Abstract
Incidental gastric subepithelial tumor (SET) is frequently found during endoscopy. Although endoscopic ultrasonography (EUS) can provide useful information, its diagnostic accuracy varies. Most of the potentially malignant tumors observed on EUS are hypoechoic lesions. Therefore, we aimed to investigate the diagnostic accuracy of EUS for hypoechoic lesions located in the submucosa or proper muscle layer. We also evaluated various characteristics for potential associations with diagnostic accuracy.
A retrospective review was conducted of the medical records of 99 patients who were diagnosed with gastric SET and who underwent EUS with pathologic confirmation between March 2008 and April 2015. After reviewing the endoscopic and pathologic findings, we attempted to analyze factors that were associated with the diagnostic accuracy of EUS.
The mean ± standard deviation size of the lesions was 20.0 ± 12.7 mm. The most common location was the upper third of the stomach (43.4%). The overall accuracy of EUS was 66.7%. No statistically significant difference in EUS accuracy was observed according to the location, size, or layer of the lesion. The following pathologic diagnostic methods were used: EUS-guided fine needle aspiration (3.0%), forceps biopsy (16.2%), deep tissue biopsy using cap-assisted mucosal resection (8.1%), endoscopic submucosal dissection (25.2%), and operation (47.5%). The accuracy of EUS according to the expected diagnosis of the lesion was 77.1% for gastrointestinal stromal tumor, 50% for neuroendocrine tumor, and 50% for ectopic pancreas.
Although EUS is a useful tool for gastric SET in clinical practice, the accuracy of diagnostic EUS is suboptimal. When considering whether to treat gastric SET, the decision should be made based on the pathologic diagnosis.
Keywords: endoscopic submucosal dissections, endoscopic ultrasonography, subepithelial tumor
1. Introduction
Subepithelial tumor (SET) is a term that is used for a mass with normal-appearing mucosa during endoscopy.[1] Most of gastric SETs are asymptomatic and are found incidentally during endoscopy. The incidence of gastrointestinal SET has been reported to be 0.36%.[2] In recent years, the detection rate of gastric SET has increased because of developments in endoscopic equipment and the increased use of endoscopic examinations. During initial endoscopy, the endoscopic features of gastric SET provide important information. The following features should be assessed: size, morphology, mobility, hardness (soft, cystic, or firm), pulsation, color, and surface appearance of the tumor.[1] However, it is difficult to confirm many tumors with initial endoscopic examination. Although conventional endoscopic forceps biopsy has been used, the diagnostic yield has been disappointing (14%–42%).[3,4] In addition, the use of bite-on-bite technique may cause complications such as bleeding in approximately 2.8% of cases.[4]
A firm gastric SET is inconsistent with a cyst, vessel, or lipoma and may necessitate endoscopic ultrasonography (EUS). One can differentiate between intramural lesions and extramural compressive lesions during EUS examination. If the lesion is intramural, one can ascertain tumor size, layer, and morphologic features, suggesting a diagnosis. By using EUS, one can examine the echogenicity of the tumor (homogenous vs inhomogeneous and hyperechoic vs hypoechoic or anechoic). Among gastric SETs, malignant and potentially malignant tumors are hypoechoic lesions, such as gastrointestinal stromal tumors (GISTs), neuroendocrine tumors, lymphomas, and metastatic carcinomas.[1] Although EUS provides a considerable amount of information, the reported accuracy of EUS is approximately 45.5%.[5] Thus, EUS alone is not a confirmative method of examination for differentiating between benign and malignant SETs.
Therefore, in the present study, we sought to analyze endoscopic–pathologic factors for potential associations with the accurate diagnosis of gastric SETs located in the submucosa (SM) or proper muscle (PM) layer.
2. Methods
2.1. Patients
We retrospectively reviewed the medical records of patients with gastric SET who visited Pusan National University Yangsan Hospital (Korea) between March 2008 and April 2015. During the study period, a total of 3003 SETs were found during endoscopy. We excluded 933 lesions that were esophageal or duodenal SETs; 1225 gastric SETs that had not been subjected to EUS examination; and 94 hyperechoic lesions, anechoic lesions, or extraluminal compressions. After making these exclusions, 751 hypoechoic lesions remained. We additionally excluded 652 lesions that had not been pathologically confirmed, leaving a total of 99 hypoechoic lesions of gastric SETs that had been histologically confirmed. These 99 lesions were included and analyzed in the present study (Fig. 1). Written informed consent was obtained from all patients before performing EUS or the diagnostic procedure. The study was approved by the Ethics Committee that belongs to our Institutional Review Board.
