Abstract
Objective:
The study objective was to investigate the protective effects of dexrazoxane (DRZ) on the cardiac autonomic nervous system (ANS) activity in anthracycline-treated breast cancer patients with diabetes.
Methods:
A total of 110 early stage breast cancer patients with type 2 diabetes were divided randomly into 2 even groups: chemotherapy alone (Chemo) and chemotherapy + DRZ (Chemo + DRZ). All patients underwent adjuvant chemotherapy (80 mg/m2 epirubicin and 500 mg/m2 cyclophosphamide) for a total of 6 cycles with 21 days/cycle. The Chemo + DRZ group patients were treated intravenously with 800 mg/m2 DRZ 30 minutes prior to the administration of epirubicin, while the Chemo group patients were given saline. The cardiac ANS function was evaluated for each patient before and after 6 cycles of chemotherapy by resting heart rate (RHR) and heart rate variability (HRV), which was evaluated by both time and frequency domains.
Results:
Before and after chemotherapy, patients in both groups showed significant decreases in HRV indices and increases in RHR and the low-frequency/high-frequency ratio. There were no significant differences between Chemo and Chemo + DRZ groups in terms of RHR and HRV indices before chemotherapy; however, after chemotherapy, patients in the Chemo group had a higher average RHR and lower HRV indices compared with patients in the Chemo + DRZ group.
Conclusion:
DRZ protects the cardiac ANS in epirubicin-treated early stage breast cancer patients with diabetes.
Keywords: breast cancer, dexrazoxane, diabetes, epirubicin, heart rate variability, resting heart rate
1. Introduction
Breast cancer is the most common cancer affecting women globally. Anthracyclines, such as doxorubicin and epirubicin, are effective clinical chemotherapeutic agents used to treat breast cancer patients. However, one of the major limitations of using anthracyclines as chemotherapy is their cardiotoxicity. Anthracycline-induced cardiotoxicity is an accumulative dose-dependent effect, which can manifest clinically as acute and potentially lethal cardiac events including pericarditis and late cardiomyopathy, which may lead to irreversible heart failure.[1,2] In addition to direct damage to cardiomyocytes, a number of studies have shown that anthracyclines cause dysfunction of the cardiac autonomic nervous system (ANS),[3–5] independent from the deterioration of cardiac contractility.[6] Hence, how to reduce or prevent the occurrence of anthracycline-linked cardiotoxicity to increase the therapeutic window for breast cancer patients has been an interesting research area for both cardiologists and oncologists in the field.[7] Dexrazoxane (DRZ) is used clinically to decrease anthracycline-induced cardiotoxicity.[8,9] A previous study demonstrated that DRZ did not reduce the therapeutic effects of anthracyclines, particularly in advanced/metastatic breast cancer patients[10]; however, the use of DRZ in combination with anthracyclines may increase the incidence of bone marrow suppression. In addition, there is limited evidence showing the benefits offered by the administration of DRZ before the initiation of anthracycline treatment in patients with early stage breast cancer. Currently, the FDA has approved only DRZ treatment for advanced/metastatic breast cancer patients who have received a 300-mg/m2 doxorubicin treatment. Whether DRZ is cardioprotective when administered prior to the treatment with anthracyclines in early stage breast cancer patients has not been well explored, but there are some preliminary findings to suggest that DRZ treatment is beneficial when used this way. One clinical study showed that patients administered DRZ before doxorubicin treatment had fewer adverse cardiac events (27.7%) compared with those in whom DRZ was not administered (52.4%).[11] Testore et al[12] also conducted a retrospective study on 318 patients who received anthracycline treatment. Among these patients, 302 had early stage breast cancers and received DRZ treatment prior to the initiation of anthracycline therapy. The authors concluded that DRZ treatment offered cardioprotective benefits for these early stage breast cancer patients.[12] Therefore, it appears that DRZ pretreatment before anthracycline administration may protect the heart from anthracycline-induced damage.
