Abstract
The study aimed to investigate the impact of different surgical margins on recurrence-free survival (RFS) of patients with solitary hepatocellular carcinoma (HCC) without macroscopic vascular invasion.
The data of 586 selected patients who underwent curative hepatectomy for HCC between 2001 and 2012 were analyzed. The patients were divided into the anatomic resection and the nonanatomic resection groups according to the surgical approaches. Each group was further divided into group A (surgical margin <5 mm), group B (5 mm ≤ surgical margin < 10 mm), and group C (surgical margin ≥10 mm). Relationship between surgical margins and RFS in different groups was established by receiver operating characteristic curve and Kaplan–Meier analyses.
The RFS of the anatomic resection group was significantly longer than that of the nonanatomic resection group (P = 0.026). There were no statistical differences in RFS between groups A, B, and C (PA VS B = 0.512, PA VS C = 0.272, PB VS C = 0.822, nA = 38, nB = 43, nC = 80) in the anatomic resection group while in the nonanatomic resection group, RFSs of groups B and C were longer than that of group A (PA VS B = 0.009, PA VS C = 0.000, PB VS C = 0.505, nA = 151, nB = 119, nC = 155).
The analytic results suggest that if the patients with solitary HCC without macroscopic vascular invasion fall in the anatomic resection group, a minimal surgical margin (≥0 mm) is probably appropriate for hepatectomy; however, in cases of the nonanatomic resection, a surgical margin ≥5 mm should be regarded suitable for surgery of HCC.
Keywords: anatomic resection, hepatocellular carcinoma, nonanatomic resection, prognosis, recurrence, surgical margin
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common and most malignant tumors with incidence rate ranking the fifth and mortality rate the third among the malignant tumors in the world.[1] The incidence rate and prevalence level of HCC are the highest in the Southeast Asia and West Africa, but both are rising in the developed countries.[2] At present, the treatments for HCC mainly include liver resection, liver transplantation, radiofrequency ablation, transcatheter arterial chemoembolization, and drug therapy while surgical resection is the preferred way among the treatments.[3] With the surgical technology being gradually improved and preoperation period of the treatment becoming better, safety of liver resection and success rate have been raised, but the recurrence rate is still very high. How to reduce the recurrence rate and prolong recurrence-free survival (RFS) remain great challenges for surgeons. Generally, surgeons think that enough surgical margins are the premise of radical operation for HCC.[4] The surgical margin has been defined as the shortest distance between the edges of the tumor and the liver incision on the liver section. However, what an enough margin should be defined as and how operative margins at different tumor stages ought to be selected are still unclear.
The purpose of this study was to investigate influencing factors of recurrence after resection of HCC. Therefore, we retrospectively analyzed the clinical and pathological data and found strong evidence that for the patients with solitary HCC without macroscopic vascular invasion, different surgical approaches led to different prognosis of HCC and a proper surgical margin can prolong RFS in the nonanatomic resection group. We think that the findings have provided important references for selection of operational manners in hepatectomy.
2. Patients and methods
2.1. Patients
From January 1, 2001, to December 31, 2012, a total of 827 patients with HCC received the operation of R0 (R0 refers to no cancerous cells seen microscopically) hepatic resection in the Affiliated Hospital of Qingdao University. Among the 827 patients, a total of 586 patients with solitary HCC without macroscopic vascular invasion were analyzed. The patients included 486 male and 100 female cases with an average age of 55.2 years (from 17 to 83 years of age). Each surgical resection specimen of all cases was given a definite pathological diagnosis of HCC. According to the seventh edition of American Joint Committee on Cancer tumor node metastasis system,[5] all of the 586 HCC patients met the criterion of T1 stage “solitary tumor, without vascular invasion.” We collected a complete set of clinical and follow-up data from all of the patients for analyses. The Affiliated Hospital of Qingdao University Ethics Committee approved this study, and informed consent was obtained from each patient according to institutional review board protocols.
2.2. Surgical methods
All 586 cases underwent liver resection according to the Couinaud segmentation method to implement hepatic segmentectomy or combined resection for adjacent liver segments (anatomic resection) or partial hepatectomy containing tumor (nonanatomic resection). R0 hepatic resection refers to no cancer cells being residual on the surgical resection margins by a microscope and no tumor existing on the margins by naked eyes.[6]
2.3. Adjuvant therapy and follow-up
In the present study, patients with hepatitis B virus–HCC took nucleoside analogues as antiviral drugs, starting either before or after surgery. Adjuvant antiviral therapy with lamivudine 100 mg, adefovir dipivoxil 10 mg, or entecavir 0.5 mg orally daily was commenced within 1 week after operation or after discharge for some patients with the high viral load. In the first 3 months after operation, liver function, α-fetoprotein, abdominal B ultrasound, and chest X-ray were checked or performed once monthly. Patients would be rechecked with upper abdominal CT, lung CT, and (or) hepatic arterial lipiodol angiography if they were suspected of being recurrent. Postoperative recurrence was diagnosed and confirmed as tumor by imaging examination. If the recurrent tumor was located, a second liver resection, radiofrequency ablation, or percutaneous ethanol injection was suggested; if the recurrent tumor was multiple or diffused, transcatheter arterial chemoembolization and multitargeted kinase inhibitor sorafenib were the choice. Treatment decision was made based on the pattern of recurrence and liver function reserve. The follow-up ended on January 31, 2015, or when patients died. The median follow-up was 46.8 months (ranging 3.0–161.3 months).
