Supplemental Digital Content is available in the text
Keywords: adefovir dipivoxil, entecavir, hepatitis B virus, kidney injury molecule-1, renal impairment
Abstract
The aim of this study was to evaluate serum kidney injury molecule-1 (KIM-1) as a new diagnostic marker of renal dysfunction in chronic hepatitis B (CHB) patients receiving long-term adefovir dipivoxil (ADV) treatment.
We retrospectively enrolled 85 patients treated with ADV and 85 patients treated with entecavir (ETV) monotherapy, for at least 6 months. The 2 groups were matched for baseline age (± 5 years), sex, and estimated glomerular filtration rate (eGFR). Serum creatinine, cystatin C, and KIM-1 concentrations were measured, and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine–cystatin C equation, at baseline and last follow-up.
eGFR decreased by 10–20% from baseline in 11/85 (14.1%) patients, 20–30% in 5/85 (5.9%), and ≥ 30% in 2/85 (2.4%) patients treated with ADV. Serum KIM-1 was more significantly increased after ADV treatment 86.53 (10.20–355.40) pg/mL than ETV treatment 61.54 (10.53–200.56) pg/mL (P < 0.01). Furthermore, serum KIM-1 was positively correlated with serum cystatin C (r = 0.47; P < 0.001) and negatively correlated with eGFR (r = -0.46; P < 0.001). The area under the receiver operating characteristic curve (AUC-ROC) of serum KIM-1 for identifying renal dysfunction in all enrolled patients was 0.94 (95% confidence interval [95% CI], 0.87 to 1.02; P < 0.001), while the AUC-ROC of serum creatinine was only 0.82 (95% CI, 0.60 to 1.03; P < 0.01).
Serum KIM-1 is a promising new diagnostic biomarker of renal dysfunction during long-term ADV therapy for CHB patients.
1. Introduction
Chronic hepatitis B virus (HBV) infection is a major public health problem, affecting more than 240 million people worldwide.[1] Infected individuals are at an increased risk of developing liver cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC), conditions that contribute to the high morbidity and mortality associated with HBV infection. Antiviral therapy can achieve sustained suppression of HBV replication and improve biochemical and histological indicators of liver disease, thereby slowing chronic hepatitis B (CHB) disease progression. Currently approved antiviral agents for CHB include interferon-alpha (IFN α) or pegylated interferon-alpha (PEG-IFN α) and nucleos(t)ide analogues (NUC). Although long-term use of oral NUC is associated with favorable efficacy and acceptable overall tolerance, drug resistance and side effects have been reported.
Adefovir dipivoxil (ADV) is an orally bioavailable prodrug of adefovir, an adenosine monophosphate analogue.[2] It is now widely used as a rescue therapy in patients infected with viruses resistant to lamivudine (LAM), telbivudine, and entecavir (ETV).[3,4] However, ADV is increasingly associated with nephrotoxicity. ADV nephrotoxicity is reported to occur in patients administered doses of 60 mg/d or 120 mg/d,[5] whereas low doses of 10 mg/d are usually considered safe for the kidney.[6,7] However, renal dysfunction was recently reported in HBV-infected patients administered 10 mg/d ADV.[8,9] ADV-associated nephrotoxicity can cause increased serum creatinine, decreased estimated glomerular filtration rate (eGFR), hypophosphatemia, and Fanconi syndrome,[9,10] and certain patients may be genetically predisposed to ADV-associated nephrotoxicity.[11] However, routine renal function tests measuring serum creatinine, blood urea nitrogen (BUN), and eGFR, are likely to lack the sensitivity and specificity required for diagnosis of renal dysfunction and structural kidney injury in patients undergoing long-term ADV treatment. Therefore, identification of reliable and sensitive biomarkers of early renal dysfunction may facilitate early diagnosis, and improve the prognosis of CHB patients.
