Abstract
Diagnosis of female genital tuberculosis (FGTB) remains a challenge. The aim of this study was to evaluate the diagnostic value of T-SPOT.TB on peripheral blood mononuclear cells (PBMCs) for diagnosing FGTB in an area with high TB burden.
Patients with suspected FGTB were enrolled consecutively between August 2010 and August 2015. T-SPOT.TB on PBMCs and histopathology were performed in all patients. T-SPOT.TB results were evaluated against patients’ final diagnosis of FGTB which was made based on clinical manifestations, radiology, microbiological and histopathological evaluation, and response to anti-TB treatment. The sensitivity, specificity, predictive value, and likelihood ratio of T-SPOT.TB were analyzed.
Among the 66 patients enrolled, 32 were diagnosed with confirmed FGTB, 33 with non-TB including ovarian tumor in 10 patients (30%), pelvic inflammatory diseases in 8 patients (24%), endometriosis in 7 patients (21%), endometrial polyps in 3 patients (9%), abscess of fallopian tube in 2 patients (6%), cyst of fallopian tube in 2 patients (6%), and endometrial carcinoma in 1 patient (3%). One patient with clinically indeterminate diagnosis was not included in the final analysis. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, and negative likelihood ratio of T-SPOT.TB on PBMCs for diagnosis of FGTB were 94%, 70%, 75%, 92%, 3.09, and 0.09, respectively. Frequencies of spot forming cells (SFCs) of T-SPOT.TB were 430 (interquartile range [IQR] 155-706) SFCs/106 PBMCs and 124 (IQR 61–313) SFCs/106 PBMCs in FGTB and non-TB patients, respectively, and the difference was statistically significant (P = 2.14 × 10−8). By receiver operating characteristic curve analysis, a cutoff value of 40 SFCs/106 PBMCs of T-SPOT.TB had a sensitivity of 94% and specificity of 76% for the diagnosis of FGTB.
T-SPOT.TB on PBMCs appeared to be a valuable and rapid diagnostic method for FGTB in TB endemic settings with high sensitivity and NPV.
Keywords: diagnosis, female genital tuberculosis, IGRA, T-SPOT.TB
1. Introduction
As a developing country, China ranks second among the 22 countries in the world with high-burden of tuberculosis (TB), accounting for 10% of the total cases worldwide.[1] Extrapulmonary TB (EPTB) is increasingly common in China and globally.[2] As a form of EPTB, female genital TB (FGTB) is an important cause of significant morbidity, short- and long-term sequelae especially infertility.[3] The incidence of FGTB varies in different countries, which was 1% in infertility clinics of USA, 6.15% to 21.1% in South Africa and 1% to 19% in various parts of India.[3–7] Timely diagnosis and prompt appropriate treatment may prevent infertility and other sequelae of the disease.[3] However, microbiological identification of mycobacterium TB (MTB) is not possible in all the cases. Diagnostic dilemma arises due to varied clinical presentation, and the low positive rate of bacteriological and histopathological tests which are also time consumption.[3] Therefore, a rapid and accurate diagnostic tool is in urgent need.
In recent years, interferon-γ release assays (IGRAs) was developed as a new diagnostic tool for TB, and it has been reported to show a favorable performance for diagnosis of EPTB.[8] However, the diagnostic value of IGRAs has rarely been evaluated in FGTB. Therefore, we aimed in this study to evaluate the utility of T-SPOT.TB for the diagnosis of FGTB.
2. Patients and methods
2.1. Patients
Patients with suspected FGTB were enrolled consecutively between August 2010 and August 2015 in Peking Union Medical College Hospital (PUMCH). The inclusion criterion was patients with any of the following: symptoms: infertility, abdominal pain, irregular menstruation and general systemic symptoms like fever, weight loss, and night sweats. Signs: mass in abdomen or ascites. Radiology: ultrasonography: ovarian cysts or mass, hydrosalpinx, endometrial thickening, or ascites; hysterosalpingography: bilateral tubal occlusion, tubal defect, or a uterine cavity filling the defect. Evidence of previous TB or contact history of active pulmonary TB. Included patients were followed up for at least 3 months from discharge to see the effect of anti-TB treatments. Patients without T-SPOT.TB test on peripheral blood mononuclear cells (PBMCs) or histopathology or complete information of the medical records were excluded. This study was reviewed and approved by the Institutional Review Board of PUMCH and waiver of consent was granted as it was a retrospective and observational study.
