Abstract
Background:
Adjunctive treatment with medication of liver-soothing-oriented method (MLSM) is one of the most commonly used approaches for subjects with depression after cerebrovascular accident (DCVA) in China. The purpose of this meta-analysis was to evaluate the outcome of MLSM treatment in subjects with DCVA using relevant published literature.
Methods:
The PubMed, Cochrane Library, Embase, Chinese databases of China National Knowledge Infrastructure, WanFang, Sinomed, and VIP were used to collect all publications until March 2016. Randomized controlled trials comparing treatments with and without MLSM for subjects with DCVA were included. The quality of each publication was assessed based on the recent Handbook (5.1 version) for Cochrane Reviewers. Cochrane Collaboration's software RevMan 5.3 software was applied for data analysis.
Results:
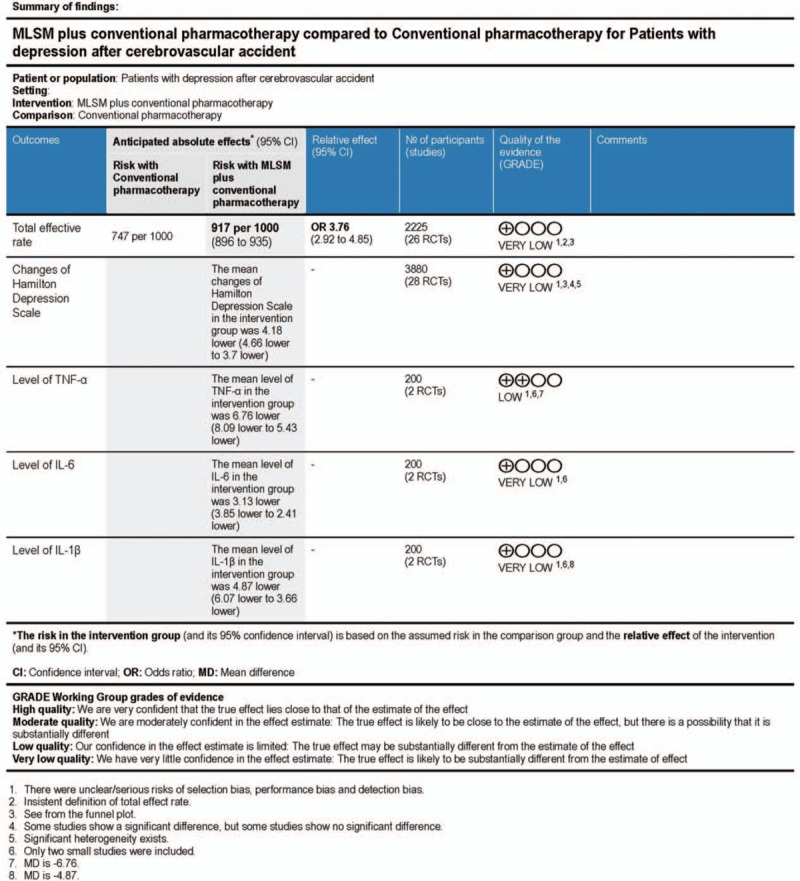
Thirty studies, including 2599 cases, were identified and collected. Adjunctive treatment with MLSM noticeably enhanced total effective rates (odds ratio 3.76; 95% confidence interval [CI] 2.92–4.85, I2 = 0%, P = 0.96) in comparison to non-MLSM conventional pharmacotherapy. Compared to non-MLSM treatment, the changes of Hamilton Depression Scale in adjunctive treatment with MLSM, respectively, decreased and showed beneficial effects after 3 weeks (weighted mean difference [WMD] −4.83; 95% CI −6.82 to −2.83; I2 = 86%, P < 0.001), 4 weeks (WMD −4.20; 95% CI −5.06 to −3.33; I2 = 78%, P < 0.001), 6 weeks (WMD −3.36; 95% CI −4.05 to −2.68; I2 = 54%, P = 0.02), 8 weeks (WMD −4.83; 95% CI −5.62 to −4.04; I2 = 73%, P < 0.001), and 12 weeks (WMD −2.88; 95% CI −4.09 to −1.67; I2 = 58%, P = 0.09). As for changes in inflammatory cytokine levels, adjunctive treatment with MLSM was associated with a significant decrease in tumor necrosis factor-α, IL-6, and interleukin-1β levels in comparison to non-MLSM treatment. Moreover, there were positive effects on score changes for National Institute of Health Stroke Scale, activities of daily living, Hamilton Anxiety Scale, Modified Edinburgh Scandinavian Stroke Scale, and Self-Rating Anxiety Scale. No serious adverse events were reported.
Conclusion:
MLSM appears to improve symptoms of depressive disorders, enhance immediate responses, and the quality of life in subjects with DCVA. The positive action of MLSM might be potentially connected with its immunoregulating effects. More prospective trials with strict design and larger sample sizes are warranted to clarify its effectiveness and safety.
Keywords: adjunctive treatment, DCVA, inflammatory cytokines, meta-analysis, MLSM, systematic review
1. Introduction
Depression after cerebrovascular accident (DCVA) is a common disorder in subjects with stroke and consequently enhances the risks of social nonparticipation, disability, and mortality.[1–2] In China, the incidence of DCVA varies between 23.0% and 76.1%.[3] Mortality in stroke subjects with depression has been observed at rates of 3.5- to 10-times higher than those with nondepression; suicide tendencies are also >10% among stroke subjects.[4–6] A daily rehabilitation regime for stroke sufferers might positively affect depression symptoms; however, most potential patients suffering from depression are ignored. Conversely, only a small proportion of patients have been definitely identified after diagnosis, and even fewer seek help or receive clinical therapy.
Recently, reports have revealed that cerebrovascular diseases—as well as immunosuppression of human body system—contribute to the risks of depression. In addition, subjects with primary immunodeficiency were more susceptible to depressive disorders.[7,8] However, most subjects diagnosed with DCVA did not manifest immunodeficiency, and only a small portion had a mild reduction in tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), or interleukin-1β (IL-1β) levels. DCVA patients are typically treated with antidepressant medications and with selective serotonin reuptake inhibitors in particular. Other therapeutic approaches for DCVA include psychotherapy, electroconvulsive therapy, or pharmacotherapy to relieve symptoms. However, there is no established consensus on the efficacy of these treatments. Furthermore, concerns have been presented about the risks of adverse effects or interactions between different drugs while treating subjects with depression in comparison to healthy individuals. Consequently, there is an imperative need to formulate alternative therapeutic agents to effectively manage DCVA.
In recent years, Chinese herbal medicine has been used as a complementary therapy to safely treat symptoms of depression in patients. Based on the hypothetical theory of ancient Chinese medicine, the etiology and pathogenesis of depression are influenced by Liver-qi stagnation—a comprehensive state exhibited with symptoms of mental strain or stress, pain of hypochondriac or hernial location, abnormal menstruation, or breast distending pain or lumps, and so on.[9,10] Despite the fact that a series of empirical formulas have been applied in clinical practice, medication that utilizes a medication of liver-soothing-oriented method (MLSM)—a herbal prescription extract of Bupleurum chinense and Rhizoma cyperi—has been chosen for optimized management of DCVA in China.[11–40] Some herbal treatments have been implicated in enhancing neuroimmunoregulation, including a potential restoration of the network balance of inflammatory cytokines in subjects with DCVA and in animal studies. Also, MLSM and its relevant components have shown to have anti-inflammatory effects in reducing TNF-α, IL-6, IL-1β, and other inflammatory factor levels in preclinical trials, while also suppressing the activity of lipoprotein-associated phospholipase A2 in cultured samples.[41–43] The findings mentioned above suggest that MLSM may potentially function as a positive modulator of immunoregulation and act as an anti-inflammatory agent.
To date, no previous reviews have focused on the additive effects of MLSM in subjects with DCVA. Therefore, the purpose of this meta-analysis was to evaluate the potential efficacy and safety of adjunctive treatment with MLSM in subjects with DCVA based on published literature.