Although the risk for cardiotoxicity caused by anthracycline treatment is largely caused by accumulation from repetitive dosing, the presence of other factors, including hypertension, aging, preexisting cardiovascular diseases, and coadministration of other treatments, may make breast cancer patients more susceptible to anthracycline-induced cardiotoxicity.[13] Diabetes is a well-defined risk factor for coronary heart disease,[14] and several studies have suggested that women with diabetes are more likely to develop breast cancer.[15–17] Although it has not been demonstrated whether the presence of diabetes increases the incidence of anthracycline-induced cardiotoxicity in breast cancer patients, given that diabetes is a strong risk factor for adverse cardiovascular events, it is likely that diabetes contributes to clinical cardiotoxicity.[18,19] In particular, cardiac ANS damage has been associated with diabetes[20] as well as with anthracyclines. A consensus has not been established regarding sustaining minimal cardiotoxicity in the treatment of breast cancer patients with comorbid diabetes. Previously, we reported that an early treatment with DRZ in combination with anthracycline administration improved diastolic function in early breast cancer patients with type 2 diabetes.[21]
In the present study, we further explored the cardioprotective effects of DRZ in early stage breast cancer patients with diabetes who had been treated with epirubicin-based adjuvant chemotherapy. Specifically, we tested whether DRZ treatment prior to the initiation of epirubicin therapy can ameliorate cardiac ANS dysfunction in early stage breast cancer patients with diabetes.
2. Materials and methods
2.1. Selection of subjects
A total of 110 patients diagnosed with both early stage breast cancer and type 2 diabetes[22] were recruited to the study between October 2012 and April 2014 at the Fourth Hospital of Hebei Medical University Breast Center. The subjects were divided randomly into 2 study groups: chemotherapy alone (Chemo) and chemotherapy + DRZ (Chemo + DRZ). The Chemo group was given saline prior to chemotherapy treatment, while the Chemo + DRZ group was administered DRZ prior to chemotherapy treatment. Final group numbers are reported in the tables. All subjects signed informed consent forms. The study protocol was approved by the Fourth Hospital of Hebei Medical University Ethics Committee (registration number ChiCTR-IPR-16007759).
2.2. Inclusion and exclusion criteria
Patients who had all of the following characteristics were included in our study: early stage breast cancer (stage I or II) as confirmed by the pathological diagnosis of the surgically removed tumor; normal liver and kidney function; no coadministration of angiotensin-converting enzyme inhibitors, β-blockers, calcium channel blockers, or steroids; no type 2 diabetes–associated acute and/or serious chronic complications 1 week before blood sampling; no trauma, surgery, infection, or other acute stress events 1 week before the study was initiated; and normal sinus rhythm.
Patients who had ≥1 of the following conditions were excluded from our study: a history of hypertension, hyperthyroidism or hypothyroidism, or kidney disease; a history of cardiac coronary disease or congestive heart failure, existing cardiovascular symptoms, left ventricular ejection fraction <50%, cardiomyopathy, or valvular disease; a history of radiotherapy or chemotherapy; and abnormal electrocardiography, such as ischemic ST-T changes, pathological Q wave, a complete left bundle branch block, atrioventricular block, persistent atrial flutter, atrial fibrillation, atrial tachycardia, and/or paced rhythm.
The patient selection process is summarized in Fig. 1.
Figure 1.
