Abstract
The objective of this study was to investigate the frequency of autoantibodies to hydroxymethylglutaryl coenzyme A reductase (HMGCR) in systemic sclerosis (SSc) and associations with inflammatory myositis and statin exposure.
This was a cross-sectional, multicenter study of 306 subjects from the Canadian Scleroderma Research Group cohort who had complete data on statin exposure and serology for anti-HMGCR antibodies assayed by an addressable laser bead immunoassay (ALBIA). Descriptive statistics were used to summarize the baseline characteristics of the patients and to compare subjects with and without anti-HMGCR antibodies.
Four (1.3%) subjects had anti-HMGCR antibodies. None of the subjects with anti-HMGCR antibodies titers had a history of an inflammatory myositis or overlap with polymyositis/dermatomyositis, compared to 8.6% and 2.0% of those without anti-HMGCR antibodies, respectively. In addition, none of the subjects with anti-HMGCR antibodies had past or current exposure to statins compared to 12% of those with negative titers.
Anti-HMGCR antibodies are rare in SSc and are not associated with inflammatory myopathy or statin exposure. Larger studies will be required to confirm these preliminary observations. Nevertheless, we conclude that anti-HMGCR antibodies are unlikely to play a major role in inflammatory myopathy in SSc and anti-HMGCR antibodies can be present in subjects without exposure to statins.
Keywords: 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), autoantibodies, systemic sclerosis
1. Introduction
Cholesterol lowering hydroxymethylglutaryl coenzyme A reductase (HMGCR) inhibitors (statins) are among the most frequently prescribed medications for the treatment of dyslipidemia. Amongst users, between 5% and 20% will develop a self-limited myopathy that is usually mild and resolves with discontinuation of the drug.[1,2] Severe myotoxicity, defined as rhabdomyolysis with elevation of creatine kinase greater than 10 times the upper limit of normal, can also occur, but is rare with an estimated rate of 0.4 to 0.9/10,000 person-years of exposure.[3–5]
In contrast to self-limited statin myopathy, a distinct subset of autoimmune necrotizing myopathy associated with anti-HMGCR antibodies that requires immunosuppression has been described.[6] This condition is characterized by progressive muscle weakness, elevated muscle enzymes (with mean creatine kinase levels before treatment of 10,000 IU/L), and anti-HMGCR autoantibodies, with or without statin exposure.[6–9] Muscle biopsies commonly show myofiber necrosis and regeneration with minimal inflammation, major histocompatibility complex (MHC) class I upregulation as well as complement membrane attack complex (MAC) deposits on necrotic fibers.[6] Myonuclear and perimysial abnormalities have also been described.[8] Anti-HMGCR antibodies have been reported to be present in approximately 5% of subjects with autoimmune myopathies, of which two-thirds had been exposed to statins[10] and emerging evidence suggests that these autoantibodies are pathogenic.
Systemic sclerosis (SSc) is a complex chronic autoimmune disease characterized by vascular damage, excessive fibrosis, and autoantibodies. It is highly heterogeneous and affects most organs. Skeletal muscle involvement is common, with weakness reported in up to 90% of patients.[11] Inflammatory myopathy has been reported in approximately 10% of subjects.[12–14] Since statins are one of the most commonly prescribed medications, and SSc is characterized by the presence of autoantibodies, muscle weakness, and inflammatory myositis, we sought to determine the frequency of anti-HMGCR antibodies in a large SSc cohort and associations with inflammatory myopathy and/or statin use.
2. Methods
2.1. Ethical considerations
Ethics committee approval for this study was obtained at McGill University (Montreal, Canada) and at all participating study sites. All subjects provided informed written consent to participate.
2.2. Design
Cross-sectional, multicenter study of 306 subjects from the Canadian Scleroderma Research Group (CSRG) cohort with baseline study visits between September 2004 and August 2011, and complete data on statin exposure and serology for anti-HMGCR antibodies (described below).