Clinical information was extracted from patients’ medical records. The diagnosis was made based on clinical manifestations, radiology, microbiological, histopathological results, and patient response to anti-TB treatment by 2 independent research physicians. The research physicians were unblinded to T-SPOT.TB results. However, they were instructed to make diagnosis independent of either the T-SPOT.TB results or the clinical diagnosis given by the treating physician. If the 2 research physicians had different opinions of the final diagnosis, a third researcher was referred.
2.2. Diagnostic category
According to previous studies,[9,10] patients were classified according to the following findings: confirmed TB (acid-fast stain or culture positive for MTB, or histological presence of caseation, granuloma/tubercles, and/or beaded/thickened tube); probable TB (histological presence of hydrosalpinx, peritubal, and/or periovarian adhesions, tubo-ovarian mass but without frank tubercles/caseation, clinical manifestations, laboratory results, and radiologic features highly suggestive of FGTB and appropriate response to anti-TB therapy); clinically indeterminate (a final diagnosis of FGTB was neither highly probable nor reliably excluded, pelvic pathology other than pelvic inflammatory disease including fibroid uterus, endometriosis, polycystic ovaries); non-TB (all microbiological samples smear and culture negative, definite alternative diagnosis identified, and clinical improvement without anti-TB treatment).
2.3. T-SPOT.TB
T-SPOT.TB became part of the routine evaluation for patients since August 2010 in our institution for the purpose of screening latent TB or ruling out active TB. Four milliliters of peripheral blood were collected from each patient and was performed within 6 hours after collection by laboratory personnel blinded to patients’ clinical data. In the T-SPOT.TB test, AIM-V (GIBCOTMAIM V Medium liquid, Invitrogen, Carlsbad, California, US) was used as negative control, phytohemagglutinin as positive control, and early-secreted antigenic target 6-kDa protein (ESAT-6) and culture filtrate protein 10 (CFP-10) as specific antigens, respectively. PBMCs were separated by Ficoil-Hypaque gradient centrifugation and obtained (2.5 × 105 per well) on a plate (Oxford Immunotec, Abingdon, UK) that was precoated with an antibody against interferon-γ. After incubation for 16 to 18 hours at 37 °C in 5% carbon dioxide, wells were washed and developed with a conjugate used and an enzyme substrate against the antibody. The number of spot-forming cells (SFCs) representing an antigen-specific T cell that secreted interferon-γ were counted with an automated ELISPOT reader (AID-ispot, Strassberg, Germany). A positive result of T-SPOT.TB on PBMCs was defined as 6 or more SFCs in the target well and had twice the number of spots than the negative control well. In addition, the background number of spots in the negative control well should be less than 10 SFCs.
2.4. Histopathological examination
According to the operating procedure, all tissues for diagnosis of FGTB were collected when patients underwent hysteroscopy and/or laparoscopy or laparotomy. Specimens with solid tissue were treated by phosphate buffer saline and fixed with 10% formalin for 8 to 36 hours. Then the specimens were paraffin-embedded, pathological section and Hematoxylin and Eosin staining. Ziehl–Neelsen acid-fast staining was done to further confirm the diagnosis of TB.
2.5. Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were calculated to evaluate diagnostic performance for T-SPOT.TB. Continuous data of normal distribution were expressed as means ± standard deviation, while data without a normal distribution were described as medians and interquartile ranges. Categorical data were expressed in percentages. Kolmogorov–Smirnov test was used to examine normal distribution. Independent Samples t test was used for the continuous variables normally distributed, and Mann–Whitney U test was used for the variables without normal distribution. Categorical variables were compared with chi-square test. Ninety-five percent confidence intervals (95% CIs) were estimated according to the binomial distribution. Significance was inferred for P < 0.05. Analyses were performed using statistical software SPSS 16.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Patients’ characteristics
A total of 71 patients with suspected FGTB were enrolled, 1 patient without histopathology, 1 patient without T-SPOT.TB on PBMCs, and 3 patients without complete medical records were excluded (the enrolling flowchart is shown in Fig. 1). Among the remaining 66 patients, 32 were diagnosed with confirmed FGTB, 33 with non-TB, and 1 was clinically indeterminate and was not included in the analysis of the diagnostic value. There were 2 patients in whom the diagnosis by research physician deviated from initial clinical diagnosis. One patient with initial TB diagnosis was classified as non-TB by research physicians, and 1 patient with initial diagnosis of non-TB was classified as a clinically indeterminate case by research physicians.