Kidney injury molecule-1 (KIM-1) is a type I transmembrane glycoprotein, highly expressed in epithelial cells in damaged regions of the renal proximal tubule. In both animal models and humans, urinary KIM-1 has been shown to be upregulated during acute kidney injury (AKI) caused by toxicity, ischemia, sepsis, and renal cell carcinoma.[12] Elevations in urinary KIM-1 are significantly associated with gentamicin, cisplatin, vancomycin, and tacrolimus–induced kidney injury.[13] Thus, KIM-1 has been qualified by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) as a highly sensitive and specific urinary biomarker for monitoring drug-induced kidney injury.[13,14] In addition to being present in urine, KIM-1 could also be released into the bloodstream as a result of altered microvascular permeability in humans, rats, and mice with AKI or chronic kidney disease (CKD).[15] Circulating KIM-1 was recently reported to serve as a sensitive prognostic indicator in patients who were overdosed with acetaminophen.[16] In this study, we aimed to investigate whether serum KIM-1 can be used as a reliable biomarker for the diagnosis of renal dysfunction in CHB patients receiving long-term ADV treatment.
2. Patients and methods
2.1. Patients
We performed a retrospective cohort study of 156 CHB patients treated for more than 6 months between January 2010 and August 2015 with 10 mg/d ADV in the Third Affiliated Hospital of Hebei Medical University and the Fifth Hospital of Shijiazhuang. An additional 169 CHB patients treated with ETV during the same period were selected as the comparison group. Both groups were matched for baseline age (± 5 years), sex, and eGFR classification (Fig. 1). The matched-pair platform consisted of 85 patients treated with ADV therapy and 85 patients with ETV monotherapy. All patients had a baseline eGFR ≥ 80 mL/min. Exclusion criteria were as follows: positivity for antibodies to human immunodeficiency virus or hepatitis C virus, diuretic users, hypertension, diabetes mellitus, HCC or other malignancy, autoimmune hepatitis, hepatic decompensation, alcoholic liver cirrhosis, severe heart and renal diseases, or the patients who take drugs that may affect the levels of KIM-1 (e.g., gentamicin, cisplatin, vancomycin, and tacrolimus).
Figure 1.
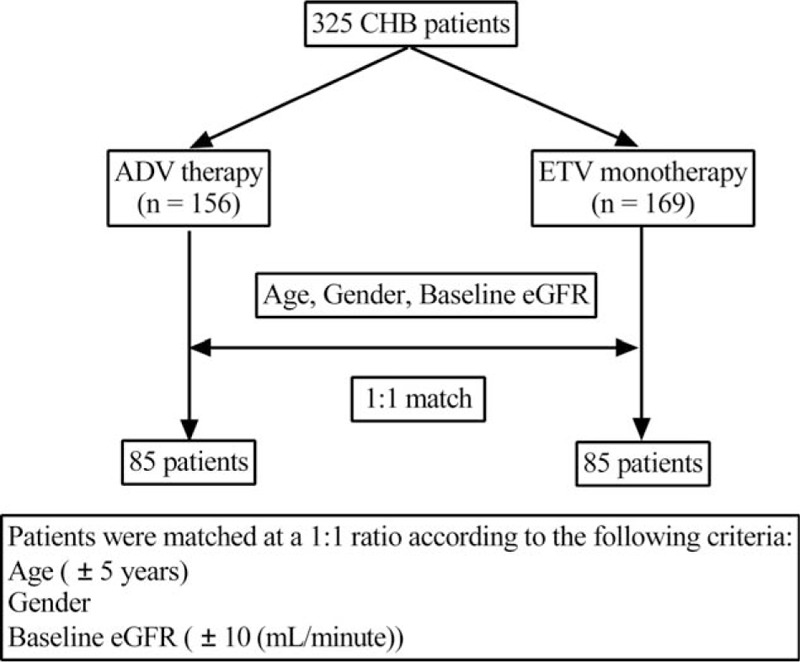
Flowchart of studied patients. ADV = adefovir dipivoxil, CHB = chronic hepatitis B, eGFR = estimated glomerular filtration rate, ETV = entecavir.
The study protocol was approved by the Ethics Committee of the Third Affiliated Hospital of Hebei Medical University, and written informed consent was obtained from each subject during the screening period.