2.3. Study subjects
SSc subjects in the CSRG cohort are recruited by rheumatologists across Canada. They are ≥18 years of age, fluent in English or French, and likely to be compliant with study procedures and visits. Approximately 98% of subjects enrolled in the CSRG registry fulfill the 2013 ACR/EULAR classification criteria for SSc.[15]
2.4. Serology
Using standard operating procedures, serum was collected and sent to a central laboratory, Mitogen Advanced Diagnostics Laboratory, University of Calgary, where aliquots were stored at −80°C until needed. Antibodies to HMGCR were detected by an addressable laser bead immunoassay (ALBIA) using a full-length recombinant human HMGCR (Sigma-Aldrich, St. Louis, MO, Catalog #H7039) which encompassed the previously described C terminal epitope. Human anti-HMGCR sera (generous gift from Dr. Andrew Mammen, Johns Hopkins University, Baltimore, MD) were used as reference standards. The protocols to establish ALBIA assays have been previously published.[16,17] Briefly, 2 μL of suspended beads in solution (Luminex Corp., Austin, TX), 35 μL of horseradish peroxidase sample diluent (INOVA Diagnostics, Inc.) and 5 μL of sera diluted 1/1000 were pipetted into the wells of light tight microtiter plates (Luminex Corp.). The plate was covered so as to limit exposure of the beads to ambient light and incubated on a shaker for 30 minutes at room temperature. After the beads were washed again as described above, 40 μL of diluted, phycoerythrin (PE) conjugated secondary antibody (goat antihuman IgG/mouse antihuman IgG, 1:50 in sample diluents, Jackson ImmunoResearch, West Grove, PA) was added and incubated with gentle agitation at 600 rpm for 30 minutes at room temperature in the dark. Control positive and negative samples were included in each run and plates were analyzed by ALBIA using the Luminex-200 flow apparatus (Luminex Corp.). The data were expressed as median fluorescence units and positivity was defined as titers >500 units based on validation against a commercially available ELISA that detects anti-HMGCR autoantibodies (QUANTA Lite HMGCR, INOVA Diagnostics, Inc.).[18]
Samples were also tested for antinuclear antibodies (ANA) by indirect immunofluorescence (IIF) performed on HEp-2 substrate (HEp-2000; ImmunoConcepts, Sacramento, CA), for antibodies against centromere (CENP-A and CENP-B), topoisomerase I, RNA polymerase III (RP11 and RP155), Pm/Scl (PM75 and PM100), Ro52/TRIM21, PDGFR, Ku, Th/To, Nor90, and fibrillarin by a SSc profile line immunoassay (Euroline: Euroimmun, Luebeck, Germany), and for antibodies against Jo-1 and U1-RNP using the (QUANTAPlex ENA 8, INOVA Diagnostics, Inc.).
2.5. Study measures
Subjects recruited into this study underwent standardized medical evaluation including medical histories, physical examinations, and laboratory investigations. Results of signs and symptoms associated with anti-HMGCR associated myopathy[19] and available in the database were extracted. These included patient self-reported myalgias and dysphagia. Muscle strength was tested by a study physician and weakness was defined as a score of ≥1 on the Medsger Disease Severity Scale muscle domain (described below)[20,21] or a score of <5 in any of 5 muscle groups (neck flexors, and right and left upper and lower proximal extremities) according to the British Medical Research Council scale.[22] History of inflammatory myositis, overlap with polymyositis/dermatomyositis or mixed connective tissue disease, and exposure to statins was recorded by study physicians. Baseline creatine kinase values from local clinical labs were also extracted.
Other study measures considered in exploratory analyses included self-reported demographic information regarding age, sex, and ethnicity. Disease duration was recorded by study physicians and defined as the interval between the onset of the first non-Raynaud disease manifestation and baseline study visit. Skin involvement was assessed using the modified Rodnan skin score, a widely used clinical assessment where the examining rheumatologist records the degree of skin thickening ranging from 0 (no involvement) to 3 (severe thickening) in 17 areas (total score range 0–51). Limited cutaneous disease (lcSSc) was defined as skin involvement distal to the elbows and knees with or without facial involvement; diffuse cutaneous disease (dcSSc) was defined as skin involvement proximal to the elbows and knees, with or without truncal involvement. To assess gastrointestinal involvement, patients answered yes/no to a series of 14 questions concerning appetite loss, difficulty swallowing, regurgitation of acid, nocturnal choking, heartburn, early satiety, abdominal bloating, nausea and vomiting, constipation, diarrhea, need for antibiotics for diarrhea, greasy stools, fecal incontinence and need for parenteral nutrition. Systolic pulmonary artery pressure (SPAP) was measured using the Doppler flow measurement of the tricuspid regurgitant jet on cardiac echocardiography. Pulmonary hypertension was defined as an estimated SPAP >45 mm Hg (an estimate that correlates strongly with right heart catheter studies[23]). The presence of interstitial lung disease (ILD) was determined using a published clinical decision rule.[24] Using this algorithm, ILD was considered present if a high-resolution computed tomography (HRCT) scan of the lung was interpreted by an experienced radiologist as showing ILD or, in the case where no HRCT is available, if either a chest X-ray was reported as showing either increased interstitial markings (not thought to be due to congestive heart failure) or fibrosis, and/or if a study physician reported the presence of typical “velcro-like crackles” on physical examination. Pulmonary function tests were obtained in local laboratories working in accordance with American Thoracic Society standards. Forced vital capacity (FVC) %predicted and single breath diffusion capacity for carbon monoxide (DLCO) %predicted were extracted from reports. Disease severity was measured using a modified Medsger Scleroderma Disease Severity Scale.[20,21] The scale assesses disease severity in 9 organ systems, namely, general health, peripheral vascular, skin, joint/tendon, muscle, gastrointestinal tract, lungs, heart, and kidneys. Each organ is scored separately from 0 to 4 depending on whether there is no, mild, moderate, severe, or end-stage involvement.