2.4. Statistical analysis
Receiver operating characteristic (ROC) curve was utilized to evaluate the accuracy of surgical margin to predict HCC recurrence. Area under the curve (AUC) was calculated as measurements of the accuracy of the test. The Kaplan–Meier survival analysis (log-rank test) was used to analyze RFS time. The factors with P < 0.05 from the Kaplan–Meier analysis were enrolled in the Cox regression hazard model. Categorical variables were compared using the χ2 test or Fisher exact test. P values were 2 tailed. Statistical significance was accepted for P values <0.05. All of the data were statistically analyzed with the software SPSS Statistics for Windows, version 13.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Independent factors influencing RFS of patients after HCC resection
Since surgical margins of HCC resection are our interest of analyses in this study, we first analyzed the patient data and compared critical points for different surgical margins of hepatectomy. Based on the ROC curve, the optimal cutoff value of surgical margin as an indicator for predicting recurrence of solitary HCC without macroscopic vascular invasion was projected to be 4.5 mm, which yielded a maximum Youden index about 0.369 with the AUC at 0.681 (95% confidence interval [CI] 0.637–0.725) (P = 0.000). As all of our surgical margin data were shown with integers, we chose 5 mm as the cutoff value for surgical margin in the following analytic performance (Fig. 1).
Figure 1.
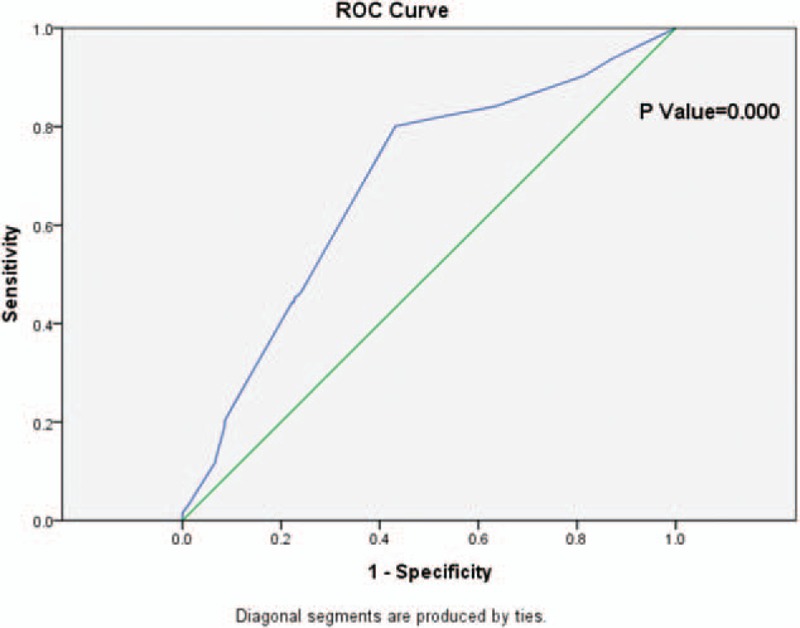
The ROC curve of surgical margin and HCC recurrence. Diagonal segments are produced by ties. HCC = hepatocellular carcinoma, ROC = receiver operating characteristic.
The data from 586 HCC patients with solitary HCC without macroscopic vascular invasion were analyzed by Kaplan–Meier analysis. The RFS rates of 1, 2, 5, and 10 years were 84.0%, 65.1%, 44.2%, and 33.8%, respectively; the median RFS time was 50.8 months. The overall survival rates of 1, 2, 5, and 10 years were 95.6%, 87.2%, 63.2%, and 38.3%, respectively; the median overall survival time was 85.4 months.
The analytic results also showed that male, preoperational alanine aminotransferase >40 U/L and γ-glutamyltransferase >64 U/L, α-fetoprotein >20 μg/L, intraoperative blood loss, blood transfusion, tumor diameter >5 cm, nonanatomic resection, and surgical margin <5 mm were important factors that had influenced the RFS time (P < 0.05). The results of Cox regression hazard model analysis showed that male, preoperational alanine aminotransferase >40 U/L, surgical margin <5 mm, and nonanatomic resection were independent risk factors influencing RFS (P < 0.05) (Table 1).
Table 1.
The influencing factors of the recurrence-free survival rate after R0 hepatectomy in 586 patients with HCC.
