Abstract
Background:
Trastuzumab targets the human epidermal growth factor receptor 2 oncogene and in combination with first-line therapy results in significantly improved survival outcomes and has thus become standard of care in both adjuvant and metastatic settings. While it is estimated that 1% to 4% of patients treated with trastuzumab will develop heart failure and ∼10% will experience a reduction in left ventricular ejection fraction (LVEF), the patient risk factors associated with trastuzumab-induced cardiotoxicity (TIC) are unclear. This meta-analysis aims to consolidate previously published data to identify the risk factors most likely leading to TIC.
Methods:
A search of the MEDLINE literature database using the keywords trastuzumab/Herceptin, risk factors, outcomes, cardiac, cardiotoxicity, cardiomyopathy, LVEF, and chemotherapy was performed. Only prospective/retrospective human studies were included, with additional studies excluded if they reported baseline LVEF > 68%, a cohort of <50 patients, or results that were not stratified based on cardiotoxic events. Pooled odds ratio (OR) and 95% confidence interval (CI) for each potential risk factor were calculated, with heterogeneity of data and samples explored using random-effects modeling.
Results:
Data were collected from 17 articles, capturing 6527 patients. Hypertension (OR 1.61, 95% CI 1.14–2.26; P < 0.01), diabetes (OR 1.62; 95% CI 1.10–2.38; P < 0.02), previous anthracycline use (OR 2.14; 95% CI 1.17–3.92; P < 0.02), and older age (P = 0.013) were all shown to be associated with TIC.
Conclusion:
Cardiac performance should be closely monitored in women treated with trastuzumab. Recognizing potential risk factors along with careful attention to symptoms/LVEF measurements could minimize the occurrence of TIC in this population.
Keywords: breast cancer, cardiotoxicity/cardiomyopathy, chemotherapy, left ventricular ejection fraction, meta-analysis, risk factors, targeted therapy, trastuzumab/herceptin
1. Introduction
Breast cancer affects 1 in 8 (12%) women in the United States and is one of the most common cancers among American women.[1] Advances in chemo- and targeted gene therapy have made treatment of breast cancer more effective than ever before as evidenced by increased survival rates; however, this success was achieved with a price, namely, additional treatment-related stress to patients’ cardiovascular system. About 2.3 million American women with a history of breast cancer are now living with a greater lifetime risk of congestive heart failure because of the received cancer treatment.[2] The risk for cardiovascular events is further magnified by an overlap of therapy-specific subclinical damages with comorbidities and unfavorable lifestyle choices like reduced physical activity and obesity.[3]
Trastuzumab, a monoclonal antibody developed by Genentech Inc. (South San Francisco, CA) targets the human epidermal growth factor receptor 2 (HER2+/neu) oncogene, which is overexpressed in ∼25% to 30% of breast cancer patients. The drug gained US Food and Drug Administration approval in September 1998. In combination with first-line chemotherapy for HER2-positive metastatic breast cancer (HER2+), trastuzumab resulted in significant improvement in survival outcomes.[4–7] Trastuzumab is also used as adjuvant drug therapy in HER2+ early-stage breast cancer and was shown to reduce recurrence and improve survival.[8–10] Previously, the recognition of cancer therapy–related cardiac dysfunction in cancer patients was predominantly focused on anthracycline-induced cardiotoxicity. However, this field became further complicated when newer drugs like trastuzumab were applied clinically, as the dose-dependent effect, short- and long-term effects, and cellular mechanisms of anthracycline-induced cardiotoxicity are relatively known compared to trastuzumab-induced cardiotoxicity (TIC).
An estimated 1% to 4% of patients treated with trastuzumab will develop overt heart failure,[11–13] and ∼10% will experience asymptomatic left ventricular ejection fraction (LVEF) reduction.[6,12,14] When combined with anthracycline, the incidence of TIC was reported to be as high as 34%.[6,15] TIC is not dose-dependent and is often reversible with discontinuation of treatment and/or standard medical therapy for congestive heart failure.[16,17] Risk factors for TIC have been studied by different groups, but results have been inconsistent. A study by Seidman et al[18] indicated that of all the factors studied (age, hypertension, previous radiation therapy to the chest wall, baseline LVEF, etc.), only age was significantly associated with increased risk of developing TIC and only in the trastuzumab plus anthracycline subgroup. In contrast, another study[19] of mostly the same factors reported that baseline LVEF might be the only significant risk factor for developing TIC. Other studies have indicated that age, hypertension, preexisting cardiac dysfunction, baseline LVEF < 50%, and previous anthracycline treatment or radiotherapy to left chest wall and concomitant chemotherapy are associated with higher incidence of cardiotoxicity.[20–22] The discrepancies among different studies may arise from overall low cardiotoxicity rates limiting size of study populations as well as use of different inclusion/exclusion criteria.