Figure 1.
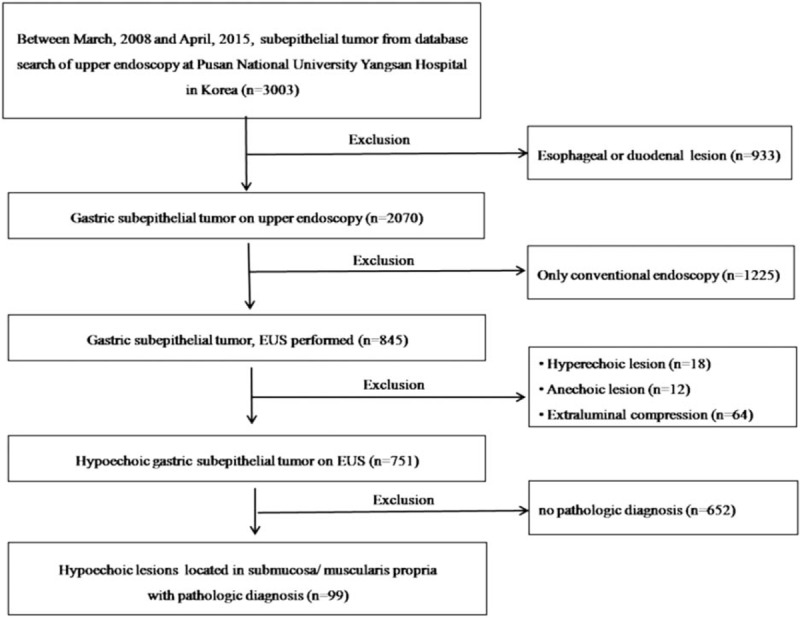
Flow diagram showing the study design and population.
2.2. Procedures
Initially, we performed diagnostic EUS using a miniprobe catheter (Olympus, Tokyo, Japan; UM DP 20-25R) with the water-filled method. If the layer of origin was uncertain or low penetration of echo was suspected, we additionally used radial EUS (Olympus; GF-UM2000). For hypoechoic lesions that were located in the SM or PM layer with surface ulceration, the histologic diagnosis was established using endoscopic forceps biopsy. If the endoscopic biopsy of an SM lesion showed a negative histological outcome, we conducted complete resection by endoscopic or surgical resection. For PM-layer tumors that were larger than 2 cm or had malignant features (such as ulcerations, erosions, or erythema), an operation was conducted. For lesions that were larger than 2 cm and covered with normal-appearing gastric mucosa, EUS-guided fine needle aspiration (EUS-FNA), deep tissue biopsy with cap-assisted mucosal resection, or submucosal dissection technique (ESD) was conducted. Deep tissue biopsy with cap-assisted mucosal resection was performed using the cap-assisted endoscopic mucosal resection (EMR) technique. After the removal of normal-appearing mucosa, endoscopic forceps biopsy was performed. For lesions that were smaller than 2 cm and covered with normal-appearing gastric mucosa, annual follow-up examination was recommended. During these follow-ups, if the size of the lesion was observed to have increased more than 50%, the histologic confirmative methods described in the above text were attempted.
Endoscopic and pathologic characteristics were assessed, including the mean size, location of the lesion, histologic confirmative modalities, and pathologic results of the SETs. We calculated the agreement between EUS findings and histologic outcomes and assessed the accuracy of EUS.
