Abstract
Hyperleukocytic acute myeloid leukemia (AML) is associated with pulmonary complications and high early mortality rate, but given its rarity, data on chest radiographic presentation are scarce.
We retrospectively reviewed the charts of 73 AML patients admitted with white blood cell count >100 × 109/L between 2003 and 2014 in order to describe the chest radiographic and computed tomography (CT) findings and to correlate them with AML subtype and respiratory symptoms.
Forty-two of the 73 patients (58%) overall and 36 of the 54 patients (67%) with clinical signs of pulmonary leukostasis had abnormal radiographs on admission. The presence of radiographic abnormalities was significantly associated with dyspnea and oxygen/ventilatory support requirements (P < 0.01) and with day 28 mortality (45% vs 13%, P = 0.005) but not with monocytic subtype of AML. Sixteen patients had isolated focal basilar airspace opacities, unilateral (n = 13) or bilateral (n = 3), while 16 patients had bilateral diffuse opacities, interstitial (n = 12) or airspace and interstitial (n = 4). Two patients had isolated pleural effusion, 2 patients had unilateral midlung airspace opacities, and 6 patients had a combination of focal airspace and diffuse interstitial opacities. Overall, 2 patterns accounted for 75% of abnormal findings: bilateral diffuse opacities tended to be associated with monocytic AML, whereas basilar focal airspace opacities were more frequent in nonmonocytic AML (P < 0.05). Eighteen patients had CT scans, revealing interlobular septal thickening (n = 12), airspace (n = 11) and ground-glass (n = 9) opacities, pleural effusions (n = 12), and acute pulmonary embolism (n = 2).
Hyperleukocytic AML is frequently associated with abnormal chest radiographs, involving mostly focal basilar airspace opacities (more frequent in nonmonocytic AML) or diffuse bilateral opacities. CT scan should be considered broadly due to the suboptimal resolution of radiographs for detecting signs of leukostasis.
Keywords: acute myeloid leukemia, acute respiratory failure, chest radiograph, computed tomography, leukostasis
1. Introduction
The incidence of acute myeloid leukemia (AML) is about 4 new cases/100,000 inhabitants per year and 10% to 20% of patients with newly diagnosed AML present with hyperleukocytosis, defined by a white blood cell (WBC) count >100 × 109 L.[1] Hyperleukocytosis per se is a laboratory abnormality, but about 30% to 40% of hyperleukocytic patients develop clinical signs of brain or pulmonary leukostasis, resulting from the obstruction of capillary vessels by leukemic cells. Overall, the presence of hyperleukocytosis carries a poor prognosis with early mortality rates reaching 20% to 30% at day 28.[2–4]
Pulmonary complications and acute respiratory failure are major causes for early mortality in hyperleukocytic AML[3,5] and have diverse etiologies. Leukemia-related pulmonary involvement mainly results from pulmonary leukostasis and lung leukemic infiltration,[6] which may be associated[7] and seem more frequent in myelomonocytic or monocytic subtypes of AML.[8] Non–leukemia-specific pulmonary complications, such as pneumonia and acute pulmonary emboli, may also contribute to acute respiratory failure, with infections accounting for up to one-third of early acute respiratory events in newly diagnosed AML patients.[9] Pulmonary leukostasis and lung leukemic infiltration may cause hypoxemia and clinical symptoms related to the vascular obstruction by leukemic cells and blast invasion of the interstitium and alveolar spaces, respectively,[1] but their clinical and radiographic manifestations are difficult to distinguish from those of nonleukemic complications such as pneumonia or pulmonary edema, which may coexist.
The pathophysiology of pulmonary leukostasis involves 2 main mechanisms accounting for the variety of radiographic findings. First, hyperleukocytosis may affect blood rheology by causing mechanical vessel obstruction and hyperviscosity; this rheological model explains why patients with autopsy-proven pulmonary leukostasis may present with normal chest radiographs[10] or perfusion defects on ventilation–perfusion scan mimicking pulmonary embolism.[11] However, the lack of clear correlation between WBC count and the incidence and severity of leukostasis suggests that additional mechanisms are also involved: leukemic cells have the ability to release cytokines (tumor necrosis factor-α [TNF-α] and interleukin-1) and induce their own adhesion on the endothelial surface, with subsequent cytokine-driven increased endothelial permeability, pulmonary edema and hemorrhage, and finally interstitial invasion by leukemic cells.[1] These mechanisms likely account for the diffuse airspace opacities and pleural effusion reported in other patients.[10] Due to the rarity of hyperleukocytic AML, data on radiographic findings at presentation are scarce and limited to small series of patients with various types of leukemias and with or without hyperleukocytosis.
Given the lack of comprehensive data, we aimed to analyze a large population of hyperleukocytic AML patients in order to describe the radiographic and CT findings on admission and to assess the correlation between radiographic findings and clinical condition. As monocytic AML is particularly associated with lung involvement and acute respiratory failure,[8] we also compared radiographic findings between monocytic and nonmonocytic AML.
