Abstract
Serum bile acids (BA) reference values are lacking for neonates. Therefore, this study aimed to determine serum BA reference values in term and preterm neonates. Furthermore, as serum BA concentrations are well-known to rise in septic adults, BA values were determined in early-onset neonatal sepsis (EOS), a common and serious disease in neonates.
Using high-performance liquid chromatography–high-resolution mass spectrometry (HPLC-HRMS), we profiled serum BA in 236 infants, including healthy term neonates (n = 84), premature infants (n = 101), and both term infants (n = 35) and preterm infants (n = 16) with EOS. We examined the impact of prematurity and EOS on BA concentrations.
The median reference values of serum BA were 8.0 μmol/L, interquartile range (IQR): 4.6 to 12.9, in healthy term neonates and 10.1 μmol/L, IQR: 5.7 to 15.7, in preterm neonates. Neonates with EOS had significantly lower median BA values, term (4.7 μmol/L, IQR: 2.7–7.6; P < 0.01) as well as preterm (6.4 μmol/L, IQR: 3.5–8.4; P < 0.01). Furthermore, primary and conjugated BA were most abundant in all groups. Taurine-conjugated BA were predominant in all neonates; glycine-conjugated BA were significantly lower in term neonates with EOS than in controls (P < 0.05). Multivariate regression analysis results obtained for BA and inflammatory parameters revealed that BA are an independent factor associated with EOS.
This is the first study to determine standard value ranges of serum BA in neonates using HPLC-HRMS. In contrast to adults with sepsis, neonates suffering from EOS exhibit significantly lower BA values than do controls of the same gestational age. These data suggest BA as a supplementary parameter within a panel of biomarkers for EOS in the future.
Keywords: bile acids, early-onset neonatal sepsis, prematurity
1. Introduction
Total bile acids (BA) in serum include several BA, principally cholic acid (CA) and chenodeoxycholic acid (CDCA), the primary BA, which are synthesized in the liver from cholesterol. Following conjugation with the amino acids taurine (T) or glycine (G), BA are transported into the intestine via the bile ducts. In the terminal ileum, BA are actively reabsorbed, returning to the liver via the portal circulation. Thus, BA mostly remain in an enterohepatic circulation (EHC).[1,2] Only 5% of primary BA reach the colon, where bacteria convert them to secondary BA, forming deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA).[3] Most secondary BA are also reabsorbed, reach the liver, and enter the EHC. A small fraction of circulating BA spills over into the systematic circulation. This small spillover of BA can be quantitated and characterized in serum samples by methods featuring high-performance liquid chromatography–high-resolution mass spectrometry (HPLC-HRMS) within hours. The results of such studies permit insight into the role of BA in neonatal physiology and pathology, an area increasingly of clinical interest.[4,5] Although peripheral serum BA reference values exist for adults, children, and adolescents, exact data for neonates using modern measuring methods have not been published.[6,7]
Early-onset neonatal sepsis (EOS) is a severe illness with a mortality rate ranging from 2% to 3% in term neonates to 20% to 30% in preterm neonates.[8,9] The signs and symptoms of EOS are nonspecific, including fever or hypothermia, respiratory distress, and lethargy.[10] Levels of various cytokines, either pro- or anti-inflammatory, have been tested as laboratory biomarkers of EOS. However, no reliable sensitive inflammatory biomarker has been found yet in ongoing research to improve timely diagnosis of EOS. Interestingly, sepsis in adults leads to alterations of serum BA levels due to sepsis-induced cholestasis.[11] Therefore, serum BA concentrations (“BA levels”) are used in adults as biomarkers for disturbed EHC secondary to intestinal, hepatic, or infectious disorders.[12–16] Until now, it is unclear if sepsis in neonates such as EOS also leads to changes in serum BA.
We sought to determine reference values of serum BA levels prospectively in healthy term and preterm neonates of varying gestational ages using HPLC-HRMS. Furthermore, we investigated BA changes in term and preterm neonates suffering from EOS.