2.3. Statistical analysis
Univariate analyses of continuous and categorical variables were performed with Student t test and the chi-square test or Fisher exact test, respectively. Values of P < 0.05 were regarded as indicating statistical significance. If variables were found to be statistically significant in univariate analyses, then they would be entered into a forward stepwise multiple logistic regression model to identify risk factors that had independent associations with early gastric cancer. In our results, continuous variables are summarized as means ± standard deviations. The statistical analyses were performed by an author of this study using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
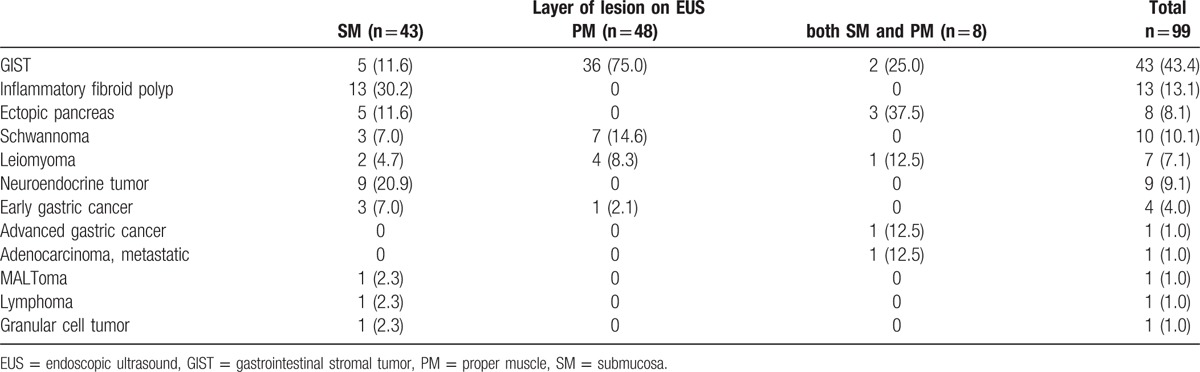
A total of 99 patients were enrolled, each of whom underwent EUS followed by pathologic diagnosis during the study period. The mean age of the patients was 59.1 ± 11.3 years. The mean size of the lesions was 20.0 ± 12.7 mm. The mean lesion sizes according to layer of origin were as follows: SM layer, 13.0 ± 8.3 mm; PM layer, 25.0 ± 12.9 mm; and SM + PM, 29.0 ± 12.5 mm. The most common location was the upper third of the stomach (43.4%, 43/99). No statistically significant difference in the EUS accuracy was observed according to the location, size, or layer of the lesion. The following pathologic diagnostic methods were used: EUS-FNA (3%, 3/99), forceps biopsy (16.2%, 16/99), deep tissue biopsy using cap-assisted mucosal resection (8.1%, 8/99), ESD (25.2%, 25/99), and operation (47.5%, 47/99) (Table 1). The diagnostic rates for each of the diagnostic methods were as follows: endoscopic biopsy, 32.7% (16/49); EUS-FNA, 75.0% (3/4); deep tissue biopsy using cap-assisted mucosal resection, 88.9% (8/9); ESD, 100% (30/30); and operation, 100% (64/64). Common pathologic diagnoses were GIST (43%, 43/99), schwannoma (10.1%, 10/99), leiomyoma (7.1%, 7/99), and neuroendocrine tumor (9.1%, 9/99) (Table 2).
Table 1.
Baseline characteristics.
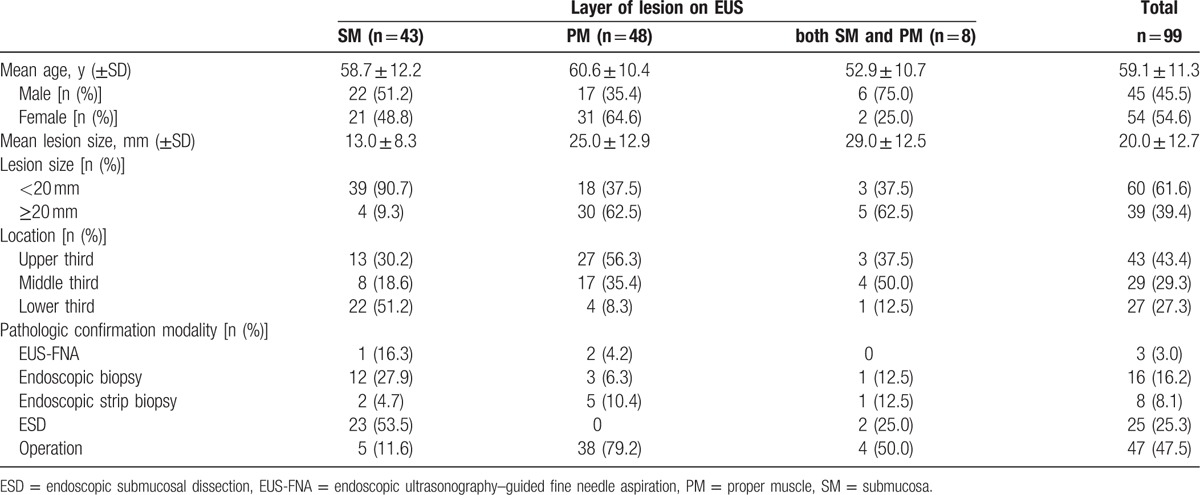
Table 2.