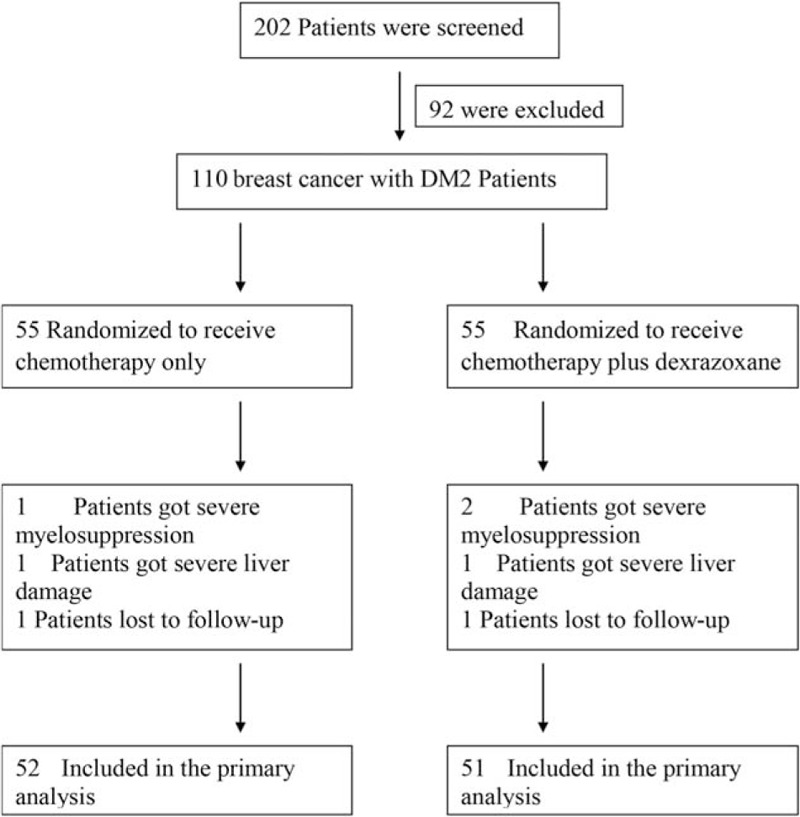
Flowchart showing the selection of patients for each study group. DM2 = type 2 diabetes.
2.3. Research protocol
All patients received epirubicin cyclophosphamide (EC) chemotherapy, consisting of 80 mg/m2 epirubicin plus 500 mg/m2 cyclophosphamide. EC chemotherapy was administered intravenously once every 3 weeks for 6 cycles (126 days total). Thirty minutes before administration of epirubicin, the patients in the Chemo group received intravenous injection of saline (0.9% NaCl solution), while the patients in the Chemo + DRZ group received intravenous injection of 800 mg/m2 DRZ.
All patients underwent general physical examinations to determine height, weight, and blood pressure. Routine blood tests were conducted on each subject to measure electrolyte, lipid, and blood glucose levels. Cardiac function was evaluated by synchronous 12-lead electrocardiography (ECG) and 24-hour Holter ECG monitoring (see subsequent text).
2.4. Regular and 24-hour Holter ECG
On the eve and the day of ECG examination, subjects were prohibited from drinking coffee, tea, or wine, smoking, exercising vigorously, engaging in stressful situations, and using any drugs that might affect cardiac ANS activity. For standard (non-Holter) ECG examination, each patient rested in a supine position for 10 minutes and a standard 12-lead ECG was recorded with a multilead ECG machine (Photoelectric Medical Electronic Instrument Co Ltd, Shanghai, China). Lead II was selected to trace 10 cardiac cycles and the average R-R interval (intervals between consecutive heartbeats) was used to calculate the resting heart rate (RHR). Subjects also underwent 24-hour ambulatory Holter ECG monitoring (model 90217-18Q, Hertford), which was capable of 24-hour monitoring of blood pressure and ECG with 3 channels. Electrodes were placed in the standard positions on disinfected skin. The recorded data were automatically analyzed by CardioVisions 1.13.0 analysis software. Heart rate variability (HRV) was evaluated for both time and frequency domains. Time-domain analysis included the following measurements: the standard deviation (SD) of all normal-to-normal intervals (i.e., the SD of R-R intervals of all sinus beats), the SD of the average of all normal-to-normal intervals during all 5-minute intervals, the root mean square of differences between adjacent normal-to-normal intervals, and the percent of number of pairs of adjacent normal-to-normal interval difference by >50 milliseconds in the total number of normal-to-normal intervals. Frequency-domain analysis included the following: low frequency (LF), 0.04 to 0.15 Hz; high frequency (HF), 0.15 to 0.40 Hz; and LF/HF, ratio of the LF band to the HF band.