2. Methods
This retrospective study was approved by the Pennsylvania State University College of Medicine Institutional Review Board (number 374) and informed consent was waived. The patients included were part of a previously published larger cohort,[12] but the present study addressed a clearly distinct question and the data presented were not previously reported except for the description of the cohort (age, gender, WBC count, presence of dyspnea, and level of oxygen/respiratory support).
2.1. Patients
The charts of all adult (>18 years old) patients admitted to our institution with newly diagnosed AML between January 2003 and December 2014 were screened and subjects who had hyperleukocytosis (WBC > 100 × 109/L) and chest radiographs available on admission were included for further analysis.
2.2. Data collected
Baseline demographic data, AML subtype (myelomonocytic or monocytic vs other types), and presence of dyspnea were collected on admission. To assess the leukostasis probability, we used the leukostasis grading score (LGS) as described by Novotny et al.[13] LGS is based on the severity of pulmonary, neurological, and other-system symptoms and grades leukostasis probability as absent (LGS = 0), possible (LGS = 1), probable (LGS = 2), or highly probable (LGS = 3). The respiratory status on presentation was collected and defined based on oxygen or ventilatory support as follows: room air, oxygen requirement, and noninvasive or invasive mechanical ventilation. The presence of fever (temperature >38.0°C), results of blood and sputum cultures, and use of antibiotics were also collected on admission. The presence of systemic inflammatory response syndrome (SIRS) was recorded if 2 of the following criteria were documented: temperature >38.0 or <36.0°C, heart rate >90 beats/min, respiratory rate >20 breaths/min, or PaCO2 <32 mm Hg (WBC count was excluded as a SIRS component, given all patients had hyperleukocytosis).[14] The results from echocardiogram were collected to evaluate left ventricular function. Day 28 mortality was also recorded.
2.3. Radiological analysis
Chest radiographs on admission and computed tomography (CT) performed within 5 days of admission were reinterpreted by a radiologist with expertise in thoracic radiology (CJ-D).
The radiographic data obtained from chest radiographs were standardized for analysis by classifying the data into the following categories: airspace opacities (focal or diffuse, unilateral or bilateral), interstitial opacities (focal or diffuse, unilateral or bilateral), and pleural effusion (unilateral or bilateral).
The radiographic data obtained from chest CT scans were standardized for analysis by classifying the data into the following categories: interlobular septal thickening (unilateral or bilateral), pleural effusion (unilateral or bilateral), presence of “halo sign” or cavitation, pulmonary nodules (centrilobular or other, unilateral or bilateral), airspace opacities (focal or diffuse, unilateral or bilateral), ground-glass opacities (focal or diffuse, unilateral or bilateral), pulmonary embolism, and emphysema.
2.4. Statistical analysis
Data were presented as median (range) unless otherwise specified. We used a Kolmogorov–Smirnov test and visual inspection of Q–Q plots (SPSS version 23; SPSS Inc, Chicago, IL) to assess the data distribution. As most data were not normally distributed and our sample size was limited, we used nonparametric Wilcoxon rank sum test and Fisher exact test to compare quantitative and categorical variables, respectively, between groups. P < 0.05 was considered for significance.
3. Results
3.1. Patients’ characteristics
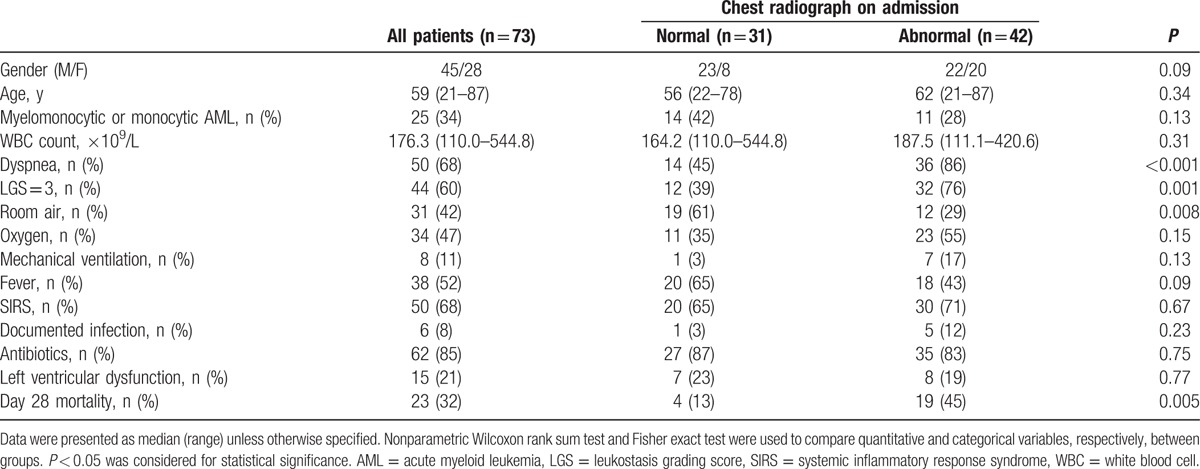
Among the 850 patients admitted with newly diagnosed AML during the study period, 80 patients had an initial WBC count >100 × 109/L. Seven of them were transferred from other hospitals without chest radiographs available, so that 73 patients (45 males, 28 females) with chest radiographs available on admission were included for further analysis. The main characteristics of the cohort are presented in Table 1. Patients were 59 (21–87) years old and had WBC count of 176.3 (110.0–544.8) × 109/L. Our population included 34% of monocytic AML subtype (n = 25) and a high proportion of patients requiring oxygen or ventilatory support on admission (58%). Fever and SIRS were present in 52% and 68% of patients, respectively; 85% of the patients were treated with antibiotics, although infection was documented in only 8% of patients. Three patients had left ventricular systolic dysfunction documented on echocardiogram, and 12 had diastolic dysfunction, mostly moderate (grade 1).