Characteristics of gastric subepithelial tumors.
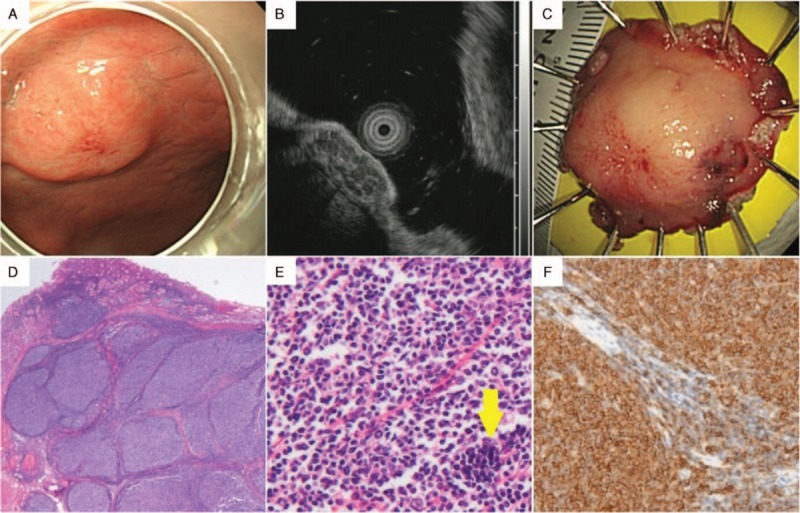
The overall accuracy of EUS was 66.7%. The location, size, and layer of origin did not show significant associations with the diagnostic accuracy of EUS (Table 3). Diagnostic accuracy is shown according to the expected diagnosis by EUS in Table 4. Although the most common expected diagnosis was GIST (48.5%, 48/99), the diagnostic accuracy for GIST was 77.1% (37/48). The accuracy was 50% for both neuroendocrine tumor (8/16) and ectopic pancreas (7/14). Among the incorrectly diagnosed lesions, 2 unexpected lesions were found: a lymphoma lesion in the SM layer, which was thought to be a neuroendocrine tumor primarily (Fig. 2), and an ectopic pancreas lesion in the PM layer, which was initially expected to be GIST (Fig. 3).
Table 3.
Associated factors with accuracy of EUS.
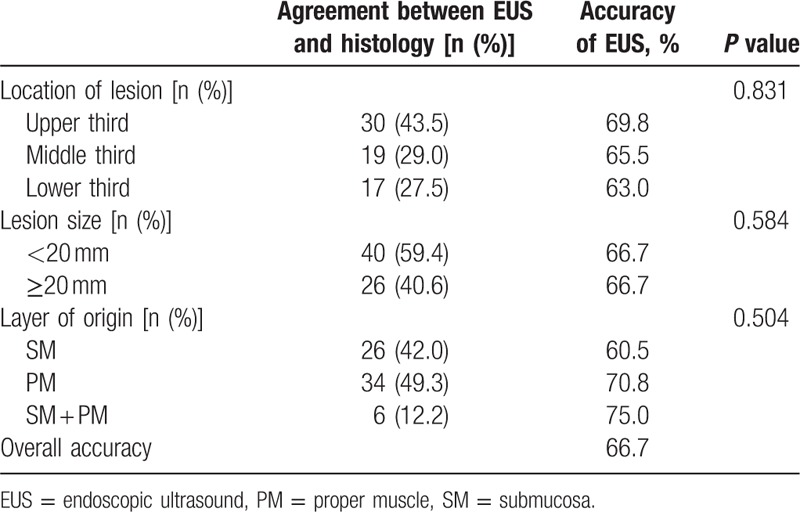
Table 4.
