.[31] Meta-analysis was performed using the comprehensive meta-analysis (CMA) V3 software (Biostat, NJ).[32]
3. Results
3.1. Flow of studies
Briefly, after multiple database searches, 844 published studies were identified and the abstracts reviewed. In total, 695 records remained after removing duplicates. However, 643 did not meet the inclusion criteria and were excluded. Also, 52 articles remained for further evaluation, of which, 32 were excluded for the following reasons: nonhuman studies, genetic, or molecular studies (n = 14); reviews or editorial articles (n = 13); not RCT (n = 2); not relevant data (3); supplementary Figure 1. Therefore, 20 studies were included in the meta-analysis. The study selection process is shown in Figure 1.
3.2. Risk of bias assessment
There is unclear risk of bias in some of items including allocation concealment, blinding of participants and personnel, incomplete outcome data, and other biases. Four studies have moderate risk of bias.[33–36] The other studies that were included had a low risk of bias according to selective outcome reporting. Details of the quality of bias assessment are shown in supplementary Table 2.
3.3. Characteristics of included studies
The 22 trials were all published between 2002 and 2015 (most of the studies were published in 2014) (Table 1). The clinical trials used different types and doses of nuts. Four studies investigated soy at an intake of 35,[37] 70,[38] or 30 g/day,[39,40] 6 studies investigated walnuts at an intake of 48,[41,42] 40 to 65,[43] 30,[36] 43,[44] 42.5 g/day,[45] 2 studies investigated almonds at an intake of 73 ± 3,[46] 34 and 68 g/day,[21] 5 studies investigated pistachio at an intake of 59–128,[47] 50,[35] 75,[48] 80,[49] 49 g/day,[50] 2 studies investigated mixed nuts; walnut (15 g/day), almond (7.5 g/day), hazelnut (7.5 g/day)[33] and another walnut (15 g/day), peanut (7.5 g/day), pine (7.5 g/day),[51] 1 study investigated Brazilian nut 0.5, 20 and 50,[34] Peanut 56,[52] hazelnut 30 and 60 g/day,[53] respectively. The range of intervention periods was from 4 day [41] up to 1 year.[36] The study designs of included studies were cross-over,[21,34,35,38–41,43–48] open label,[49] parallel-group [33,36,37,49–53] and double-blinded.[42] Selected trials enrolled subjects with metabolic syndrome,[33,37,41,42,50,51] postmenopausal and metabolic Syndrome,[38,39] type 2 diabetes,[35,36,47] overweight/obese,[52,53] healthy subjects,[21,34,48] healthy hyperlipidemic men and postmenopausal women,[46] hypercholesterolemic,[43] healthy men and postmenopausal women,[44] mild dyslipidemia[49] and mild hyperlipidemic.[45] The number of participants included in studies ranged from 10[34] to 107.[53] The range of ages of the participants was from 18[52,53] to 86.[46] Three of these studies were carried out on female subjects only.[37,39,51] Moreover, details each of these studies are listed in Table 1.
3.4. Pooled estimate of the effect of nuts consumption
Pooled estimate of the effect of nuts consumption on inflammatory and anti-inflammatory indexes are shown in Fig. 2. Our results showed that nut consumption have no significant effect on serum CRP level 0.17(mg/L) (95% CI –0.67 to 0.33); subgroup analysis is also shown in Fig. 2. We also found no significant effects of nut consumption on other inflammatory and anti-inflammatory factors apart from leptin 0.71 pg/mL (95% CI –1.11 to –0.30). We have failed to find a significant effect on serum markers of endothelial function (shown in Fig. 3). Our analysis showed that nut consumption improved HDL-c, LDL-c, total cholesterol, ApoAI, and fasting blood glucose significantly (Fig. 4).
Figure 2.