2. Material and methods
2.1. Search strategy
The review was conducted in accordance with the recommendations from preferred reporting items for systematic reviews and meta-analyses.[44] A systematic search was conducted using the following databases: PubMed, Cochrane Library, Embase, Chinese databases of China National Knowledge Infrastructure, WanFang, Sinomed, and VIP up to March 2016. The terms of retrieval were restricted in randomized controlled trials (RCTs) and using the following keywords: (liver-soothing San OR liver-soothing Tang OR shugansan OR liver-soothing OR Shu-gan OR Liver-soothing-oriented method) AND (depression after cerebrovascular accident OR post-stroke depression OR depression after stroke OR stroke with depressive disorders). The references of included publications were also manually checked to clarify potentially eligible studies.
2.2. Study selection
The inclusion criteria were as follows—trial design: RCTs; recruitment population: subjects with DCVA diagnosis are referred to in the diagnostic criteria; intervention approaches: MLSM plus conventional pharmacotherapy versus conventional pharmacotherapy alone or versus the conventional pharmacotherapy plus placebo (the placebo was a simulated treatment of MLSM with identical dosage forms [i.e., capsule], weight, labeling, etc. in the literature.); outcome measurement: the primary outcome indexes were total effective rate based on Hamilton Depression Scale (HAM-D) score changes.[45] The second outcome indexes were based on the levels of inflammatory cytokines (e.g., TNF-α, IL-6, and IL-1β), score changes of National Institute of Health Stroke Scale (NIHSS),[46] activities of daily living (ADL),[47] Hamilton Anxiety Scale (HAM-A),[48] Modified Edinburgh Scandinavian Stroke Scale (MESSS),[49] Self-Rating Anxiety Scale (SAS),[50] and adverse events; and the treatment periods were no less than 3 weeks. The studies were excluded if publications did not use the original formula of MLSM; subjects had taken any antidepressants up to 4 weeks before recruitment; and enrolled subjects had liver and renal insufficiency, mental disease, type 2 diabetes mellitus, or other cardiovascular disorders.
2.3. Definition
DCVA diagnosis must be conducted using standards established by the international classification of diseases (ICD-9[51] or ICD-10[52]), Chinese classification of mental disorders-3[53], Diagnostic and Statistical Manual of Mental Disorders (DSM-III,[54] DSM-IIIR,[55] DSM-IV,[56] or DSM-5[57]), or other well recognized criteria. Conventional pharmacotherapy for symptomatic treatment included fluoxertine hydrochloride, deanxit, paroxetine hydrochloride, venlafaxine, or other routine antidepressants. The total effective rate was calculated using the number of subjects who obtained markedly effective responses (≥50% reduction in HAM-D scores) and effective responses (≥25% reduction in HAM-D scores), divided by the total number of subjects.
2.4. Data extraction and quality assessment
Two reviewers gathered the informative sources from all qualified studies. The extracted items included the publication year, first author, sample size, target subjects’ gender and age, MLSM interventions, control therapies, diagnoses, treatment durations, follow-up periods, outcome measurement, and adverse events. Once incomplete data or potentially duplicated documents were identified, attempts were made to contact the corresponding authors or other potential leading members via telephone or by e-mail. Publications were excluded from the review if the intervention strategy or control group settings were inconsistent with the selection criteria, in addition to other incomplete data that could not be verified by consultation with publication authors. The quality of the eligible studies was estimated based on the tools for risk bias assessment recommended by the Cochrane Collaboration (Revman5.3; www.cochrane.org/training/cochrane-handbook). Key items in the assessment included selection bias, reporting bias, performance bias, attrition bias, detection bias, and other sources of bias.
2.5. Statistical methods
Dichotomous data were presented by odds ratio (OR) with a 95% confidence interval (CI), while the continuous variables were estimated by weighted mean difference (WMD) with a 95% CI. OR values with a total effective rate >1 indicated that MLSM from pooled effects was better in comparison to the control. Using the synthesized results of ADL score changes, WMD values that were >0 indicated that MLSM was superior to those in control. However, WMD values >0 revealed that MLSM was better than those in control, while taking into account pooled effects of score changes of HAM-D, NIHSS, ADL, HAM-A, MESSS, and SAS, and the levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β). Furthermore, that heterogeneity of risk factors among trials was analyzed using the I2 and Cochrane Q statistical models. The corresponding data were evaluated based on the fixed-effect model (FEM) if there was no statistical heterogeneity observed (I2 < 50% or P > 0.10). Once certain statistical heterogeneity was determined (I2 > 50% or P < 0.10), pooled results were analyzed by to the random-effects model (REM). When ≥9 publications presented similar outcome indexes, funnel plots were applied to verify the potential publication bias. Intervention studies that involved different treatment durations or follow-up periods of MLSM, subgroup analyses were also performed. Grading evidence for quality evaluation of this review was conducted using the Grades of Recommendations Assessment Development and Evaluation (GRADE) profiler software (https://gradepro.org). The RevMan 5.3 software recommended by Cochrane Collaboration was utilized for data analyses (http://tech.cochrane.org/revman/).
2.6. Ethical review
The study is a systemic literature review that did not involve experimental participants or human subjects, and there was no collection of any private data or sensitive information during the review process.
3. Results
3.1. Description of included trials
The initial retrieval of publications resulted in the collection of 768 potential studies. After reviewing the full texts, a total of 30 studies qualified for review using the predetermined criteria of inclusive recruitment (Fig. 1). In all, 2599 subjects with DCVA were included in this review. All of the publications included were written in the Chinese language (Mandarin), and the experimental work was performed in China. The duration of therapies was between 4 and 12 weeks. Outcome measures were collected both before and at the end of the relevant treatments. Target subjects in the control groups were given conventional pharmacotherapy of symptomatic treatment, while subjects in the MLSM treatment groups were given MLSM plus routine pharmacotherapy. MLSM was formulated by herbal decoction, particle, powder, capsule, or oral liquid. The baseline characteristics from the 30 studies are presented in Tables 1 and 2. The evaluation of methodological quality for each included trial is listed in Figs. 2 and 3. In general, most of the enrolled studies were observed with obvious bias from unclear to high risks. The grading evidence of quality was calculated using GRADEprofile software (Fig. 4).
Figure 1.
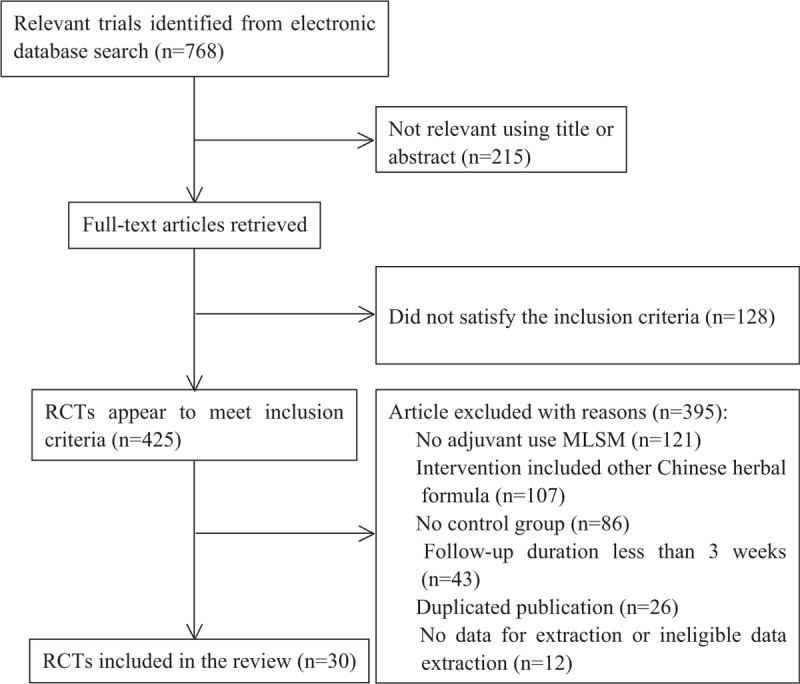
Flowchart of trial selection process.
Table 1.
Characteristics of trials included in the meta-analysis.