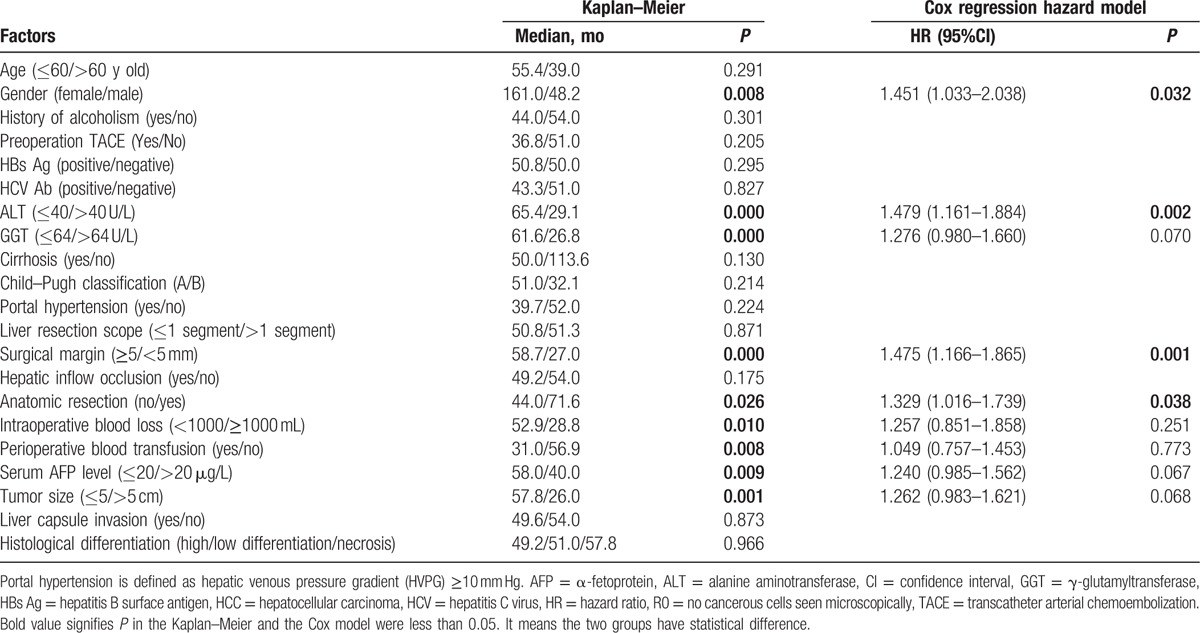
The analytic results indicate that surgical margin <5 mm is an important and independent factor influencing the RFS. Therefore, we focused on this factor and performed further analyses.
3.2. The RFS time of the patients through anatomic resection and nonanatomic resection
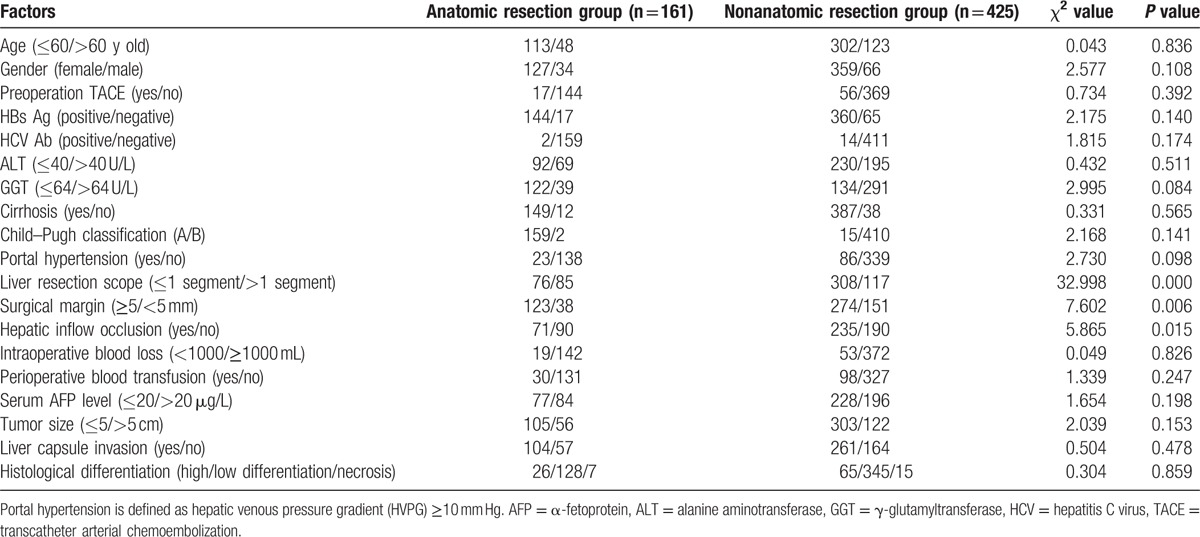
The 586 patients were classified into 2 groups: anatomic resection (n = 161) and nonanatomic resection (n = 425) according to the surgical approaches applied. The results of Kaplan–Meier analysis showed that in the anatomic resection group, the median RFS time was 71.6 months. The 1-, 2-, and 5-year RFS rates were 86.9%, 69.5%, and 49.9%, respectively. In the nonanatomic resection group, the median RFS time was 44.0 months. The 1-, 2-, and 5-year RFS rates were 82.9%, 63.2%, and 41.8%, respectively. The analysis showed that there was statistically significant difference in the RFS time between the 2 groups (P = 0.026). The RFS time of the anatomic resection group was significantly longer (Fig. 2). The result suggests that operational manner is important for survival of the HCC patients. Compared with the nonanatomic resection group, we also noticed that the resection scope for the anatomic resection group was always larger than 1 hepatic segment and more liver tissue was resected while less hepatic inflow occlusion occurred in the group (P < 0.05). Other clinicopathological data showed no obvious difference between the 2 groups (Table 2).