2.6. Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics of the subjects. The number of subjects with missing data for each variable of interest were indicated in the tables. Fisher exact test for categorical variables and Mann–Whitney U test for continuous variables were used to compute P values. Bonferroni corrections were made to adjust for multiple testing. Further statistical analysis was not performed due to the exploratory nature of the study. All statistical analyses were performed with SAS v.9.2 (SAS Institute, Cary, North Carolina, USA).
3. Results
This study included 306 SSc subjects, of which 88% were women, mean age was 56 ± 12 years, mean disease duration was 11 ± 9 years, and 91% were White (Table 1). Four (1.3%) subjects had anti-HMGCR antibodies.
Table 1.
Baseline characteristics of the study cohort, stratified by anti-HMGCR antibody status.
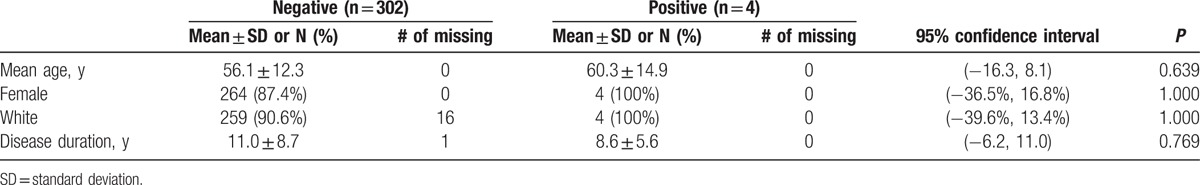
There were no clinically meaningful differences in the rates of myalgias, muscle weakness, or dysphagia between subjects with and without anti-HMGCR antibodies (Table 2). None of the subjects with anti-HMGCR antibodies had a history of inflammatory myositis, overlap with polymyositis/dermatomyositis, or overlap with mixed connective tissue disease compared to 8.6%, 2.0%, and 1.3% of those with negative titers, respectively. Subjects with anti-HMGCR antibodies had significantly lower baseline CK levels (median 38 μ/L [interquartile range (IQR) 30, 44] vs 83 μ/L [IQR 57, 112]), but also had lower body mass indices (22.1 ± 1.8 vs 26.1 ± 5.9 kg/m2) compared to negative subjects. Finally, none of those with anti-HMGCR antibodies had past or current exposure to statins compared to 12% of those with negative titers.
Table 2.
Associations between anti-HMGCR antibodies and markers of muscle disease in SSc (P values < 0.005 are considered to be statistically significant after Bonferroni correction).
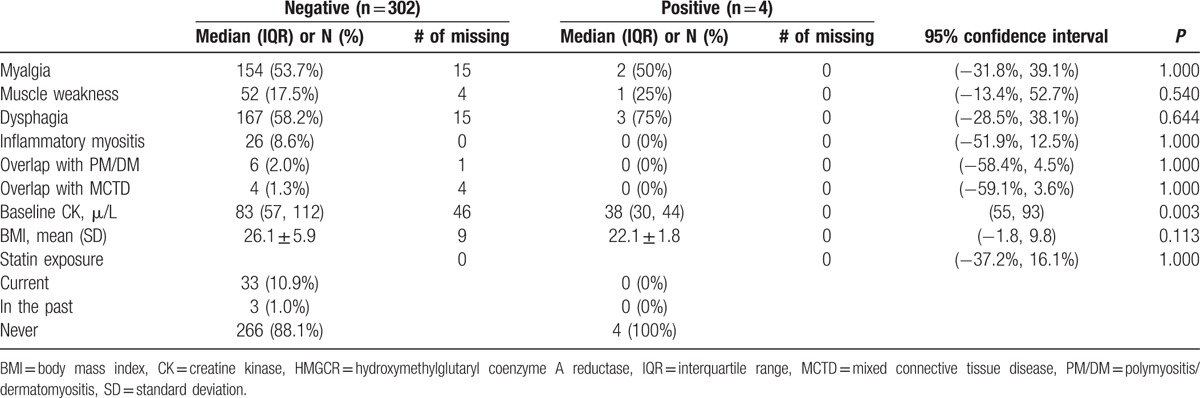
Other clinical and serological correlates of anti-HMGCR antibodies were explored (Table 3). Of note, there was a strong numerical trend for subjects with anti-HMGCR antibodies to have ILD (75% vs 35%) compared to negative subjects. Mean FVC %predicted was lower in subjects with anti-HMGCR antibodies compared to negative subjects (73% vs 89%). In addition, subjects with anti-HMGCR antibodies were more likely to have pulmonary hypertension compared to negative subjects (67% vs 10%). However, %predicted DLCO was similar in the 2 groups (73% vs 70%).