2.5. Statistical analysis
All data were presented as mean ± SD. Statistical analyses were performed with SPSS 9.0 statistical software. Paired t test was used to compare data obtained before and after the treatment of each individual. Two-sample t test was used to compare data between 2 groups. The nonparametric rank sum test was used to compare data that did not meet the assumption of normality. The χ2 test was used for count data analysis. P values ≤0.05 were considered statistically significant.
3. Results
3.1. General outcomes of participants after treatment in the Chemo and Chemo + DRZ groups
As summarized in Fig. 1, 3 patients from the Chemo group were excluded from data analysis; after treatment, 1 patient had severe bone marrow suppression, 1 exhibited severe liver damage, and 1 was lost to follow-up. Four patients from the Chemo + DRZ group were excluded from data analysis; 2 patients had severe bone marrow suppression, 1 exhibited severe liver damage, and 1 was lost to follow-up. There were no tumor-related deaths, tumor recurrences, or symptoms of heart failure in any participant from either group.
3.2. Comparison of demographics, baseline characteristics, and medical history of participants between Chemo and Chemo + DRZ groups
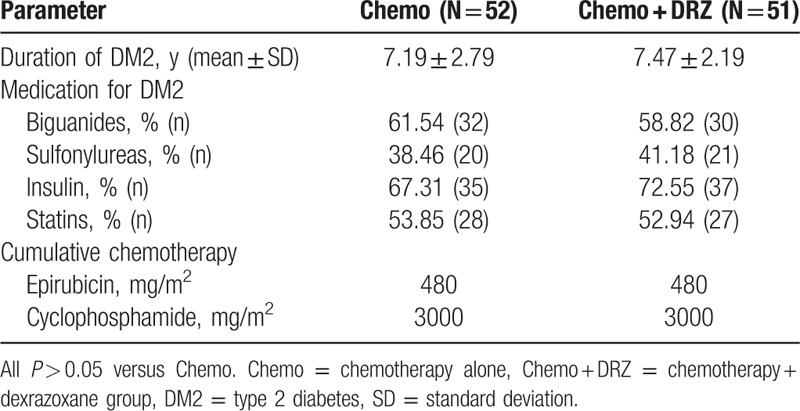
There were no significant differences in demographics or baseline characteristics of patients between the Chemo and Chemo + DRZ groups including age, observation duration, body mass index, the percentage of smokers, Eastern Cooperative Oncology Group Performance Status scores, or tumor staging (Table 1). Also, no significant differences were observed with regard to the duration of diabetes, diabetes-related medical histories between the patients of these 2 groups including duration of diabetes, medications for diabetes, or cumulative dosages of chemotherapeutic agents such as epirubicin and cyclophosphamide (Table 2).
Table 1.
Comparison of demographics, baseline, and basic clinical characteristics of participants between Chemo and Chemo + DRZ groups.
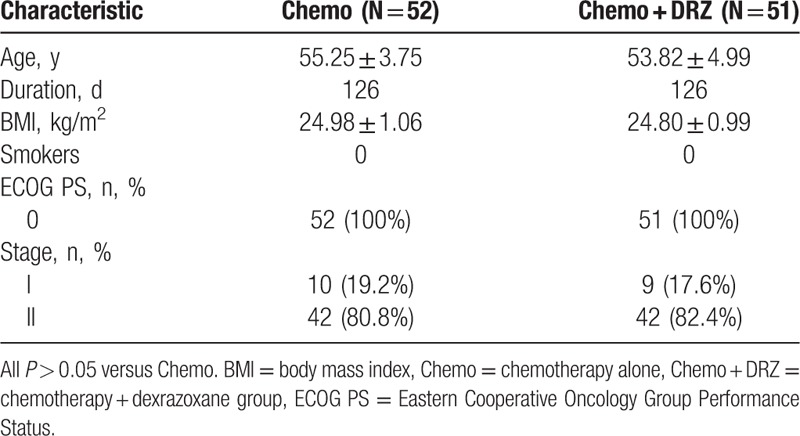
Table 2.