2. Patients and methods
2.1. Study design and patient characteristics
We prospectively studied neonates without disordered EHC at the Department of Pediatrics and Adolescent Medicine from March 2013 until May 2015. The clinical study was approved by the Medical University of Graz ethics committee (24–549 ex 11/12 and 26–215 ex 13/14). Parental consent was obtained for each subject. All neonates (term and preterm) born at the Medical University of Graz ages 1 to 3 days were included. Neonates of <37 weeks’ GA were considered preterm. Exclusion criteria comprised elevated serum transaminase activity levels, primary hepatic diseases, asphyxia, or death within 1 month after birth. In controls, EOS was excluded. The layout of the study groups is displayed in Fig. 1.
Figure 1.
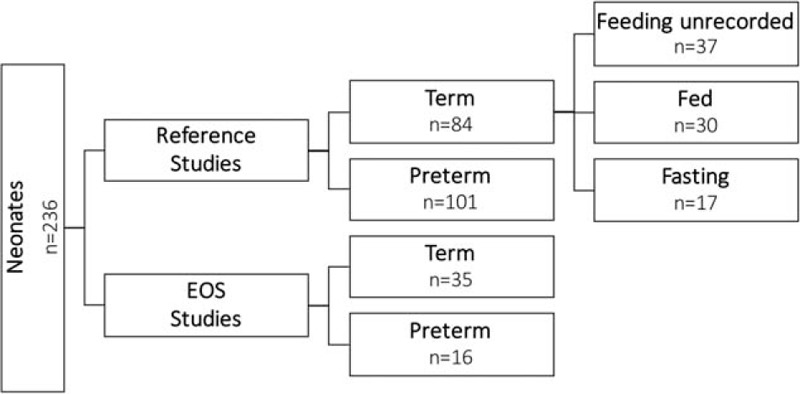
Overview of groups and numbers of overall studied neonates. Neonates were divided into a reference study group and an early-onset neonatal sepsis study group, and further divided into term and preterm. In a part of the healthy term group, feeding status was recorded.
2.2. Early-onset neonatal sepsis
In EOS studies, only neonates with proven EOS were included. Diagnosis was confirmed by successful culture of microorganisms from blood, cerebrospinal fluid, or tracheal aspirate. In cases of clinically suspected sepsis, C-reactive protein (CRP) concentrations were above 5 mg/L during the first 72 h of life. Concentrations of CRP in serum, of procalcitonin (PCT) and interleukin 6 (IL-6) in cord blood, and of bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GGTP) in serum were measured by standard laboratory methods.
2.3. Sample collection
Obtaining blood solely to define reference values of serum BA in neonates is ethically moot. Hence, blood sampling in healthy term neonates was performed during routine screening for phenylketonuria. Fasting blood sample collection in neonates is difficult, since neonates are fed at least every 4 h. However, feeding status was identified retrospectively when possible (Table 1). Patients not fed within 2 h before blood sampling were considered “fasted.” In the EOS group, blood samples were collected within 24 h after first clinical signs of EOS and before first antibiotic administration. All serum samples were stored at −80°C until batch analysis.
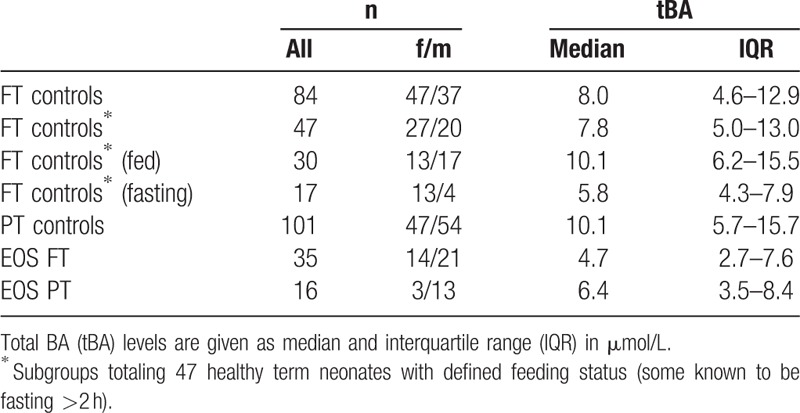
Table 1.
Serum BA concentrations (tBA levels) in healthy term neonates (FT controls; ≥37 weeks gestational age), healthy preterm neonates (PT), PT neonates with early-onset sepsis (EOS), and FT neonates with EOS; all neonates age 1 to 3 days.