Table 3.
Exploratory analyses of associations between anti-HMGCR antibodies and other clinical and serological manifestations of SSc (P values < 0.002 are considered to be statistically significant after Bonferroni correction).
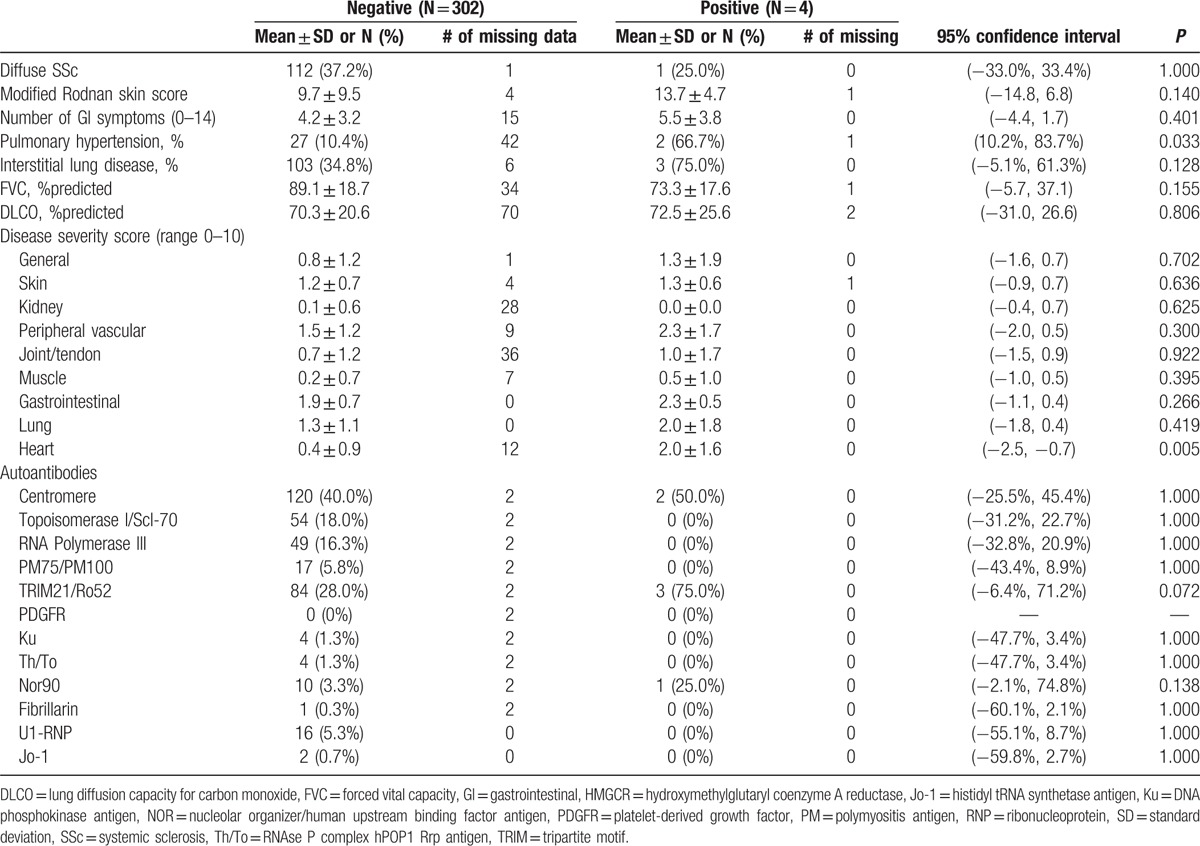
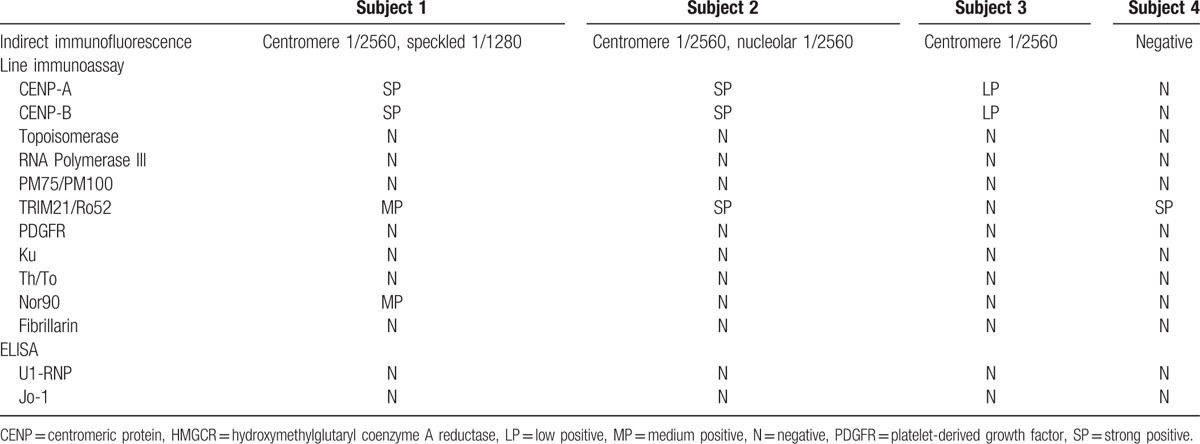
Three of the 4 subjects with anti-HMGCR antibodies had strongly positive ANA by IIF with a centromere pattern (CENP) at titers of 1/2560 (Table 4). One of these also had nuclear speckled staining at 1/1280 and another with nucleolar staining at 1/2560. The fourth subject had a negative ANA. Three subjects with anti-HMGCR had anti-CENP autoantibodies but none of the subjects with anti-HMGCR antibodies had anti-topoisomerase I, anti-RNA polymerase III, anti-PM75/100, anti-PDGFR, anti-Ku, anti-Th/To, or anti-fibrillarin antibodies by line immunoassay. Three subjects with anti-HMGCR antibodies were positive for anti-Ro52/TRIM21 antibodies by line immunoassay, including the 1 with a negative ANA by IIF. All 3 had ILD. One subject was also positive for anti-NOR90 (NOR, Nucleolar ORganizer/human upstream binding factor) autoantibodies by line immunoassay. None of the subjects with anti-HMGCR antibodies were positive for anti-U1RNP or anti-Jo1 by ELISA.
Table 4.
Detailed serology of subjects with anti-HMGCR antibodies.
4. Discussion
In this study of 306 SSc subjects, anti-HMGCR antibodies were rare (1.3%) and were not associated with a clinically apparent inflammatory myopathy, overlap with polymyositis/dermatomyositis and/or statin exposure.
The history of the detection of anti-HMGCR antibodies and their association with an immune mediated necrotizing myopathy (IMNM) has recently been reviewed.[6,25,26] Commercially available immunoassays for the detection of anti-HMGCR antibodies have been developed to aid in the diagnosis of IMNM[9,26–29] and a significant difference between statin-exposed and statin-unexposed anti-HMGCR antibody positive patients has been reported (reviewed in Ref.