Figure 1.
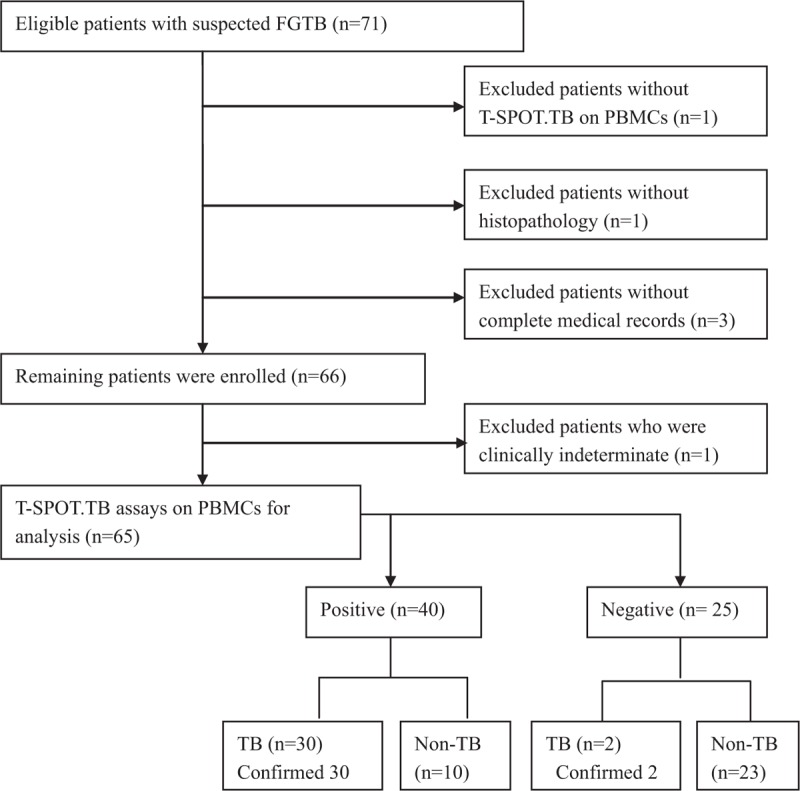
Flowchart of enrolling patients.
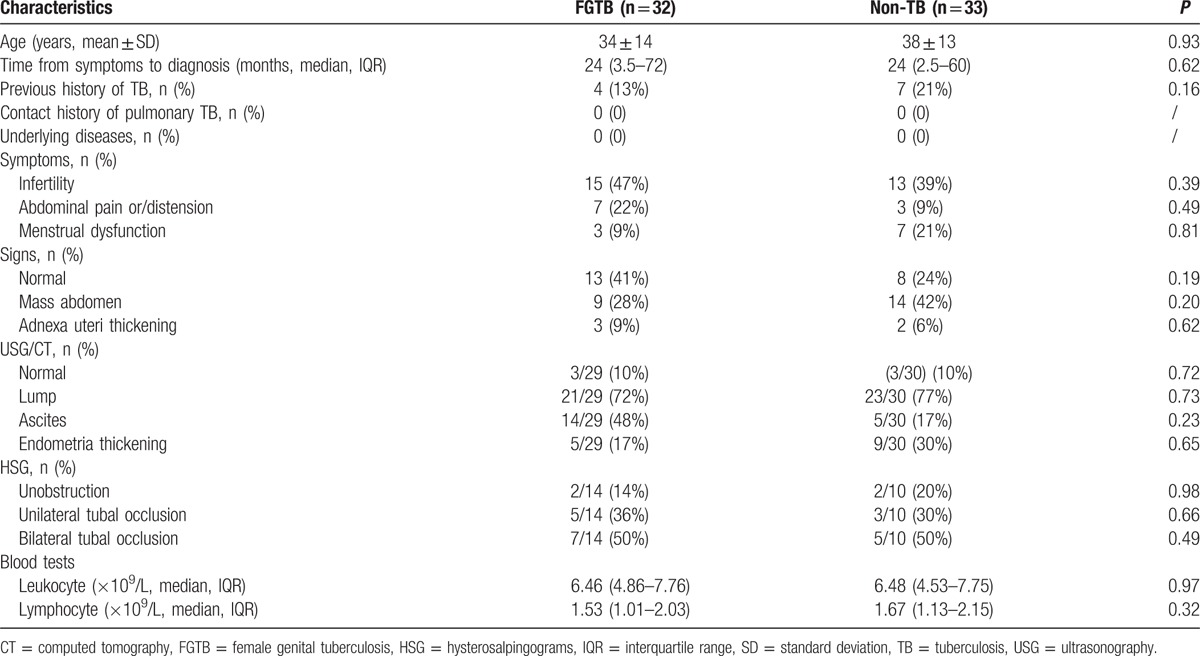
Among patients with confirmed FGTB, the most common organs involved were fallopian (25, 78%), followed by uterus (17, 53%), and ovaries (13, 41%). Non-TB included ovarian tumor in 10 patients (30%), pelvic inflammatory diseases in 8 patients (24%), endometriosis in 7 patients (21%), endometrial polyps in 3 patients (9%), abscess of fallopian tube in 2 patients (6%), cyst of fallopian tube in 2 patients (6%), and endometrial carcinoma in 1 patient (3%). In FGTB group, Acid fast bacilli smear of specimen from the surgery was performed in 21 patients and was positive in 2 patients. Culture of TB was performed in 2 patients and was positive in 1 patient. Histopathologic diagnosis of FGTB was made in all the 32 patients. Histopathologic findings included epithelioid granuloma in 30 patients (94%), caseous necrosis in 2 (6%) patients, tubercles in 10 patients (31%), and thickened tube in 12 patients (38%). Some patients had more than 1 finding. All the 32 patients responded well to anti-TB treatment. Patients’ demographic and clinical characteristics are shown in Table 1. The difference of age, course of disease, evidence of previous TB, and count of lymphocyte was not statistically significant.
Table 1.
Demographic and clinical characteristics of patients with and without female genital tuberculosis.
4. Diagnostic value of T-SPOT.TB
4.1. Sensitivity and specificity of T-SPOT.TB
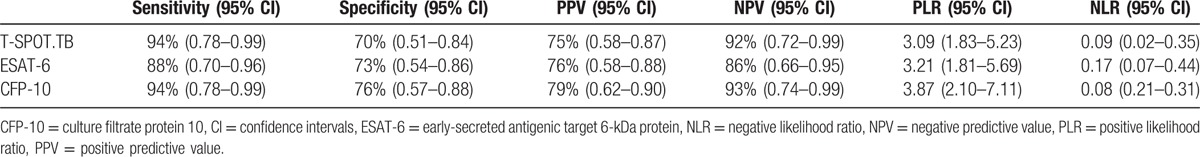