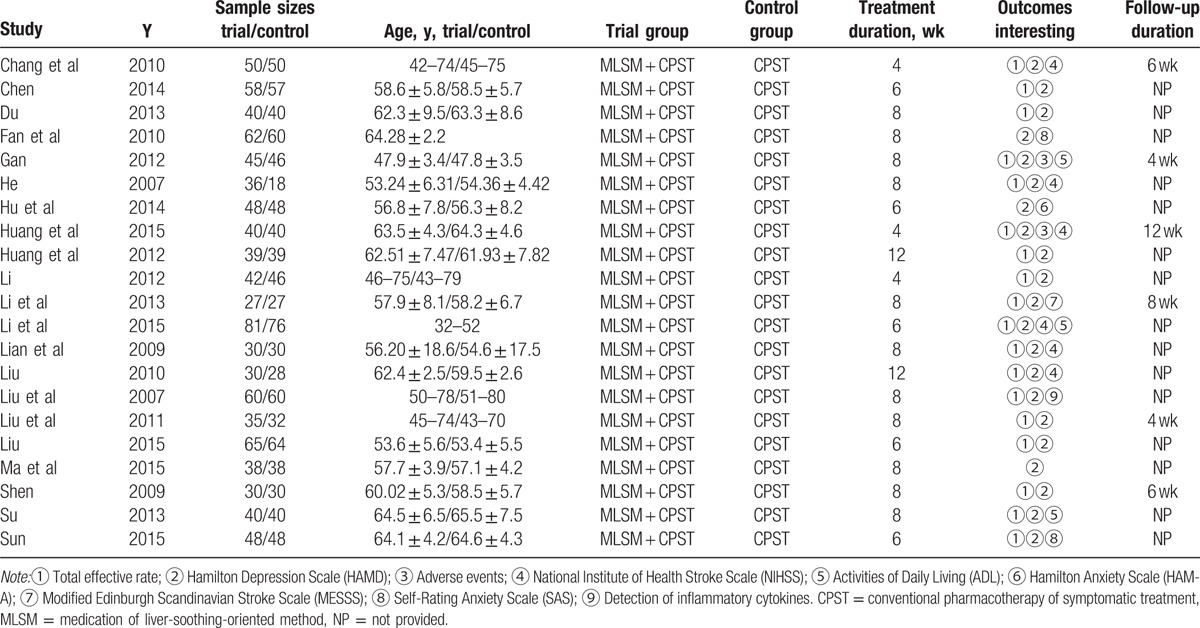
Table 2.
Characteristics of trials included in the meta-analysis.
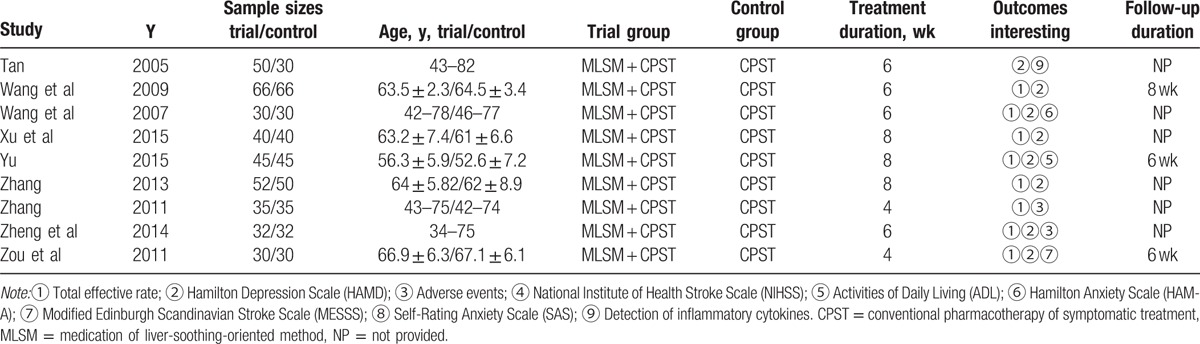
Figure 2.
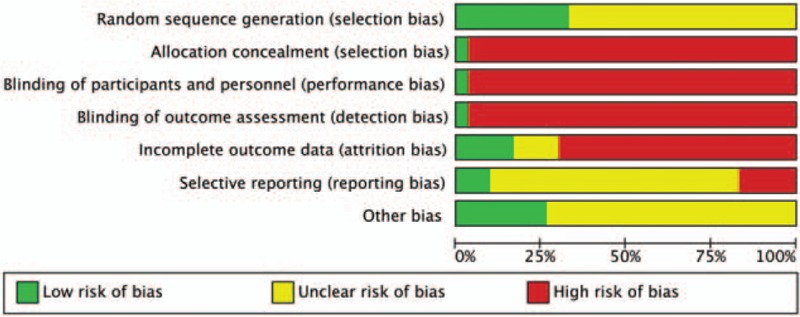
Risk of bias graph with items presented as percentages across all included studies.
Figure 3.
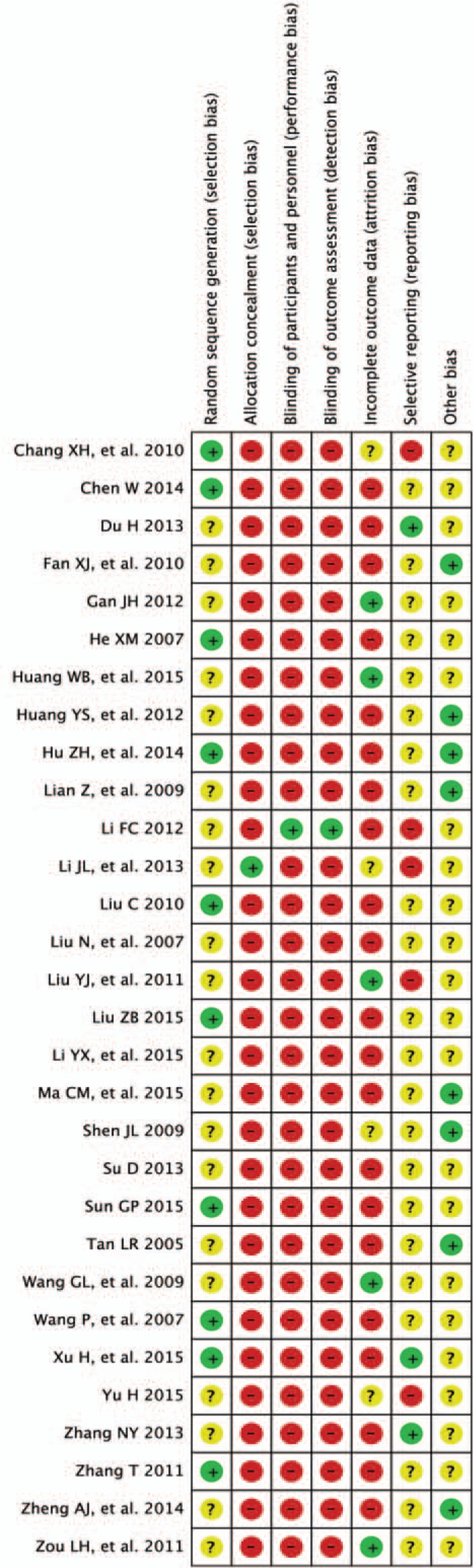
Risk of bias summary for the studies based on the Cochrane Handbook.
Figure 4.
Grades of recommendations assessment development and evaluation quality grading evaluation of this systematic review.
3.2. Effects of the interventions: primary outcomes
3.2.1. Total effective rates with or without MLSM
Twenty-six trials[11–13,15,16,18–27,29–31,33–40] reported the total effective rates as key measurement of outcomes. A total of 1126 cases were allocated to MLSM group, while 1099 cases were assigned to the control group based on conventional pharmacotherapy alone. Adjunctive treatment with MLSM significantly enhanced the total effective rate (OR 3.76; 95% CI 2.92–4.85, I2 = 0%, P = 0.96) in comparison to the control in the FEM (Fig. 5).
Figure 5.
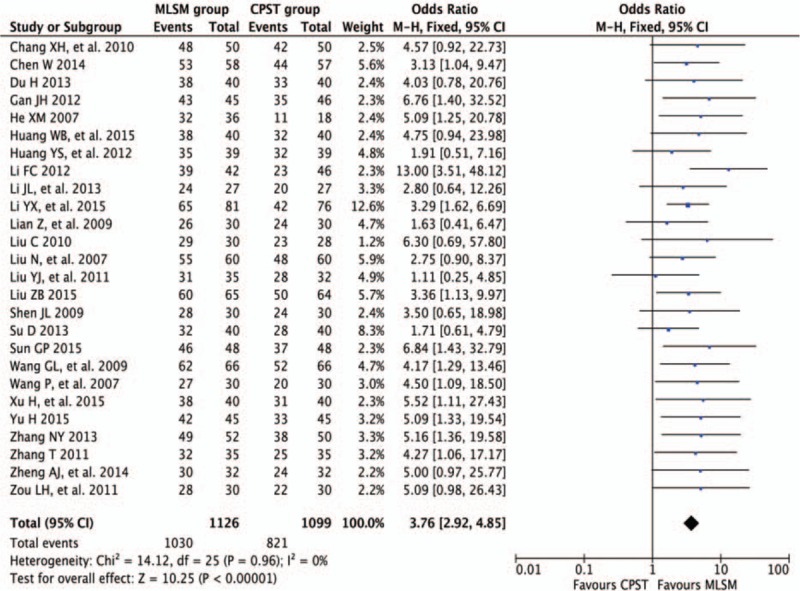
Forest plots showing odds ratio with 95% confidence interval for the total effective rate comparing with or without medication of liver-soothing-oriented method in a fixed-effect model.