Figure 2.
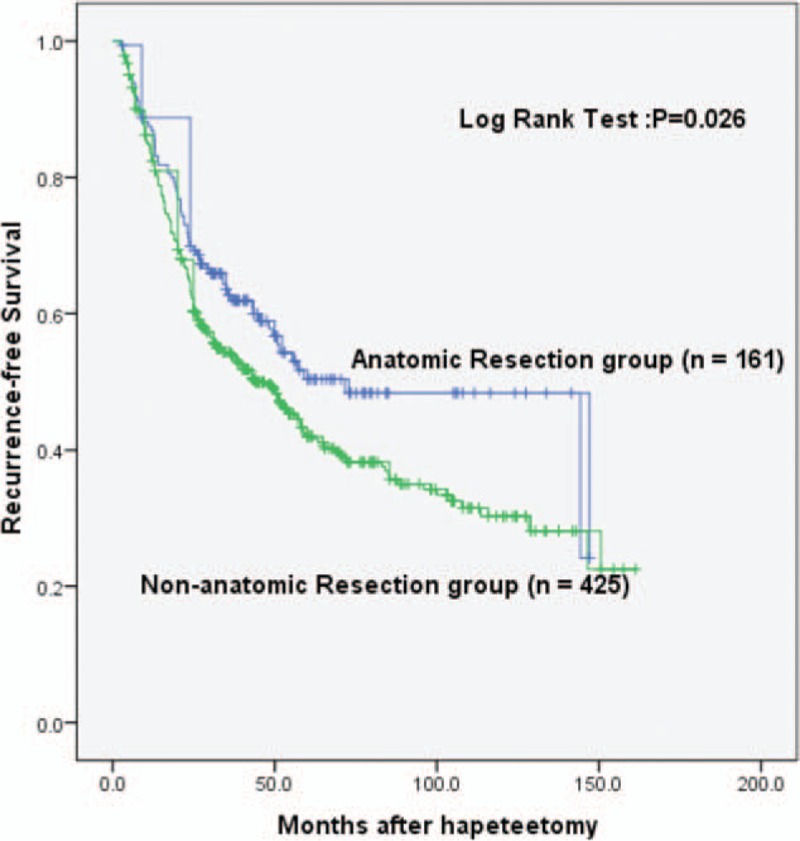
Comparison of RFS between the anatomic resection group and the nonanatomic resection group. RFS = recurrence-free survival.
Table 2.
Clinical pathological data in the anatomic resection group and the nonanatomic resection group.
3.3. RFS in the nonanatomic resection group with different surgical margins
From the ROC curve for surgical margins and HCC recurrence in the anatomic resection group, which yielded a maximum Youden index about 0.105, with the AUC at 0.567 (95% CI 0.478–0.656) (P = 0.142), there was no optimal cutoff value of surgical margin selected as an indicator for predicting recurrence (Fig. 3).
Figure 3.
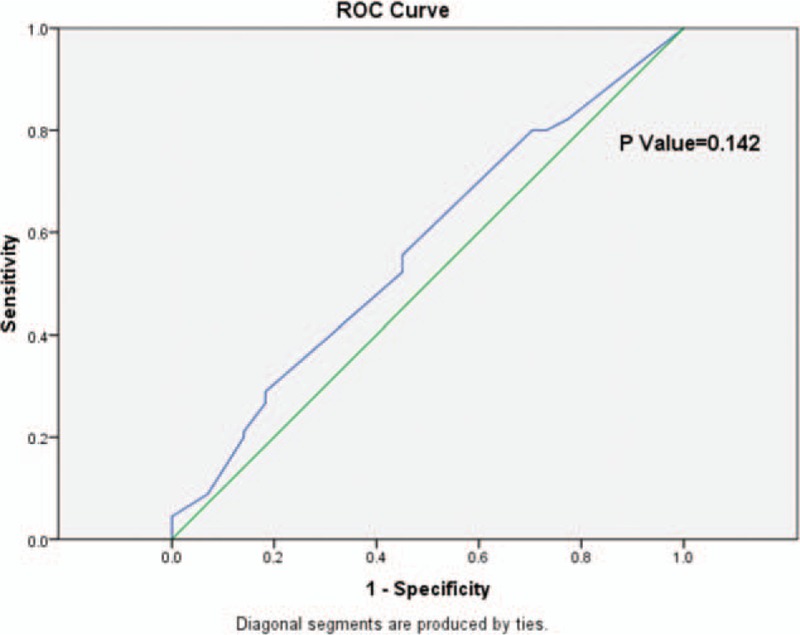
The ROC curve of surgical margins and HCC recurrence in the anatomic resection group. Diagonal segments are produced by ties. HCC = hepatocellular carcinoma, ROC = receiver operating characteristic.