T-SPOT.TB test was positive in 30 of 32 FGTB cases, giving a sensitivity of 94%. In addition, ESAT-6 and CFP-10 antigen were positive in 28 and 30 cases respectively, giving the sensitivity of 88% and 94%, respectively.
Among 33 non-TB cases, T-SPOT.TB was negative in 23 patients, the specificity was 70%. ESAT-6 and CFP-10 antigen were negative in 24 and 25 cases, respectively, giving the specificity of 73% and 76%, respectively. Ten patients with non-TB had positive T-SPOT.TB, among whom 7 had a previous history of TB (Table 2).
Table 2.
Diagnostic parameters of T-SPOT.TB.
4.2. Predictive value and likelihood ratio of T-SPOT.TB
We also analyzed the predictive values and likelihood ratios of T-SPOT.TB in 65 patients with a definitive diagnosis (Table 2). Among 40 patients who had positive T-SPOT.TB results, 30 were diagnosed with FGTB, giving the PPV of 75%. Among 25 patients with negative T-SPOT.TB results, 23 were diagnosed with non-FGTB, giving the NPV of 92%. The PLR and NLR of T-SPOT.TB was 3.09 and 0.09, respectively.
4.3. Comparison of frequencies of ESAT-6 and CFP-10-IFN-γ secreting T cells on peripheral blood in FGTB and non-TB patients
There was a significant difference of frequencies of SFCs of T-SPOT.TB between FGTB and non-TB group (P < 0.01). The frequencies of SFCs responding to ESAT-6 and CFP-10 antigens in FGTB patients were both significantly different from those observed in non-TB patients (P < 0.01 and P < 0.01). The range for positive T-SPOT.TB test was 52–4772 SFCs/106 PBMCs in FGTB group and 28–548 SFCs/106 PBMCs in non-TB group. In addition, the spots of IFN-γ secreting T cells specific for ESAT-6 appeared higher than CFP-10 in FGTB patients, but the difference was not statistically significant (P = 0.15) (Table 3, Fig. 2).