3.2.2. Publication bias of total effective rates with or without MLSM
In order to evaluate potential publication bias, funnels plot analyses were performed using total effective rates from 26 trials[11–13,15,16,18–27,29–31,33–40] that compared treatment with or without MLSM. Significant asymmetry was observed, indicating the presence of possible publication bias and inclusion of low-quality trials (Fig. 6).
Figure 6.
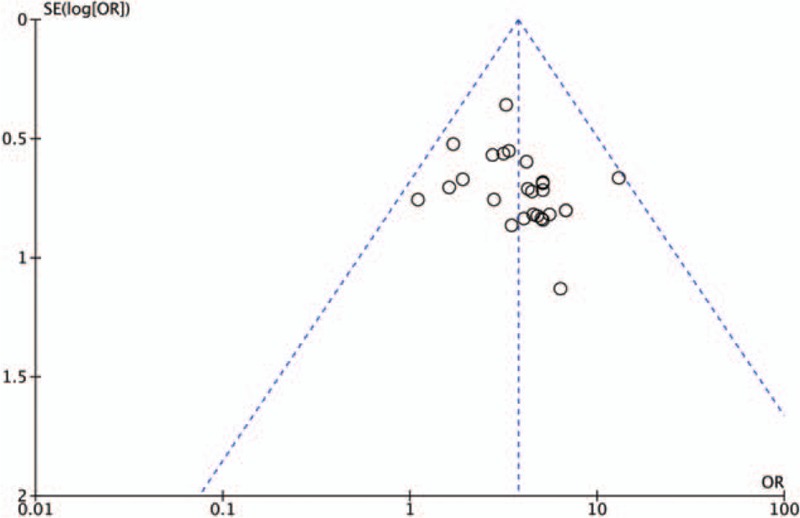
Funnel plot showing for the total effective rate comparing with or without medication of liver-soothing-oriented method.
3.2.3. Score changes in HAM-D with or without MLSM
Twenty-eight studies[11,13–37,39,40] that compared with or without MLSM treatment were assessed using the mean change from baseline in HAM-D scores, resulting in substantial heterogeneity (P < 0.10, I2 > 50%) due to variability in the timing point of the synthesized results. Therefore, pooled estimates were conducted based on REM models, with subgroup analyses between studies using different intervention periods of 3, 4, 6, 8, and 12 weeks. Results of subgroup analyses on the changes in HAM-D scores are displayed in Fig. 7. Compared with non-MLSM, there were positive effects on HAM-D score changes after 3 weeks (WMD −4.83; 95% CI −6.82 to −2.83; I2 = 86%, P < 0.001) in 5 trials,[17,32–34,39] 4 weeks (WMD −4.20; 95% CI −5.06 to −3.33; I2 = 78%, P < 0.001) in 13 trials,[11,15,18,20,23,25–28,35–37,40] 6 weeks (WMD −3.36; 95% CI −4.05 to −2.68; I2 = 54%, P = 0.02) in 10 trials,[17,22,26,27,31–35,39] 8 weeks (WMD −4.83; 95% CI −5.62 to −4.04; I2 = 73%, P < 0.001) in 14 trials,[13–16,21,23,25,26,28–30,35–37] 12 weeks (WMD −2.88; 95% CI −4.09 to −1.67; I2 = 58%, P = 0.09) in 3 trials,[18,19,24] and an overall effect observed (WMD −4.18; 95% CI −4.66 to −3.70; I2 = 80%, P < 0.001) (Fig. 7). The outcomes of sensitivity analyses revealed that no changes were observed in effect sizes when any single trial was excluded (data not included), which supported the reliability of these results.
Figure 7.
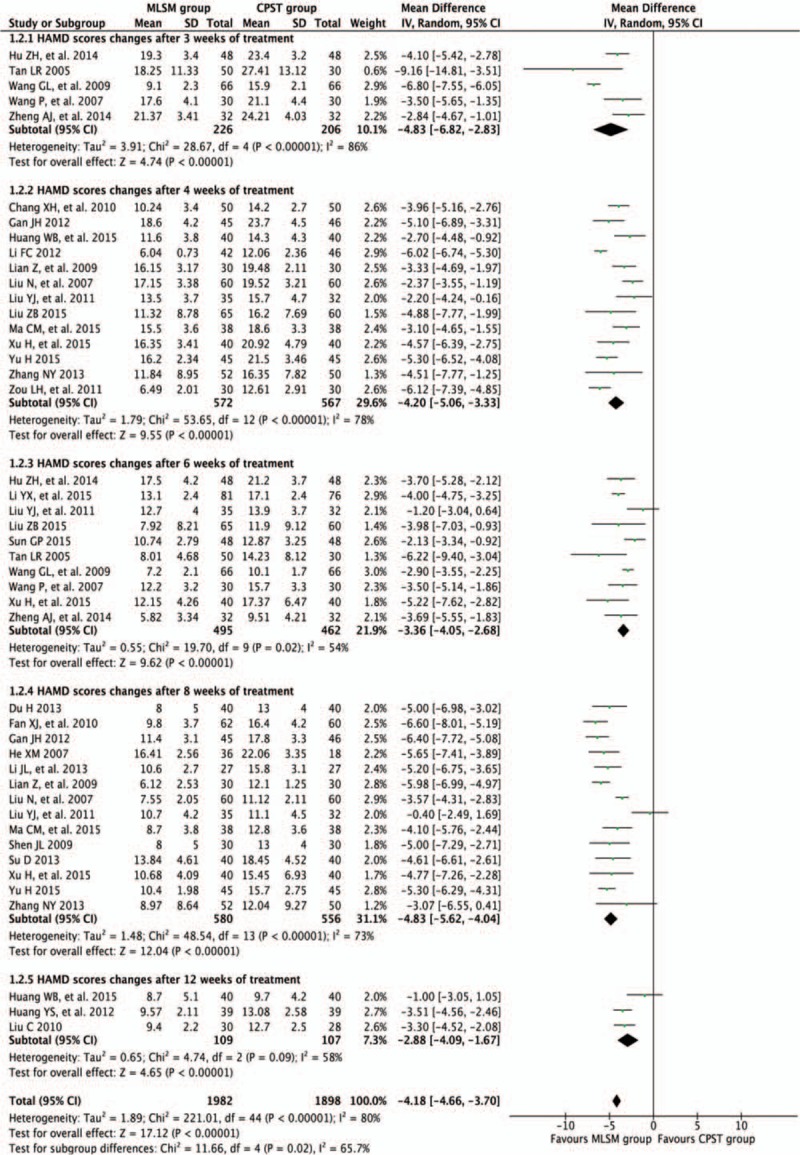
Forest plots showing weighted mean difference with 95% confidence interval for changes of Hamilton Depression Scale comparing with or without medication of liver-soothing-oriented method in a random-effect model.
3.2.4. Publication bias of HAM-D comparing with or without MLSM
In order to estimate potential publication bias, a funnel plot analysis of 28 trials[11,13–37,39,40] was performed that compared studies with or without MLSM adopting HAM-D score changes, and significant asymmetry was observed (Fig. 8). This could indicate publication bias among the included studies.
Figure 8.