2.4. Bile acids analysis
Unconjugated and T- and G-conjugated BA were determined using HPLC-HRMS, including CA, CDCA, LCA, DCA, and UDCA and their conjugates TCA, TCDCA, TLCA, TDCA, TUDCA, GCA, GCDCA, GLCA, GDCA, and GUDCA. Before tandem mass spectrometry analysis (Q Exactive MS/MS; Thermo Fisher Scientific, Waltham, MA) serum was mixed with acetonitrile and target analytes were separated by HPLC on a reversed phase (C18) column using a methanol/water gradient and d4 deuterated internal standards for quantification.
2.5. Statistical analysis
Patient characteristics and biochemical variables are presented as median and interquartile range (IQR). Nonparametric tests (Mann–Whitney U test) were performed when data were not normal-distributed. Correlations between BA and liver parameters were assessed using Spearman correlation analysis. Multivariate regression analysis was used on combined results for BA and inflammation parameters to identify variables independently associated with EOS. All statistical tests were 2-tailed and P values of <0.05 were considered statistically significant. The SPSS Statistics Package 23.0.0 (IBM SPSS, Armonk, NY) was used for all analyses.
3. Results
3.1. Reference values of serum bile acids depend on feeding status
Reference values for serum BA in healthy term neonates were defined in a group of 84 participants (Fig. 1). The median BA levels were 8.0 μmol/L (IQR: 4.6–12.9; Table 1). Influence of fasting condition was determined in 47 healthy term neonates (median BA levels 7.8 μmol/L; IQR: 5.0–13.0); in 37 cases the feeding status was unclear. Interestingly, BA values differed significantly between the 2 feeding statuses: Levels were significantly higher in the fed group (n = 30; 10.1 μmol/L; IQR: 6.2–15.5) than in the fasting group (n = 17; 5.8 μmol/L; IQR: 4.3–7.9; P < 0.01; Fig. 2A).
Figure 2.
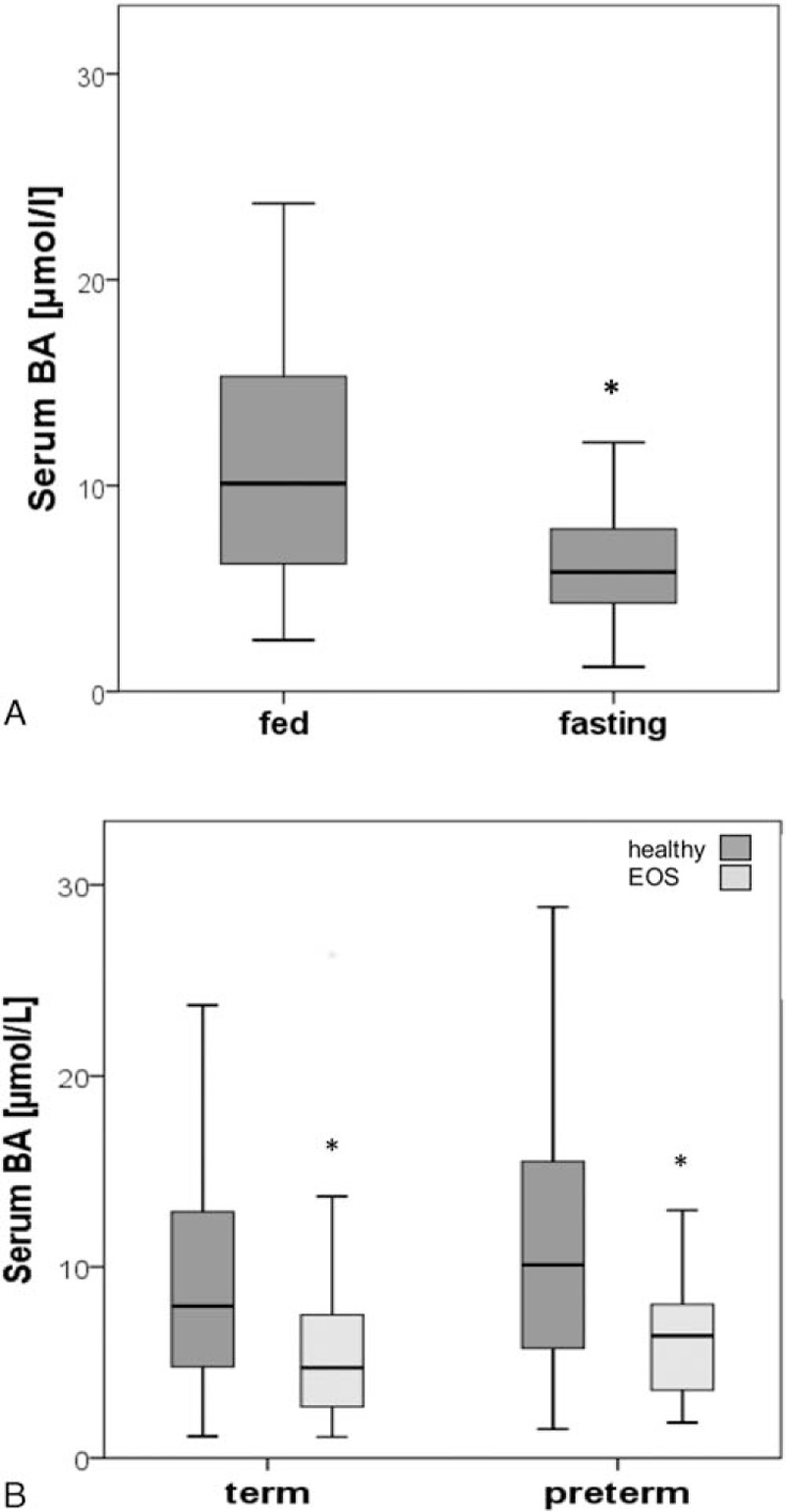