Table 3.
Frequencies of SFCs in patients with and without female genital tuberculosis.
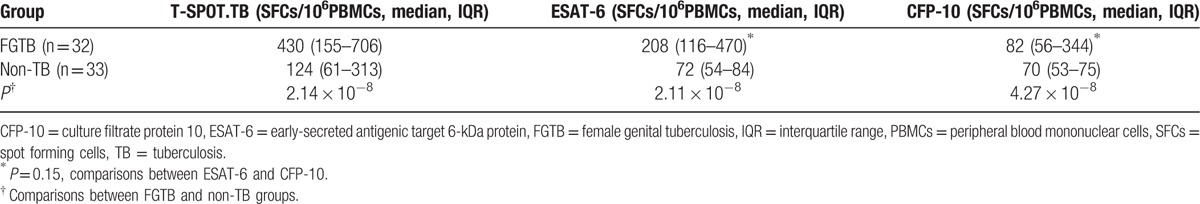
Figure 2.
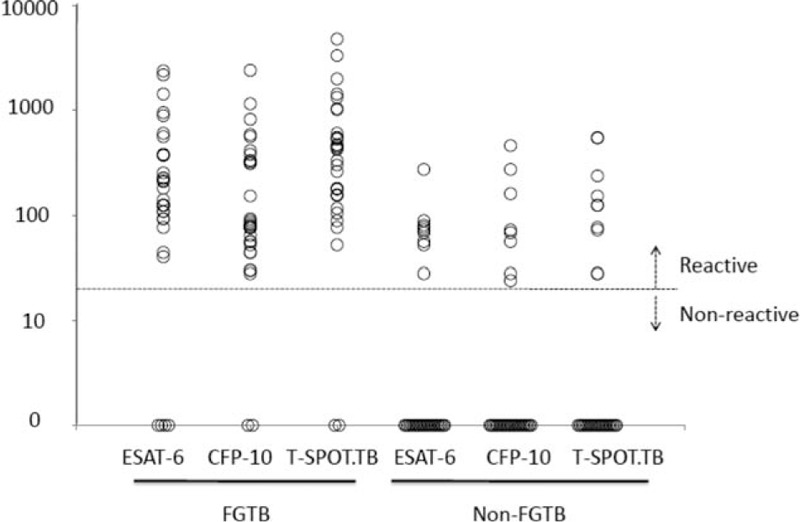
Frequencies of mycobacterium tuberculosis specific IFN-γsecreting T cells in peripheral blood. In patients with female genital tuberculosis, the frequencies of spots forming cells (SFCs) responding to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10, respectively, and in total were all significantly different from those observed in non-TB patients (P < 0.01). The dividing line indicating the cutoff value of a positive T-SPOT.TB test (24 SFCs/106 PBMCs). PBMCs = peripheral blood mononuclear cells, SFCs = spots forming cells.
4.4. Receiver operating characteristic (ROC) curve of T-SPOT.TB
Receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of T-SPOT.TB. The area under the ROC curve was 0.893 (95% CI, 0.791–0.956, P < 0.01) for T-SPOT.TB. According to ROC curve, the cutoff value of T-SPOT.TB for the diagnosis of FGTB was 40 SFCs/106 PBMCs, with a sensitivity of 94%, a specificity of 76%, PPV of 80%, NPV of 92%, PLR of 3.87, and NLR of 0.083 (Fig. 3).
Figure 3.