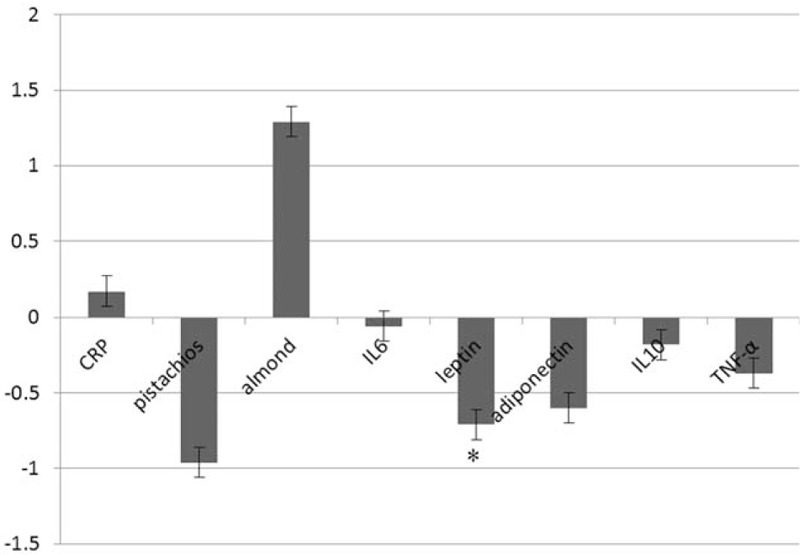
Plot to display weighted mean difference and 95% confidence intervals for the impact of nuts on inflammatory and anti-inflammatory factors.
Figure 3.
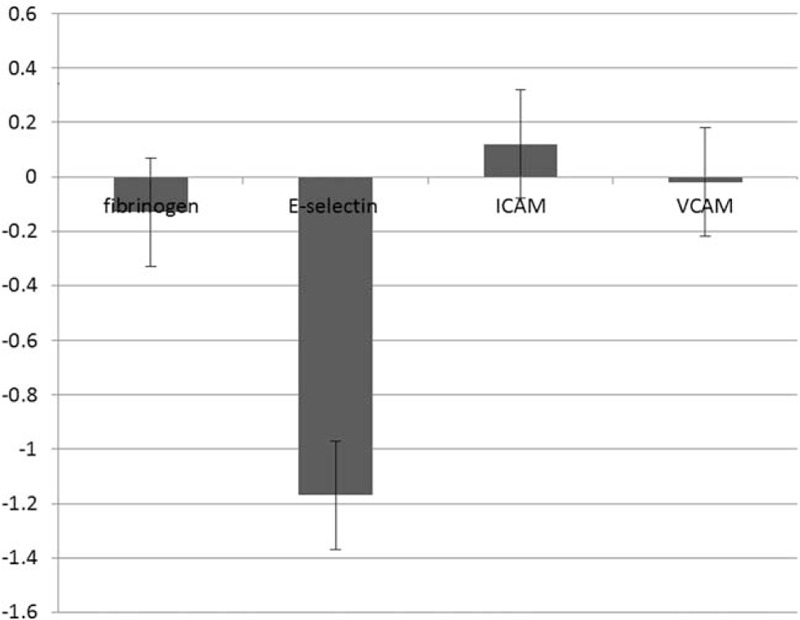
Plot to display weighted mean difference and 95% confidence intervals for the impact of nuts on endothelial function parameters.
Figure 4.

Plot to display weighted mean difference and 95% confidence intervals for the impact of nuts on lipid profile and glycemia.
3.5. Sensitivity analysis
In leave-one-out sensitivity analyses, the pooled effect estimates remained similar across all studies and within subgroups (Table 2).
Table 2.
Sensitivity analysis across all studies and within BAS-specific subgroups.
3.6. Publication bias
Visual inspection of funnel plot symmetry suggested no potential publication bias for the comparison of CRP levels between nuts consumed group and placebo (Fig. 5). That is in line with Begg's rank correlation test (tau with continuity correction = –0.03, z-value = 0.24, P-value = 0.809) and Egger's linear regression (intercept = –1.25, standard error = 2.96, 95% CI = –7.45 to 4.94, t-value = 0.423, df = 9, 2-tailed P = 0.676). After adjustment of effect size for potential publication bias using the Duval and Tweedie “trim and fill” correction, no potentially missing studies were imputed in the funnel plot (WMD 0.17 (mg/L), 95% CI –0.67, 0.33) (Fig. 6).
Figure 5.
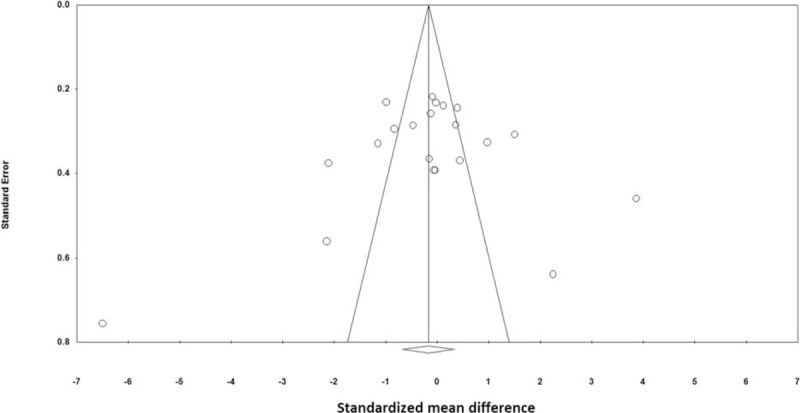
Funnel plots detailing publication bias in the studies selected for analysis. Open circles represent observed published studies; open diamond represents observed effect size.