Accuracy of EUS findings according to expected diagnosis of gastric subepithelial lesions.
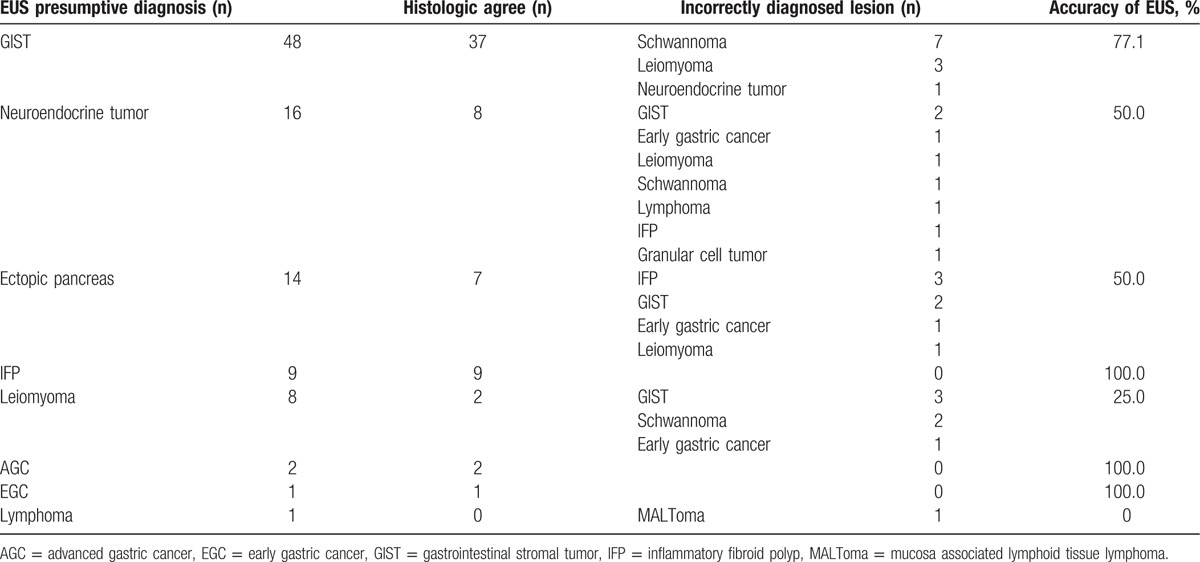
Figure 2.
A lesion showing the histology of follicular lymphoma after endoscopic submucosal dissection (ESD). (A) Upper endoscopy showed a well defined, polypoid gastric subepithelial lesion with a size of about 1.5 cm in the middle body, anterior wall. This lesion had a central mucosal erythema that is believed to be a scar created by a previous biopsy that was performed at another medical center. (B) On endoscopic ultrasonography, the lesion appeared as a 15 mm × 5 mm hypoechoic mass in the submucosa layer without infiltration of the muscularis propria. It was presumed to be a neuroendocrine tumor, even though the lesion had inhomogeneous and multiseptated morphological features. (C) The ESD specimen of this lesion appeared to be 2.8 × 2.4 × 0.6 cm3 in size after ESD, which had been performed without complications. (D) Histopathological analysis of the ESD specimen showed lymphoid hyperplasia below the normal gastric mucosa (hematoxylin and eosin stain, original magnification ×12). (E) This structure showed back-to-back arrangement and a very thin mantle zone (hematoxylin and eosin, original magnification ×200). (F) Lymphocytes of the abnormal structure were positive for CD20; this finding was compatible with follicular lymphoma (hematoxylin and eosin, original magnification ×200).
Figure 3.
