Abstract
Introduction:
Hypoxic–ischemic brain injury (HI-BI), a result of oxygen deprivation of the brain, is accompanied by memory impairment. In this study, we report on a patient with neural injury of the Papez circuit following HI-BI, demonstrated by diffusion tensor tractography (DTT).
Clinical Findings/Patient Concerns:
A 48-year-old male patient suffered spontaneous cardiopulmonary arrest and underwent cardiopulmonary resuscitation for approximately 20 minutes with the concomitant oxygen deprivation leading to HI-BI. The patient showed severe memory impairment at 10 weeks after onset: a Mini-Mental State Examination score of 11 (full score: 30, cut-off score < 24), and examination using the memory function test was not possible due to severe cognitive deficit.
Outcomes:
On 10-week DTT, discontinuation of the fornical column was observed in both hemispheres and thinning of the thalamocingulate tract was observed in the right hemisphere and nonreconstruction in the left hemisphere.
Conclusion:
Using DTT, neural injury of the Papez circuit was demonstrated in a patient with memory impairment following HI-BI. These results suggest that analysis of the Papez circuit using DTT could provide beneficial information by detecting injury of the Papez circuit that cannot be detected on conventional brain MRI in patients with HI-BI.
Keywords: diffusion tensor imaging, hypoxic–ischemic brain injury, memory, Papez circuit
1. Introduction
Hypoxic–ischemic brain injury (HI-BI) occurs by oxygen deprivation of the brain, with survival rates in out-of-hospital cardiopulmonary arrest of less than 10%.[1,2] HI-BI is accompanied by neurological symptoms including motor and somatosensory dysfunction, and cognitive impairment.[2–4] Over 69% of patients with HI-BI suffer from memory impairment,[5,6] and the critical period for recovery of memory function is within 3 months after onset of HI-BI.[3,7] Therefore, elucidation of the cause of memory impairment at early stage in HI-BI would be important for successful rehabilitation, although this topic is little understood.
The Papez circuit, as a closed neural circuit in the limbic system, was first postulated by James Papez and involved in control of emotional expression.[8] However, recent studies have reported that it has a more important role in memory function than control of emotional expression, particularly spatial and episodic memory.[9–12] The Papez circuit is comprised of various brain structures and neural tracts; hippocampus—fornix—mammillary body—anterior thalamic nucleus—cingulate gyrus—cingulum—parahippocampal gyrus—hippocampus.[8] Because of its complexity and deep location in the white matter, examination of the Papez circuit in the live human brain has been limited. Recently developed diffusion tensor imaging (DTI) produces images of biological tissues weighted with the local microstructural characteristics of water diffusion.[13] Base on DTI, diffusion tensor tractography (DTT) enables 3-dimensional reconstruction and evaluation of the Papez circuit.[14–17] In particular, probabilistic DTT, based on the multitensor model, can reflect the dominant and nondominant orientations of water diffusion in each voxel whereas deterministic DTT, based on the single-tensor model, allows tracing only a dominant orientation of water diffusion in each voxel.[18,19] A few studies have reported on injury of the Papez circuit in patients with brain injury; however, no study on injury of the Papez circuit following HI-BI has been reported.[14–17]
In this study, we report on a patient with neural injury of the Papez circuit following HI-BI, demonstrated by DTT.
2. Case report
A 48-year-old male patient who suffered HI-BI induced by spontaneous cardiopulmonary arrest underwent cardiopulmonary resuscitation for approximately 20 minutes. Ten weeks later, he was admitted to the department of rehabilitation of a university hospital. The patient's memory was severely impaired: Mini-Mental State Examination score of 11 (full score: 30, cut-off score <24), and examination using the memory function test was not possible due to severe cognitive deficit. T2-weighted brain MR images taken 10 weeks after onset showed no specific abnormality (Fig. 1A). The patient's wife provided signed, informed consent, and the study protocol was approved by Yeungnam University hospital institutional review board.
Figure 1.