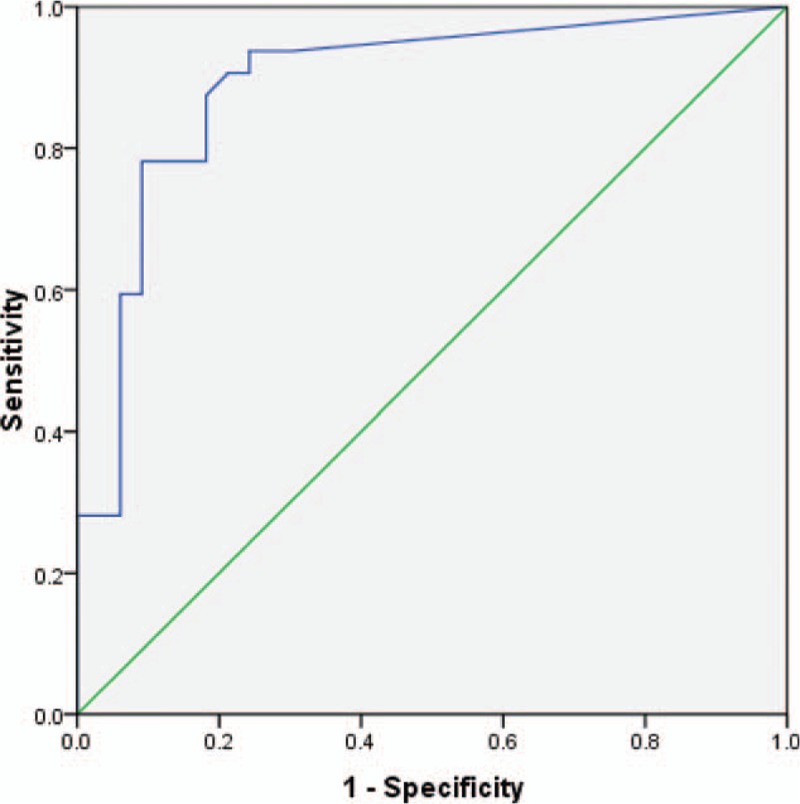
Receiver operating characteristic (ROC) curve for T-SPOT.TB on peripheral blood mononuclear cells (PBMCs) in patients with suspected female genital tuberculosis (FGTB). The area under the ROC curve was 0.893 (95% confidence interval: 0.791–0.956, P < 0.001) for T-SPOT.TB. The cutoff value of T-SPOT.TB for the diagnosis of FGTB was 40 SFCs/106 PBMCs, with a sensitivity of 94%, a specificity of 76%, PPV of 80%, NPV of 92%, PLR of 3.87, and NLR of 0.083. ROC = receiver operating characteristic.
5. Discussion
The diagnostic value of T-SPOT.TB for diagnosis of FGTB was rarely reported. We found both sensitivity and NPV were high, and a cutoff value of 40 SFCs/106 PBMCs for T-SPOT.TB showed a sensitivity of 94%, and a specificity of 76% for diagnosis of FGTB.
TB is still a major public health issue resulting in 1.5 million deaths in 2014 worldwide.[1] Females with genitourinary TB comprise approximately 0.5% of all TB cases, and among genitourinary cases less than 50% are genital TB cases.[11] In infertility patients, incidence of FGTB varies in different countries.[3] In China, FGTB (24.5%) was the leading cause of infertility in the period of 1985 to 1995, and accounted for 6.7% of infertility in the period of 1996 to 2006.[12]
In our study, the most common organs involved in FGTB were fallopian (78.1%), followed by uterus (53.1%) and ovaries (40.6%). Similar results were shown in other studies that fallopian tubes were involved in 90% to 100% FGTB cases, endometrium was involved in 50% to 80% cases, and ovaries were involved in 20% to 30% cases with tubo-ovarian masses. Cervical TB (in 5%–15% cases) and TB of vagina and vulva was relatively rare (1%–2%).[3,13]
Early diagnosis of FGTB is critical as it can save the patient from a series of unnecessary invasive diagnostic or/and therapeutic procedures; moreover, in some cases, it leads to timely therapy and avoidance of fibrosis. Once fibrosis is established, it is very difficult to restore fertility, even with appropriate treatment.[11] So an efficient method for diagnosis is in great need.