According to the difference of surgical margins, the anatomic or the nonanatomic resection group was further divided into 3 groups: group A (surgical margin <5 mm), group B (5 mm ≤ surgical margin < 10 mm), and group C (surgical margin ≥10 mm). The results of Kaplan–Meier analysis showed that in the anatomic resection group, the 1-, 2-, and 5-year RFS rates were 84.7%, 67.7%, and 40.9%, respectively, for group A; 87.7%, 71.1%, and 49.2%, respectively, for group B; and 86.2%, 69.4%, and 55.0%, respectively, for group C. There were no statistical differences between the 3 groups (PA VS B = 0.512, PA VS C = 0.272, PB VS C = 0.822, nA = 38, nB = 43, nC = 80) (Fig. 4). The results suggest that a surgical margin as narrow as <5 mm was not related to the RFS time. Therefore, a minimum surgical margin (surgical margins probably 0 to <5 mm) in the anatomic resection could be considered appropriate for hepatectomy.
Figure 4.
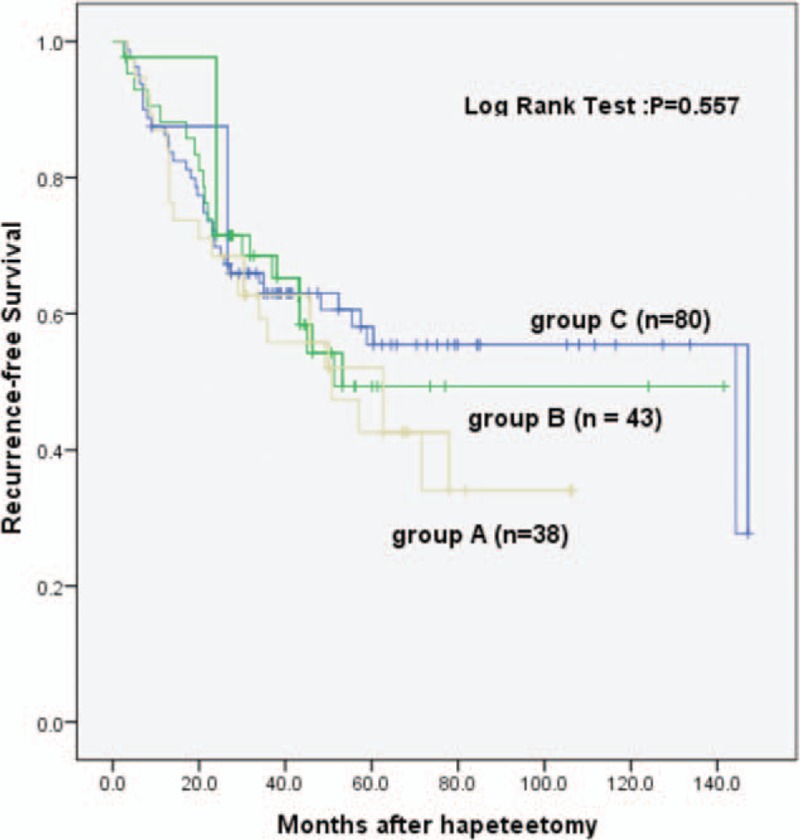
Recurrence-free survival analysis of different surgical margins in the anatomic resection group.
In the nonanatomic resection group, based on the ROC curve, the optimal cutoff value of surgical margin as an indicator for predicting recurrence was projected to be 4.5 mm, which, however, yielded a sensitivity of 64.8% and a specificity of 80.1%, with the AUC at 0.719 (95% CI 0.669–0.769), giving a maximum Youden index about 0.449 (P = 0.000) (Fig. 5).
Figure 5.
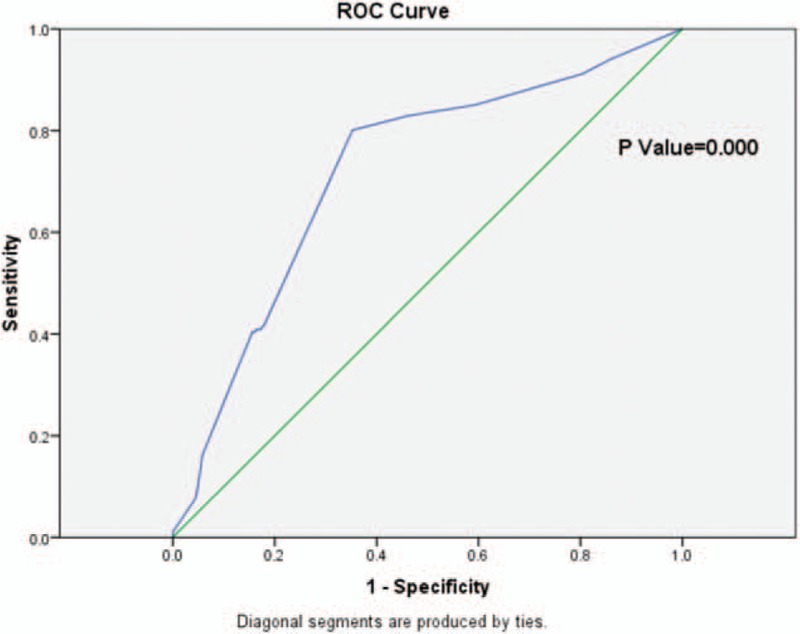
The ROC curve of surgical margin and HCC recurrence in the nonanatomic resection group. Diagonal segments are produced by ties. HCC = hepatocellular carcinoma, ROC = receiver operating characteristic.