2.2. Study protocol
Patients visited our hospital every 3 months after the initiation of ADV or ETV treatment and routine virological and biochemical markers were measured. eGFR level was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine–cystatin C equation as follows: 135 × min (Scr/κ, 1)α × max (Scr/κ, 1)−0.601 × min (Scys/0.8, 1)−0.375 × max (Scys/0.8, 1)−0.711 × 0.995 Age [× 0.969 if female] [× 1.08 if black], where Scr is serum creatinine, Scys is serum cystatin C, κ is 0.7 for females and 0.9 for males, α is −0.248 for females and −0.207 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.[17] Serum cystatin C was measured by particle-enhanced nephelometric immunoassay. Renal impairment was classified as follows: unimpaired (eGFR ≥ 80 mL/min), mildly impaired (50 mL/min ≤ eGFR < 80 mL/min), moderately impaired (30 mL/min ≤ eGFR < 50 mL/min), and severely impaired (eGFR ≤ 30 mL/min).[8]
2.3. Serum KIM-1 quantification
Serum KIM-1 concentration was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (RayBiotech, Norcross, GA). The detection range of the kit was 2–3000 pg/mL. Intra-assay and inter-assay precision were % coefficients of variation: <10% and <12%, respectively. The minimum detectable dose of KIM-1 was 2 pg/mL. Mean (95% confidence interval [CI]) plasma KIM-1 concentration in healthy volunteers was reported to be 64.4 (51.0–77.7) pg/mL.[15]
2.4. Statistical analysis
Data were analyzed using PRISM 6.0 (GraphPad Software, Inc., San Diego, CA) and SPSS 17.0 (SPSS, Chicago, IL). Descriptive statistics were reported as proportion (%) for categorical variables, and mean ± standard deviation or median (range) for continuous variables. Categorical variables were evaluated using the χ2 test. Normally distributed data were analyzed using Student's t-test. For non-normally distributed data, differences between groups were analyzed using Mann–Whitney U test. Bivariate correlation analysis was assessed by Spearman correlation test. Receiver operating characteristic curves (ROC) and the area under the ROC curve (AUC) were calculated to analyze the predictive value of the biomarkers. The Cox proportional hazard regression model was used to estimate univariate and multivariate risk factors for serum KIM-1 abnormality. Statistical significance was defined with a 2-tailed P-value < 0.05.
3. Results
3.1. Baseline clinical characteristics
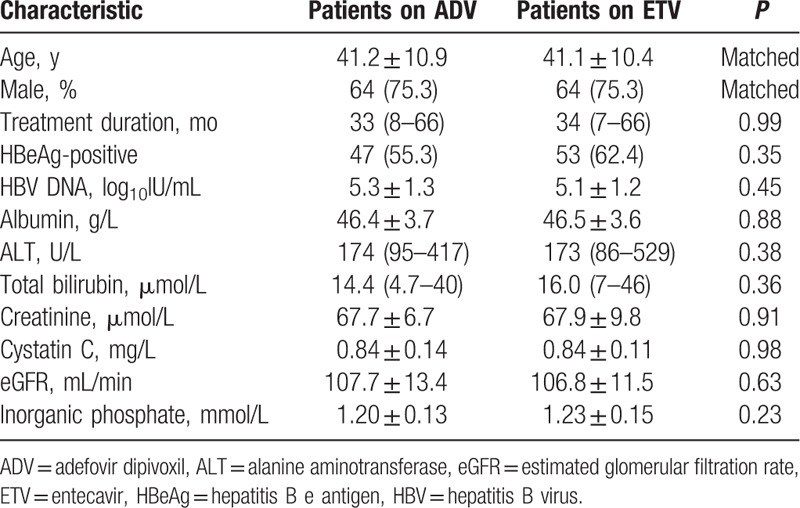
A total of 85 CHB patients prescribed ADV and 85 CHB patients prescribed ETV were included in this retrospective cohort study. Baseline characteristics of the groups are presented in Table 1. The median duration of ADV therapy was 33 months (range: 8–66) and the median duration of ETV monotherapy was 34 months (range: 7–66). The ADV group and ETV group were well-matched with a similar baseline mean age, sex ratio, and eGFR classification.
Table 1.
Baseline characteristics of all patients.