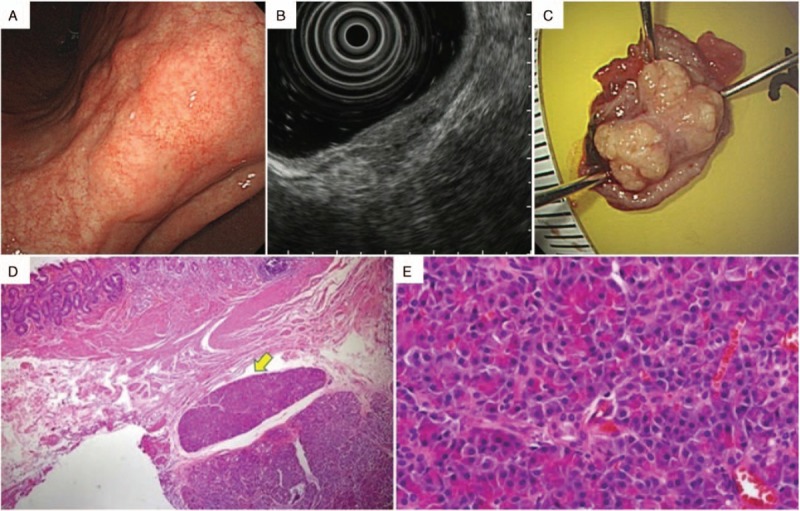
A lesion showing the histology of ectopic pancreas after endoscopic strip biopsy. (A) Upper endoscopy showed a gastric subepithelial lesion in the lesser curvature of the lower body. The lesion had an irregular margin and surface. (B) On endoscopic ultrasonography, it appeared as a well defined hypoechoic mass, mainly in the muscularis propria. The lesion was therefore presumed to be a gastrointestinal stromal tumor, even though it had heterogeneous echotexture. (C) The specimen from the endoscopic strip biopsy had a yellowish inner portion, which was presumed to be pancreatic tissue. (D) Histopathological analysis of the biopsy specimen showed the lesion in the muscularis propria layer with intact overlying mucosa and submucosa (hematoxylin and eosin, original magnification ×15) (yellow arrow). (E) Viewed at greater magnification, the specimen showed the presence of pancreatic acini and ductal structures. The features were compatible with ectopic pancreas (hematoxylin and eosin, original magnification ×400).
Diagnostic discrepancy was found in 33.3% (n = 33) of the 99 patients. Among the discrepancies, 21 cases were clinically significant misdiagnoses (presumptive diagnosis benign lesion vs malignant or potentially malignant lesions, or vice versa). In 7 cases, the presumptive diagnosis was benign, but the histologic diagnosis was malignant or premalignant lesion (2 gastric cancers and 5 GISTs). All cases were treated with curative surgical resection. In 13 cases, the presumptive diagnosis was malignant or potentially malignant lesion, but the pathologic diagnosis was benign (8 schwannomas, 4 leiomyomas, 1 inflammatory fibrinoid polyp, and 1 granular cell tumor). The inflammatory fibrinoid polyp and the granular cell tumor were resected using ESD techniques. The schwannomas and leiomyomas were initially suspected to be GIST after diagnostic EUS. However, these tumors were resected via laparoscopic wedge resection because of surface ulceration or increased tumor size during follow-up.
4. Discussion
Recently, there have been many cases of incidental diagnoses of gastric SETs during endoscopy. The detection of gastric SET raises several concerns: What is the pathologic diagnosis? Can we differentiate the lesions that require therapy from those that do not? What is the best treatment plan? After gastric SETs are found, it is important to differentiate between benign and malignant or potentially malignant lesions. EUS has been a popular endoscopic procedure for evaluating SET and provides a considerable amount of information. The first role of EUS is to distinguish intramural and extrinsic compressive lesions. The most common causes of extraluminal compression are the spleen and splenic vessels, while the other causes are the hepatic left lobe, gallbladder, colon, and pancreas. In addition, abscesses, cysts of the pancreas or kidney, aneurysms, and enlarged lymph nodes might be suspected of being extraluminal compression.[1] If an intramural lesion is identified, EUS can provide more information than conventional endoscopy. For example, EUS can provide the lesion size, layer of origin, tumor margin, and echogenicity.[1] After conventional endoscopy or EUS, if typical findings of benign tumors are suspected, such as lipoma (hyperechoic lesion), vascular structures, or cyst (anechoic lesion), then no further diagnostic tests or treatments are required. However, most malignant or potentially malignant lesions are hypoechoic. Thus, in the present study, we only enrolled subjects with hypoechoic tumors that originated from the SM or PM layer.