Influence of fasting condition on serum BA levels was determined in 47 healthy term neonates (A); levels were significantly higher in the fed group (n = 30) than in the fasting group (n = 17; ∗P < 0.01). In (B), serum BA levels were determined in healthy term neonates (n = 84) and preterm neonates (n = 101), term neonates with EOS (n = 35), and preterm neonates with EOS (n = 16). Bile acids values were significantly decreased in both term and preterm neonates with EOS compared to term and preterm controls (∗P < 0.01), respectively. BA = bile acids, EOS = early-onset neonatal sepsis.
3.2. Reference values of serum bile acids levels are similar in term and preterm neonates
In a second step, we determined if prematurity influences BA levels. Reference values for serum BA in healthy preterm neonates were defined in a group of 101 participants and compared to those in healthy term neonates. Feeding status in all preterm neonates was defined as “fed” since they were fed regularly. Bile acids levels in preterm neonates were similar to fed term controls (10.1 μmol/L; IQR: 5.7–15.7 vs 10.1 μmol/L; IQR: 6.2–15.5, respectively).
3.3. Serum bile acids levels are significantly decreased in term neonates with early-onset neonatal sepsis
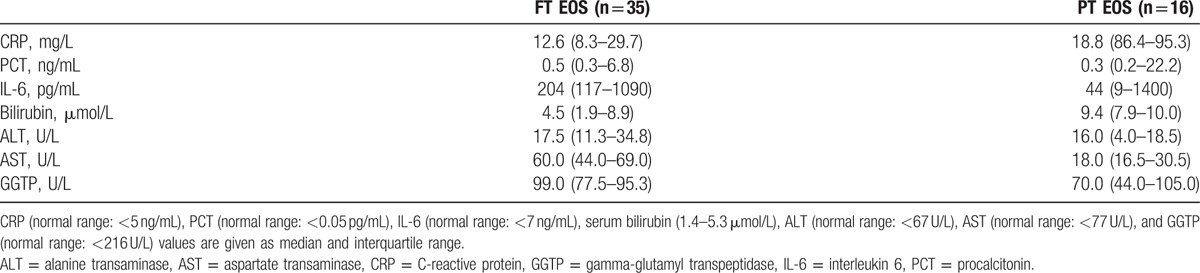
Furthermore, we aimed to study alterations of BA levels in septic neonates, since BA levels are elevated in septic adults. Thirty-five term neonates with EOS presented with inflammation marker concentrations above normal ranges, including CRP at 12.6 mg/L (IQR: 8.3–29.7), PCT at 0.5 ng/mL (IQR: 0.3–6.8), and IL-6 at 204 pg/mL (IQR: 117–1090). Median levels of total serum bilirubin—a marker of cholestasis—were within the normal range at 4.5 μmol/L but 20% were above the reference range (IQR: 1.9–8.9). Serum activities of ALT, AST, and GGTP were within the normal range at 17.5 U/L (IQR: 11.3–34.8), 60.0 U/L (IQR: 44.0–69.0), and 99.0 U/L (IQR: 77.5–95.3), respectively. All laboratory parameters and reference ranges are listed in Table 2. Interestingly, serum BA levels were significantly lower in term neonates with EOS than in term controls (4.7 μmol/L; IQR: 2.7–7.6 vs 8.0 μmol/L; IQR: 4.6–12.9, P < 0.01; Fig. 2B).