The objective of this meta-analysis is to consolidate the published data and identify potential risk factors for developing strategies to minimize TIC occurrence in clinical practice.
2. Methods
A literature search using the MEDLINE database was executed using the following keywords trastuzumab/herceptin, risk factors, outcomes, cardiac, cardiotoxicity, cardiomyopathy, LVEF, and chemotherapy. Articles were limited to those in the English language and published within the past 15 years (1999–2014). We included prospective and retrospective studies of patients with breast cancer who were treated with anthracycline and trastuzumab. Systematic reviews or meta-analyses, along with basic science, animal, and modeling studies, were excluded. Also excluded were study populations <50 patients, cohorts that included subjects aged <18 years, or cohorts with a mean baseline LVEF > 68%. As the goal of our analysis was to determine the risk factors for TIC, included studies were required to stratify by those who had or had not experienced cardiotoxic events. This review incorporated interventional randomized clinical trial and observational studies that provided the necessary and pertinent data for analysis. The primary outcome of interest was the development of TIC, manifested as heart failure or deterioration in LVEF based on the criteria presented within each study.
This meta-analysis met the criteria for waiving Institutional Review Board review and approval.
2.1. Data extraction
The following data were independently collected by 3 reviewers (RMP, LG, and ZJ): age; smoking history; LVEF; evidence of hypertension, diabetes, coronary artery disease, and dyslipidemia/hyperlipidemia; body mass index; use of radiotherapy or anthracycline; and location of tumor (right/left breast). Discrepancies were reviewed and resolved by consensus.
2.2. Statistical analysis
Pooled odds ratio (OR) and 95% confidence interval (CI) for each variable were calculated. To explore heterogeneity, a random-effect model was used. Heterogeneity among studies was assessed using Cochran Q test and also by measuring consistency (I2). For variables showing high heterogeneity, funnel plots were used to assess visual asymmetry. In addition, Egger test was used for analysis. No judgments were made comparing sample sizes between studies. As such, random-effect modeling was used to capture any true difference as well as the effect size while considering a heterogeneous sample. Statistical analysis was performed using Comprehensive Meta-Analysis Version 2.0 software (Biostat, Englewood, NJ), and P < 0.05 was considered significant.
3. Results
Initial MEDLINE search resulted in 635 citations. After excluding based on study criteria (Fig. 1), a total of 17 studies[15,19,23–37] were included for analysis, characteristics for which can be found in Table 1 along with details on treatment regimens and tumor types. Included articles were a mix of retrospective and prospective studies. The total number of breast cancer patients treated with trastuzumab in all 17 studies combined was 6527 patients. Patients were treated both in adjuvant and metastatic settings. All studies had patients with HER2+ breast cancer, and all were treated with trastuzumab. Treatment for adjuvant/neoadjuvant therapy varied, as did dosing and regimen. The primary endpoints given for these studies were manifestation of a cardiac event, decline in LVEF greater than 10%, or occurrence of cardiotoxicity. Studies included 1 or more of the following risk factors for cardiotoxicity (Table 2): race, age, familial history of cardiac disease, smoking history, hypertension, diabetes, history of coronary artery disease, LVEF, body mass index, dyslipidemia, history of radiotherapy, anthracycline use, and originating site of breast cancer (left/right breast).
Figure 1.
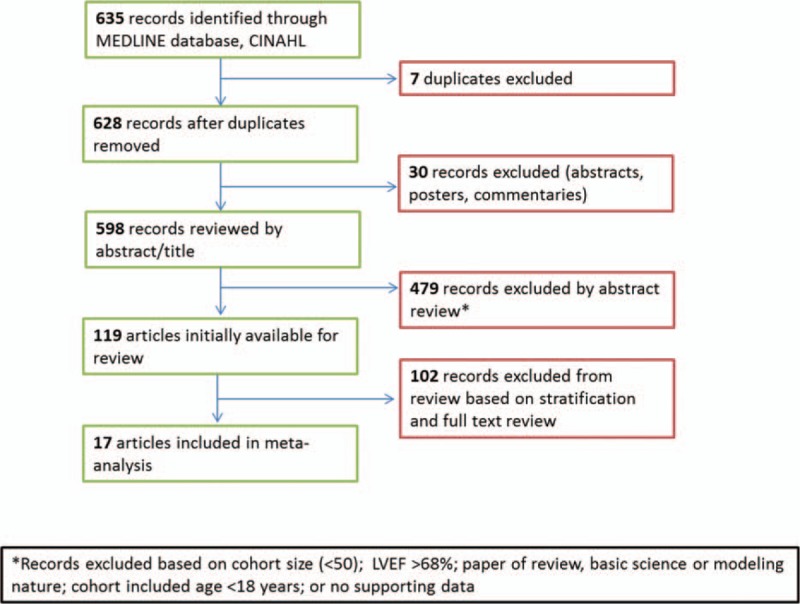
Flowchart of review design. LVEF = left ventricular ejection fraction.