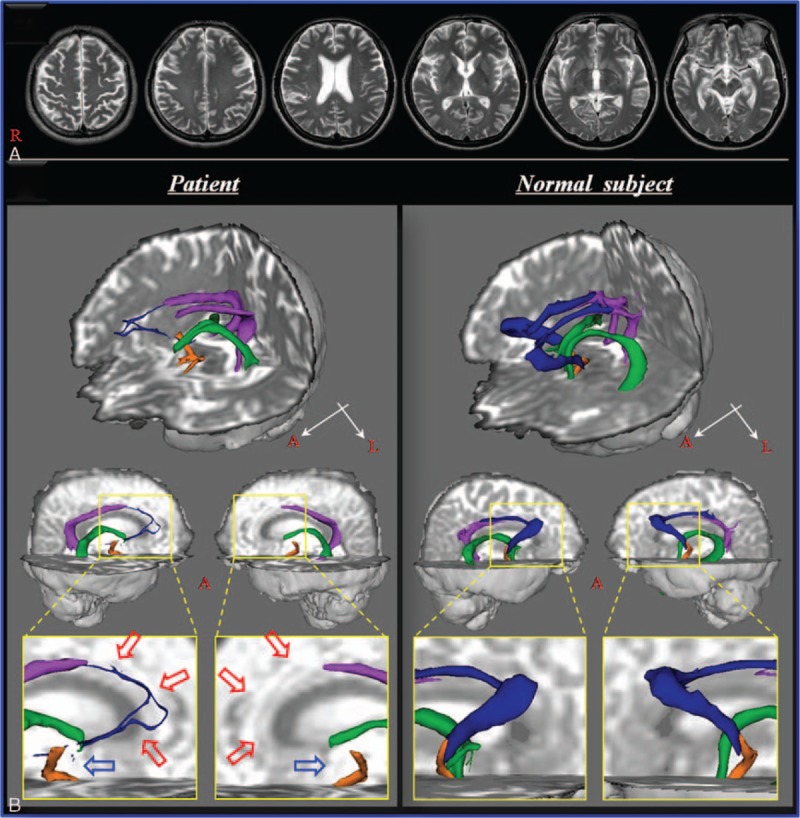
(A) T2-weighted brain MR images at 10 weeks after onset show no abnormality. (B) Results of diffusion tensor tractography of the Papez circuit. On 10-week diffusion tensor tractography, discontinuation of the fornical column is observed in both hemispheres (blue arrows), and thinning of the thalamocingulate tract is observed in the right hemisphere and nonreconstruction in the left hemisphere (red arrows). MR = magnetic resonance.
DTI data were acquired at 10 weeks after onset on a 1.5T Philips Gyroscan Intera (Philips, Ltd., Best, the Netherlands) with 32 gradients. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; repetition time = 10,398 ms; echo time = 72 ms; parallel imaging reduction factor = 2; echo-planar imaging factor = 59; b = 1000 s/mm2; and a slice thickness of 2.5 mm. Head motion effect and image distortion due to eddy current were corrected using affine multiscale 2-dimensional registration. Fiber tracking was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Diffusion Software (default option: 5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2).[18] Each neural tract of the Papez circuit was determined by selection of fibers passing through seed and target regions of interest (ROIs) as follows[20–22]; thalamocingulate tract—the cingulate gyrus on the axial image (ROI-1), anterior limb of the internal capsule on the axial image (ROI-2), and the anterior thalamic nuclei on the coronal image (ROI-3); fornix—the mammillary body on the axial image (ROI-1) and the crus of the fornix (ROI-2); mammillothalamic tract—the anterior thalamic nuclei on the axial image (ROI-1) and the isolated mammillothalamic tract (ROI-2), and the mammillary body on the axial image (ROI-3); cingulum—the middle (ROI-1) and posterior (ROI-2) of the cingulum on the coronal images and the hippocampal cortex on the axial image (ROI-3). The threshold of 2 streamlines was applied for the results of fiber tracking.
On 10-week DTT, discontinuation of the fornical column was observed in both hemispheres and thinning of the thalamocingulate tract was observed in the right hemisphere and nonreconstruction in the left hemisphere (Fig. 1B).
3. Discussion
In this study, using DTT, we attempted to demonstrate neural injury of the Papez circuit in a patient with HI-BI. We found neural injury of the Papez circuit (discontinuation of both fornical columns and thinning (right side) and nonreconstruction (left side) of the thalamocingulate tracts). It appeared that injury of these neural tracts was at least in part associated with the severe memory impairment because no specific brain region was observed on conventional MRI. Therefore, our results suggest the necessity of examination of the Papez circuit in patients who show memory impairment after HI-BI, even when no definite brain lesion is observed on conventional brain MRI.
A few studies have reported on injury of a portion of the Papez circuit in patients with brain injury.[14–17] Kwon et al[14] [2015] and Chang et al[15] [2016] reported an injury of the thalamocingulate tract within the Papez circuit in a patient with memory impairment following a putaminal hemorrhage and encephalitis. In 2016, Yeo and Jang reported a severe injury of the entire Papez circuit in a patient with severe memory impairment and confabulation following a spontaneous subarachnoid hemorrhage, compared with 5 normal control subjects.[16] During the same year, Yang et al[17] reported injury of the thalamocingulate tract within the Papez circuit in 2 patients following mild traumatic brain injury. However, to the best of our knowledge, this is the first study to demonstrate injury of the Papez circuit following HI-BI.