[26]). Although the nomenclature of the IMNM clinical phenotype is still not fully established, several studies use the term necrotizing autoimmune myopathy (NAM) or autoimmune inflammatory myopathy (AIM). In our study, we used an ALBIA that had been established with the use of index anti-HMGCR sera (kind gift of Dr. A. Mammen, Johns Hopkins University, Baltimore, MD) and subsequently validated by a commercially available ELISA. While there is good agreement between the various assays (90%), in order to fully understand the clinical utility of anti-HMGCR autoantibody tests, studies of large disease cohorts have been initiated.[26]
While the frequency of anti-HMGCR in our SSc cohort is admittedly low, it is important to point out that in an extensive study of community-based Atherosclerosis Risk in Communities (ARIC) Study and 98 French Canadian subjects with familial hypercholesterolemia, including 51 with documented statin intolerance, none (0%) had anti-HMGCR.[30] Similarly, in our own centre using the ALBIA described in this study, 0% of normal blood donor controls and 0% of osteoarthritis patients had anti-HMGCR (data not shown). In addition, elevated creatine kinase levels are not a uniform feature of anti-HMGCR autoimmune myopathy,[31] although normal levels are in fact the exception, present in only 4% of subjects in one study.[30]
Anti-CENP antibodies were seen in three of the four subjects with anti-HMGCR autoantibodies. Anti-HMGCR did not associate with antibodies against other SSc-specific and SSc-associated autoantibodies, with the exception of anti-Ro52/TRIM21, which was also present in 3 out of 4 subjects with anti-HMGCR antibodies, including one with negative ANA by IIF. Anti-Ro52/TRIM21 is the second most common autoantibody in SSc[12,32] and has been associated with other autoimmune myopathy-related autoantibodies[33] and ILD.[34] All 3 subjects with anti-Ro52/TRIM21 antibodies in this study also had ILD.