3.2. ADV-related nephrotoxicity
The incidence and severity of decreased eGFR in the ADV group differed significantly from that in the ETV group. In the ADV group, eGFR decreased by 10–20% from baseline in 12/85 (14.1%) patients, 20–30% in 5/85 (5.9%), and ≥ 30% in 2/85 (2.4%) patients, and 8 patients (9.4%) developed mildly impaired renal function. In the ETV group, serum creatinine, serum cystatin C, and eGFR remained stable over the treatment period (Fig. 2A–C). Serum KIM-1 was significantly higher in the ADV group, 86.53 (10.20–355.40) pg/mL, than the ETV group, 61.54 (10.53–200.56) pg/mL (P < 0.01) (Fig. 2D). Serum KIM-1 was positively correlated with serum cystatin C (r = 0.47; P < 0.001) (Fig. 2E) and negatively correlated with eGFR (r = -0.46; P < 0.001) (Fig. 2F). In the ADV group, there were 37 patients treated with ADV monotherapy and 48 patients treated with ADV combination (Supplementary Table 1). There was no significant difference of kidney injury in the patients either treated with ADV alone or in combination with LAM (Supplementary Figure 1). Therefore, we did not probe further.
Figure 2.
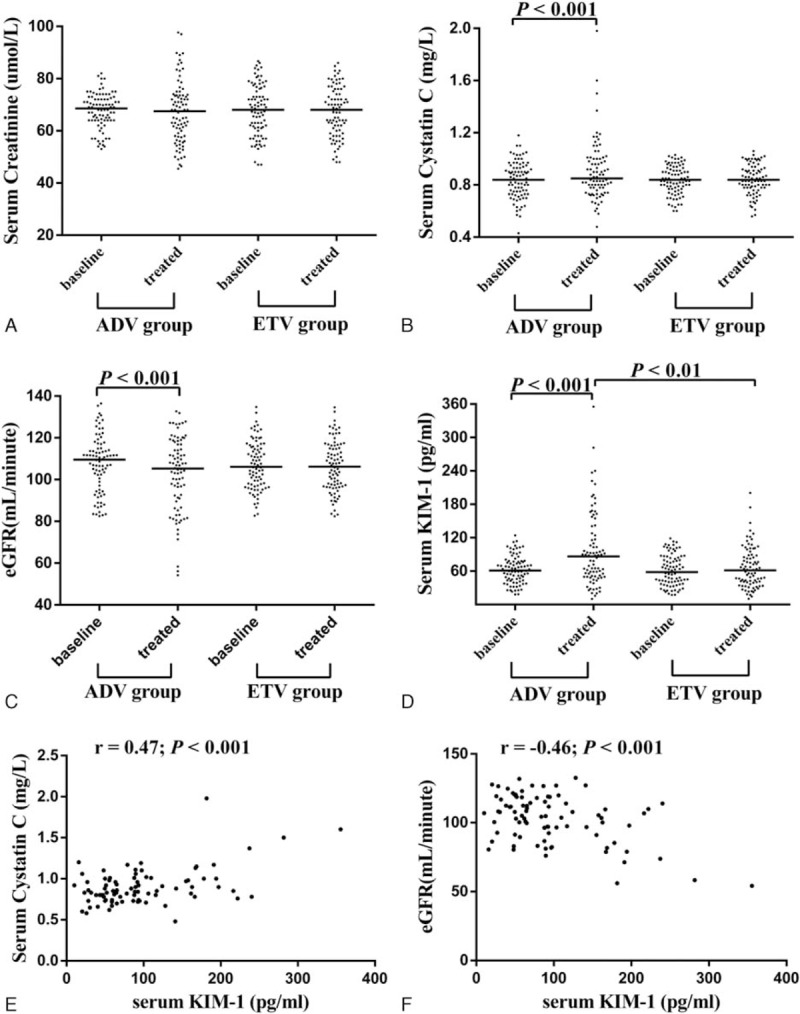
Evolution of various renal dysfunction indicators from baseline. Dot plots indicate serum creatinine (A), serum cystatin C (B), estimated glomerular filtration rate (eGFR) (C), and serum kidney injury molecule-1 (KIM-1) (D) for each patient. Scatterplot demonstrating a correlation between serum KIM-1 levels and serum cystatin C levels (r = 0.47; P < 0.001) (E), scatterplot demonstrating a correlation between serum KIM-1 levels and eGFR levels (r = -0.46; P < 0.001) (F).