The diagnostic role of a positive tuberculin skin test (TST) is controversial. Raut et al reported that almost 45% of infertile women with strong indirect evidence of FGTB, such as laparoscopic findings (thickened tubes, areas of caseation, etc.), had a negative TST.[14,15] Acid fast stain of smears is not often useful since 105 mycobacteria/mL must be present in the specimen to give a positive result. On the other hand, solid and liquid mycobacterial cultures usually need at least 2 weeks for the isolation of MTB. The polymerase chain reaction (PCR) is a very useful method for establishing the diagnosis of FGTB, as it can increase the yield in clinical samples and detect genital TB earlier in the process; however, proper sampling for PCR requires invasive techniques (e.g., laparoscopy), and it does not have the ability to distinguish live from killed organisms.[11]
Our study demonstrated a favorable sensitivity (94%) and specificity (70%) of T-SPOT.TB for diagnosis of FGTB. According to Cho et al,[8] the overall sensitivity and specificity of the blood T-SPOT.TB in patients with suspected extrapulmonary TB were 84% (129/153, 95% CI, 78–89%) and 51% (87/172, 95% CI, 43–58%), respectively. And another study by Kim[16] which analyzed the diagnostic value of T-SPOT.TB for diagnosis of abdominal TB suggested that the sensitivity and specificity of the PBMC ELISPOT assay were 89% (95% CI, 71–98%) and 78% (95% CI, 52–94%), respectively. Our sensitivity was relatively higher than those in the previous studies, which might be due to the reason that all the patients enrolled in our study were immunocompetent. Interestingly, Cho et al[8] showed in their study that the sensitivity of T-SPOT.TB increased from 73% in immunocompromised patients to 88% in immunocompetent patients. They also found that chronic forms of extrapulmonary TB were independently associated with a low incidence of false-negative blood T-SPOT.TB results,[8] which might explain the negative results in the FGTB patients. Testing with both a TST and an IGRA is highly recommended in cases where, although the initial TST test is negative, the clinical suspicion for active TB is very strong (such as in persons with symptoms, signs, and/or radiographic evidence suggestive of active TB).[17]
Among the non-TB group, 3 patients without evidence of previous TB also had positive T-SPOT.TB. We speculate that these cases were latent TB infection (LTBI), since patients with LTBI may also have a positive IGRA result. Gao et al[18] investigated the prevalence of LTBI in China and showed that the baseline of QuantiFERON (QFT) positivity rates ranged from 13% to 20%, indicating high prevalence. This LTBI epidemiological data might also explain the relatively low specificity in our study.
Subtractive deoxyribonucleic acid (DNA) hybridization of pathogenic Mycobacterium bovis and Bacillus Calmette–Guerin (BCG) and comparative DNA-microarray analysis of MTB H37Rv and BCG have identified 1 region of difference (RD1), designated RD1, that was found to be present in all MTB and pathogenic M. bovis strains but lacking in all BCG vaccine strains and almost all environmental mycobacteria. One antigen encoded by RD1 is the ESAT-6, and then a second RD1-encoded protein was designated as CFP-10.[19,20] We found that frequencies of cells responding to ESAT-6 appeared higher than CFP-10 in FGTB patients, but the difference was not statistically significant (P = 0.15). A study of T-SPOT.TB for diagnosis of extrapulmonary TB also demonstrated that response to ESAT-6 was higher for samples from patients with chronic than with acute forms of extrapulmonary TB. However, they found no significant associations between responses to ESAT-6 (P = 0.29) and CFP-10 (P = 0.19) and immunosuppressive conditions, neither.[8] Another study also supported the result that no significant differences in sensitivity, specificity, and median numbers of SFCs were found between ESAT-6 and CFP-10 in active TB group.[21] Further studies may be done to have a better understanding of this.
There are also some limitations in our study. This is a retrospective study, and some bias may exist, which may overestimate or underestimate the final result. However, as few researches reported the diagnostic value of T-SPOT.TB for diagnosis of FGTB, this study still provided some valuable information. Moreover, this study was done in a national referral center where cases tended to be severe and complex, which suggest caution when the finding of study is extrapolated to centers with potentially different patient characteristics. A multicenter study will be valuable to understand the performance of T.SPOT-TB in different settings.
In summary, this study suggested that T-SPOT.TB could be considered as a potential triage test for FGTB, which is rapid, practical and appears to have high sensitivity and NPV.
Acknowledgments
We are grateful to all the patients participating in this study at PUMCH. We thank all the healthcare staff of relevant district at PUMCH for supporting site implementation.
Footnotes
Abbreviations: CFP-10 = culture filtrate protein 10, CI = confidence interval, EPTB = extra pulmonary tuberculosis, ESAT-6 = early-secreted antigenic target 6-kDa protein, FGTB = female genital tuberculosis, IGRAs = interferon-γ release assays, IQR = interquartile range, MTB = mycobacterium tuberculosis, NLR = negative likelihood ratio, NPV = negative predictive value, PBMCs = peripheral blood mononuclear cells, PLR = positive likelihood ratio, PPV = positive predictive value, ROC = receiver operating characteristic, SFCs = spot-forming cells.
XL and SB contributed equally to the article.