Table 1.
Patients’ characteristics on admission according to the chest radiograph appearance.
3.2. Radiographic findings
Forty-two patients (58%) had abnormal chest radiographs on admission. Radiographic findings are detailed in Table 2. Two main patterns emerged and accounted for 75% of the 42 patients with abnormal radiographs: 16 patients had isolated focal basilar airspace opacities, unilateral (n = 13) or bilateral (n = 3) (Fig. 1), while 16 patients had bilateral diffuse opacities, interstitial (n = 12) or airspace and interstitial (n = 4) (Fig. 2). The remainder of the patients were distributed as follows: 2 patients had isolated pleural effusion, 2 patients had unilateral midlung airspace opacities, and 6 patients had a combination of focal airspace and diffuse interstitial opacities.
Table 2.
Radiographic findings for the 42 patients with abnormal radiographs according to the presence of myelomonocytic or monocytic AML subtype.
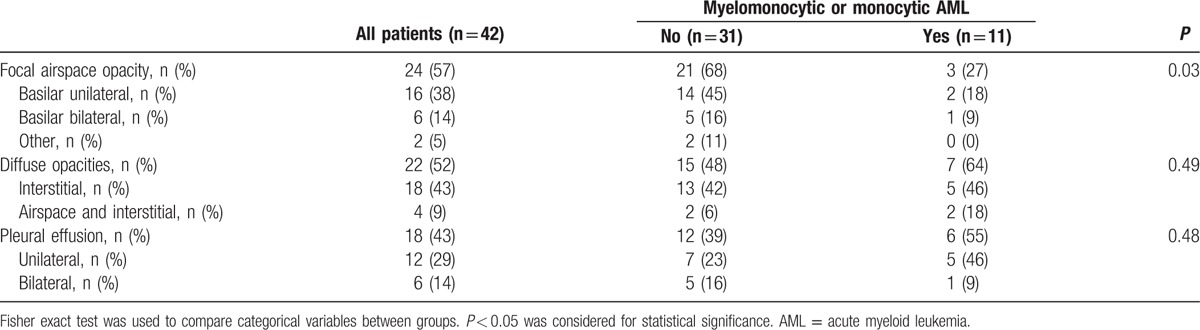
Figure 1.
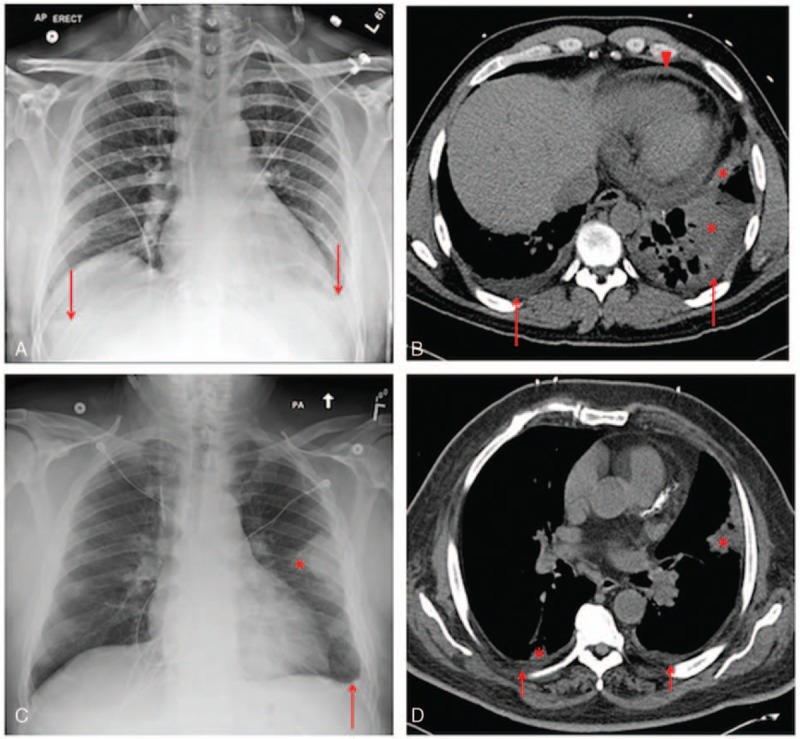
Examples of chest radiographs (left panels) and corresponding CT scans (right panels) in 2 hyperleukocytic AML patients with unilateral (A and B) or bilateral (C and D) focal airspace opacities observed on radiographs. In the first patient, the radiograph (A) showed small bilateral pleural effusions (arrows) and left lower lobe airspace opacities. A CT scan performed 9 hours later (B) showed small bilateral pleural effusions (arrows), consolidations in the left lower lobe and lingula (asterisks), and a small pericardial effusion (arrowhead). Additional findings (not seen in the presented images) included minimal patchy ground-glass opacities and airspace opacities at the periphery of the right upper lobe, right lower lobe and lingula, diffuse bilateral bronchial wall thickening, and mildly enlarged mediastinal nodes. In the second patient, the radiograph (C) showed a small left pleural effusion (arrow) and an airspace opacity along the periphery of the left midlung (asterisk). A CT scan obtained 5 hours later (D) showed small bilateral pleural effusions (arrows), and small airspace opacities in the lingula and right lower lobe (asterisks). AML = acute myeloid leukemia, CT = computed tomography.