In exploratory analyses, we found that subjects with anti-HMGCR antibodies were more likely to have ILD and lower predicted % FVC compared to negative subjects. This is in contrast to findings of a review that reported that ILD did not appear to be a feature of HMGCR-associated autoimmune myopathy.[19] Pulmonary hypertension was also more common in subjects with anti-HMGCR antibodies compared to negative subjects, but %predicted DLCO was similar in the 2 groups. Hence, it is possible that the pulmonary hypertension was secondary to ILD, rather than pulmonary vascular disease.
The finding of anti-HMGCR autoantibodies in patients that neither had a history of statin exposure nor clinical features of IMNM raises some interesting questions. First, it is increasingly appreciated that despite lack of evidence of prescribed statin exposure in IMNM and in patients with anti-HMGCR antibodies, there is a possibility of exposure to “environmental” statins or statin-like substances.[26] For example, the history of the discovery of statins is traced back to the studies of Akira Endo and colleagues in 1972 who isolated compactin, the first statin compound, from a blue-green mold, Penicillium citrinum Pen-51, isolated from contaminated rice.[35] Compactin, and other statins that were developed thereafter, have structural similarities to HMG-CoA making it a potent competitive inhibitor of HMG-CoA reductase, the rate controlling enzyme in cholesterol biosynthesis. Indeed, there are a number of “naturally occurring statins” in foods such as red yeast rice (food coloring used in oriental cuisine), soy (i.e., soy tempeh), grains (i.e., wheat germ, phytosterols, grain oils), and oyster mushrooms (the source of oyster sauce used in cooking). Therefore, the interesting pathogenic link of statins to the induction of anti-HMGCR autoantibodies makes it important to consider sources of statin exposure other than prescription drugs. Asians are more likely to consume or be exposed to the natural statins outlined above, but in our study all SSc patients with anti-HMGCR were Caucasian.
Statins are known to upregulate the expression of the HMGCR, which is believed to trigger a B cell response in some patients leading to the development of pathogenic anti-HMGCR autoantibodies.[10] This autoimmune response is perpetuated by high levels of HMGCR expression in regenerating muscle and upregulation of MHC class I even after statins are discontinued. Muscle biopsies of anti-HMGCR positive patients after statin cessation show upregulated HMGCR expression in cells expressing neural cell adhesion molecule, a marker of muscle regeneration.[10] In a recent multicentre international study, anti-HMGCR antibodies characterized a subpopulation of autoimmune mediated myopathy patients with a history of statin exposure who are frequently of older age and present predominantly with necrosis on muscle biopsy.[26] Future studies are needed to understand why apparently statin-naïve patients develop anti-HMGCR antibodies.
Equally important are the well-known but somewhat underappreciated pleiotropic effects of statins that, in addition to lipid lowering effects, include antithrombotic, antiinflammatory, and antioxidant effects,[36,37] which makes them an interesting consideration for adjunct therapy of SSc. Last, there are a number of known risk factors related to the establishment of adverse responses to statins and they include demographics, gene markers, comorbidities, drug interactions, and dosing regimens.[38] Hence, the possible association of anti-HMGCR with ILD may be related to pathogenic pathways that differ from those observed in IMNM.
This study is not without limitations. First, results should be interpreted with caution because of the small number of patients with anti-HMGCR antibodies and the extensive exploratory analyses, with positive or negative findings possibly related to either type I or type II error. Second, a history of inflammatory myositis and overlap with PM/DM were not defined using specific criteria and the presence or absence thereof was not confirmed with electromyography, magnetic resonance imaging, or muscle biopsies. However, the fact these were recorded by study physicians, all of whom were experienced rheumatologists, provides some support for the validity of these diagnoses. Third, creatine kinase levels were only recorded at baseline study visits and may not have corresponded to episodes of inflammatory muscle disease. In fact, if anything, this may have underestimated the true frequency of muscle disease. Finally, subjects in this cohort have established SSc, with mean disease duration over 10 years. Thus, survival bias is possible and the results are therefore generalizable to the sampling frame. Longitudinal follow-up and muscle biopsies of a large, unselected sample of incident SSc subjects would be required to overcome these limitations and confirm these preliminary observations.