Funding: This work was supported by grants from the National Major Science and Technology Research Projects for the Control and Prevention of Major Infectious Diseases in China (2014ZX10003003), and Health Research &Special Projects Grant (201402001).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: Conceived and designed the study: XL. Performed the experiments: WW, QT, LZ, YZ, and ZL. Analyzed the data: XL, SB, XC, and LZ. Wrote the paper: XL, SB, XC, WW, and YZ. Managed clinical information and diagnosis: XL, QT, ZL, XS, and SB. Discussed and revised the manuscript: XL, QT, and YZ.
The authors have no conflicts of interest to disclose.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. 20th ed.Geneva:World Health Organization; 2015. [Google Scholar]
- 2.Zhou XX, Liu YL, Zhai K, et al. Body fluid interferon-gamma release assay for diagnosis of extrapulmonary tuberculosis in adults: a systematic review and meta-analysis. Sci Rep 2015; 5:15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma JB. Current diagnosis and management of female genital tuberculosis. J Obstet Gynaecol India 2015; 65:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oosthuizen AP, Wessels PH, Hefer JN. Tuberculosis of the female genital tract in patients attending an infertility clinic. S Afr Med J 1990; 77:562–564. [PubMed] [Google Scholar]
- 5.Tripathy SN, Tripathy SN. Infertility and pregnancy outcome in female genital tuberculosis. Int J Gynaecol Obstet 2002; 76:159–163. [DOI] [PubMed] [Google Scholar]
- 6.Jindal UN. An algorithmic approach to female genital tuberculosis causing infertility. Int J Tuberc Lung Dis 2006; 10:1045–1050. [PubMed] [Google Scholar]
- 7.Gupta N, Sharma JB, Mittal S, et al. Genital tuberculosis in Indian infertility patients. Int J Gynaecol Obstet 2007; 97:135–138. [DOI] [PubMed] [Google Scholar]
- 8.Cho OH, Park KH, Kim SM, et al. Diagnostic performance of T-SPOT.TB for extrapulmonary tuberculosis according to the site of infection. J Infect 2011; 63:362–369. [DOI] [PubMed] [Google Scholar]
- 9.Bhanu NV, Singh UB, Chakraborty M, et al. Improved diagnostic value of PCR in the diagnosis of female genital tuberculosis leading to infertility. J Med Microbiol 2005; 54 (pt 10):927–931. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Zhang Y, Shi X, et al. Utility of T-cell interferon-gamma release assays for diagnosing tuberculous serositis: a prospective study in Beijing, China. PLoS One 2014; 9:e85030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neonakis IK, Spandidos DA, Petinaki E. Female genital tuberculosis: a review. Scand J Infect Dis 2011; 43:564–572. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WH, Hao M. Pelvic inflammatory disease: a retrospective clinical analysis of 1,922 cases in North China. Gynecol Obstet Invest 2014; 77:169–175. [DOI] [PubMed] [Google Scholar]
- 13.Sharma JB, Roy KK, Pushparaj M, et al. Genital tuberculosis: an important cause of Asherman's syndrome in India. Arch Gynecol Obstet 2008; 277:37–41. [DOI] [PubMed] [Google Scholar]
- 14.Hadzibegovic DS, Maloney SA, Cookson ST, et al. Determining TB rates and TB case burden for refugees. Int J Tuberc Lung Dis 2005; 9:409–414. [PubMed] [Google Scholar]
- 15.Raut VS, Mahashur AA, Sheth SS. The Mantoux test in the diagnosis of genital tuberculosis in women. Int J Gynaecol Obstet 2001; 72:165–169. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Cho OH, Park SJ, et al. Diagnosis of abdominal tuberculosis by T-cell-based assays on peripheral blood and peritoneal fluid mononuclear cells. J Infect 2009; 59:409–415. [DOI] [PubMed] [Google Scholar]
- 17.Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep 2010; 59 (RR–5):1–25. [PubMed] [Google Scholar]
- 18.Gao L, Lu W, Bai L, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2015; 15:310–319. [DOI] [PubMed] [Google Scholar]
- 19.Arend SM, Andersen P, van Meijgaarden KE, et al. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis 2000; 181:1850–1854. [DOI] [PubMed] [Google Scholar]
- 20.Berthet FX, Rasmussen PB, Rosenkrands I, et al. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 1998; 144 (pt 11):3195–3203. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Yu Y, Chen W, et al. Evaluation of the characteristics of the enzyme-linked immunospot assay for diagnosis of active tuberculosis in China. Clin Vaccine Immunol 2015; 22:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]