3.3. Diagnostic value of serum KIM-1
The AUC-ROC of serum KIM-1 for identifying renal dysfunction from all these populations, including both ADV and ETV treatment groups, was 0.94 (95% CI, 0.87 to 1.02; P < 0.001), while the AUC-ROC of serum creatinine was only 0.82 (95% CI, 0.60 to 1.03; P < 0.01) (Fig. 3). The cut-off of serum KIM-1 for diagnosis of renal dysfunction was 166.8 pg/mL, and sensitivity, specificity, positive predictive value, and negative predictive value were 88%, 95%, 47%, and 99%, respectively.
Figure 3.
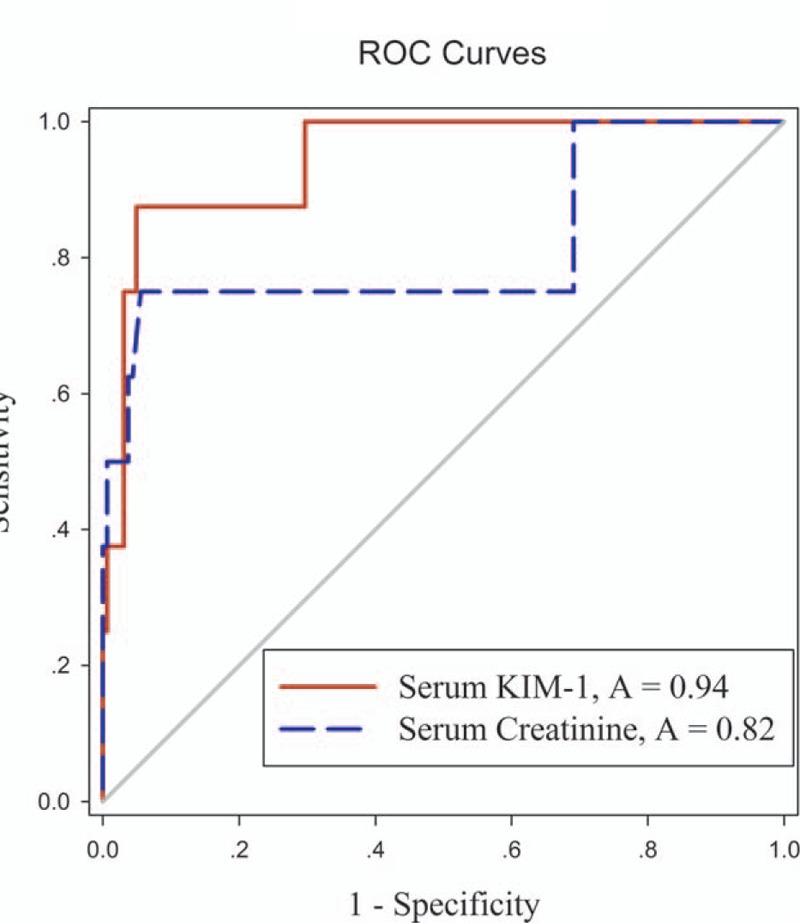
ROC curve analysis comparing performance of serum KIM-1 (solid red line, AUC 0.94) and serum creatinine (dashed blue line, AUC 0.82) levels. AUC = area under the ROC curve, KIM-1 = kidney injury molecule-1, ROC = receiver operating characteristic curve.
3.4. Predictive factors for serum KIM-1 abnormality
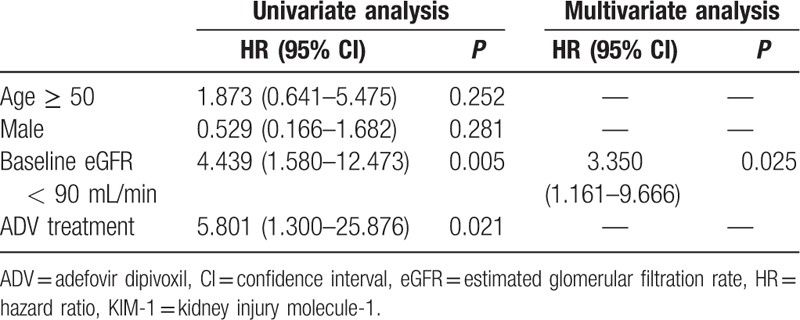
Serum levels of KIM-1 over 166.8 pg/mL were considered abnormal. Univariate analysis (Table 2) indicated that baseline eGFR < 90 mL/min (P = 0.005), and ADV treatment (P = 0.021) were significantly associated with the development of serum KIM-1 abnormality (P < 0.05). Multivariate analysis (Table 2) indicated that baseline eGFR < 90 mL/min (P = 0.025) was a significant predictor of serum KIM-1 abnormality.