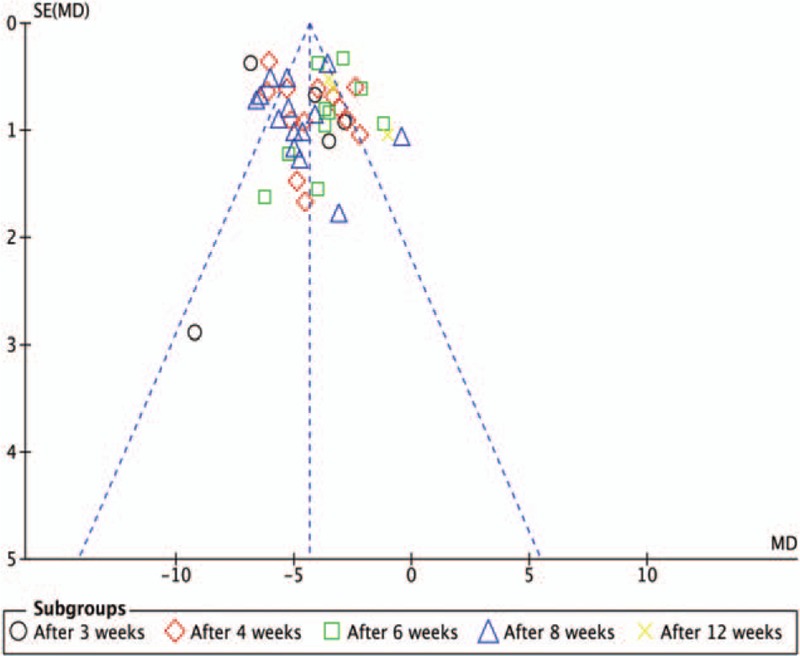
Funnel plot showing for changes of Hamilton Depression Scale comparing with or without medication of liver-soothing-oriented method.
3.3. Effects of the interventions: secondary outcomes
3.3.1. Changes in inflammatory cytokines levels with or without MLSM
Two trials[25,32] evaluated the change in inflammatory cytokine levels based on TNF-α, IL-6, and IL-1β counts. Adjunctive treatment with MLSM was associated with a significant decrease in TNF-α levels (WMD −6.76; 95% CI −8.09 to −5.43; I2 = 0%, P = 0.42), IL-6 levels (WMD −3.13; 95% CI −3.85 to −2.41; I2 = 0%, P = 1.00), and IL-1β levels (WMD −4.87; 95% CI −6.07 to −3.66; I2 = 0%, P = 0.63) in a FEM when compared to the conventional treatment group (Fig. 9).
Figure 9.
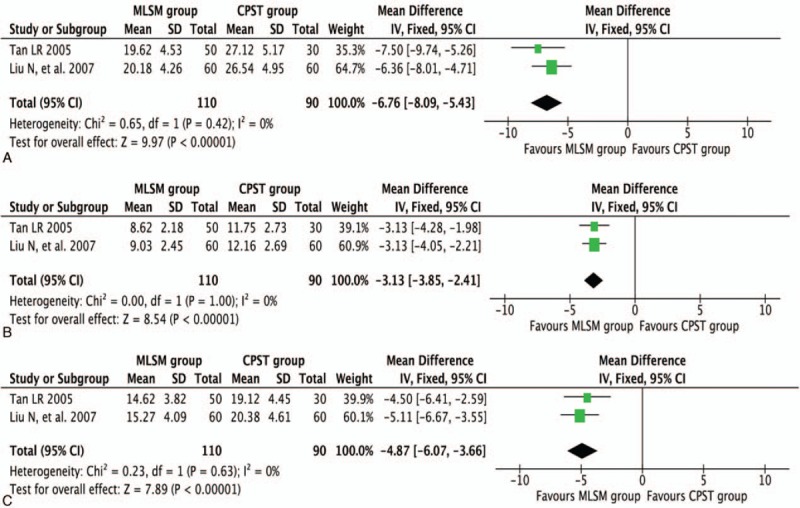
Forest plots showing weighted mean difference with 95% confidence interval for changes of detection of inflammatory cytokines comparing with or without medication of liver-soothing-oriented method in a fixed-effect model: (A) TNF-α, (B) IL-6, and (C) IL-1β.
3.3.2. Score changes of NIHSS with or without MLSM
Pooled results were calculated using REM model analysis of data from 6 trials[11,16,18,22–24] that compared control versus MLSM treatment by evaluating differences in mean change from baseline in NIHSS scores, and subgroup analyses were performed using follow-up periods of 4, 6, 8, and 12 weeks. There were significant decreases in NIHSS scores in regard to the MLSM group after 4 weeks (WMD −5.81; 95% CI −7.03 to −4.59; I2 = 0%, P = 0.48) in 2 trials,[11,18] 6 weeks (WMD −2.00; 95% CI −2.80 to −1.20) in 1 trial,[22] 8 weeks (WMD −6.94; 95% CI −7.99 to −5.89; I2 = 0%, P = 0.64) in 2 trials,[16,23] 12 weeks (WMD −5.33; 95% CI −7.57 to −3.09; I2 = 55%, P = 0.13) in 2 trials,[18,24] and for an overall effect observed (WMD −5.34; 95% CI −7.21 to −3.47; I2 = 91%, P < 0.001) (Fig. 10).
Figure 10.
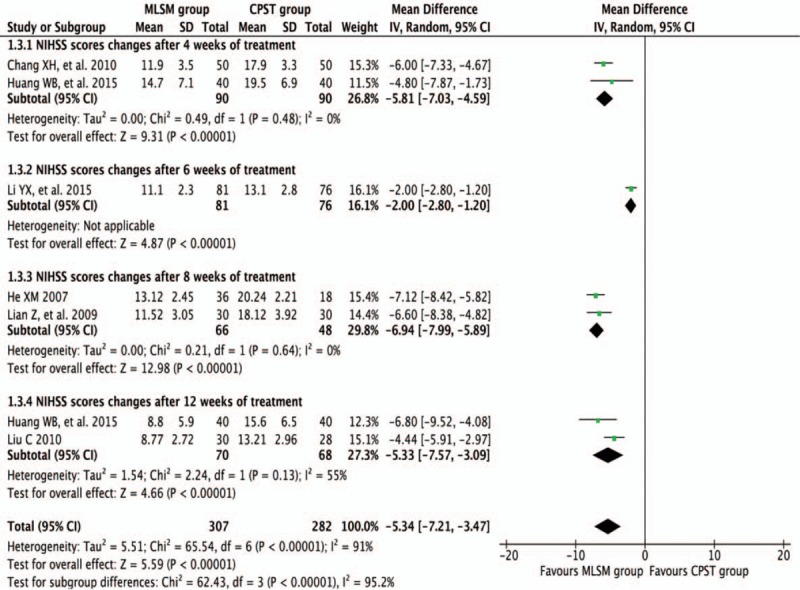
Forest plots showing weighted mean difference with 95% confidence interval for changes of National Institute of Health Stroke Scale comparing with or without medication of liver-soothing-oriented method in a random-effect model.
3.3.3. Score changes of ADL with or without MLSM
Pooled results were calculated using REM model analysis of data from 4 trials[15,22,30,36] that compared MLSM with non-MLSM by assessing differences in mean change from baseline in ADL scores, and subgroup analyses between trials were performed using follow-up durations of 4, 6, and 8 weeks. In comparison to non-MLSM treatment, there were significant improvements in ADL scores observed that were in favor of the MLSM group after 4 weeks (WMD 12.95; 95% CI 10.60–15.30; I2 = 24%, P = 0.25) in 2 trials,[15,36] 6 weeks (WMD 17.00; 95% CI 15.59–18.41) in 1 trial,[22] 8 weeks (WMD 16.09; 95% CI 13.74–18.45; I2 = 0%, P = 0.43) in 3 trials,[15,30,36] and an overall effect observed (WMD 15.02; 95% CI 12.98–17.06; I2 = 62%, P = 0.02) (Fig. 11).
Figure 11.