The primary aim of the present study was to identify the diagnostic accuracy of EUS, while the secondary aim was to evaluate endoscopic and pathologic features associated with the diagnostic accuracy of EUS. In the present study, the accuracy of EUS was 66.7%. This result is similar to that of a previous study.[5] However, after analyzing the data, we could not find any significant associations between endoscopic features and the diagnostic accuracy of EUS. The main problem with diagnostic EUS is that the expected diagnosis is operator dependent. In cases of lipoma, cystic lesion, and extrinsic compression, the diagnostic accuracy of EUS is acceptable; however, EUS does not show satisfactory value with regard to GIST and other SETs.[6] In our experience, during EUS for gastric SETs, the expected diagnosis of the lesion is influenced by the reported frequencies of lesions associated with different layers of origin and locations. For example, GISTs may be expected in PM-layer cases because it is the most common SET originating from the PM layer, and neuroendocrine tumors may be expected in SM-layer cases because it is a frequent or important SET originating from the SM layer. Therefore, it is important to obtain SET tissues to identify the pathology of the lesions.
To date, various tissue acquisition methods have been reported. Although the first step may be conventional endoscopic forceps biopsy, the diagnostic rate of this method is low because the lesion is located beneath the mucosa. Endoscopic biopsy techniques, such as the bite-on-bite technique or the use of jumbo biopsy forceps, yield definite diagnosis rates of 14% to 58.9%, but they are associated with bleeding complication rates of 2.8% to 35.7%.[4,7] In the present study, the diagnostic rate of endoscopic forceps biopsy was 32.6% (16/49). The diagnostic rate of endoscopic forceps biopsy may be affected by the originating layer of the tumor and the surface mucosal appearance. If endoscopic forceps biopsy is used for SM-layer tumors and lesions with overlying mucosal changes (such as color changes or ulceration), the diagnostic rate of forceps biopsy may increase. EUS-FNA and biopsy may represent the most recent and well defined procedure. EUS-FNA and Tru-Cut biopsy allow the cellular evaluation of GISTs with reported accuracies of 63% to 94%.[8–10] In the era of ESD, many different endoscopic resection and biopsy techniques have been introduced. Endoscopic resection using a ligation device or a transparent cap yields definite diagnosis in approximately 90% of SM-layer tumors.[11] Endoscopic partial resection with the unroofing technique or single-incision needle-knife biopsy yields a 92.8% to 93.7% definite diagnosis rate.[12–14] The associated procedure-related bleeding rate was 56% (9/16), but could be controlled easily through endoscopic bleeding techniques.[13] In the present study, the diagnosis rates for the modalities were as follows: EUS-FNA, 75.0% (3/4); endoscopic biopsy, 32.7% (16/49); deep tissue biopsy using cap-assisted mucosal resection, 88.9% (8/9); ESD, 100.0% (30/30); and operation, 100.0% (64/64).
In this study, we limited the study cohort to patients who had submucosa or PM-layer tumors with hypoechogenicity because the hypoechoic lesion might have malignant potential. In addition, the mean size of the PM-layer tumors was more than 2 cm.
The most popular deep tissue techniques are EUS-FNA and Tru-Cut biopsy. Some difficulties can occur during the application of these techniques, such as misfire of the needle inside the lesion and procedural difficulties when the lesion is in the distal antrum. However, when applying ESD techniques or deep biopsy after mucosa unroofing techniques (endoscopic partial resection), the diagnostic yields have been reported to be more than 90%.[12–14] Further, in the present study, there were neither cases of significantly delayed bleeding, nor cases of serious complications.
Differentiation between benign and potentially malignant gastric SETs is important. However, diagnostic discrepancy was observed for 33.3% (33/99) of the lesions in the present study. This means that applied management strategies may be inappropriate for 1/3 of patients. Therefore, we propose that the deep tissue acquisition techniques should be chosen according to the preference of the endoscopist.