Table 2.
Determinants of serum KIM-1 abnormality.
4. Discussion
Although major international guidelines recommend both ETV and tenofovir as first-line therapies for CHB,[18–20] ADV is widely used in some Asian countries due to the low cost and medical convention, as in the early 2000s ADV was the only alternative therapy for patients who developed LAM resistance. Accumulating evidence that ADV nephrotoxicity is dose-related has since caused the daily oral dose of ADV to be reduced from 120 mg to 10 mg.[5,7,21]
ADV is known to enter proximal kidney tubule cells through the basolateral human organic anion transporter 1 (hOAT1) and to be transported from tubule cells to the tubular lumen through multidrug resistance protein 2 (MRP2). As HBV DNA integrates into the human hepatocyte genomic DNA, long-term therapy is required, but extensive intracellular drug accumulation is associated with ADV nephrotoxicity.[22] In a study of long-term therapy with ADV in 125 HBeAg-negative CHB patients for up to 5 years, 5% of patients experienced slight elevations in serum creatinine levels.[23] In an open-label trial of long-term ADV therapy in 226 liver transplant candidates and 241 transplanted patients with LAM-resistant HBV infection, nephrotoxicity was observed in 6% and 21% of cases, respectively.[24] In another retrospective study of 687 patients, over a median treatment duration of 27 months, 10.5% of patients developed renal impairment, defined by a decrease of >20% from baseline eGFR.[25]
In this study, we found that during a median of 33 months treatment with ADV, eGFR was decreased ≥ 10% from baseline in approximately 22.0% patients. Furthermore, only 8 patients developed adefovir-related mild renal dysfunction. The rates of eGFR depression are less pronounced than those reported previously,[8,9,25] likely due to differences in patient number and ethnicity, in eGFR calculation methodology, and in the definition of renal dysfunction. Indeed, routine renal function was generally estimated by the rise in serum creatinine, a parameter known to be affected by many factors such as age, sex, race, muscle mass, metabolism, hydration status, nutrition status, medications. Recently, several urinary proteins such as β2-M, retinol-binding protein (RBP), neutrophil gelatinase-associated lipocalin (NGAL), and interleukin-18 (IL-18) have been evaluated as noninvasive indicators of kidney injury.[26–28] However, these markers may not make ideal indicators as they can be unstable in the urine, modified as a result of urine physicochemical properties, or appear relatively late following kidney injury, and high-throughput methods for their detection are not well established.[29] In addition, the consistency of their upregulation may vary between models of nephrotoxicity and upregulation may not be sustained throughout the time course of renal dysfunction, preventing accurate monitoring of injury progression and regression.[29] KIM-1 is one of the most promising biomarkers for renal dysfunction due to the consistent results of preclinical and clinical trials. Measurement of KIM-1 is noninvasive, as KIM-1 is easily detectable in accessible body fluids (e.g., serum or urine), and highly sensitive and specific methods for detecting AKI rapidly and reliably may facilitate early detection of AKI and prediction of AKI severity and prognosis, unaffected by other biological variables.[30]
KIM-1 is a membrane receptor for human hepatitis A virus (HHAV) and T-cell immunoglobulin and mucin domain containing 4 (TIMD 4). KIM-1 is a single pass type I cell membrane glycoprotein that is expressed at a low level in normal kidney tissue but becomes highly expressed in dedifferentiated proximal tubule epithelial cells in human and rodent kidneys after ischemic or toxic injury.[31,32]
Since KIM-1 upregulation was detected in a rat model of renal ischemia, numerous animal and human studies have been performed in order to examine the diagnostic role of KIM-1 in AKI models. Ichimura et al[29] examined tissue and urinary KIM-1 expression in a rat model of cisplatin-induced nephrotoxicity and found that KIM-1 is a faster and superior marker of nephrotoxicity than serum creatinine. In another rat study, the diagnostic value of urinary KIM-1 significantly exceeded traditional biomarkers (serum creatinine and BUN) as predictors of kidney tubular histopathological changes.[33] In preclinical and clinical studies, urinary KIM-1 is reported to be a more sensitive diagnostic indicator of kidney injury than conventional biomarkers including serum creatinine, BUN, glycosuria, and increased proteinuria.[13,34] Additional studies have confirmed that urinary KIM-1 concentration is upregulated in various kidney diseases including diabetic nephropathy, focal glomerulosclerosis, membranoproliferative glomerulonephritis, IgA nephropathy, and even renal cell carcinoma.[35] Sabbisetti et al[15] demonstrated that both in rodent and human AKI and mouse and human CKD, increased levels of KIM-1 can be detected in the blood, and KIM-1 serves as a biomarker of kidney injury.