Table 1.
Characteristics of the studies and patients included in the meta-analysis.
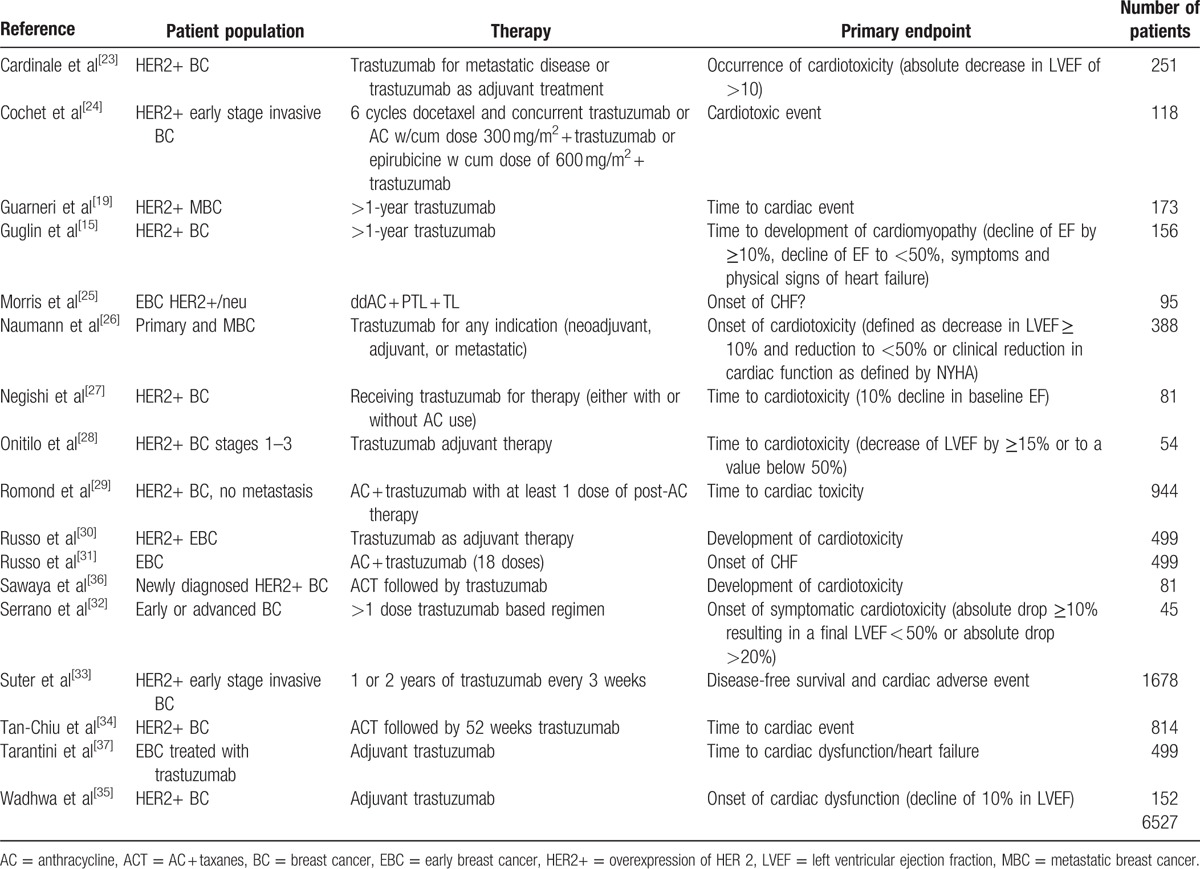
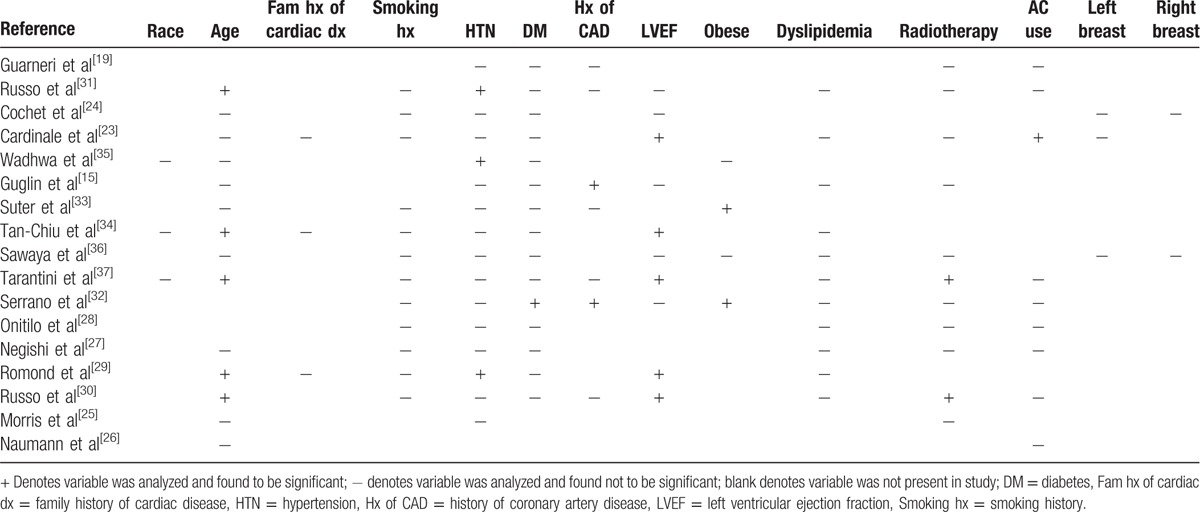
Table 2.
Cardiotoxicity risk factors evaluated in the included literatures. The plus sign signifies that information was included for that variable and that it was found to be significant. The minus sign signifies that information was included for that variable and it was not found to be significant. The blanks are variables not included within that particular study.
3.1. Incidence of TIC
The majority of studies included in this meta-analysis defined cardiotoxicity as a 10% to 20% decline in LVEF, a decline in LVEF to a level <50%, or patients exhibiting signs and symptoms of heart failure. All studies used either echocardiography or a multigated acquisition scan (MUGA) as a tool to serially assess LVEF. The overall incidence of cardiotoxicity was 12.0% with 95% CI 11.3% to 12.9%. A total of 786 patients (out of 6527 patients) experienced TIC; however, only a small portion of patients had overt trastuzumab-induced heart failure, with the rest being asymptomatic (i.e., had a decline in LVEF without experiencing any signs or symptoms of heart failure). Only 6 patients (about 0.09%) died due to cardiac complications of trastuzumab.
3.2. Risk factors associated with TIC