Most gastric SETs are asymptomatic and have small sizes (<2 cm).[14–16] Although 3.6% to 8.5% of gastric SET increase in size, most of the lesions are benign in nature.[14,15] Therefore, histologic confirmation is not necessary for all gastric SETs. The potentially malignant features during endoscopy and EUS are surface ulceration, size larger than 2 to 5 cm, rapid growth, irregularity of the extraluminal border, presence of cystic spaces, heterogeneity,[16] and echogenicity—particularly, hypoechoic lesion with lower echogenicity in the muscle. Thus, in cases of gastric SET with known malignant features, we must consider performing a histologic diagnosis. In the present study, most of the tumors located in the SM layer were resected using endoscopic techniques (ESD techniques were used for 25 lesions). For tumors that were located in the PM layer with surface changes, a large size of more than 5 cm, and inhomogeneous echogenicity, we recommended an operation (47/99 lesions).
Our study is subject to several limitations. First, it was conducted retrospectively in a single center. The accumulation of data from multiple centers and prospective studies may provide more rigorous and generalizable assessments of the diagnostic accuracy of EUS. Second, there may have been some technical differences between EUSs in this study because EUS is an operator-dependent examination. Third, the methods of pathologic diagnosis differed because they were selected based on each operator's individual preference.
In summary, the overall accuracy of diagnostic EUS was 66.7% (66/99) for hypoechoic gastric SETs, meaning that diagnostic discrepancy occurred in 1 of 3 cases. Although the aim of the present study was to determine endoscopic features that were associated with diagnostic accuracy, no statistically significant differences in accuracy were observed according to the location, size, or layer of the lesion. When incidental gastric SET is found during endoscopy, one should attempt to differentiate benign lesions and potentially malignant lesions primarily based on their endoscopic features. When the gastric SET is firm, conventional endoscopic forceps biopsy and EUS should be considered to ascertain any change in surface or size. Considering the substantial differences that have been observed between the presumptive diagnosis and pathologic results, one should obtain a pathologic diagnosis. Each endoscopist can select from the available diagnostic methods, as per his or her individual preference.
Footnotes
Abbreviations: ESD = endoscopic submucosal dissection, EUS = endoscopic ultrasonography, EUS-FNA = endoscopic ultrasonography–guided fine needle aspiration, GIST = gastrointestinal stromal tumor, PM = proper muscle, SET = gastric subepithelial tumor, SM = submucosa.
The authors have no conflicts of interest to disclose.
References
- 1.Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006; 130:2217–2228. [DOI] [PubMed] [Google Scholar]
- 2.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991; 5:20–23. [DOI] [PubMed] [Google Scholar]
- 3.Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 2003; 57:68–72. [DOI] [PubMed] [Google Scholar]
- 4.Ji JS, Lee BI, Choi KY, et al. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med 2009; 24:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc 2010; 71:722–727. [DOI] [PubMed] [Google Scholar]
- 6.Gress F, Schmitt C, Savides T, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc 2001; 53:71–76. [DOI] [PubMed] [Google Scholar]
- 7.Buscaglia JM, Nagula S, Jayaraman V, et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc 2012; 75:1147–1152. [DOI] [PubMed] [Google Scholar]
- 8.Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc 2009; 69:1218–1223. [DOI] [PubMed] [Google Scholar]
- 9.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy 2009; 41:329–334. [DOI] [PubMed] [Google Scholar]
- 10.Vander Noot MR, 3rd, Eloubeidi MA, Chen VK, et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2004; 102:157–163. [DOI] [PubMed] [Google Scholar]
- 11.Alkhatib AA, Faigel DO. Endoscopic ultrasonography-guided diagnosis of subepithelial tumors. Gastrointest Endosc Clin N Am 2012; 22:187–205.vii. [DOI] [PubMed] [Google Scholar]
- 12.de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, et al. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc 2011; 74:672–676. [DOI] [PubMed] [Google Scholar]
- 13.Lee CK, Chung IK, Lee SH, et al. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc 2010; 71:188–194. [DOI] [PubMed] [Google Scholar]
- 14.Kim MY, Jung HY, Choi KD, et al. Natural history of asymptomatic small gastric subepithelial tumors. J Clin Gastroenterol 2011; 45:330–336. [DOI] [PubMed] [Google Scholar]
- 15.Song JH, Kim SG, Chung SJ, et al. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy 2015; 47:675–679. [DOI] [PubMed] [Google Scholar]
- 16.Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol 2010; 16:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]