We evaluated renal function using eGFR, calculated using prediction equations that take into account serum cystatin C and creatinine concentration as well as age, sex, and race. Cystatin C is a basic 13 kDa protein synthesized in all nucleated cells, and estimation of eGFR using cystatin C levels has clinical interest as it is not influenced by muscle mass, sex, or age.[36] eGFR calculated by combining creatinine and cystatin C is reported to more accurately reflect measured GFR than either marker alone.[17,37] Our results indicate that serum KIM-1 was correlated with serum cystatin C (r = 0.47; P < 0.001) and eGFR (r = -0.46; P < 0.001). Moreover, serum KIM-1 was significantly higher in the ADV group than the ETV group (P < 0.01). The superior diagnostic performance of serum KIM-1 was supported by AUC-ROC curve data.
We also analyzed risk factors for serum KIM-1 abnormality after long-term ADV treatment. Our univariate analysis indicated that baseline eGFR < 90 mL/min, and long-term ADV administration were significant and independent risk factors for serum KIM-1 abnormalities. Multivariate analysis also suggested that baseline eGFR < 90 mL/min was an independent risk factor for serum KIM-1 abnormality.
Our conclusions are, however, limited by the scope of this study. We enrolled a limited number of patients, did not perform continuous serum KIM-1 monitoring in CHB patients, and did not compare KIM-1 levels with other renal damage indicators. Despite its preliminary nature, this study clearly indicates that serum KIM-1 is a promising diagnostic biomarker for renal impairment during long-term adefovir therapy for CHB.
Supplementary Material
Footnotes
Abbreviations: β2-M = β2-microglobulin, ADV = adefovir dipivoxil, AKI = acute kidney injury, BUN = blood urea nitrogen, CHB = chronic hepatitis B, CI = confidence interval, CKD = chronic kidney disease, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR = estimated glomerular filtration rate, ELISA = enzyme-linked immunosorbent assay, EMA = European Medicines Agency, ETV = entecavir, FDA = Food and Drug Administration, HCC = hepatocellular carcinoma, HHAV = human hepatitis A virus, hOAT1 = human organic anion transporter 1, IL-18 = interleukin-18, KIM-1 = kidney injury molecule-1, LAM = lamivudine, MRP2 = multidrug resistance protein 2, NGAL = neutrophil gelatinase-associated lipocalin, NUC = nucleos(t)ide analogues, PEG-IFN α = pegylated interferon-alpha, RBP = retinol-binding protein, ROC = characteristic curves, TIMD 4 = T-cell immunoglobulin and mucin domain containing 4.
ZYL and CS contributed equally to this work.