Our analysis of the 17 included studies revealed that diagnoses of hypertension (OR: 1.61; 95% CI: 1.14–2.26; P < 0.01; Fig. 2A), diabetes (OR: 1.62; 95% CI: 1.10–2.38; P < 0.02; Fig. 2B), and previous anthracycline use (OR: 2.14; 95% CI: 1.17–3.92; P < 0.02; Fig. 2C) were all associated with risk of TIC (Fig. 3). Increased age (P = 0.013; Fig. 2D) also was found to be significantly associated with TIC. Familial history of cardiac disease showed significance (P < 0.001), but this finding was not reliable as too few studies reported these results (n = 3). Diabetes and age showed the least amount of heterogeneity (I2 = 1% and 22%, respectively), whereas hypertension and previous anthracycline use showed the most evidence (I2 = 60% and 76%, respectively). Due to the evidence of heterogeneity, funnel plots were used for a visual representation of asymmetry. However, when we formally tested for asymmetry using Egger test, asymmetry did not appear to be significant (P = 0.706 and 0.340, respectively; Fig. 4A and B) for hypertension and anthracycline use. Our results failed to show any significant association between TIC and a patient's race, body mass index, dyslipidemia, or history of coronary artery disease. There was not a sufficient number of articles describing cumulative anthracycline dosage, metastatic disease, familial history of cardiac disease, or left/right breast tumor site to allow for statistical analysis.
Figure 2.