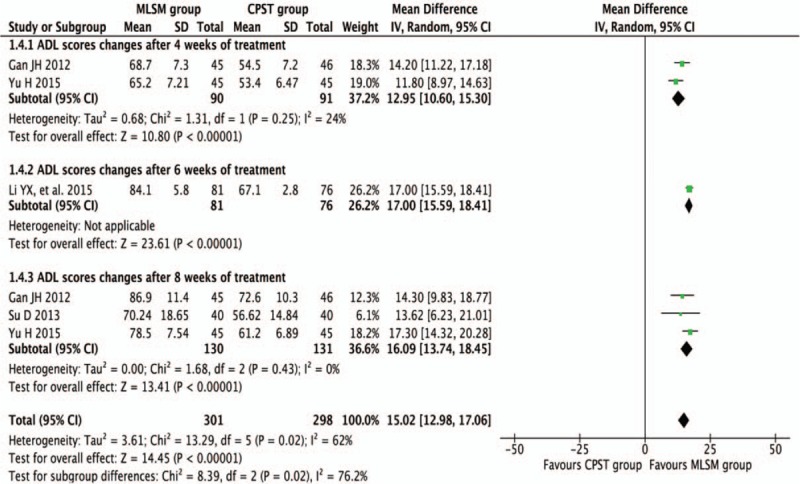
Forest plots showing weighted mean difference with 95% confidence interval for changes of Activities of Daily Living comparing with or without medication of liver-soothing-oriented method in a random-effect model.
3.3.4. Score changes of HAM-A comparing with or without MLSM
Pooled results were calculated using REM model analysis of data from 2 trials[17,34] that compared control versus MLSM treatment by evaluating differences in mean change from baseline in HAM-A scores, and subgroup analyses were conducted between trials that used either 3- or 6-week duration times. In comparison to non-MLSM treatment, there were additive benefits from MLSM in terms of HAM-A scores after 3 weeks (WMD −2.96; 95% CI −4.34 to −1.59; I2 = 0%, P = 0.44) in 2 trials,[17,34] 6 weeks (WMD −2.62; 95% CI −3.74 to −1.50; I2 = 0%, P = 0.86) in 2 trials,[17,34] and for an overall effect observed (WMD −2.76; 95% CI −3.62 to −1.89; I2 = 0%, P = 0.86) (Fig. 12).
Figure 12.
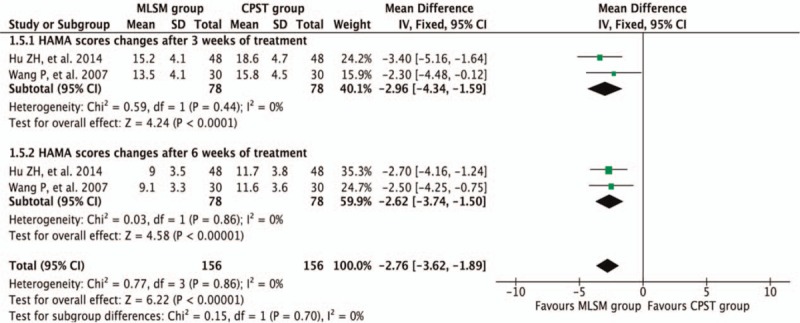
Forest plots showing weighted mean difference with 95% confidence interval for changes of Hamilton Anxiety Scale-A) comparing with or without medication of liver-soothing-oriented method in a fixed-effect model.
3.3.5. Score changes of MESSS with or without MLSM
Two trials[21,40] reported a change in MESSS scores during the treatment periods. Adjunctive treatment with MLSM significantly decreased the MESSS scores (WMD −4.04; 95% CI −5.49 to −2.59; I2 = 0%, P = 0.55) when compared to the conventional treatment alone (Fig. 13).
Figure 13.
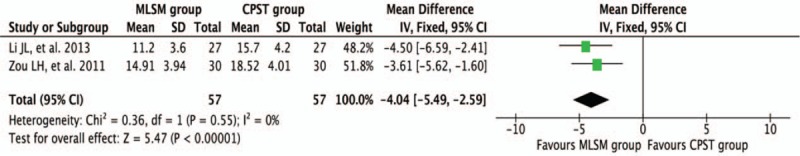
Forest plots showing weighted mean difference with 95% confidence interval for changes of Modified Edinburgh Scandinavian Stroke Scale comparing with or without medication of liver-soothing-oriented method in a fixed-effect model.
3.3.6. Score changes of SAS with or without MLSM
Two trials[14,31] reported a change in SAS scores during the treatment period. The pooled results suggested that changes in SAS scores (WMD −1.82; 95% CI −2.60 to −1.03; I2 = 0%, P = 0.51) were significantly lower in MLSM-treated subjects during the follow-up duration (Fig. 14).
Figure 14.
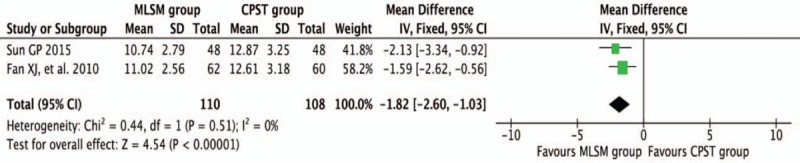
Forest plots showing weighted mean difference with 95% confidence interval for changes of Self-Rating Anxiety Scale comparing with or without medication of liver-soothing-oriented method in a fixed-effect model.
3.3.7. GRADE quality of evidence
GRADEprofiler software was utilized to evaluate the evidence used for this meta-analysis. The quality of present evidence, based on the GRADEprofiler analysis, revealed that the results were “Low/Very low”. This occurred due to relatively high risk of bias between studies and low-quality trials with small sample sizes (Fig. 4).
3.3.8. Adverse events
Four trials[15,18,38,39] reported the safety evaluation as outcome indexes. The Treatment Emergent Symptom Scale was mentioned in 1 trial,[15] while the other 3 trials[18,39] only covered the number of adverse events. For the MLSL treatment group, 1 trial[15] reported 2 cases with dry mouth and 1 case with dizziness. One trial[18] reported 4 cases with loss of appetite and 2 cases with mild diarrhea. One trial[38] reported 5 cases with epigastric discomfort. One trial[39] reported 2 cases with palpitation, 1 case with dry mouth, and 1 case with dizziness. For the conventional pharmacotherapy group, 1 trial[15] reported 5 cases with nausea and vomiting, 3 cases with insomnia, 2 cases with dry mouth, and 2 cases with dizziness. One trial[18] reported 8 cases with stomach upset, 3 cases with dizziness, and 1 case with headache. One trial[38] reported 9 cases with dry mouth, 8 cases with loss of appetite, 5 cases with nausea, 3 cases with fatigue, and 2 cases with headache. One trial[39] reported 3 cases with palpitation, 2 cases with dry mouth, 2 cases with stomach upset, and 2 cases with dizziness. No serious or frequently occurring adverse effects were reported among the literature reviewed.
4. Discussion
The review revealed that adjunctive treatment with MLSM could improve total effective rates, as well as HAM-D score changes in DCVA subjects when compared to non-MLSM treatments. Despite the fact that most of the included literatures were of relatively poor methodological quality, the pooled outcomes suggested beneficial effects of MLSM when combined with conventional pharmacotherapy as demonstrated by score changes in NIHSS, ADL, HAM-A, MESSS, and SAS when comparing to the conventional treatment group. Furthermore, stratified analyses indicated that the positive actions of MLSM regarding score changes of HAM-D, NIHSS, ADL, and HAM-A were found in similar magnitudes among the subgroups. In addition, significant differences were noted in the decreased levels of TNF-α, IL-6, and IL-1β in subjects with or without MLSM.
When a patient with stroke attacks found a higher susceptibility to depression, the etiology and pathology of primary immunodeficiency might be taken into account initially. A vital approach for therapies is to enhance the positive immune responses or restore innate defense mechanisms of patients’ themselves. Immune system modulated through proper channels, to some extents, could decrease the risks of progression and recurrence of target conditions.[7,8,58] In this review, apart from enhancing the total effective rates, and reducing the score changes of HAM-D or other self-rating indexes during the follow-up period, adjunctive treatment with MLSM also obviously lower the levels of TNF-α as well as levels of IL-6 and IL-1β counts, which in turn improved anti-inflammatory actions. Recent literatures claimed that certain domains in MLSM enhanced the immune effects based on subjects’ active self-functions involved immune organs, specific immunity, and nonspecific immunity.[59,60] In all, the findings abovementioned revealed that the antidepressive effects of MLSM might be associated with its immunomodulating effects. Unfortunately, no consensus has been drawn on the levels of immunoglobulin or inflammatory cytokines and conditions of recurrence or symptom enhancement in subjects with DCVA.