Figure 2.
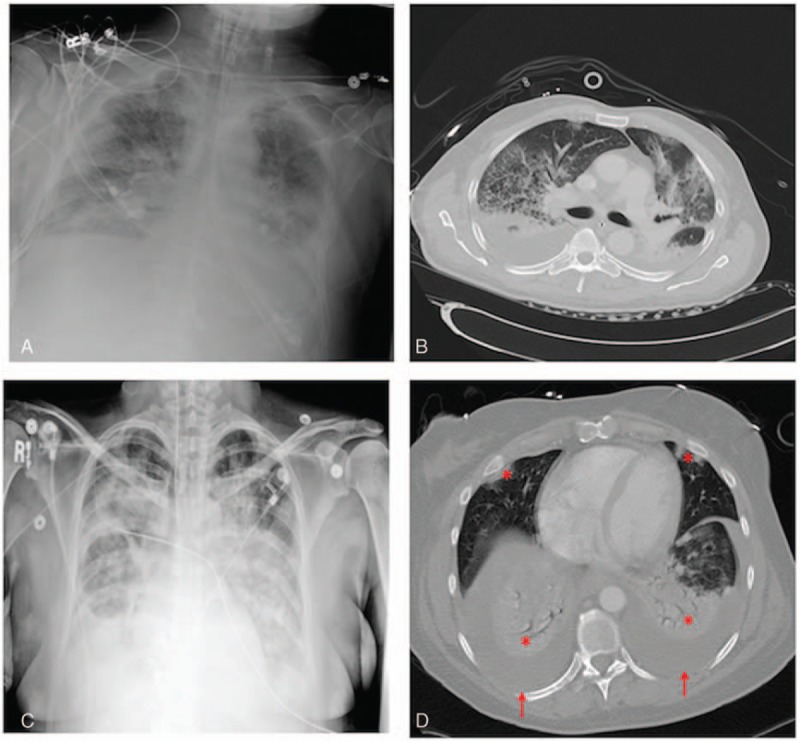
Examples of chest radiographs (left panels) and corresponding CT scans (right panels) in 2 hyperleukocytic AML patients with diffuse bilateral opacities observed on radiographs. In the first patient, the radiograph (A) showed diffuse bilateral interstitial and airspace opacities while a CT scan performed 3 days later (B) showed bilateral pleural effusions, diffuse bilateral interstitial and airspace opacities in the upper lobes, and dependent bilateral lower lobe consolidations. In the second patient, the radiograph (C) showed a moderate right pleural effusion and diffuse bilateral interstitial and airspace opacities, while a CT scan obtained 3 hours earlier (D) showed moderate bilateral pleural effusions (arrows), bilateral lower lobe consolidations, and small foci of airspace disease in the right middle lobe and lingula (asterisks). AML = acute myeloid leukemia, CT = computed tomography.
Among the 31 patients with normal chest radiographs on admission, 18 had repeat radiographs between days 3 and 5: 8 were still normal, 5 showed new bilateral interstitial opacities, 2 showed focal airspace opacity, and 3 showed isolated pleural effusion.
In our whole cohort of 73 patients, 54 presented with clinical signs of pulmonary leukostasis (defined as dyspnea or requirement for supplemental oxygen) and 19 were asymptomatic. Among the 54 patients with respiratory symptoms, the proportion of abnormal radiographs increased to 67% (n = 36) but 33% of them (n = 18) had normal radiographs. Conversely, 6 of 19 asymptomatic patients (no dyspnea or oxygen requirements) had abnormal radiographs, 4 presenting with diffuse bilateral interstitial opacities and 2 with unilateral basilar airspace opacities.
Among 25 patients with myelomonocytic or monocytic subtypes of AML, 11 patients had abnormal radiographs, a proportion not significantly different from nonmonocytic AML (44% vs 65%, P = 0.13). However, focal airspace opacities were significantly more frequent in nonmonocytic AML (68% vs 27%, P = 0.03), whereas monocytic AML patients tended to have more diffuse bilateral opacities (Table 2).
As compared to patients with focal airspace opacities, patients with diffuse bilateral opacities were more likely to have pleural effusion (55% vs 20%, P = 0.03), but no significant difference was observed between the 2 groups in terms of age, WBC count, left ventricular dysfunction, fever, SIRS, documented infection, antibiotic administration, or respiratory status.