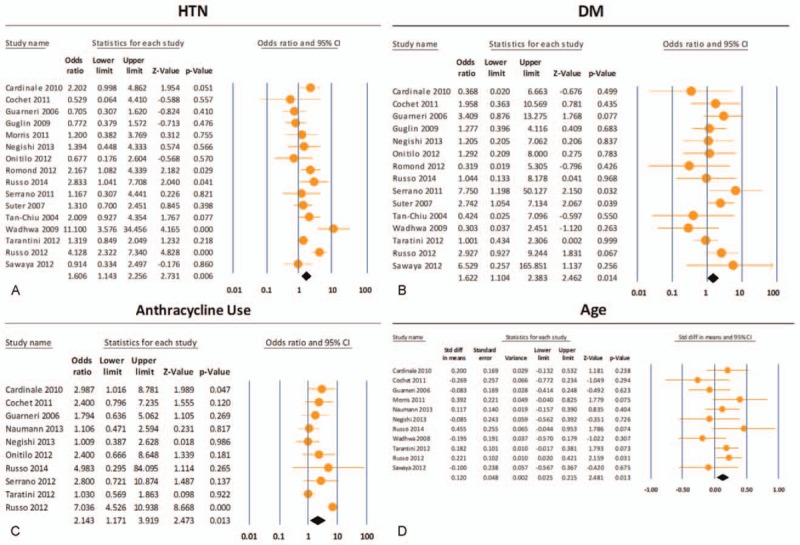
(A)–(D) Data analysis of risk factors associated with trastuzumab-induced cardiotoxicity (TIC). Hypertension (odds ratio [OR]: 1.61; 95% confidence interval [CI]: 1.14–2.26; P < 0.01), diabetes mellitus (OR: 1.62; 95% CI: 1.10–2.38; P < 0.02), and previous anthracycline use (OR: 2.14; 95% CI: 1.17–3.92; P < 0.02) were all shown to be associated with TIC. DM = diabetes mellitus, HTN = hypertension.
Figure 3.
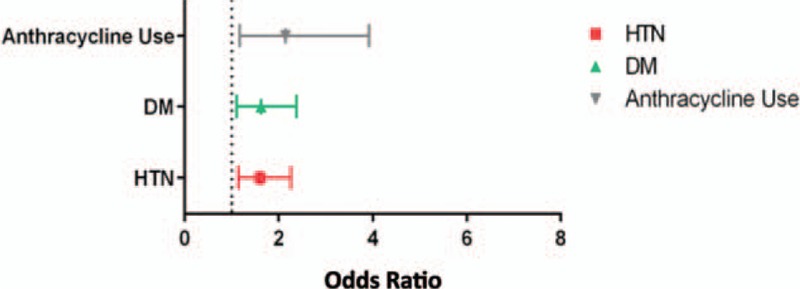
Data analysis of significant variables reporting effect measures for each included study.
Figure 4.
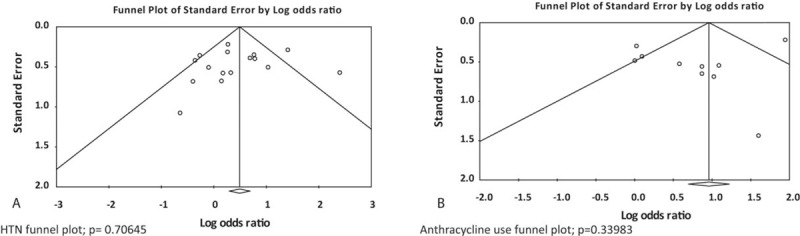
Testing for bias and asymmetry for hypertension and anthracycline use by funnel plot and Egger test.
3.3. Study bias
After reviewing our results and subsequent funnel plots, we determined that reporting bias may be evident when interpreting the data on family history of cardiac disease as only 3 studies[23,34,35] reported this variable in a manner usable to our analysis. Of the 3 studies, the one reporting significant result was weighted at approximately the cumulative of the other 2. Publication bias was assessed and determined acceptable for all collected variables. Results obtained from all other factors indicate that increased age, a diagnosis of hypertension or diabetes, and anthracycline use are the most substantial risk factors for cardiotoxicity in women treated with trastuzumab. Familial history of cardiac disease may contribute additional risk; however, the evidence supporting this conclusion is quite heterogeneous, primarily due to small sample size.