Comparison of medical history of participants between Chemo and Chemo + DRZ groups.
3.3. No significant differences in basic clinical data for individual patients before and after chemotherapy
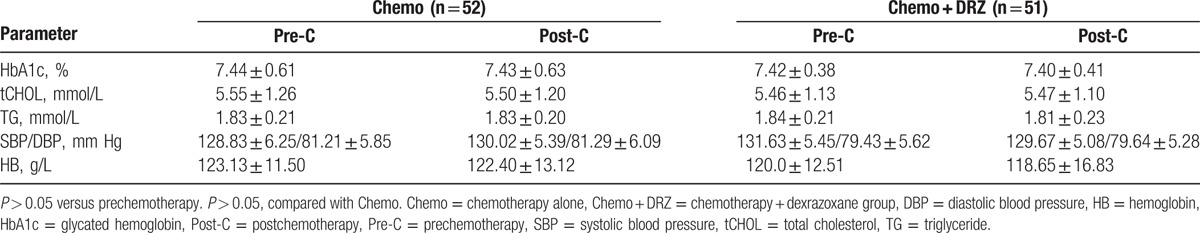
Blood pressure, serum hemoglobin, glycated hemoglobin, total cholesterol, and triglyceride levels were measured from each subject before and after 6 cycles of chemotherapy. As shown in Table 3, no significant differences were observed in any of these indices in the patients of either group, suggesting that treatment with DRZ did not significantly alter the basic clinical characteristics of patients with both breast cancer and diabetes.
Table 3.
Comparison of basic clinical characteristics of participants before and after chemotherapy.
3.4. DRZ attenuated the increase in RHR of participants in Chemo + DRZ group
To evaluate whether chemotherapy caused damage to the cardiac ANS, we measured the RHR for all patients before and after chemotherapy. RHR is a well-recognized indicator for ANS function.[23] Patients in both groups showed comparable baseline RHR before chemotherapy and, indeed, all patients had higher RHR after chemotherapy (P < 0.05; Tables 4 and 5), suggesting that chemotherapy significantly increased RHR. However, subjects in the Chemo + DRZ group exhibited a significantly lower RHR than those in the Chemo group (P < 0.05; Tables 4 and 5), indicating that DRZ treatment attenuated the increase in RHR of patients that was induced by chemotherapy.
Table 4.
DRZ attenuated the increase in RHR but decrease in HRV of participants after chemotherapy.
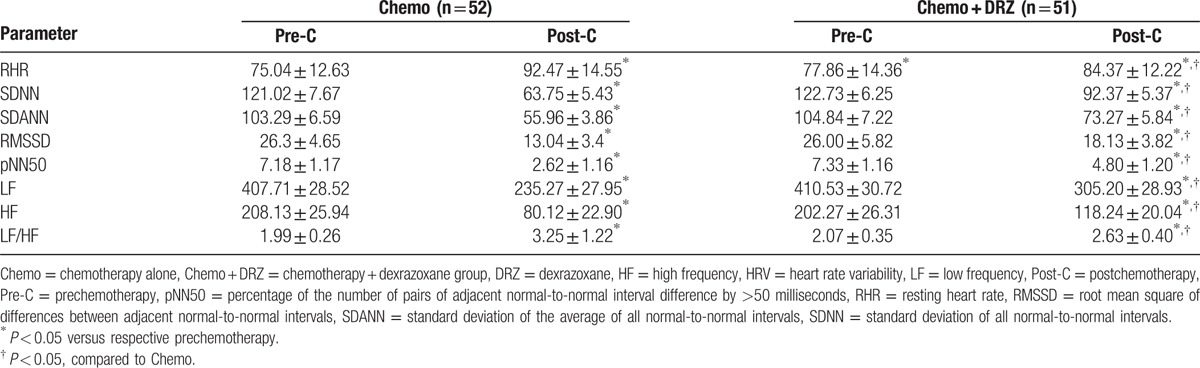
Table 5.