Syndrome differentiation and individualized treatment in accordance with diseases patterns are well considered as the most fundamental principles of traditional clinical medicine in China. MLSM is composed of herbal prescription focus on liver soothing, for example, Bupleurum chinense, by certain weight of dried plants or other natural products.[61,62] Syndrome of Liver-qi stagnation is one of the most key manifestations in subjects with DCVA. Despite the additive benefits of MLSM for DCVA, an increasing concern for decision-making of doctors and patients is the possible toxic or side effects of MLSM. In this review, MLSM appeared to be safely applied and widely tolerable for subjects with DCVA. This situation occurred because few studies included reported relevant adverse events during the treatment or follow-up. Instead, there were just 4 studies[15,18,38,39] that mentioned adverse events of MLSM as outcome measurements for safety. Furthermore, combined uses of medicinal products regarding biological and pharmacological activities might produce a series of potentially synergistic actions or side effect–neutralizing responses. Thus, the safety of MLSM calls for further study with strict designs.
There are some potential limitations as follows. First, the methods of randomization could not found details in most the included studies. Second, traditional clinical medicine in China was connected with pattern or syndrome differentiation. However, the studies included did not clarify subjects’ syndrome diagnosis, which could contribute to selection bias of participants. Third, definitions of DCVA are conformed to patients’ reported outcomes of symptoms or other self-rating scales of emotional disorders. Thus, misclassification in a proportion of subjects could not be ruled out. Fourth, previous data on the distribution of apathy, or other similar psychological disorders were not mentioned in the original documents, differences in severity of DCVA could also have biased effects for the reliable results. Last but not the least, substantial heterogeneities were found in the process of pooling results. In the subgroup analyses, treatment periods (dosage of MLSM), follow-up durations, formula of MLSM (herbal decoction, particle, powder, capsule, or oral liquid), and MLSM combined with immunomodulating or other active components only interpreted partial causes of heterogeneity in this review. The subjects’ severity of DCVA, characteristics of each study at baselines, or other potential confounders might result in the additional heterogeneity.
Given the methodological limitations of the studies included, there were some implications of improvement for further research as follows: informative descriptions of study design, methods of randomization, concealment of allocation, and other specified data should be provided; withdrawal, dropout, and adverse effects also should be attempted to clarify; mean number of episode periods, reduction of symptom, inflammatory cytokines, and adverse effects should also be included in the measurement of outcome indexes; and syndrome or pattern differentiation should be taken into account while conducting the diagnostic process, especially MLSM is better matching for the type of Liver-qi stagnation.
5. Conclusion
The review indicates that adjunctive treatment with MLSM could improve symptoms of depressive disorders, enhance immediate response and quality of life in subjects with DCVA. The positive action of MLSM might be potentially connected with its immunoregulating effects. More prospective trials with strict design and larger sample sizes are warranted to clarify its effectiveness and safety.
Acknowledgments
We thank Prof Patrick Wall and other editors/reviewers for the helpful comments and suggestions. We thank LetPub (www.letpub.com) for its linguistic assistance during the proofreading of this manuscript.
Footnotes
Abbreviations: ADL = activities of daily living, CI = confidence interval, DCVA = depression after cerebrovascular accident, DSM = diagnostic and statistical manual of mental disorders, FEM = fixed-effect model, GRADE = grades of recommendations assessment development and evaluation, HAM-A = Hamilton Anxiety Scale, HAM-D = Hamilton Depression Scale, ICD = international classification of diseases, IL-1β = interleukin-1β, IL-6 = interleukin-6, MESSS = Modified Edinburgh Scandinavian Stroke Scale, MLSM = medication of liver-soothing-oriented method, NIHSS = National Institute of Health Stroke Scale, RCTs = randomized controlled trials, REM = random-effects model, SAS = Self-Rating Anxiety Scale, TNF-α = tumor necrosis factor-α, WMD = weighted mean difference.
L-FZ and W-XL have contributed equally to the article.
Author's contribution: QW and Z-PL conceived and designed the study. The literature searches, trial selection, critical appraisal, data extraction, as well as contacting authors for additional data were conducted and performed by L-FZ, J-CL, X-YC, W-YD, K-ZW, and C-RM. L-FZ, YC, and LW carried out analysis and interpretation of the data. L-FZ, W-XL, and N-SW drafted the article. L-FZ and QW further revised the manuscript. The final version of the manuscript was read and approved by QW, Z-PL, L-FZ, J-CL, X-YC, W-YD, YC, LW, C-RM, K-ZW, W-XL, and N-SW.
The work was supported by National Natural Science Foundation of China (no. 81373883), the Workshop/Studio of Ling-Nan Needle-Medicine of Mutual Reinforcement School (Construction Project of Ling-Nan Zhen-yao Xiang-xu Liu-pai); the Cultivation Project for Excellent Doctoral Dissertation of Guangdong Province (no. A1-AFD015141Z10504), National Science and Technology Major Project (no. 2012ZX09303009-003), Characteristic Key Discipline Construction Fund of Chinese Internal Medicine of Guangzhou University of Chinese Medicine and South China Chinese Medicine Collaborative Innovation Center (no. A1-AFD01514A05).
The authors have no conflicts of interest to disclose.
References
- 1.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry 2016; 173:221–231. [DOI] [PubMed] [Google Scholar]
- 2.Hachinski V. Post-stroke depression, not to be underestimated. Lancet 1999; 353:1728. [DOI] [PubMed] [Google Scholar]
- 3.Huang BY, Liao ZA, Fang MH. The clinical research overview of post stroke depression. Chin J Phys Med Rehabil 2005; 27:315–317. [Google Scholar]
- 4.Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol 2008; 164:837–840. [DOI] [PubMed] [Google Scholar]
- 5.Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002; 52:253–264. [DOI] [PubMed] [Google Scholar]
- 6.Allan LM, Rowan EN, Thomas AJ, et al. Long-term incidence of depression and predictors of depressive symptoms in older stroke survivors. Br J Psychiatry 2013; 203:453–460. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Ling S, Yang Y, et al. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett 2014; 35:104–109. [PubMed] [Google Scholar]
- 8.Fang J, Cheng Q. Etiological mechanisms of post-stroke depression: a review. Neurol Res 2009; 31:904–909. [DOI] [PubMed] [Google Scholar]
- 9.Han H, Wu LM, Yang WM, et al. Characteristics of traditional Chinese medicine syndromes in post-stroke depression. J Chin Integr Med 2010; 8:427–431. [DOI] [PubMed] [Google Scholar]
- 10.Xia JB, Li NN. Treatment progress in post-stroke depression. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology 2015; 27:1734–1738. [Google Scholar]
- 11.Chang XH, Zhang LZ. Fifty cases study on Chai-hu Shu-gan power treating post-stroke depression. Liaoning J Tradit Chin 2010; 37:1973–1974. [Google Scholar]
- 12.Chen W. Clinical observation on Shu-gan Jie-yu capsule in the treatment of post-stroke depression. J Med Theor Prac 2014; 27:2005–2006. [Google Scholar]
- 13.Du H. Observation of the curative effect of radix bupleuri liver powder in the treatment of depression after cerebral apoplexy for 40 cases. J Qiqihar Univ Med 2013; 34:2247–2248. [Google Scholar]
- 14.Fan XJ, Chang YF. Sixty-two cases study on post-stroke depression treated by Shu-gan Jie-yu decoction combined with fluoxetine. Shaanxi J Tradit Chin Med 2010; 31:678–679. [Google Scholar]