Table 2.
Laboratory values in term (FT) and preterm (PT) neonates with diagnosis of early-onset sepsis (EOS).
3.4. Serum bile acids levels are lower in preterm neonates with early-onset neonatal sepsis than in healthy preterm neonates
We investigated if and to which extent EOS in combination with prematurity affects BA levels. Bile acids levels were determined in 16 preterm neonates with diagnosed EOS (Table 1). These neonates presented with elevated concentrations of CRP at 18.8 mg/L (IQR: 6.4–95.3), PCT at 0.3 ng/mL (IQR: 0.2–22.2), and IL-6 at 44 pg/mL (IQR: 9–1400) as well as elevated concentrations of bilirubin at 9.4 ng/mL (IQR: 7.9–10.0) (Table 2). Liver and biliary enzymes ALT, AST, and GGTP were within the reference range at 16.0 U/L (IQR: 4.0–18.5), 18.0 U/L (IQR: 16.5–30.5), and 70.0 U/L (IQR: 44.0–105.0), respectively. Interestingly, BA levels in preterm neonates with EOS were significantly lower than in preterm controls (6.4 μmol/L [IQR: 3.5–8.4] vs 10.1 μmol/L [IQR: 5.7–15.7]; P < 0.01; Fig. 2B]).
3.5. Serum bile acids levels are independently associated with early-onset neonatal sepsis
No correlation was found between BA and liver parameters ALT (ρ = −0.22; P = 0.40), AST (ρ = −0.11; P = 0.68), and GGTP (ρ = −0.27; P = 0.29). Moreover, multivariate regression analysis of BA, CRP, PCT, IL-6, and bilirubin revealed that serum BA levels are an independent factor associated with EOS (r = 0.85, P = 0.11); BA are not to be predicted by CRP (P = 0.14), PCT (P = 0.56), IL-6 (P = 0.34), and bilirubin (P = 0.18).
3.6. Early-onset neonatal sepsis influences bile acids levels but not bile acids composition
The primary conjugated BA TCA, TCDCA, GCA, and GCDCA were the most abundant BA, and T-conjugates predominated over G-conjugates in all 4 groups (Fig. 3). Secondary and unconjugated BA levels did not exceed 0.1 μmol/L. Bile acids profiles of EOS patients and controls were similar in the term and preterm groups. However, low BA levels in term neonates with EOS were due to a significant decrease in G-conjugated BA (P < 0.05). Differences in both T- and G-conjugates between healthy and EOS preterm neonates were not significant. Only minor differences in BA profiles were observed in healthy term neonates between fed and fasting conditions; unconjugated BA were slightly higher in fed status than in fasting (9% vs 3%, respectively).
Figure 3.