Several limitations of this study should be considered. First, because it is based on a single case report, this study is limited. Second, although examination of memory function using the Memory Assessment Scale was attempted, detailed memory function could not be determined due to severe cognitive deficit (Mini-Mental State Examination: 11 score). Third, the relation between neural tracts in the Papez circuit and characteristics of memory function could not be determined. Fourth, DTT, which is sensitive to complexity in a voxel, can show both false positive and negative results.[23] Therefore, we suggest that further studies, including large numbers of patients, on the characteristics of memory function following injury of the Papez circuit should be encouraged.
In conclusion, using DTT, neural injury of the Papez circuit was demonstrated in a patient with memory impairment following HI-BI. These results suggest that because DTT enables invisible or micro damage of neural tracts, analysis of the Papez circuit using DTT could provide beneficial information in detecting injury of the Papez circuit which cannot be detected on conventional brain MRI in patients with HI-BI.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A2A2A01004073).
Footnotes
Abbreviations: DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, HI-BI = Hypoxic–ischemic brain injury, ROI = region of interest.
The authors have no conflicts of interest to declare.
References
- 1.Herlitz J, Andersson E, Bang A, et al. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Goteborg. Eur Heart J 2000; 21:1251–1258. [DOI] [PubMed] [Google Scholar]
- 2.Chiota NA, Freeman WD, Barrett KM. Hypoxic-ischemic brain injury and prognosis after cardiac arrest. Continuum (Minneap Minn) 2011; 17:1094–1118. [DOI] [PubMed] [Google Scholar]
- 3.Lim C, Alexander MP, LaFleche G, et al. The neurological and cognitive sequelae of cardiac arrest. Neurology 2004; 63:1774–1778. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CA, Arciniegas DB. Cognitive sequelae of hypoxic-ischemic brain injury: a review. NeuroRehabilitation 2010; 26:47–63. [DOI] [PubMed] [Google Scholar]
- 5.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA 1993; 269:237–242. [PubMed] [Google Scholar]
- 6.Pusswald G, Fertl E, Faltl M, et al. Neurological rehabilitation of severely disabled cardiac arrest survivors. Part II. Life situation of patients and families after treatment. Resuscitation 2000; 47:241–248. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren-Nilsson A, Rosen H, Hofgren C, et al. The first year after successful cardiac resuscitation: function, activity, participation and quality of life. Resuscitation 2005; 66:285–289. [DOI] [PubMed] [Google Scholar]
- 8.1995; Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. 7:103–112. [DOI] [PubMed] [Google Scholar]
- 9.Beglinger LJ, Haut MW, Parsons MW. The role of the Mammillary bodies in memory: a case of amnesia following bilateral resection. Eur J Psychiatr 2006; 20:88–95. [Google Scholar]
- 10.Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics 2011; 31:1107–1121. [DOI] [PubMed] [Google Scholar]
- 11.Albo Z, Di Prisco GV, Vertes RP. Multisite Spike-Field Coherence, Theta Rhythmicity, and Information Flow within Papez's Circuit. In: Vertes PR, Stackman Jr WR, eds. Electrophysiological Recording Techniques Totowa, NJ: Humana Press, 2011. [Google Scholar]
- 12.Nishio Y, Hashimoto M, Ishii K, et al. Neuroanatomy of a neurobehavioral disturbance in the left anterior thalamic infarction. J Neurol Neurosurg Psychiatry 2011; 82:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 2005; 360:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon HG, Chang CL, Jang SH. Is thalamocortical tract injury responsible for memory impairment in a patient with putaminal hemorrhage? Neural Regen Res 2015; 10:321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang MC, Yeo SS, Do Lee H, Jang SH. Diffusion tensor tractography in a patient with memory impairment following encephalitis. Acta Neurol Belg. 2016. [Epub ahead of print]. PMID: 26732618. [DOI] [PubMed] [Google Scholar]
- 16.Jang SH, Yeo SS. Injury of the Papez circuit in a patient with provoked confabulation following subarachnoid hemorrhage: a diffusion tensor tractography study. Acta Neurol Belg. 2016. [Epub ahead of print]. PMID: 26830649. [DOI] [PubMed] [Google Scholar]
- 17.Yang DS, Kwon HG, Jang SH. Injury of the thalamocingulate tract in the Papez circuit in patients with mild traumatic brain injury. Am J Phys Med Rehabil 2016; 95:e34–e38. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 (suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 19.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007; 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol 2005; 26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon HG, Hong JH, Jang SH. Mammillothalamic tract in human brain: diffusion tensor tractography study. Neurosci Lett 2010; 481:51–53. [DOI] [PubMed] [Google Scholar]
- 22.Jang SH, Yeo SS. Thalamocortical tract between anterior thalamic nuclei and cingulate gyrus in the human brain: diffusion tensor tractography study. Brain Imaging Behav 2013; 7:236–241. [DOI] [PubMed] [Google Scholar]
- 23.Jeurissen B, Leemans A, Tournier JD, et al. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 2013; 34:2747–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]