DRZ attenuated the changes of RHR and HRV of participants of Chemo and Chemo + DRZ groups before and after chemotherapy.

3.5. DRZ attenuated the decrease in HRV
Next, we used time and frequency domains to measure patients’ HRV. Changes in HRV can indicate the extent of cardiac ANS damage.[24] Patients in both groups showed comparable baseline HRV before chemotherapy (Tables 4 and 5). After chemotherapy, the subjects from both groups had significantly lower HRV indices including SD of all normal-to-normal intervals, SD of the average of all normal-to-normal intervals, root mean square of differences between adjacent normal-to-normal intervals, percent of number of pairs of adjacent normal-to-normal interval difference by >50 milliseconds, LF, and HF, but a higher LF/HF compared with those before chemotherapy (P < 0.05 for all indices; Tables 4 and 5). However, subjects in the Chemo + DRZ group exhibited significantly higher HRV indices and lower LF/HF ratios than those in the Chemo group (P < 0.05; Tables 4 and 5), suggesting that DRZ attenuated the decrease in HRV induced by chemotherapy.
4. Discussion
In this prospective study, we measured cardiac ANS activity to evaluate the protective effects of DRZ on the hearts of epirubicin-treated early stage breast cancer patients with diabetes, and showed that DRZ significantly improved the activity of cardiac ANS in these patients as assessed by RHR and indices of HRV.
HRV, that is, beat-to-beat variations in heart rate, has been used widely to quantify cardiovascular ANS activity and its regulatory function.[24] Abnormal HRV is considered a risk factor for cardiovascular mortality independent of traditional indicators such as left ventricular ejection fraction, the number of ventricular premature beats, late potential, and average heart rate.[25] HRV reflects both sympathetic and parasympathetic tones of the heart. A number of measures may be used to evaluate HRV,[26] and in the present study, we employed the 2 most frequently used types of measurement, time domain and frequency domain measurements, to evaluate HRV; both of them may be used to assess the cardiac sympathovagal balance. A decrease in HRV represents impaired cardiac autonomic activity. Consistent with previous findings showing damaged cardiac sympathovagal balance by anthracyclines,[3–5,27] our study revealed indeed that patients in both the Chemo and Chemo + DRZ groups exhibited decreased HRV after epirubicin treatment, suggesting that epirubicin impaired cardiac ANS activity. However, this decrease was attenuated in patients in the Chemo + DRZ group, indicating that DRZ ameliorated the ANS dysfunction induced by epirubicin. In the present study, we also used RHR to evaluate the degree of cardiac ANS dysfunction caused by epirubicin and the cardioprotective effect of DRZ. RHR is also an indicator of ANS activity, and its abnormal increase is a risk factor for adverse cardiovascular events.[23] A previous study showed that an increase in RHR by 10 beats/min was equivalent to an increase in systolic blood pressure of 10 mm Hg, resulting in an 8% increase in the incidence of major cardiovascular events, and an 11% increase in the risk for heart failure specifically.[28] Indeed, we found that after chemotherapy, all patients exhibited higher RHR, suggestive of impaired ANS activity. The DRZ treatment attenuated the increase in RHR significantly (Tables 4 and 5). Thus, consistent with the above-mentioned HRV data, DRZ improved cardiac ANS function in epirubicin-treated breast cancer patients as evaluated by RHR changes.