4. Discussion
Research has shown that trastuzumab significantly improves outcomes in women with HER2+ breast cancers, but its use is associated with the potential risk of cardiotoxicity. Historically, the question to balance the success of cancer therapy at the expense of the “heart” has laid a lot of emphasis on early diagnosis. Some studies have tested the role of cardiac biomarkers (troponin, pro-B-type Natriuretic Peptide) and various imaging modalities (echocardiography, MUGA, cardiac magnetic resonance imaging, etc.) to aid in preclinical diagnosis. Indeed, it is imperative to identify the at-risk population from the pool of all exposed patients and monitor them more vigorously. Knowledge of various clinical risk factors associated with development of TIC can help clinicians immensely in this regard. Published reports have identified various risk factors for developing cardiotoxicity, including age, comorbidities, and use of radiotherapy. Our meta-analysis (which included strictly studies that stratified those who had experienced cardiotoxic events to those that had not) found that age, hypertension, diabetes, and previous anthracycline use are risk factors for a cardiac event.
Several studies have identified increased age as an important cardiotoxic risk factor for patients treated with trastuzumab. According to surveillance epidemiology and end results (SEER) Cancer Statistics Review, a report published by National Cancer Institute's Surveillance, Epidemiology, and End Results Program,[38] the risk of breast cancer increases with age; at age 30 years, a woman has a 0.4% chance of developing breast cancer, when she reaches age 60, this jumps to 3.6%, and at age 70, the chance is about 3.8%. The cumulative age range in our meta-analysis was 25 to 100 years, and most of the studies reported a range cluster of 28 to 80 years. The mean age of patients in the studies that included age as a risk factor was 54 years, which may represent a slightly younger than average population of breast cancer, a disease most commonly diagnosed in women aged 55 to 64 years.[38] Interestingly, cardiovascular disease and breast cancer incidence are likely to overlap, as major cardiovascular disease also increases with age. Since older age is likely associated with increased presence of cardiovascular comorbidities and unfavorable lifestyle choices that decrease overall physical fitness, such as reduced physical activity and obesity, it is unclear whether age, which was identified as a risk factor in our study, is an independent risk factor or inherently associated with other cardiovascular comorbidities for developing TIC.
Large clinical trials testing trastuzumab included relatively younger and healthier patients with limited comorbidities and therefore may not reflect the true breast cancer population. Our study identified the comorbidities hypertension and diabetes as significant risk factors for a cardiac event in patients who were treated with trastuzumab. A recent study[39] using the SEER-Medicare and Texas Cancer Registry Medicare-linked databases reported a retrospective cohort with older breast cancer patients (median age of 71 years). This study found that among patients treated with trastuzumab, those with cardiac comorbidities and older age may be at a higher risk. Prospective studies with more patients are needed to confirm the roles specific cardiac comorbidities play in the development of TIC. Strategies for monitoring, intervention, and management of cardiac comorbidities for those who are receiving trastuzumab need to be developed. Another recent study[40] showed that older women receiving trastuzumab tend to have suboptimal cardiac monitoring, with only a third of the patients getting recommended screening per current guidelines. Patients undergoing trastuzumab therapy should at least have cardiac monitoring at baseline and then at 3, 6, and 9 months, respectively. Routine monitoring is really imperative as TIC, unlike anthracycline-induced cardiotoxicity, is not dose-dependent, mostly occurs earlier in the course of treatment, and is reversible in the vast majority of cases via drug withdrawal and conventional heart failure therapy.
As treatment plans for breast cancer patients are generally complex, with more than 1 modality, it is often hard to dissect the cardiotoxic effect of each individual regimen. These treatments may interact with each other and have added effect to the cardiovascular system. Indeed, our study found that previous anthracycline use is a significant risk factor for developing TIC. When we further tried to determine if accumulated dosage of previous anthracycline use contributed to increased cardiotoxicity, we were unable to draw any solid conclusion, as the study data were limited (only 4 studies collected data for accumulated dosage of anthracycline) and produced large standard deviation. Our institution's own experience with retrospective studies of chemotherapy-related cardiotoxicity has found that obtaining accurate records of accumulated anthracycline dosage is difficult since treatment history may not be well documented or easily obtained. However, accumulated dosage of previous anthracycline use is an important factor that should not be overlooked and has the potential to serve a crucial role in the monitoring of TIC. Therefore, it is highly recommended that records of accumulated anthracycline dosage be better monitored by providers.