Figure 6.
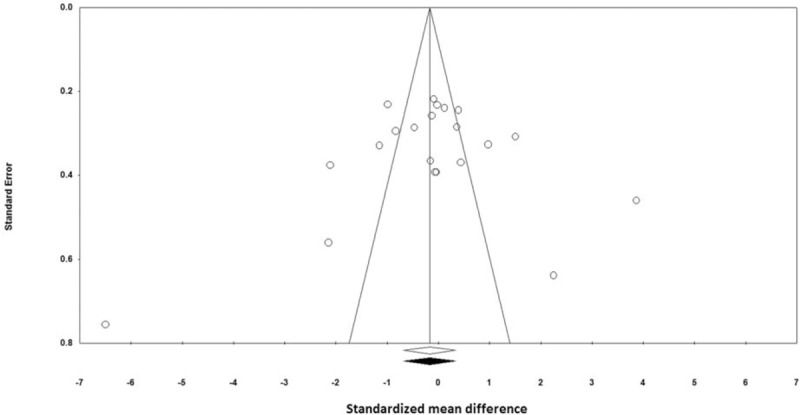
Trim and fill method was used to impute for potentially missing studies; no potentially missing study was imputed in the funnel plot. Open circles represent observed published studies; open diamond represents observed effect size; closed diamond represents imputed effect size.
4. Discussion
As far we are aware, this is the first meta-analysis of randomized controlled trials specifically considered to evaluate the effects of nuts consumption on inflammatory markers and endothelial function parameters. Meta-analysis presented consuming variable doses of nuts significantly improved inflammatory and anti-inflammatory indexes compared with baseline. However, we found that nut consumption did not have a significant effect on serum CRP levels, though it was associated with a small nonsignificant increase. Other serum markers of inflammation and anti-inflammatory that we reviewed were not influenced by the nut consumption, except leptin which was associated with a significant increase with nut consumption. We surveyed several endothelial markers too in this study such as plasma fibrinogen, and E-selectin, ICAM and VCAM; our analysis did not reveal any significant changes in their serum concentration after nut consumption. Although we did not find a significant association between nut and seed consumption with most inflammatory index independently previously, various components of nuts and seeds it possible have anti-inflammatory properties. Numerous cross-sectional studies revealed lower concentrations of circulating levels of pro-inflammatory cytokines or endothelial cell adhesion molecules in subjects consuming nuts, for example, it has been described that α-linolenic acid (18:3(n-3)), resulting from nuts was inversely related with levels of CRP, IL-6, soluble tumor necrosis factor receptors 1 and 2, and fibrinogen in healthy individuals and/or patients with stable coronary artery disease .[13,54,55] Moreover, it has been reported individuals with the more consuming of nuts and virgin olive oil presented the lower level of VCAM-1, ICAM-1, IL-6, and CRP.[56] They detailed that tree nuts, particularly almonds, pistachios, and walnuts, have higher antioxidant and anti-inflammatory effect.[57,58] These helpful effects are supposed to be attribute to the configuration of nuts, which is recognized by a greater level of MUFAs, lesser saturated fatty acids, no cholesterol, and a suitable amount of proteins, fiber, phytosterols, antioxidants, and numerous minerals and vitamins.[59,60] Soy also have a fiber, PUFA, and phytoestrogens, which are separately related with lesser concentration of inflammatory parameters and improved endothelial function factors.[61] In this meta-analysis, we focused on these types of nuts. In contrast with our findings, some other individual studies showed different effects of nuts on inflammatory and endothelial markers. One study reported that a high Isoflavone soy diet augmented IL-6 in women.[62] Also, a long-term observational study stated that nuts consumption is related with greater adiponectin level.[63] It might be supposed that a mechanism relating between different effect of nuts and seed intake on elements involved in the procedure of inflammation and endothelial function is certain diseases at the baseline, various ethnicity, and various doses of nuts.