We conclude that anti-HMGCR antibodies are unlikely to play a major role in inflammatory myopathy in SSc and that these autoantibodies can be present in subjects without exposure to statins.
Acknowledgment
The technical assistance of Haiyan Hou and Meifeng Zhang at the University of Calgary is gratefully acknowledged.
Footnotes
Abbreviations: AIM = autoimmune inflammatory myopathy, ALBIA = addressable laser bead immunoassay, ANA = antinuclear antibody, ARIC = Atherosclerosis Risk in Communities, CENP = centromeric protein, CSRG = Canadian Scleroderma Research Group, dcSSc = diffuse cutaneous systemic sclerosis, DLCO = diffusion capacity of the lung for carbon monoxide, FVC = forced vital capacity, HMGCR = hydroxymethylglutaryl coenzyme A reductase, HRCT = high resolution computed tomography, IIF = indirect immunofluorescence, ILD = interstitial lung disease, IMNM = immune mediated necrotizing myopathy, lcSSc = limited cutaneous systemic sclerosis, MAC = membrane attack complex, NAM = necrotizing autoimmune myopathy, NOR = Nucleolar ORganizer/human upstream binding factor, SPAP = systolic pulmonary artery pressure, SSc = systemic sclerosis.
Investigators of the Canadian Scleroderma Research Group: M. Baron, Montreal, Quebec; M. Hudson, Montreal, Quebec; G. Gyger, Montreal, Quebec; J. Pope, London, Ontario; M. Larché, Hamilton, Ontario; N. Khalidi, Hamilton, Ontario; A. Masetto, Sherbrooke, Quebec; E. Sutton, Halifax, Nova Scotia; D. Robinson, Winnipeg, Manitoba; T.S. Rodriguez-Reyna, Mexico City, Mexico; D. Smith, Ottawa, Ontario; C. Thorne, Newmarket, Ontario; P.R. Fortin, Quebec, Quebec; M. Fritzler, Mitogen Advanced Diagnostics Laboratory, Cumming School of Medicine, Calgary, Alberta, Canada.
Funding: This study was funded in part by the Canadian Institutes of Health Research (CIHR), the Scleroderma Society of Canada and its provincial Chapters, Scleroderma Society of Ontario, Scleroderma Society of Saskatchewan, Sclérodermie Québec, Cure Scleroderma Foundation, Dr. Fooke Laboratorien GmbH (Neuss, Germany), Euroimmun (Lubeck, Germany), Mikrogen GmbH (Neuried, Germany), the Canadian Arthritis Network (CAN), and the Lady Davis Institute of Medical Research of the Jewish General Hospital, Montreal, QC. The CSRG has also received educational grants from Pfizer and Actelion pharmaceuticals. Autoantibody diagnostic kits were a gift of INOVA Diagnostics Inc. (San Diego, CA), ImmunoConcepts (Sacramento, CA). Dr. Hudson was funded by the Fonds de la recherche en Santé du Québec. The funding sources had no role in the design of the study, analysis of the data, preparation of the manuscript, and decision to submit for publication.
This research project was also supported by the CIHR, the CIHR-CSRG 2012 Summer Studentship program, and funds from the Arthritis Society Research Chair at the University of Calgary held by MJF.
The authors have no conflicts of interest to disclose.