4.1. Limitations
There are inherent limitations to systematic reviews and meta-analysis. The main limitation in our analysis is the definition of cardiotoxic event. Our analysis was based on the stratification of those having experienced a cardiotoxic event versus those who had not. Within the 17 articles analyzed, a cardiotoxic event was defined as any one of the following: a decline in cardiac function as measured by a decrease in LVEF of 10%, 15%, or 20% or the onset of heart failure. To date, there is no 1 clear definition of cardiotoxicity. Sengupta et al[11] reviewed and evaluated clinical trials and defined chemotherapy-induced cardiotoxicity as one of the following: reduction of LVEF; symptoms or signs associated with heart failure; reduction in LVEF from baseline by ≥5% to <55% in the presence of signs or symptoms of heart failure, or a reduction in LVEF ≥10% to <55% without signs or symptoms of heart failure. Plana et al[41] documented a new definition of cardiac toxicity in 2014, which both the American Society of Echocardiography and European Association of Cardiovascular Imaging have endorsed. This definition states that cardiac toxicity is a decrease in LVEF of >10% to a value <53% and if the value is confirmed by repeated cardiac imaging at 2 to 3 weeks after initial decline. Moving forward, these organizations have encouraged researchers to use this definition when qualifying a patient as having TIC or not. The second limitation in our study is the small sample size of subgroup analyses in testing for significance of variables, such as the case for familial history of cardiac disease. In addition, publication bias could account for the overall effects measured.
As with any treatment, it is imperative for patients and providers to have an open conversation on the risks/benefits of any therapy before commencing. For patients with multiple comorbidities, such as hypertension and diabetes, or those with previous anthracycline use, it is important for both cardiologists and oncologists to partner in the care of patients being treated with trastuzumab. The intention of this study was not to prescribe or deter providers from using trastuzumab but to highlight the importance of closely monitoring a patient's cardiac function when on trastuzumab and assess when the potential risks of this treatment outweigh the benefits. The recent study posed by Gavila et al[42] outlines a plan in which both cardiologists and oncologists work together in managing the potential of cardiotoxicity, an approach we believe should become common practice for taking care of cancer patients who undergo treatment with potential for cardiotoxicity[43] and will ultimately maximize both quality of life and survival.
5. Conclusion
Our study identified hypertension, diabetes, age, and previous anthracycline use as risk factors for a cardiac event, but this should not be taken as exhaustive. Also, the weight of each risk factor is unknown. Even though our study included 17 independent studies and more than 6500 patients, numbers were still limited when trying to dissect the role of each risk factor in TIC. Still, we feel this review of the risks that can accompany trastuzumab therapy provides valuable support to the comprehensive teams of caregivers responsible for treating breast cancer patients.
Footnotes
Abbreviations: CI = confidence interval, HER2+ = human epidermal growth factor receptor 2, I2 = Cochran Q test, LVEF = left ventricular ejection fraction, MUGA = multigated acquisition scan, OR = odds ratio, SEER = surveillance epidemiology and end results, TIC = trastuzumab-induced cardiotoxicity.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Laing C. LVAD: left ventricular assist devices for end-stage heart failure. Nurse Pract 2014; 39:42–47. [DOI] [PubMed] [Google Scholar]
- 2.Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007; 50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Herman EH, Lipshultz SE, et al. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 2008; 26:3777–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang RY, Finn RS. Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. Br J Cancer 2012; 106:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dores H, Abecasis J, Correia MJ, et al. Detection of early sub-clinical trastuzumab-induced cardiotoxicity in breast cancer patients. Arq Bras Cardiol 2013; 100:328–332. [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792. [DOI] [PubMed] [Google Scholar]
- 7.Murthy RK, Varma A, Mishra P, et al. Effect of adjuvant/neoadjuvant trastuzumab on clinical outcomes in patients with HER2-positive metastatic breast cancer. Cancer 2014; 120:1932–1938. [DOI] [PubMed] [Google Scholar]
- 8.Viani GA, Afonso SL, Stefano EJ, et al. Adjuvant trastuzumab in the treatment of HER-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer 2007; 7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang I, Hitre E. [Trastuzumab (Herceptin) in the adjuvant treatment of HER-2-positive early breast cancer]. Magy Onkol 2006; 50:293–302. [PubMed] [Google Scholar]