Interestingly, some studies examined how quickly after beginning of walnut consumption promising effects on factors of inflammation can be seen.[41,42] In this regard, it has been stated that intake of walnuts (48 g per day for 4 days) was associated with a substantial enhance in the apolipoprotein level, nevertheless did not alter the concentration of CRP, IL-6, IL-8, and tumor necrosis factor-α (TNF-α).[42] It might be concluded that >4 days are essential for detecting the beneficial effects of the walnut consumption on inflammatory factors and different results between investigations of short- and long-term walnut consumption are perhaps attributing to the length of the intervention (≤4 days vs to ≥4 weeks).
Additionally, though it has been stated that there was no significant alterations in LDL, HDL, total cholesterol, or triglyceride levels on walnut consumption in 4 days period,[41] but it has been reported that that short-term intake of walnuts have beneficial effects on lipid profile and lipid metabolism even within 4 days of intake.
Some limitations of this meta-analysis should be noted. First, most of the included studies had a moderately small sample size, theoretically causing unstable estimates of treatment effects.[64,65] Another point would that we have used some studies which they did not count the leptin level as an end point which may be affect our results. Potential explanations for the heterogeneity in the results, might be because each study had its own follow-up periods, inclusion criteria, basic health condition, gender, varied periods of life, drug usage, amount of the nut. Accordingly, because of the heterogeneity, we have performed our analysis by using the random-effects model. Moreover because the original studies we have used were lack of the real composition of the nuts, and hence may be our finding is somehow biased. As we all know that even there are differences in composition of same nuts in different part of the world and even different part of the same country.
5. Conclusion
This meta-analysis suggests that while nut consumption appears to be associated with a reduction in serum leptin in our selected publications, it had no significant effect on serum CRP, IL6, adiponectin, IL10, and TNF-α.
Supplementary Material
Footnotes
Abbreviations: CCTR = Cochrane Central Register of Controlled Trials, CDSR = Cochrane Database of Systematic Reviews, CI = confidence interval, CMA = comprehensive meta-analysis, CRP = C-reactive protein, CVD = cardiovascular disease, ICAM-1 = intracellular adhesion molecule-1, IL-6 = interleukin-6, PRISMA = systematic reviews and meta-analyses, RCT = randomized control trials, SD = standard deviation, SEM = standard error of the mean, VCAM-1 = vascular cell adhesion molecule-1, [WMD] = weighted mean difference.
Authorship: MM designed the study.MM and PR searched databases, performed the selection of studies, and wrote the manuscript. MM analyzed the data; MM, HKG, and GF contributed to writing the manuscript and commented on it and approved the last version. All authors reviewed and approved the final manuscript.
Ethical approval: Because the nature of our work we did not need to take any ethical approval.
Funding: MM was supported by a TWAS studentship of the Chinese Academy of Sciences, during the preparation of this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan A, Ridker P. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J 2002; 23:831–834. [DOI] [PubMed] [Google Scholar]
- 3.Salas-Salvadó J, Casas-Agustench P, Murphy MM, et al. The effect of nuts on inflammation. Asia Pac J Clin Nutr 2008; 17 (suppl 1):333–336. [PubMed] [Google Scholar]
- 4.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol 2006; 97:3–11. [DOI] [PubMed] [Google Scholar]
- 5.Hermsdorff HHM, Zulet MÁ, Abete I, et al. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr 2011; 50:61–69. [DOI] [PubMed] [Google Scholar]
- 6.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008; 87:114–125. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo M, Rizvi AA, Rini GB, et al. The therapeutic modulation of atherogenic dyslipidemia and inflammatory markers in the metabolic syndrome: what is the clinical relevance? Acta Diabetologica 2009; 46:1–11. [DOI] [PubMed] [Google Scholar]
- 8.Fredrikson GN, Hedblad B, Nilsson J-Å, et al. Association between diet, lifestyle, metabolic cardiovascular risk factors, and plasma C-reactive protein levels. Metabolism 2004; 53:1436–1442. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Int Med 2007; 167:31–39. [DOI] [PubMed] [Google Scholar]