3.3. CT findings
Eighteen (25%) of the 73 patients had a chest CT scan performed within 5 days of admission; 8 of them received intravenous contrast. One CT scan was normal. The most frequent findings (Figs. 1–4) were interlobular septal thickening (n = 12, mostly diffuse and bilateral), pleural effusion (n = 12), focal (n = 8) or diffuse (n = 3) consolidations, and ground-glass opacities (n = 9). Other abnormalities included bilateral nodules (n = 2) and emphysema (n = 2), and 2 patients were found to have acute pulmonary emboli (1 of them with normal radiographs, the other one with focal consolidation). Thirteen patients had mildly enlarged mediastinal lymph nodes, and 1 was noted to have pulmonary artery enlargement.
Figure 4.
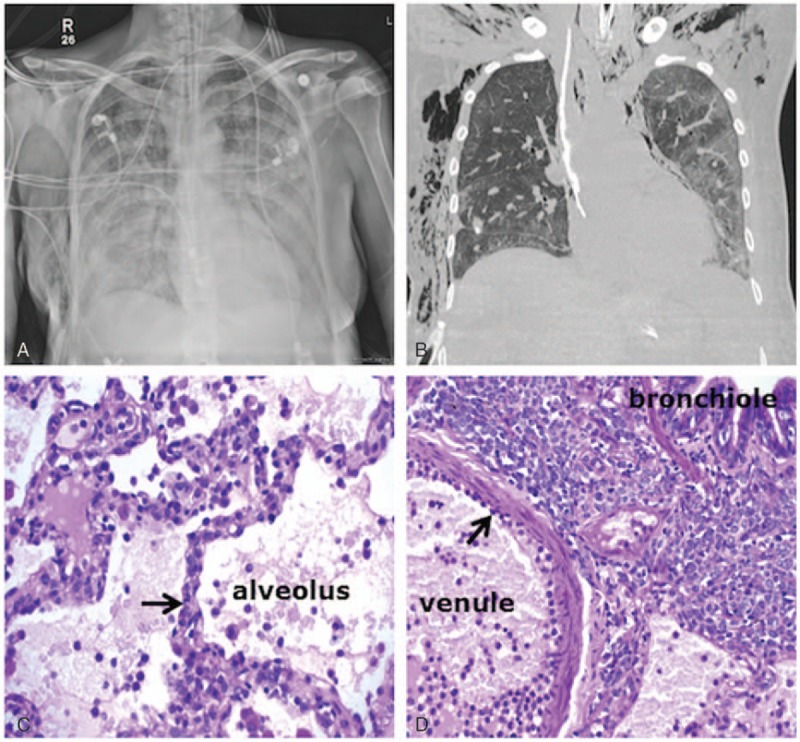
Radiological–pathological correlation in a 55-year-old female patient diagnosed with monocytic AML and presenting with hyperleukocytosis (WBC = 254 × 109/L) and pulmonary leukostasis with acute hypoxic respiratory failure requiring mechanical ventilation. Initial chest radiograph showed diffuse, bilateral airspace and interstitial opacities (A). The patient underwent open lung biopsy and a CT scan obtained at day 5 for air leakage showed persistent diffuse ground-glass opacities, more confluent on the left side and dependently (B). Bottom panels: periodic acid–Schiff stain of the lung biopsy. (C) Leukostasis with capillary obstruction by leukemic cells (blue stain, black arrow) within the alveolar–capillary barrier. (D) Leukemic cells adhering to the endothelium (black arrow) and massively infiltrating the interstitium between the venule and the bronchiole. AML = acute myeloid leukemia, CT = computed tomography, WBC = white blood cell.
Figure 3.
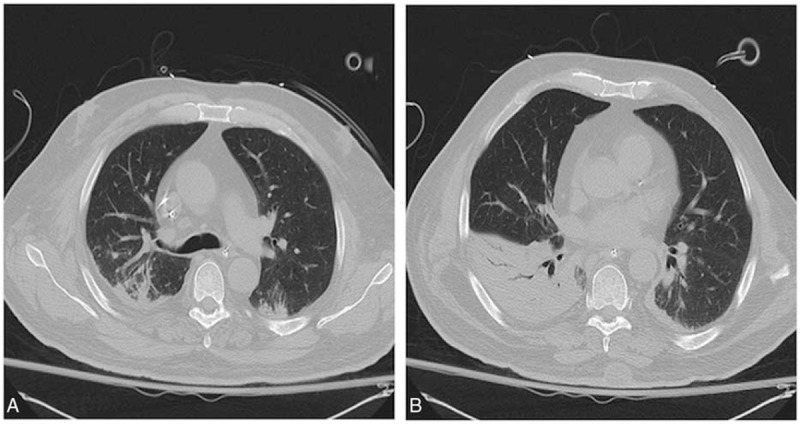
(A and B) Example of a chest CT scan combining the most frequent CT findings in hyperleukocytic AML patients. The CT scan showed dependent bilateral airspace opacities involving the right upper lobe and bilateral lower lobes, patchy ground-glass opacities, and bronchial wall thickening in the posterior segment of the right upper lobe. AML = acute myeloid leukemia, CT = computed tomography.