- 10.Baselga J, Perez EA, Pienkowski T, et al. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist 2006; 11 (suppl 1):4–12. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta PP, Northfelt DW, Gentile F, et al. Trastuzumab-induced cardiotoxicity: heart failure at the crossroads. Mayo Clin Proc 2008; 83:197–203. [DOI] [PubMed] [Google Scholar]
- 12.Farolfi A, Melegari E, Aquilina M, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart 2013; 99:634–639. [DOI] [PubMed] [Google Scholar]
- 13.Leyland-Jones B, Gelmon K, Ayoub JP, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 2003; 21:3965–3971. [DOI] [PubMed] [Google Scholar]
- 14.Fried G, Regev T, Moskovitz M. Trastuzumab-related cardiac events in the treatment of early breast cancer. Breast Cancer Res Treat 2013; 142:1–7. [DOI] [PubMed] [Google Scholar]
- 15.Guglin M, Hartlage G, Reynolds C, et al. Trastuzumab-induced cardiomyopathy: not as benign as it looks? A retrospective study. J Card Fail 2009; 15:651–657. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med 2007; 357:39–51. [DOI] [PubMed] [Google Scholar]
- 17.Floyd JD, Nguyen DT, Lobins RL, et al. Cardiotoxicity of cancer therapy. J Clin Oncol 2005; 23:7685–7696. [DOI] [PubMed] [Google Scholar]
- 18.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002; 20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 19.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience. J Clin Oncol 2006; 24:4107–4115. [DOI] [PubMed] [Google Scholar]
- 20.Huszno J, Les D, Sarzyczny-Slota D, et al. Cardiac side effects of trastuzumab in breast cancer patients—single centere experiences. Contemp Oncol (Pozn) 2013; 17:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamo V, Ricciardi GR, Adamo B, et al. The risk of toxicities from trastuzumab, alone or in combination, in an elderly breast cancer population. Oncology 2014; 86:16–21. [DOI] [PubMed] [Google Scholar]
- 22.Spano JP, Falandry C, Chaibi P, et al. Current targeted therapies in breast cancer: clinical applications in the elderly woman. Oncologist 2011; 16:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 2010; 28:3910–3916. [DOI] [PubMed] [Google Scholar]
- 24.Cochet A, Quilichini G, Dygai-Cochet I, et al. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Res Treat 2011; 130:845–854. [DOI] [PubMed] [Google Scholar]
- 25.Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res 2011; 17:3490–3499. [DOI] [PubMed] [Google Scholar]
- 26.Naumann D, Rusius V, Margiotta C, et al. Factors predicting trastuzumab-related cardiotoxicity in a real-world population of women with HER2+ breast cancer. Anticancer Res 2013; 33:1717–1720. [PubMed] [Google Scholar]
- 27.Negishi K, Negishi T, Hare JL, et al. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013; 26:493–498. [DOI] [PubMed] [Google Scholar]
- 28.Onitilo AA, Engel JM, Stankowski RV, et al. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat 2012; 134:291–298. [DOI] [PubMed] [Google Scholar]
- 29.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012; 30:3792–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo G, Cioffi G, Di Lenarda A, et al. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern Emerg Med 2012; 7:439–446. [DOI] [PubMed] [Google Scholar]
- 31.Russo G, Cioffi G, Gori S, et al. Role of hypertension on new onset congestive heart failure in patients receiving trastuzumab therapy for breast cancer. J Cardiovasc Med (Hagerstown) 2014; 15:141–146. [DOI] [PubMed] [Google Scholar]
- 32.Serrano C, Cortés J, De Mattos-Arruda L, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol 2012; 23:897–902. [DOI] [PubMed] [Google Scholar]
- 33.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J Clin Oncol 2007; 25:3859–3865. [DOI] [PubMed] [Google Scholar]
- 34.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2—overexpressing breast cancer: NSABP B-31. J Clin Oncol 2005; 23:7811–7819. [DOI] [PubMed] [Google Scholar]
- 35.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat 2009; 117:357–364. [DOI] [PubMed] [Google Scholar]
- 36.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012; 5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarantini L, Cioffi G, Gori S, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail 2012; 18:113–119. [DOI] [PubMed] [Google Scholar]
- 38.Sparrow CT, Nassif ME, Raymer DS, et al. Pre-operative right ventricular dysfunction is associated with gastrointestinal bleeding in patients supported with continuous-flow left ventricular assist devices. JACC: Heart Fail 2015; 3:956–964. [DOI] [PubMed] [Google Scholar]
- 39.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol 2013; 31:4222–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavez-MacGregor M, Niu J, Zhang N, et al. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol 2015; 33:2176–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014; 27:911–939. [DOI] [PubMed] [Google Scholar]
- 42.Gavila J, Seguí M, Calvo L, et al. Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol 2016; 1–14.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol 2010; 7:564–575. [DOI] [PubMed] [Google Scholar]