- 10.Nakano S, Kuboki K, Matsumoto T, et al. Small, dense LDL and high-sensitivity C-reactive protein (hs-CRP) in metabolic syndrome with type 2 diabetes mellitus. J Atheroscler Thromb 2010; 17:410–415. [DOI] [PubMed] [Google Scholar]
- 11.Erlinger TP, Miller ER, Charleston J, et al. Inflammation modifies the effects of a reduced-fat low-cholesterol diet on lipids results from the DASH-sodium trial. Circulation 2003; 108:150–154. [DOI] [PubMed] [Google Scholar]
- 12.Zhao G, Etherton TD, Martin KR, et al. Dietary α-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 2004; 134:2991–2997. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Garcia E, Schulze MB, Manson JE, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 2004; 134:1806–1811. [DOI] [PubMed] [Google Scholar]
- 14.Wells BJ, Mainous AG, Everett CJ. Association between dietary arginine and C-reactive protein. Nutrition 2005; 21:125–130. [DOI] [PubMed] [Google Scholar]
- 15.Ros E. Nuts: consumption, composition, health benefits and safety. CAB Rev 2008; 3:1–12. [Google Scholar]
- 16.Kelly JH, Sabaté J. Nuts and coronary heart disease: an epidemiological perspective. Brit J Nutr 2006; 96 (S2):S61–S67. [DOI] [PubMed] [Google Scholar]
- 17.Jiang R, Jacobs DR, Mayer-Davis E, et al. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2006; 163:222–231. [DOI] [PubMed] [Google Scholar]
- 18.Kris-Etherton PM, Hu FB, Ros E, et al. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008; 138:1746S–1751S. [DOI] [PubMed] [Google Scholar]
- 19.Blomhoff R, Carlsen MH, Andersen LF, et al. Health benefits of nuts: potential role of antioxidants. Brit J Nutr 2006; 96:S52–S60. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein AM, Roizen MF, Martinez L. Purified palmitoleic acid for the reduction of high-sensitivity C-reactive protein and serum lipids: a double-blinded, randomized, placebo controlled study. J Clin Lipidol 2014; 8:612–617. [DOI] [PubMed] [Google Scholar]
- 21.Rajaram S, Connell KM, Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Brit J Nutr 2010; 103:907–912. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 23.Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015; 4:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiley Online Library, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5. 2008. [Google Scholar]
- 25.Mohsen Mazidi EK, Peyman rezaie, Gordon A. Ferns. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Journal of Diabetes and Its Complications. 2016. 10.1016/j.jdiacomp.2016.05.022, http://www.jdcjournal.com/article/S1056-8727(16)30175-1/abstract. [DOI] [PubMed] [Google Scholar]
- 26.Joh Wiley & Sons Ltd., Higgins J, Green S. Cochrane Handbook for Systematic Reviews, version 5.0.2 The Cochrane Collaboration. 2009. [Google Scholar]
- 27.Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. Vol. 348. Chichester, UK:Wiley; 2000. [Google Scholar]
- 29.Ferretti G, Bacchetti T, Sahebkar A. Effect of statin therapy on paraoxonase-1 status: a systematic review and meta-analysis of 25 clinical trials. Progr Lipid Res 2015; 60:50–73. [DOI] [PubMed] [Google Scholar]
- 30.Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? evidence from a meta-analysis. Phytother Res 2014; 28:633–642. [DOI] [PubMed] [Google Scholar]
- 31.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 32.Borenstein M, Hedges L, Higgins J, et al. Comprehensive Meta-analysis, version 2. 2005; Englewood, NJ:Biostat, 104. [Google Scholar]
- 33.Casas-Agustench P, López-Uriarte P, Bulló M, et al. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis 2011; 21:126–135. [DOI] [PubMed] [Google Scholar]
- 34.Colpo E, Vilanova CDD, Reetz LGB, et al. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition 2014; 30:459–465. [DOI] [PubMed] [Google Scholar]
- 35.Parham M, Heidari S, Khorramirad A, et al. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: a randomized crossover trial. Rev Diabet Stud 2013; 11:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapsell LC, Batterham M, Teuss G, et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 2009; 63:1008–1015. [DOI] [PubMed] [Google Scholar]
- 37.Bakhtiary A, Yassin Z, Hanachi P, et al. Effects of soy on metabolic biomarkers of cardiovascular disease in elderly women with the metabolic syndrome. J Family Reprod Health 2010; 4:95–104. [Google Scholar]
- 38.Reverri EJ, LaSalle CD, Franke AA, et al. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol Nutr Food Res 2015; 59:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azadbakht L, Kimiagar M, Mehrabi Y, et al. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr 2007; 85:735–741. [DOI] [PubMed] [Google Scholar]
- 40.Azadbakht L, Kimiagar M, Mehrabi Y, et al. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care 2007; 30:967–973. [DOI] [PubMed] [Google Scholar]