One concern in our study was that our chemotherapy regime contained both epirubicin and cyclophosphamide; theoretically, the role of cyclophosphamide on the insult to cardiac ANS function could not be ruled out completely. However, a previous study revealed that cyclophosphamide caused heart damage only at a high dosage (>1.55 g/m2/d) after 1 to 3 weeks of administration.[29] Also, cyclophosphamide treatment alone was ever used as a control to explore the impairment of the cardiac ANS activity in the anthracycline-treated breast cancer patients.[6] The cyclophosphamide dosage used in our study (0.5 g/m2/d) was much lower than the above-mentioned heart-damaging dosage, and none of the patients in our study showed any signs of heart failure at any time in the observation period. Therefore, it is unlikely that the cardiac ANS damage observed in these chemotherapy patients was due to cyclophosphamide; instead, we believe that the cardiac ANS damage reflected by the increase in RHR and decrease in HRV is attributable to epirubicin.
Diabetes is a widely recognized risk factor for cardiovascular disease,[14] and autonomic neuropathy is one of the most common complications of diabetes with an incidence rate >60% for diabetic patients.[30] HRV is reduced in diabetic patients with autonomic neuropathy and has been used extensively to evaluate the degree of autonomic neuropathy in patients.[31] Our study suggested that DRZ improved HRV in epirubicin-treated breast cancer patients with diabetes; however, we did not recruit a control (nondiabetic) group. Therefore, we did not know whether these patients had impaired cardiac autonomic function linked to their diabetes prior to commencing chemotherapy. Also, diabetic patients were not further divided into subgroups based on their blood sugar levels, and we did not pursue the question of whether DRZ combined with an antidiabetic treatment would benefit epirubicin-treated breast patients with diabetes synergistically with regards to cardiac ANS function. In addition, the sample size of our study was small, and the length of the observation period was relatively short. Therefore, our findings need to be further corroborated in studies with larger sample sizes and longer observation periods.
In conclusion, we demonstrated that DRZ protects the cardiac ANS from epirubicin-induced impairment in early stage breast cancer patients with diabetes, as evidenced by ameliorated RHR and HRV. Therefore, we recommend administration of DRZ prior to the initiation of chemotherapy to effectively reduce the incidence of cardiotoxicity in anthracycline-treated breast cancer patients with diabetes.
Acknowledgment
The authors would like to express their thanks to Health and Family Planning Commission of Hebei Province, 2016, key projects of medical science research in Hebei Province (project no. 20160646) for their support.
Footnotes
Abbreviations: ANS = autonomic nervous system, Chemo = chemotherapy alone, DRZ = dexrazoxane, ECG = electrocardiography, HF = high frequency, HRV = heart rate variability, LF = low frequency, RHR = resting heart rate, SD = standard deviation.
The authors have no conflicts of interest to disclose.
References
- 1.Hayek ER, Speakman E, Rehmus E. Acute doxorubicin cardiotoxicity. N Engl J Med 2005; 352:2456–2457. [DOI] [PubMed] [Google Scholar]
- 2.Kaklamani VG, Gradishar WJ. Epirubicin versus doxorubicin: which is the anthracycline of choice for the treatment of breast cancer? Clin Breast Cancer 2003; 4 (suppl 1):S26–S33. [DOI] [PubMed] [Google Scholar]
- 3.Postma A, Elzenga NJ, Haaksma J, et al. Cardiac status in bone tumor survivors up to nearly 19 years after treatment with doxorubicin: a longitudinal study. Med Pediatr Oncol 2002; 39:86–92. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer CA, Gietema JA, van den Berg MP, et al. Long-term cardiac follow-up in survivors of a malignant bone tumour. Ann Oncol 2006; 17:1586–1591. [DOI] [PubMed] [Google Scholar]
- 5.Syvanen K, Ekholm E, Anttila K, et al. Immediate effects of docetaxel alone or in combination with epirubicin on cardiac function in advanced breast cancer. Anticancer Res 2003; 23:1869–1873. [PubMed] [Google Scholar]