3.4. Clinicoradiological correlation
As compared to patients with normal tests, patients with abnormal radiographs presented more frequently with dyspnea and a LGS of 3 (Table 1). The patients with abnormal chest radiographs were significantly less likely to breathe on room air (P = 0.008) and more likely to require supplemental oxygen or mechanical ventilation (71% vs 39%, P = 0.008), the proportions of patients on room air, oxygen, or mechanical ventilation being 29%, 55%, and 17%, respectively, in this group versus 61%, 35%, and 3%, respectively, in subjects with normal chest radiographs. Day 28 mortality was significantly higher for patients with abnormal radiographs as compared to that for patients with normal tests (45% vs 13%, P = 0.005) (Table 1). There was no difference in the incidence of fever, SIRS, documented infection, antibiotic administration, or left ventricular dysfunction between patients with normal radiographs and those with abnormal radiographs (Table 1).
4. Discussion
This study is the first to systematically report chest radiographic abnormalities in a large cohort of hyperleukocytic AML patients. The main findings were as follows: about 60% of the patients overall and 70% of the patients with dyspnea or oxygen requirement had abnormal radiographs on admission with heterogeneous findings; the 2 main patterns, accounting for 75% of the patients with abnormal radiographs, were focal basilar airspace opacities and diffuse bilateral interstitial opacities; monocytic AML was not associated with a higher proportion of abnormal radiographs, but tended to present with diffuse bilateral opacities, whereas nonmonocytic AML patients had more focal airspace opacities; and CT scan often revealed ground-glass opacities and interlobular septal thickening, whose detection with radiographs may be difficult due to suboptimal resolution.
Although AML is the most frequent acute leukemia in adults, its incidence is about 4 new cases/100,000 inhabitants per year, and only 10% to 20% of patients present with hyperleukocytosis. [2,15,16] Data on radiographic findings are therefore scarce despite a high incidence of respiratory complications in these patients.[6] The incidence of radiographic abnormalities in this population is unknown; we observed that abnormal radiographs were relatively frequent (58% of patients) but poorly correlated with clinical condition, as about one-third of patients with dyspnea or oxygen requirement had normal radiographs, whereas one-third of asymptomatic patients had abnormal tests. However, the presence of abnormal radiographic findings has a prognostic significance as it is associated with higher day 28 mortality. Chest radiograph should therefore be considered during the initial evaluation of hyperleukocytic AML patients, even in asymptomatic subjects.
As far as radiographic abnormalities are concerned, in a series of 10 AML patients with autopsy-proven leukostasis, van Buchem et al [10] described 4 cases with normal chest radiographs, 4 cases with diffuse, bilateral consolidations, and 2 cases with focal abnormalities, with rapid progression of radiographic findings in some patients. Although all patients had leukostasis and vascular occlusions, those with normal radiographs had macroscopically normal lungs on autopsy while the others had edematous lungs, suggesting that leukostasis may coexist with pulmonary edema/hemorrhage. Case reports of hyperleukocytic myeloid leukemias with pulmonary leukostasis, minor radiographic findings, and perfusion defects on ventilation–perfusion scans were also described,[11,17] highlighting the role of vascular occlusion by leukemic cells in the pathogenesis of leukostasis.[13] In agreement with these studies, we observed a variety of radiographic abnormalities. Focal basilar airspace opacities, diffuse interstitial or airspace and interstitial opacities, and pleural effusion were all observed in about half of the patients with abnormal radiographs, and 2 main patterns (basilar airspace opacities and diffuse bilateral opacities) accounted for 75% of abnormal findings.
Most of our patients likely had pulmonary leukostasis, as attested by high LGS.[18] As the clinical manifestations of pulmonary leukostasis are not specific, Novotny et al developed a score to assess the clinical probability of leukostasis.[13] LGSs of 3 (high probability of leukostasis) have been reported in 44% to 50% of hyperleukocytic AML patients[13,18] and a slightly higher proportion (60%) of our patients had a LGS of 3. The pathogenesis of pulmonary leukostasis involves not only mechanical vessel obstruction by leukemic cells but also their ability to release cytokines (TNF-α and interleukin-1), and induce the expression by endothelial cells of vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and selectins, with subsequent adhesion of blasts on the endothelial surface, increased endothelial permeability with pulmonary edema and hemorrhage, and finally interstitial invasion by leukemic cells.[1] Accordingly, although pulmonary leukostasis and leukemic infiltration are classically described as distinct syndromes accounting for leukemia-specific respiratory complications,[6] authors have already discussed them as a continuum rather than 2 separate entities.[7] The variety of radiographic presentations observed in our population, from normal radiographs to focal opacities and diffuse findings, reinforces the idea of a continuum between pulmonary leukostasis and leukemic infiltration. Both entities may coexist, as shown by the lung biopsy example in Fig. 4; one might hypothesize that among our patients, those with predominant leukostasis and capillary obstruction by leukemic cells within the alveolar–capillary barrier were hypoxemic due to impaired oxygen diffusion but with minimal radiographic changes (Fig. 1), while patients with more interstitial infiltration displayed diffuse interstitial and airspace opacities (Figs. 2 and 4). A wide spectrum of presentations may exist between these 2 extremes, depending on the degree of hyperleukocytosis, the phenotype of leukemic cells, and their ability to infiltrate interstitium.