Unlike the anatomic resection, however, in this group, the 1-, 2-, and 5-year RFS rates were 77.7%, 51.3%, and 33.4%, respectively, for group A; 83.1%, 66.5%, and 43.5%, respectively, for group B; and 86.9%, 71.5%, and 48.2%, respectively, for group C. The RFSs of groups B and C were significantly longer than that of group A while there were no statistical differences between groups B and C (PA VS B = 0.009, PA VS C = 0.000, PB VS C = 0.505, nA = 151, nB = 119, nC = 155) (Fig. 6). The results suggest that in the nonanatomic resection, a narrow surgical margin (<5 mm) was associated with a shortened RFS. And we have further analyzed that in the nonanatomic resection group, surgical margin <5 mm has an early recurrence of HCC than surgical margin ≥5 mm (the recurrence of HCC within 2 years) (P < 0.05) (Table 3).
Figure 6.
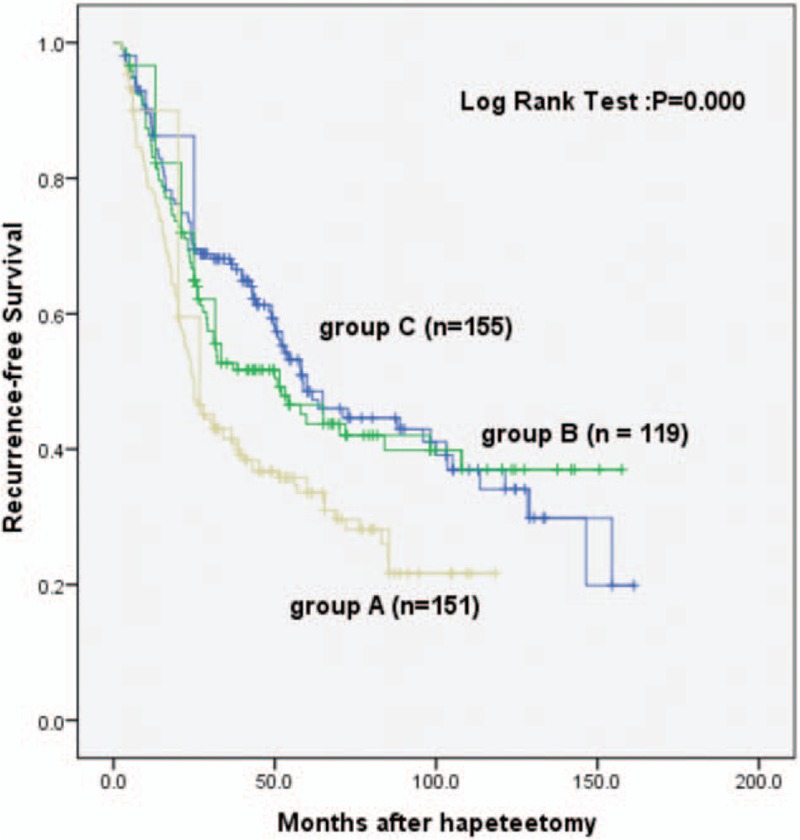
Recurrence-free survival analysis of different surgical margins in the nonanatomic resection group.
Table 3.
The relationship between different surgical margins and time of HCC recurrence in the nonanatomic resection group.

4. Discussion
4.1. Different viewpoints on the selection of surgical margins in HCC resection
Previous studies identified that such factors as tumor and surgical treatments were associated with higher incidences of tumor recurrence.[7,8] The effect of surgical margins in hepatectomy on prognosis has always been a hot spot in the field of surgery. Several studies have confirmed the surgical safety margin as a crucial factor that influences the survival of patients undergoing traditional or laparoscopic liver resection.[9,10] Shi et al [11] reported the effect of surgical margins, which included <10, 10, 10 to 20, and >20 mm on postoperative recurrence of HCC, tumor-free survival rate, and cumulative survival rate. The conclusion of the study is that determination of a surgical margin should be based on the direction of blood flow in the portal vein, and a standard extent of hepatic resection with surgical margins 10 mm at the proximal end but 20 mm at distal end of the portal vein extended tumor-free survival time and overall survival time of postoperative patients with HCC.[12] Poon reported that the postoperative recurrence analysis of 288 cases of HCC patients without residual cancer in the naked eye showed that a 10-mm surgical margin would not affect the recurrence rate. Majority of intrahepatic recurrence was due to intravascular dissemination of tumor cells and even a larger surgical margin could not avoid its occurrence.[13] Zhou et al [14] reported that the minimal lengths of the resection margin were required to be 5.5 and 6 mm to achieve 99% and 100% micrometastasis clearance, respectively, in the surrounding liver of HCC patients without macroscopic tumor thrombi or macrosatellites. Lee et al[15] reported that there were no differences in the prognosis between the patients with cancer 5 mm off the cut edge and those with cancer 10 mm off the cut edge.[15] Nara et al [16] reported that marginal resection was acceptable in most HCC patients. Ker et al[17] reported that no significant differences were observed in the survival rates of the patients with 5 to 9 and >10 mm safety margins. From the previous discussion, it seems that controversies about the surgical margins of HCC resection exist in different research institutes.