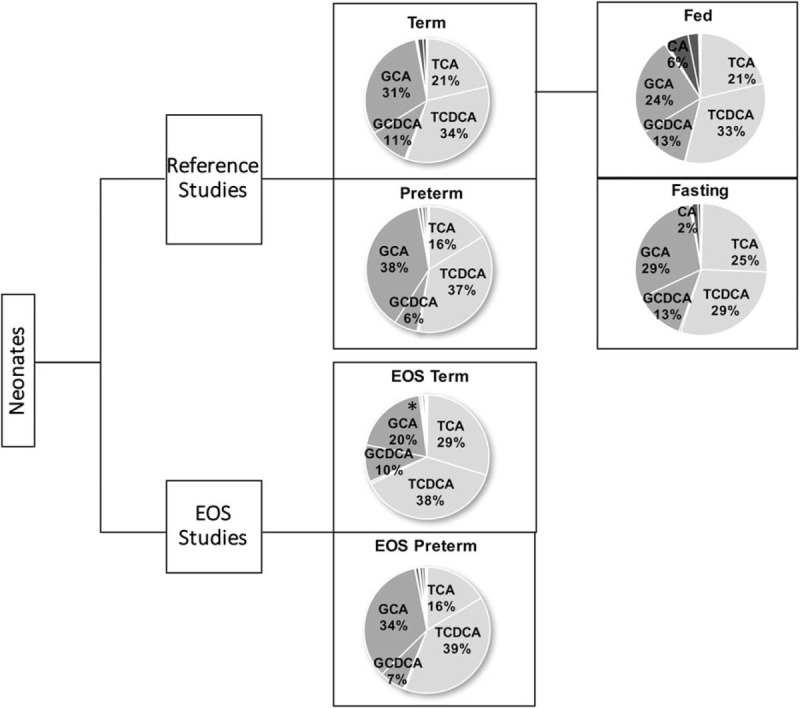
BA profiles of both healthy and EOS neonates. T-conjugates outweighed G-conjugates in healthy term and preterm neonates. TCA (taurocholic acid), TCDCA (taurochenodeoxycholic acid), GCA (gylchocholic acid), and GCDCA (glycodeoxycholic acid) were the most frequent BA in all groups. In term neonates with EOS T-conjugates outweighed G-conjugates, which were significantly (∗P < 0.05) lower in EOS neonates than in term controls. In preterm neonates with EOS T-conjugates outweighed G-conjugates and BA profile was similar to that in preterm controls. In all conditions, unconjugated and secondary BA constituted <10% of total BA. BA = bile acids, EOS = early-onset neonatal sepsis.
4. Discussion
In this study, we measured reference serum BA values (BA levels) in healthy term and preterm neonates within 72 h after birth using highly sensitive HPLC-HRMS to determine if prematurity affects BA levels in neonates. In addition, we sought to investigate whether EOS affects BA levels in term and preterm neonates. Finally, we compared BA composition between selected groups. We found that BA levels were significantly lower in both term and preterm neonates with EOS than in healthy age-matched controls. We also found significant differences in G-conjugates between BA profiles of term neonates with EOS and healthy term neonates, but not between BA profiles of preterm neonates with EOS and healthy preterm neonates.
We initially found that serum BA levels are higher in healthy term neonates than in healthy adults (0.28–6.50 μmol/L).[6] High levels of serum BA in neonates have been reported previously by Polkowska et al measured by enzymatic-colorimetric test, with peak values of 22.2 ± 5.1 μmol/L at the age of 1 month, and Niijima et al, who measured serum BA levels between the ages of 0 and 4 weeks (11.0 ± 8.7 μmol/L) by HPLC.[17,18] In our study, serum BA levels were measured using HPLC-HRMS, a state-of-the-art method that has numerous advantages, including substantially higher sensitivity, selectivity, and reproducibility resulting in better comparison between laboratories, and higher validity of our standard values. We also showed that in healthy term neonates postprandial BA levels were significantly higher than fasting levels (>2 h fasting). This accords with the work of LaRusso et al[19] in adults, who found peak levels of BA and conjugates 90 min after a meal. Suchy et al[20] reported high BA levels in term neonates; they postulated that frequent food intake induces de novo BA synthesis and stimulates enterohepatic BA circulation.