- 41.Brennan AM, Sweeney LL, Liu X, et al. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity 2010; 18:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aronis KN, Vamvini MT, Chamberland JP, et al. Short-term walnut consumption increases circulating total adiponectin and apolipoprotein A concentrations, but does not affect markers of inflammation or vascular injury in obese humans with the metabolic syndrome: data from a double-blinded, randomized, placebo-controlled study. Metabolism 2012; 61:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ros E, Núñez I, Pérez-Heras A, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects a randomized crossover trial. Circulation 2004; 109:1609–1614. [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Piotrowski K, Rau T, et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism 2014; 63:382–391. [DOI] [PubMed] [Google Scholar]
- 45.Chiang Y-L, Haddad E, Rajaram S, et al. The effect of dietary walnuts compared to fatty fish on eicosanoids, cytokines, soluble endothelial adhesion molecules and lymphocyte subsets: a randomized, controlled crossover trial. Prostaglandins Leukot Essent Fatty Acids 2012; 87:111–117. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins DJ, Kendall CW, Marchie A, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein (a), homocysteine, and pulmonary nitric oxide a randomized, controlled, crossover trial. Circulation 2002; 106:1327–1332. [DOI] [PubMed] [Google Scholar]
- 47.Sauder KA, McCrea CE, Ulbrecht JS, et al. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: a randomized trial. Metabolism 2015; 64:1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernández-Alonso P, Salas-Salvadó J, Baldrich-Mora M, et al. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care 2014; 37:3098–3105. [DOI] [PubMed] [Google Scholar]
- 49.Kasliwal RR, Bansal M, Mehrotra R, et al. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition 2015; 31:678. [DOI] [PubMed] [Google Scholar]
- 50.Gulati S, Misra A, Pandey RM, et al. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition 2014; 30:192–197. [DOI] [PubMed] [Google Scholar]
- 51.Lee YJ, Nam GE, Seo JA, et al. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr Res 2014; 34:814–820. [DOI] [PubMed] [Google Scholar]
- 52.Moreira Alves RD, Boroni Moreira AP, Macedo VS, et al. High-oleic peanuts: new perspective to attenuate glucose homeostasis disruption and inflammation related obesity. Obesity 2014; 22:1981–1988. [DOI] [PubMed] [Google Scholar]
- 53.Tey SL, Gray AR, Chisholm AW, et al. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J Nutr 2013; 143:1254–1262. [DOI] [PubMed] [Google Scholar]
- 54.Pischon T, Hankinson SE, Hotamisligil GS, et al. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003; 108:155–160. [DOI] [PubMed] [Google Scholar]
- 55.Madsen T, Skou HA, Hansen VE, et al. C-reactive protein, dietary n-3 fatty acids, and the extent of coronary artery disease. Am J Cardiol 2001; 88:1139–1142. [DOI] [PubMed] [Google Scholar]
- 56.Salas-Salvadó J, Garcia-Arellano A, Estruch R, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr 2008; 62:651–659. [DOI] [PubMed] [Google Scholar]
- 57.Albert CM, Gaziano JM, Willett WC, et al. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch Intern Med 2002; 162:1382–1387. [DOI] [PubMed] [Google Scholar]
- 58.Ellsworth J, Kushi L, Folsom A. Frequent nut intake and risk of death from coronary heart disease and all causes in postmenopausal women: the Iowa Women's Health Study. Nutr Metab Cardiovasc Dis 2001; 11:372–377. [PubMed] [Google Scholar]
- 59.Dreher ML. Pistachio nuts: composition and potential health benefits. Nutr Rev 2012; 70:234–240. [DOI] [PubMed] [Google Scholar]
- 60.Kocyigit A, Koylu A, Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr Metab Cardiovasc Dis 2006; 16:202–209. [DOI] [PubMed] [Google Scholar]
- 61.Esposito K, Giugliano D. Diet and inflammation: a link to metabolic and cardiovascular diseases. Eur Heart J 2006; 27:15–20. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins DJ, Kendall CW, Connelly PW, et al. Effects of high-and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism 2002; 51:919–924. [DOI] [PubMed] [Google Scholar]
- 63.Mantzoros CS, Williams CJ, Manson JE, et al. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am J Clin Nutr 2006; 84:328–335. [DOI] [PubMed] [Google Scholar]
- 64.Nuesch E, Trelle S, Reichenbach S, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ (Clinical Res Ed) 2010; 341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53:1119–1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