4.2. Selection of HCC patients for anatomic or nonanatomic resection
Although different kinds of retrospective studies have been reported to date, each study reported a different result. We will never reach “the truth” regarding this issue unless a well-designed randomized control trial is performed. As is known, the selection biases are inevitable in retrospective comparisons of postoperative outcomes in patients who underwent either anatomic resection or nonanatomic resection for HCC, in that patients with poorer liver function tended to undergo nonanatomic resection, whereas patients with macroscopic vascular invasion tended to undergo anatomic resection.[18–20] In order to eliminate the selection biases as much as possible, we excluded the patients with multiple HCCs and macroscopic vascular invasion for screening the clinicopathological characteristics of patients. In this research, we selected 586 cases of HCC patients who had a solitary tumor with no vascular invasion. We conducted a stratified study of patients who were divided into anatomic liver resection group and nonanatomic liver resection group according to the surgical approaches.
4.3. Explanation of outcomes due to different surgical margins in anatomic and nonanatomic resection
For the anatomic resection, postoperative RFS was unaffected when the resection margin was <5 mm. This is explained with the blood supply of HCC and the characteristics of microvascular invasion (MVI).[21] Studies have showed that HCC forms vascular plexus, which is double supplied by hepatic artery and portal vein in the early growth stage. Central locations of tumors are mainly supplied by hepatic arteries but peripheral locations mainly by portal veins. Since the peripheral tissues of HCC grow actively, a portal vein is important for invasive growth of HCC. The cancer cells adhere to the portal vein wall and gradually form a micro–portal vein tumor thrombus.[22] Therefore, in the anatomic resection of liver, since the Glisson pipelines are ligated and cut off in advance, more MVI lesions can be removed regardless of surgical margins when liver parenchyma is dissected.[11] This probably explains why postoperative RFS for the anatomic resection was unaffected when the resection margins were from 0 to 5 mm.
For the nonanatomic resection, if a surgical margin is narrow and little amount of micrometastasis of hepatic portal vein system is removed, this may lead to more micrometastasis in liver or distant organs.[14] Due to the presence of immune system, only a minority of patients eventually advance to clinical metastasis. But the presence of MVI is still the premise and origin of overt metastases in clinic, while in the nonanatomic liver resection, it may accelerate desquamation, intrahepatic dissemination, and distant metastasis of cancer tissue or cell because of tumor compression in operation. According to the theory of “seed and soil,”[23] the paraneoplastic tumor microenvironment is one of the limitations that decide whether or not a tumor transfers and recurs. A study has found that the presence of inflammation in the liver tissue microenvironment can selectively clone and amplify variant cells and form a tumor lesion.[24] At the same time, because of the narrow surgical margin, the operation could not clean up as much as possible micrometastases in intrahepatic portal venous system, which likely causes relapse after surgery as “seed and soil.” In our study, a narrow surgical margin (<5 mm) is connected with early recurrence of HCC (P < 0.05). There is a tendency that intrahepatic recurrence due to disseminated cells occurs within 2 years after hepatectomy and multicentric recurrence occurs 2 years after hepatectomy.[25] A narrow surgical margin could not be effective for prevention of intrahepatic recurrence due to disseminated cells. These may be the reasons for the short RFS of a narrow surgical margin (<5 mm) after operation in the nonanatomic resection.
Another aspect that is worth considering is that there are many HCC patients who frequently suffer from other hepatic complications. Nonanatomic liver resection with less hepatic resection volume is gradually replacing the anatomic liver resection and has become a main type of operation in order to reduce possible liver dysfunction of postoperation. Some tumors, especially small ones, are mostly located in liver parenchyma or close to the important pipeline in the liver. In the nonanatomic liver resection, a larger volume of liver tissue may be removed in order to obtain a larger surgical margin. Here, the priority should be considered to ensure the integrity of the important pipeline of liver. Since resection of excessive liver tissue may lead to postoperative liver dysfunction,[27] a 5- mm margin in the nonanatomic resection group may be regarded as the suitable surgical margin for the surgery of HCC.
In general, the prognosis of solid tumor is related to the stage of tumor and to the clinical treatment. However, the prognosis of HCC is very complicated, because other hepatic complications also affect the prognosis of HCC.[28] Most of the primary HCC occurs from original chronic liver disease or cirrhosis. Liver cirrhosis, portal hypertension, and liver dysfunction exert a significant adverse effect on the prognosis of HCC.[26,29] Therefore, most HCC patients actually suffer from 2 kinds of diseases that affect each other. The progression of HCC exacerbated the deterioration of liver function and poor liver function of the patients cannot tolerate active treatment. Furthermore, studies have shown that patients with chronic liver diseases increase the likelihood of recurrence after resection of HCC, compared with patients with noncirrhotic, nonfibrotic, seronegative liver.[30] In this study, both single-factor and multiple-factor analyses showed that cirrhosis was not a risk factor for the prognosis of HCC. This may be related to the proportion of patients with cirrhosis, which accounted for 91.5% (536/586), while the ratio of patients without cirrhosis accounted for only 8.5% (50/586). Incidentally, this study belongs to a single center and has certain limitations that may cause some deviation of the results.