Secondly, we found that BA levels do not differ between those born at term and those born preterm. (Since preterm neonates are fed regularly their levels were compared to BA levels of fed term neonates.) During this study we also investigated the influence of GA on BA levels. We observed a trend of higher BA levels in preterm neonates born before 29 weeks; however, this trend did not achieve statistical significance due to a relatively wide range and small group sizes (data not shown). In summary, BA levels are similar in neonates born term and preterm, but are higher than reference values in adults.[6] Despite a lower BA synthesis rate and a decreased BA pool size, serum BA levels were reported to be increased in healthy term and preterm neonates, and may reach values as high as found in adults with clinical cholestasis.[20] The elevated serum BA levels during this period are termed physiological cholestasis and are ascribed to poor hepatic extraction of bile salts from the portal circulation. An improvement in the hepatic uptake of BA occurs over the first years of life and corresponds to a decrease in the peripheral serum BA levels during childhood.[7]
Thirdly, we found that BA levels in term and preterm neonates suffering from EOS were significantly lower than in age-matched controls. Given that the liver plays a major role in host defense against bacterial infection and sepsis this might be caused by impaired hepatic BA synthesis, conjugation, or secretion.[21] Interestingly, liver and biliary parameters were within the normal range in all patients with EOS and no correlation was observed between BA and liver parameters arguing against liver damage as underlying cause for lower BA levels. Furthermore, when evaluated in multivariate regression analysis with other inflammation parameters, serum BA levels were identified as an independent factor associated with EOS which emphasizes the role of serum BA measurements in this patient group. Also of interest is that our study results are not in agreement with the behavior of BA levels in septic adults; these increase (sepsis-induced cholestasis).[11] BA levels in our patients were measured early in sepsis, whereas BA levels in septic adults may be measured only when sepsis is advanced, with frank icterus among its manifestations. Lipopolysaccharide, a substance found in the walls of bacteria, can affect BA metabolism: Cholesterol-7-alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in BA synthesis, is repressed in C57BL/6 mice and Wistar rats at the transcriptional level by lipopolysaccharide treatment. Under normal conditions, hydrophobic BA repress CYP7A1 transcription via binding to the farnesoid X receptor and interaction with the BA response element II in the CYP7A1 promoter.[22–24] Hepatobiliary alterations during sepsis, thus may share pathways that mediate negative feedback regulation of CYP7A1 by BA, including those involving farnesoid X receptor. We hypothesize that this is the predominant mechanism at an early stage of sepsis, resulting in lower BA levels as seen in neonatal EOS. However, with progressing inflammation a severe cholestasis might shift this balance to increased BA levels, as reported in adult sepsis.
Our fourth finding is that BA profiles in neonates differ substantially from those in healthy adults, where G-conjugates are the predominant species.[6] In our neonatal patients, TCA, TCDCA, and GCA were the 3 most abundant BA, whereas unconjugated and secondary BA were present only in low concentrations. Our findings accord with results obtained by Barbara et al, who reported high primary BA levels in serum of fetuses and neonates determined by radioimmunoassay with CA as the predominant BA in term neonates.[25] Low serum concentrations of secondary BA in infancy might be explained by immaturity of the colonic microbiome, which develops within the first year.[26] Of further note are the high concentrations of T-conjugates in neonates. This may be nutrition-determined: T is the most abundant free amino acid in breast milk and, at present, is a standard supplement in formula milk.[27] Indeed, we found no differences in BA profiles between neonates fed breast milk and neonates fed formula (data not shown). Interestingly, we observed a drop in G-conjugated BA levels in term neonates diagnosed with EOS, but not in preterm neonates. We theorize that at lower GA the liver may not be able to respond to EOS with a drop in G conjugation. In term neonates with a more mature liver, however, hepatic BA transporters might handle G-conjugates during sepsis differently from T-conjugates, altering relative proportions of the 2 in nonportal serum.
Our findings support the concept that BA levels and profiles vary among neonates, infants, children, and adults. They suggest that significantly decreased serum BA levels and shifts in BA profiles mark EOS. Since they are an independent factor associated with EOS, serum BA levels could be considered as a supplementary parameter within a panel of biomarkers for EOS in both term and preterm neonates in the future. This possibility awaits clarification by further studies.
Acknowledgments
The excellent technical assistance of Maria Schäffer is gratefully acknowledged. The authors thank A.S. Knisely for helpful comments on the manuscript and Heidrun Hargassner for assistance.