- 6.Viniegra M, Marchetti M, Losso M, et al. Cardiovascular autonomic function in anthracycline-treated breast cancer patients. Cancer Chemother Pharmacol 1990; 26:227–231. [DOI] [PubMed] [Google Scholar]
- 7.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation 2012; 126:2749–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremer LC, van Dalen EC. Dexrazoxane in children with cancer: from evidence to practice. J Clin Oncol 2015; 33:2594–2596. [DOI] [PubMed] [Google Scholar]
- 9.Conway A, McCarthy AL, Lawrence P, et al. The prevention, detection and management of cancer treatment-induced cardiotoxicity: a meta-review. BMC Cancer 2015; 15:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marty M, Espie M, Llombart A, et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane®) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol 2006; 17:614–622. [DOI] [PubMed] [Google Scholar]
- 11.Choi HS, Park ES, Kang HJ, et al. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J Korean Med Sci 2010; 25:1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testore F, Milanese S, Ceste M, et al. Cardioprotective effect of dexrazoxane in patients with breast cancer treated with anthracyclines in adjuvant setting: a 10-year single institution experience. Am J Cardiovasc Drugs 2008; 8:257–263. [DOI] [PubMed] [Google Scholar]
- 13.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004; 109:3122–3131. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37 (suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 15.Michels KB, Solomon CG, Hu FB, et al. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care 2003; 26:1752–1758. [DOI] [PubMed] [Google Scholar]
- 16.Lipscombe LL, Fischer HD, Austin PC, et al. The association between diabetes and breast cancer stage at diagnosis: a population-based study. Breast Cancer Res Treat 2015; 150:613–620. [DOI] [PubMed] [Google Scholar]
- 17.Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer 2012; 107:1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambetti M, Moliterni A, Materazzo C, et al. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol 2001; 19:37–43. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013; 31:3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinik AI, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care 2003; 26:1553–1579. [DOI] [PubMed] [Google Scholar]
- 21.Sun F, Qi X, Geng C, et al. Dexrazoxane protects breast cancer patients with diabetes from chemotherapy-induced cardiotoxicity. Am J Med Sci 2015; 349:406–412. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg R, Punthakee Z. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 2013; 37 (suppl 1):S8–S11. [DOI] [PubMed] [Google Scholar]
- 23.Nauman J, Janszky I, Vatten LJ, et al. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA 2011; 306:2579–2587. [DOI] [PubMed] [Google Scholar]
- 24.Appel ML, Berger RD, Saul JP, et al. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol 1989; 14:1139–1148. [DOI] [PubMed] [Google Scholar]
- 25.Schuster AK, Fischer JE, Thayer JF, et al. Decreased heart rate variability correlates to increased cardiovascular risk. Int J Cardiol 2016; 203:728–730. [DOI] [PubMed] [Google Scholar]
- 26.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93:1043–1065. [PubMed] [Google Scholar]
- 27.Nousiainen T, Vanninen E, Jantunen E, et al. Neuroendocrine changes during the evolution of doxorubicin-induced left ventricular dysfunction in adult lymphoma patients. Clin Sci (Lond) 2001; 101:601–607. [PubMed] [Google Scholar]
- 28.Pfister R, Michels G, Sharp SJ, et al. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC-Norfolk study. Eur J Heart Fail 2012; 14:1163–1170. [DOI] [PubMed] [Google Scholar]
- 29.Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev 2011; 37:300–311. [DOI] [PubMed] [Google Scholar]
- 30.Khoharo HK, Halepoto AW. QTc-interval, heart rate variability and postural hypotension as an indicator of cardiac autonomic neuropathy in type 2 diabetic patients. J Pak Med Assoc 2012; 62:328–331. [PubMed] [Google Scholar]
- 31.Li X, Jiang YH, Jiang P, et al. Analysis of heart rate variability and cardiac autonomic nerve remodeling in streptozotocin-induced diabetic rats. Exp Clin Endocrinol Diabetes 2015; 123:272–281. [DOI] [PubMed] [Google Scholar]