In summary, the results of this study showed that different surgical approaches led to different prognosis of HCC. HCC patients who have a solitary tumor without macroscopic vascular invasion are suggested to undergo anatomic hepatectomy with a minimal surgical margin being sufficient for hepatectomy, even if surgical margin is as small as ≥0 mm. For a nonanatomic hepatic resection, surgeons should pay attention to the surgical margin and make sure that the surgical margin is >5 mm, which is considered as a safe and feasible surgical margin.
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, HCC = hepatocellular carcinoma, MVI = microvascular invasion, R0 = no cancerous cells seen microscopically, RFS = recurrence-free survival, ROC = receiver operating characteristic.
This study was supported by the Funding for Excellent Departments (Qingdao University) to LW and the National Natural Science Foundation of China (31371152) to ZQ.
The authors have no conflicts of interest to disclose.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Galun D, Basaric D, Zuvela M, et al. Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol 2015; 7:2274–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graf D, Vallböhmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med 2014; 25:430–437. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, Pang X, Guo W, et al. Relationship of different surgical margins with recurrence-free survival in patients with hepatocellular carcinoma. Int J Clin Exp Pathol 2015; 8:3404–3409. [PMC free article] [PubMed] [Google Scholar]
- 5.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 6.Paul Hermanek MD, Christian Wittekind MD. Residual tumor (R) classification and prognosis. Semin Surg Oncol 1994; 10:12–20. [DOI] [PubMed] [Google Scholar]
- 7.Makuuchi M, Imamura H, Sugawara Y, et al. Progress in surgical treatment of hepatocellular carcinoma. Oncology 2016; 62 (suppl 1):74–81. [DOI] [PubMed] [Google Scholar]
- 8.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol 2003; 38:200–207. [DOI] [PubMed] [Google Scholar]
- 9.Jeng KS, Jeng WJ, Sheen IS, et al. Is less than 5 mm as the narrowest surgical margin width in central resections of hepatocellular carcinoma justified? Am J Surg 2013; 206:64–71. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X-B, Wang J-H, Yan Z-P, et al. Hepatocellular carcinoma with main portal vein tumor thrombus. Cancer 2009; 115:1245–1252. [DOI] [PubMed] [Google Scholar]
- 11.Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007; 245:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakon M, Ogawa H, Fujita M, et al. Hepatic resection for hepatocellular carcinoma based on tumor hemodynamics. Hepatol Res 2013; 43:155–164. [DOI] [PubMed] [Google Scholar]
- 13.Poon RTP. Significance of resection margin in hepatectomy for hepatocellular carcinoma. Ann Surg 2000; 231:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou XP, Quan ZW, Cong WM, et al. Micrometastasis in surrounding liver and the minimal length of resection margin of primary liver cancer. World J Gastroenterol 2007; 13:4498–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KT, Wang SN, Su RW, et al. Is wider surgical margin justified for better clinical outcomes in patients with resectable hepatocellular carcinoma? J Formos Med Assoc 2012; 111:160–170. [DOI] [PubMed] [Google Scholar]
- 16.Nara S, Shimada K, Sakamoto Y, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery 2012; 151:526–536. [DOI] [PubMed] [Google Scholar]
- 17.Ker CG, Chen HY, Chen HJ, et al. Challenge of safety margin in laparoscopic liver resection for hepatocellular carcinoma. Formos J Surg 2014; 47:183–188. [Google Scholar]
- 18.Dahiya D, Wu TJ, Lee CF, et al. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery 2010; 147:676–685. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa K, Kokudo N, Imamura H, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 2005; 242:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno S, Kubo F, Sakoda M, et al. Efficacy of anatomic resection vs nonanatomic resection for small nodular hepatocellular carcinoma based on gross classification. J Hepatobiliary Pancreat Surg 2008; 15:493–500. [DOI] [PubMed] [Google Scholar]
- 21.Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008; 15:1375–1382. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki K, Matsuda M, Yu O, et al. Minimum resection margin should be based on tumor size in hepatectomy for hepatocellular carcinoma in hepatoviral infection patients. Hepatol Res 2013; 43:1295–1303. [DOI] [PubMed] [Google Scholar]
- 23.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3:453–458. [DOI] [PubMed] [Google Scholar]
- 24.Leonardi GC, Candido S, Cervello M, et al. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol 2012; 40:1733–1747. [DOI] [PubMed] [Google Scholar]
- 25.Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006; 243:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology 2007; 132:1261–1269. [DOI] [PubMed] [Google Scholar]
- 27.Tomimaru Y, Eguchi H, Marubashi S, et al. Equivalent outcomes after anatomical and non-anatomical resection of small hepatocellular carcinoma in patients with preserved liver function. Dig Dis Sci 2012; 57:1942–1948. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013; 144:512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127 (5 suppl 1):S35–S50. [DOI] [PubMed] [Google Scholar]
- 30.Young AL, Adair R, Prasad KR, et al. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. J Am Coll Surg 2012; 214:174–183. [DOI] [PubMed] [Google Scholar]