Funding/support: This work was supported by the Key Program of Natural Science Foundation of Hebei Province (H2016206550) and the Innovation Project Fund Designated for Graduate Student of Academic Degree Commission of Hebei Provincial Education Department, China.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30:2212–2219. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev 2003; 16:569–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrillo R, Schiff E, Yoshida E, et al. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 2000; 32:129–134. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Xie Q, Tan D, et al. The 104-week efficacy and safety of telbivudine-based optimization strategy in chronic hepatitis B patients: a randomized, controlled study. Hepatology 2014; 59:1283–1292. [DOI] [PubMed] [Google Scholar]
- 5.Kahn J, Lagakos S, Wulfsohn M, et al. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA 1999; 282:2305–2312. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou Y, Bochet M, Thibault V, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet 2001; 358:718–723. [DOI] [PubMed] [Google Scholar]
- 7.Izzedine H, Hulot JS, Launay-Vacher V, et al. Renal safety of adefovir dipivoxil in patients with chronic hepatitis B: two double-blind, randomized, placebo-controlled studies. Kidney Int 2004; 66:1153–1158. [DOI] [PubMed] [Google Scholar]
- 8.Ha NB, Ha NB, Garcia RT, et al. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology 2009; 50:727–734. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Suzuki F, Seko Y, et al. Renal dysfunction and hypophosphatemia during long-term lamivudine plus adefovir dipivoxil therapy in patients with chronic hepatitis B. J Gastroenterol 2014; 49:470–480. [DOI] [PubMed] [Google Scholar]
- 10.Xu LJ, Jiang Y, Liao RX, et al. Low-dose adefovir dipivoxil may induce Fanconi syndrome: clinical characteristics and long-term follow-up for Chinese patients. Antivir Ther 2015; 20:603–611. [DOI] [PubMed] [Google Scholar]
- 11.Su M, Zhao C, Zhang L, et al. Association of SLC34A1 and RGS14 polymorphisms with serum phosphorus levels in chronic hepatitis B patients following long-term treatment of adefovir dipivoxil. Chin J Clin Infect Dis 2014; 7:516–520.(in Chinese). [Google Scholar]
- 12.Simsek A, Tugcu V, Tasci AI. New biomarkers for the quick detection of acute kidney injury. ISRN Nephrol 2013; 2013:394582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 2010; 28:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int 2009; 76:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 2014; 25:2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoine DJ, Sabbisetti VS, Francis B, et al. Circulating Kidney Injury Molecule 1 Predicts Prognosis and Poor Outcome in Patients With Acetaminophen-Induced Liver Injury. Hepatology 2015; 62:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinese Society of Hepatology, Diseases. SoI. Guidelines for prevention and treatment of chronic hepatitis B: a 2015 update. Chin J Clin Infect Dis 2015; 8:481–502.(in Chinese). [Google Scholar]
- 19.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Furusyo N, Ikezaki H, et al. Predictors of kidney tubular dysfunction induced by adefovir treatment for chronic hepatitis B. World J Gastroenterol 2015; 21:2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006; 131:1743–1751. [DOI] [PubMed] [Google Scholar]
- 24.Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl 2007; 13:349–360. [DOI] [PubMed] [Google Scholar]
- 25.Kim YJ, Cho HC, Sinn DH, et al. Frequency and risk factors of renal impairment during long-term adefovir dipivoxil treatment in chronic hepatitis B patients. J Gastroenterol Hepatol 2012; 27:306–312. [DOI] [PubMed] [Google Scholar]
- 26.Jia HY, Ding F, Chen JY, et al. Early kidney injury during long-term adefovir dipivoxil therapy for chronic hepatitis B. World J Gastroenterol 2015; 21:3657–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003; 14:2534–2543. [DOI] [PubMed] [Google Scholar]
- 28.Parikh CR, Jani A, Melnikov VY, et al. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 2004; 43:405–414. [DOI] [PubMed] [Google Scholar]
- 29.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 2006; 290:F517–F529. [DOI] [PubMed] [Google Scholar]
- 30.Soni SS, Ronco C, Katz N, et al. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif 2009; 28:165–174. [DOI] [PubMed] [Google Scholar]
- 31.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 1998; 273:4135–4142. [DOI] [PubMed] [Google Scholar]
- 32.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62:237–244. [DOI] [PubMed] [Google Scholar]
- 33.Martensson J, Martling CR, Bell M. Novel biomarkers of acute kidney injury and failure: clinical applicability. Br J Anaesth 2012; 109:843–850. [DOI] [PubMed] [Google Scholar]
- 34.Huo W, Zhang K, Nie Z, et al. Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando) 2010; 24:143–146. [DOI] [PubMed] [Google Scholar]
- 35.Lucarelli G, Mancini V, Galleggiante V, et al. Emerging urinary markers of renal injury in obstructive nephropathy. Biomed Res Int 2014; 2014:303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem 2015; 68:57–69. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012; 157:471–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