References
- 1.Baigent C, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Rodriguez LA, Masso-Gonzalez EL, Wallander MA, et al. The safety of rosuvastatin in comparison with other statins in over 100,000 statin users in UK primary care. Pharmacoepidemiol Drug Saf 2008; 17:943–952. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Kusama M, Ono S, et al. Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information. BMJ Open 2013; 3:e002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C. [DOI] [PubMed] [Google Scholar]
- 6.Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med 2016; 374:664–669. [DOI] [PubMed] [Google Scholar]
- 7.Mohassel P, Mammen AL. The spectrum of statin myopathy. Curr Opin Rheumatol 2013; 25:747–752. [DOI] [PubMed] [Google Scholar]
- 8.Alshehri A, Choksi R, Bucelli R, et al. Myopathy with anti-HMGCR antibodies: perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm 2015; 2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allenbach Y, Drouot L, Rigolet A, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore) 2014; 93:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medsger TA, Jr, Rodnan GP, Moossy J, et al. Skeletal muscle involvement in progressive systemic sclerosis (scleroderma). Arthritis Rheum 1968; 11:554–568. [DOI] [PubMed] [Google Scholar]
- 12.Hudson M, Pope J, Mahler M, et al. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res Therapy 2012; 14:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhansing KJ, Lammens M, Knaapen HK, et al. Scleroderma-polymyositis overlap syndrome versus idiopathic polymyositis and systemic sclerosis: a descriptive study on clinical features and myopathology. Arthritis Res Ther 2014; 16:R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domsic R, Medsger T. Silver R, Denton C. Two Scleroderma Patients with Differing Patterns of Muscle Disease. Springer, Case Studies in Systemic Sclerosis. London:2011. [Google Scholar]
- 15.Alhajeri H, Hudson M, Fritzler M, et al. 2013 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Systemic Sclerosis Outperform the 1980 Criteria: data from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 2015; 67:582–587. [DOI] [PubMed] [Google Scholar]
- 16.Selak S, Mahler M, Miyachi K, et al. Identification of the B-cell epitopes of the early endosome antigen 1 (EEA1). Clin Immunol 2003; 109:154–164. [DOI] [PubMed] [Google Scholar]
- 17.Eystathioy T, Chan EK, Takeuchi K, et al. Clinical and serological associations of autoantibodies to GW bodies and a novel cytoplasmic autoantigen GW182. J Mol Med (Berl) 2003; 81:811–818. [DOI] [PubMed] [Google Scholar]
- 18.Kassardjian CD, Lennon VA, Alfugham NB, et al. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72:996–1003. [DOI] [PubMed] [Google Scholar]
- 19.Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep 2015; 17:72. [DOI] [PubMed] [Google Scholar]
- 20.Medsger TA, Jr, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol 1999; 26:2159–2167. [PubMed] [Google Scholar]
- 21.Medsger TA, Jr, Bombardieri S, Czirjak L, et al. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003; 21 (3 suppl 29):S42–S46. [PubMed] [Google Scholar]
- 22.Medical Research Council, Aids to the examination of the peripheral nervous system, in Memorandum no. 45. 1976, Her Majesty's Stationery Office: London. [Google Scholar]
- 23.Hsu VM, Moreyra AE, Wilson AC, et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol 2008; 35:458–465. [PubMed] [Google Scholar]
- 24.Steele R, Hudson M, Lo E, et al. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012; 64:519–524. [DOI] [PubMed] [Google Scholar]
- 25.Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373:1680–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musset L, Allenbach Y, Benveniste O, et al. Anti-HMGCR antibodies as a biomarker for immune mediated necrotizing myopathies: a history of statins and experience from a large international multi-center study. Autoimmun Rev 2016; 15:983–993. [DOI] [PubMed] [Google Scholar]
- 27.Mohassel P, Mammen AL. Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies. Muscle Nerve 2013; 48:477–483. [DOI] [PubMed] [Google Scholar]
- 28.Musset L, Miyara M, Benveniste O, et al. Analysis of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase using different technologies. J Immunol Res 2014; 2014:405956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drouot L, Allenbach Y, Jouen F, et al. Exploring necrotizing autoimmune myopathies with a novel immunoassay for anti-3-hydroxy-3-methyl-glutaryl-CoA reductase autoantibodies. Arthritis Res Ther 2014; 16:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585. [DOI] [PubMed] [Google Scholar]
- 32.Mehra S, Walker J, Patterson K, et al. Autoantibodies in systemic sclerosis. Autoimmun Rev 2013; 12:340–354. [DOI] [PubMed] [Google Scholar]
- 33.Dugar M, Cox S, Limaye V, et al. Diagnostic utility of anti-Ro52 detection in systemic autoimmunity. Postgrad Med J 2010; 86:79–82. [DOI] [PubMed] [Google Scholar]
- 34.Wodkowski M, Hudson M, Proudman S, et al. Monospecific anti-Ro52/TRIM21 antibodies in a tri-nation cohort of 1574 systemic sclerosis subjects: evidence of an association with interstitial lung disease and worse survival. Clin Exp Rheumatol 2015; 33 (4 suppl 91):S131–S135. [PubMed] [Google Scholar]
- 35.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci 2010; 86:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006; 6:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bifulco M, Endo A. Statin: new life for an old drug. Pharmacol Res 2014; 88:1–2. [DOI] [PubMed] [Google Scholar]
- 38.Chatzizisis YS, Koskinas KC, Misirli G, et al. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187. [DOI] [PubMed] [Google Scholar]