- 15.Gan JH. Efficacy analysis of treating 45 cases of post-stroke depression with Shu-gan Jie-yu decoction plus fluoxetine. Clin J Chin Med 2012; 4:14–15. [Google Scholar]
- 16.He XM, Zhang YK. Thirty-six cases study on post-stroke depression treated by modified powder of Chai-hu Shu-gan combined with fluoxetine. J N Chin Med 2007; 39:22–23. [Google Scholar]
- 17.Hu ZH, Lan P. Clinical study on effects of Chai-hu Jie-yu decoction combined with deanxiton for post-stroke depression. Pract Clin J Integr Tradit Chin West Med 2014; 14:35–36. [Google Scholar]
- 18.Huang WB, Zeng YQ. Clinical observation on Chai-hu Shu-gan powder combined with fluoxetine in the treatment of post-stroke depression. Guiding J Tradit Chin Med Pharm 2015; 21:79–81. [Google Scholar]
- 19.Huang YS, Luo RH, Yuan ZX. Clinical study on modified powder of Chai-hu Shu-gan treating post-stroke depression. J Pract Tradit Chin Intern Med 2012; 26:36–37. [Google Scholar]
- 20.Li FC. Clinical research on patients with post-stroke depression treated by Chai-hu Shu-gan san combined with fluoxetine. Clin J Tradit Chin Med 2012; 24:730–731. [Google Scholar]
- 21.Li JL, Mou K. Study on post-stroke depression treated through integrated Chinese and Western medicine. Pract J Card Cereb Pneumal Vasc Dis 2013; 21:57–58. [Google Scholar]
- 22.Li YX, Shen Y, He WT, et al. Clinical observation on patients with post-stroke depression treated by Shu-gan Jie-yu capsule. Ningxia Med J 2015; 37:58–59. [Google Scholar]
- 23.Lian Z, Wu Q, Tian KW. Efficacy analysis of post-stroke depression treated with Chai-hu Shu-gan san plus fluoxetine. Guangming J Chin Med 2009; 24:101–102. [Google Scholar]
- 24.Liu C. Thirty cases of post-stroke depression treated with modified powder of Chai-hu Shu-gan. Shaanxi J Tradit Chin Med 2010; 31:1315–1316. [Google Scholar]
- 25.Liu N, Ma L. Study on patients with post-stroke depression treated by modified powder of Chai-hu Shu-gan combined with fluoxetine. Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease 2007; 5:894–895. [Google Scholar]
- 26.Liu YJ, Su LH, Wang SM. Clinical observation on modified powder of Chai-hu Shu-gan plus fluoxetine in the treatment of post-stroke depression. Chin J Integr Med Cardio/Cerebrovasc Dis 2011; 9:182–183. [Google Scholar]
- 27.Liu ZB. Clinical observation on treating 65 cases of post-stroke depression with Chai-hu Shu-gan san plus fluoxetine capsules. Clin J Chin Med 2015; 7:54–56. [Google Scholar]
- 28.Ma CM, Zhu LT, Cui SK. Study on post-stroke depression treated with liver-soothing Jie-yu decoction plus deanxit. Biotech World 2015; 10:137–139. [Google Scholar]
- 29.Shen JL. Thirty cases of post-stroke depression treated by Chai-hu Shu-gan decoction combined with deanxit. J Pract Tradit Chin Med 2009; 25:392–393. [Google Scholar]
- 30.Su D. Clinical study on 40 cases with post-stroke depression treated by modified powder of Chai-hu Shu-gan. Guangming J Chin Med 2013; 28:2554–2555. [Google Scholar]
- 31.Sun GP. Clinical observation on depression patients after stroke treated by Shu-gan Jie-yu decoction. Guangming J Chin Med 2015; 30:2606–2607. [Google Scholar]
- 32.Tan LR. Research on fluoxetine plus Chai-hu Shu-gan san in the treatment of post-stroke depression. Chin J Integr Med Cardio/Cerebrovasc Dis 2005; 3:182. [Google Scholar]
- 33.Wang GL, Jin YB. Clinical observation on patients with post-stroke depression treated by Chai-hu Shu-gan powder combined with paroxetine. Chin J Integr Med Cardio/Cerebrovasc Dis 2009; 7:1428–1429. [Google Scholar]
- 34.Wang P, Li X, Lou T, et al. Thirty cases patients with post-stroke depression treated by Shu-gan Jie-yu decoction plus clomipramine. Shaanxi J Tradit Chin Med 2007; 28:666–667. [Google Scholar]
- 35.Xu H, Chen JH, Zhang HM. Clinical study on post-stroke depression treated with Jie-yu Shu-gan plus fluoxetine. J Emerg Tradit Chin Med 2015; 24:367–368. [Google Scholar]
- 36.Yu H. Efficacy analysis of Shu-gan Jie-yu capsules plus routine medication in the treatment of post-stroke depression. Chin J Pract Neruous Dis 2015; 18:121–122. [Google Scholar]
- 37.Zhang NY. Clinical observation on post-stroke depression treated with Chai-hu Shu-gan powder. J Zhejiang Chin Med Univ 2013; 37:566–567. [Google Scholar]
- 38.Zhang T. Clinical research of Chai-hu Shu-gan san joint prozac in the treatment of depression after stroke. Chin J Chin Med 2011; 26:1350–1351. [Google Scholar]
- 39.Zheng AJ, Zhao F, Zheng SP. Clinical observation of curative effect on Shu-gan Jie-yu capsules combined with paroxetine in the treatment of post-stroke depression. Guide Chin Med 2014; 12:26–28. [Google Scholar]
- 40.Zou LH, Li H, Zeng ZQ, et al. Chai-hu Shu-gan powder combined with venlafaxine for treatment of post-stroke depression. Chin J Integr Med Cardio/Cerebrovasc Dis 2011; 9:1330–1332. [Google Scholar]
- 41.Shen XM, Han N, Ma YZ, et al. The effect of Shu-yu granules on monoamine neurotransmitters and inflammatory factors in hippocampus in post-stroke depression rats. Chin J Exp Tradit Med Formulae 2011; 17:177–1780. [Google Scholar]
- 42.Jin L, Yang QH, Zhang YP, et al. Effects of soothing liver recipes of Chinese herbal medicine on hepatic inflammatory cytokines (TNF-α, IL-6 and IL-1) of rats with nonalcoholic steatosis hepatitis. J Jinan Univ Nat Sci Med 2012; 33:161–166. [Google Scholar]
- 43.Li XH. Study on correlation between brain derived neurotrophic factor and inflammatory factor in patients with post-stroke depression. Lab Med Clin 2014; 11:1410–1411. [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20:864–870. [DOI] [PubMed] [Google Scholar]
- 47.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 3:179–186. [PubMed] [Google Scholar]
- 48.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959; 1:50–55. [DOI] [PubMed] [Google Scholar]
- 49.Scandinavian Stroke Study Group. Multicenter trial of hemodilution in ischemic stroke—background and study protocol. Stroke 1985; 16:885–890. [DOI] [PubMed] [Google Scholar]
- 50.Zung WW. A rating instrument for anxiety disorders. Psychosomatics 1965; 12:371–379. [DOI] [PubMed] [Google Scholar]
- 51.Slee VN. The international classification of diseases: ninth revision (ICD-9). Ann Intern Med 1978; 3:424–426. [DOI] [PubMed] [Google Scholar]
- 52.Brämer GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q 1988; 41:32–36. [PubMed] [Google Scholar]
- 53.Chen YF. Chinese classification of mental disorders (CCMD-3): towards integration in international classification. Psychopathology 2002; 35:171–175. [DOI] [PubMed] [Google Scholar]
- 54.Kasahara Y. DSM-III (diagnostic and statistical manual of mental disorders, the 3d edition), a new diagnostic criteria in the United States. Seishin Shinkeigaku Zasshi 1981; 10:607–611. [PubMed] [Google Scholar]
- 55.Kendell RE. Diagnostic and statistical manual of mental disorders, 3rd ed., revised (DSM-III-R). Am J Psychiatry 1988; 145:1301–1302. [Google Scholar]
- 56.Bell CC. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA 1994; 272:828–829. [Google Scholar]
- 57.First MB. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-V). J Nerv Ment Dis 2013; 201:727–729. [DOI] [PubMed] [Google Scholar]
- 58.Swardfager W, Winer DA, Herrmann N, et al. Interleukin-17 in post-stroke neurodegeneration. Neurosci Biobehav Rev 2013; 37:436–447. [DOI] [PubMed] [Google Scholar]
- 59.Qian RQ, Zhang CY, Yang Y. Comparative study on effects of liver-soothing Chinese medicinals on immune functions in stressed mice. Chin Tradit Pat Med 2000; 22:645–647. [Google Scholar]
- 60.Yang DH, Li JB, Zheng AH, et al. Changes of T cellular immune function in the rats with liver-qi stagnation syndrome and effect of powder of Chai-hu Shu-gan on it. J Sichuan Tradit Chin Med 2006; 24:14–16. [Google Scholar]
- 61.Law BY, Mo JF, Wong VK. Autophagic effects of Chai-hu (dried roots of Bupleurum Chinense DC or Bupleurum scorzoneraefolium WILD). Chin Med 2014; 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeung WF, Chung KF, Ng KY, et al. Prescription of Chinese herbal medicine in pattern-based traditional Chinese medicine treatment for depression: a systematic review. Evid Based Complement Alternat Med 2015; 15:160189. [DOI] [PMC free article] [PubMed] [Google Scholar]