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase, BA = bile acids, CA = cholic acid, CDCA = chenodeoxycholic acid, CRP = C-reactive protein, CYP7A1 = cholesterol-7-alpha-hydroxylase, DCA = deoxycholic acid, EOS = early-onset neonatal sepsis, G = glycine, HPLC-HRMS = high-performance liquid chromatography–high-resolution mass spectrometry, IL-6 = interleukin 6, LCA = lithocholic acid, PCT = procalcitonin, T = taurine, UDCA = ursodeoxycholic acid.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Montagnani M, Aldini R, Roda A, et al. New insights in the physiology and molecular basis of the intestinal bile acid absorption. Ital J Gastroenterol Hepatol 1998; 4:435–440. [PubMed] [Google Scholar]
- 2.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009; 10:1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galatola G, Jazrawi RP, Bridges C, et al. Direct measurement of first-pass ileal clearance of a bile acid in humans. Gastroenterology 1991; 4:1100–1105. [DOI] [PubMed] [Google Scholar]
- 4.Javitt NB. Cholestasis in infancy. Status report and conceptual approach. Gastroenterology 1976; 6:1172–1181. [PubMed] [Google Scholar]
- 5.Sondheimer JM, Bryan H, Andrews W, et al. Cholestatic tendencies in premature infants on and off parenteral nutrition. Pediatrics 1978; 6:984–989. [PubMed] [Google Scholar]
- 6.Tagliacozzi D, Mozzi AF, Casetta B, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med 2003; 12:1633–1641. [DOI] [PubMed] [Google Scholar]
- 7.Jahnel J, Zohrer E, Scharnagl H, et al. Reference ranges of serum bile acids in children and adolescents. Clin Chem Lab Med 2015; 53:1807–1813. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011; 5:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B Streptococcal disease in the United States, 1999–2005. JAMA 2008; 17:2056–2065. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 1:2–8. [DOI] [PubMed] [Google Scholar]
- 11.Kosters A, Karpen SJ. The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis 2010; 2:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013; 5:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie G, Zhong W, Li H, et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J 2013; 9:3583–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka N, Matsubara T, Krausz KW, et al. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012; 1:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucangioli SE, Castano G, Contin MD, et al. Lithocholic acid as a biomarker of intrahepatic cholestasis of pregnancy during ursodeoxycholic acid treatment. Ann Clin Biochem 2009; 1:44–49. [DOI] [PubMed] [Google Scholar]
- 16.Ye L, Liu S, Wang M, et al. High-performance liquid chromatography-tandem mass spectrometry for the analysis of bile acid profiles in serum of women with intrahepatic cholestasis of pregnancy. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 1:10–17. [DOI] [PubMed] [Google Scholar]
- 17.Polkowska G, Polkowski W, Kudlicka A, et al. Range of serum bile acid concentrations in neonates, infants, older children, and in adults. Med Sci Monit 2001; 7:268–270. [PubMed] [Google Scholar]
- 18.Niijima S. Studies on the conjugating activity of bile acids in children. Pediatr Res 1985; 3:302–307. [DOI] [PubMed] [Google Scholar]
- 19.LaRusso NF, Korman MG, Hoffman NE, et al. Dynamics of the enterohepatic circulation of bile acids. Postprandial serum concentrations of conjugates of cholic acid in health, cholecystectomized patients, and patients with bile acid malabsorption. N Engl J Med 1974; 14:689–692. [DOI] [PubMed] [Google Scholar]
- 20.Suchy FJ, Balistreri WF, Heubi JE, et al. Physiologic cholestasis: elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology 1981; 80:1037–1041. [PubMed] [Google Scholar]
- 21.Dhainaut JF, Marin N, Mignon A, et al. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 2001; 7:S42–S47. [DOI] [PubMed] [Google Scholar]
- 22.Dikopoulos N, Weidenbach H, Adler G, et al. Lipopolysaccharide represses cholesterol 7-alpha hydroxylase and induces binding activity to the bile acid response element II. Eur J Clin Invest 2003; 1:58–64. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999; 5:543–553. [DOI] [PubMed] [Google Scholar]
- 24.Laffitte BA, Kast HR, Nguyen CM, et al. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem 2000; 14:10638–10647. [DOI] [PubMed] [Google Scholar]
- 25.Barbara L, Lazzari R, Roda A, et al. Serum bile acids in newborns and children. Pediatr Res 1980; 11:1222–1225. [DOI] [PubMed] [Google Scholar]
- 26.Groer MW, Luciano AA, Dishaw LJ, et al. Development of the preterm infant gut microbiome: a research priority. Microbiome 2014; 2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesney RW, Helms RA, Christensen M, et al. An updated view of the value of taurine in infant nutrition. Adv Pediatr 1998; 45:179–200. [PubMed] [Google Scholar]