Respiratory complications in this setting are also a dynamic process, as suggested by the rapid radiographic changes observed.[10] It is possible that different time points of a common pathogenetic process account for the variety of radiographic findings observed in our population. Finally, some of the radiographic findings described earlier may reflect non–leukemia-specific complications. Several patients had heart failure as documented by echocardiogram and may have presented with acute pulmonary edema. Although large volumes of intravenous fluids are usually administered in AML patients to prevent tumor lysis syndrome, it is unlikely to account for our radiographic findings, as we analyzed only chest radiographs obtained on admission, prior to fluid administration. Likewise, we attempted to document infections but we cannot exclude that some of the radiographic findings observed were related to infectious processes. Although no pneumonia was documented by a positive culture, two-thirds of our patients had fever and SIRS criteria, and 85% of them received antibiotics, regardless of the normal radiographic findings. The clinical and radiographic criteria used to define infections are therefore poorly reliable in hyperleukocytic AML and the true incidence of infections difficult to assess.
CT findings were reported in patients with lung leukemic infiltration and involved thickening of bronchovascular bundles, reflecting the predilection of leukemic cells for the perilymphatic pulmonary interstitium, as well as prominence of peripheral pulmonary arteries and ground-glass opacities.[19,20] Eighteen of our patients also underwent an early (within 5 days) chest CT scan. As radiographs and CT scan were not performed simultaneously, we were unable to perform a reliable comparison, but CT overall added valuable information as compared to chest radiography. One reason is that the resolution of radiographs is suboptimal to detect interlobular septal thickening, prominence of peripheral pulmonary arteries, and ground-glass opacities, which are the most frequent CT findings in leukostasis or leukemic infiltration.[19] In 2 patients, CT scan also confirmed acute pulmonary embolism. Knowing that AML is a risk factor for venous thromboembolic disease[21] and that subtle findings such as ground-glass opacities and interlobular septal thickening may be difficult to detect on chest radiograph, CT scans should be considered broadly in hyperleukocytic AML patients, even though the risk/benefit ratio of intravenous contrast should be carefully weighted in patients already at risk for tumor lysis syndrome and acute kidney injury. Although we observed that patients with abnormal radiographs had more dyspnea and required higher respiratory support, 33% of patients requiring oxygen or mechanical ventilation had normal radiographs. CT scan is probably useful in this specific subset of patients or whenever there is a discrepancy between the clinical condition and the chest radiograph, by showing undetected ground-glass opacities or interlobular septal thickening.
We analyzed monocytic AML as a subgroup as it tends to be associated with leukemia-specific respiratory complications.[8] A possible explanation for this specificity is that monocytes are larger and less deformable (“stiffer”) than other leukocytes, including neutrophils, and therefore more likely to be retained in pulmonary capillaries.[22] Monocytes also express more TNF-α than other lineages,[23] which might account for the increased incidence of SIRS and cardiopulmonary complications observed in this AML subtype.[8,24] We observed a higher proportion of airspace opacities in nonmonocytic AML, but these were mostly focal opacities, whereas monocytic AML tended to be associated with diffuse, bilateral findings. We confirmed here the propensity of monocytic AML to cause diffuse bilateral radiographic abnormalities, as observed by Azoulay et al [8] in a series of patients with monocytic AML and acute respiratory failure, where 14 of 20 patients presented with bilateral airspace opacities.
The main limitation of our study is the lack of biopsies or autopsies to correlate the radiographic findings with pathological entities. However, based on LGS, we may assume that most of our patients, and by definition all of those with dyspnea or oxygen requirements, had a high probability of leukostasis. We collected data on cardiac function and signs of infection and did not observe significant differences between patients with normal and abnormal chest radiographs that would have suggested a major contribution of cardiogenic pulmonary edema or pneumonias to our findings. As discussed previously, leukostasis and lung leukemic infiltration may also be intricated and cause the filling of lung alveoli by edema and leukemic cells, which even with biopsies may be difficult to distinguish from cardiogenic pulmonary edema or infectious processes. Finally, our objective was not to describe the radiographic abnormalities specifically associated with leukostasis but to report the radiographic findings in hyperleukocytic AML patients with respect to their clinical presentation and AML subtype. Interpretation of the chest radiographs and CT scans was performed by a single radiologist (CJ-D) with a 13-year expertise exclusively in thoracic radiology, and as our study was a purely qualitative description of radiographic findings, it is unlikely that the interpretation by 2 independent radiologists would have affected our results.
5. Conclusion
In a large cohort of hyperleukocytic AML patients, we report that 58% of the patients (and 67% of those with dyspnea or oxygen requirement) had abnormal chest radiographs on presentation. Although heterogeneous findings were observed, 2 main patterns emerged: nonmonocytic AML presented more often with focal basilar airspace opacities, whereas monocytic AML tended to cause diffuse bilateral opacities. CT scan may add valuable information by showing interlobular septal thickening or ground-glass opacities, suggestive of leukostasis or leukemic infiltration and not detected on chest radiographs.
Footnotes
Abbreviations: AML = acute myeloid leukemia, CT = computed tomography, LGS = leukostasis grading score, SIRS = systemic inflammatory response syndrome, TNF-α = tumor necrosis factor-α, WBC = white blood cell.
MS and CJ-D contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood 2015; 125:3246–3252. [DOI] [PubMed] [Google Scholar]
- 2.Kuo KH, Callum JL, Panzarella T, et al. A retrospective observational study of leucoreductive strategies to manage patients with acute myeloid leukaemia presenting with hyperleucocytosis. Br J Haematol 2015; 168:384–394. [DOI] [PubMed] [Google Scholar]
- 3.Marbello L, Ricci F, Nosari AM, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia: a single-center retrospective study and review of literature. Leuk Res 2008; 32:1221–1227. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira LC, Romano LG, Prado-Junior BP, et al. Outcome of acute myeloid leukemia patients with hyperleukocytosis in Brazil. Med Oncol 2010; 27:1254–1259. [DOI] [PubMed] [Google Scholar]
- 5.Bug G, Anargyrou K, Tonn T, et al. Impact of leukapheresis on early death rate in adult acute myeloid leukemia presenting with hyperleukocytosis. Transfusion 2007; 47:1843–1850. [DOI] [PubMed] [Google Scholar]
- 6.Moreau AS, Lengline E, Seguin A, et al. Respiratory events at the earliest phase of acute myeloid leukemia. Leuk Lymphoma 2014; 55:2556–2563. [DOI] [PubMed] [Google Scholar]
- 7.Porcu P, Cripe LD, Ng EW, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma 2000; 39:1–18. [DOI] [PubMed] [Google Scholar]
- 8.Azoulay E, Fieux F, Moreau D, et al. Acute monocytic leukemia presenting as acute respiratory failure. Am J Respir Crit Care Med 2003; 167:1329–1333. [DOI] [PubMed] [Google Scholar]
- 9.Chaoui D, Legrand O, Roche N, et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia 2004; 18:670–675. [DOI] [PubMed] [Google Scholar]
- 10.van Buchem MA, Wondergem JH, Kool LJ, et al. Pulmonary leukostasis: radiologic–pathologic study. Radiology 1987; 165:739–741. [DOI] [PubMed] [Google Scholar]
- 11.Kaminsky DA, Hurwitz CG, Olmstead JI. Pulmonary leukostasis mimicking pulmonary embolism. Leuk Res 2000; 24:175–178. [DOI] [PubMed] [Google Scholar]
- 12.Van de Louw A, Schneider CW, Desai RJ, et al. Initial respiratory status in hyperleukocytic acute myeloid leukemia: prognostic significance and effect of leukapheresis. Leuk Lymphoma 2016; 57:1319–1326. [DOI] [PubMed] [Google Scholar]
- 13.Novotny JR, Muller-Beissenhirtz H, Herget-Rosenthal S, et al. Grading of symptoms in hyperleukocytic leukaemia: a clinical model for the role of different blast types and promyelocytes in the development of leukostasis syndrome. Eur J Haematol 2005; 74:501–510. [DOI] [PubMed] [Google Scholar]
- 14.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 15.Giles FJ, Shen Y, Kantarjian HM, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long-term survival. Leuk Lymphoma 2001; 42:67–73. [DOI] [PubMed] [Google Scholar]
- 16.Hug V, Keating M, McCredie K, et al. Clinical course and response to treatment of patients with acute myelogenous leukemia presenting with a high leukocyte count. Cancer 1983; 52:773–779. [DOI] [PubMed] [Google Scholar]
- 17.Szyper-Kravitz M, Strahilevitz J, Oren V, et al. Pulmonary leukostasis: role of perfusion lung scan in diagnosis and follow up. Am J Hematol 2001; 67:136–138. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo N, Laurenti L, Chiusolo P, et al. Reliability of leukostasis grading score to identify patients with high-risk hyperleukocytosis. Am J Hematol 2009; 84:381–382. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka N, Matsumoto T, Miura G, et al. CT findings of leukemic pulmonary infiltration with pathologic correlation. Eur Radiol 2002; 12:166–174. [DOI] [PubMed] [Google Scholar]
- 20.Heyneman LE, Johkoh T, Ward S, et al. Pulmonary leukemic infiltrates: high-resolution CT findings in 10 patients. AJR Am J Roentgenol 2000; 174:517–521. [DOI] [PubMed] [Google Scholar]
- 21.Mohren M, Markmann I, Jentsch-Ullrich K, et al. Increased risk of venous thromboembolism in patients with acute leukaemia. Br J Cancer 2006; 94:200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downey GP, Doherty DE, Schwab B, 3rd, et al. Retention of leukocytes in capillaries: role of cell size and deformability. J Appl Physiol 1990; 69:1767–1778. [DOI] [PubMed] [Google Scholar]
- 23.Kurzrock R, Kantarjian H, Wetzler M, et al. Ubiquitous expression of cytokines in diverse leukemias of lymphoid and myeloid lineage. Exp Hematol 1993; 21:80–85. [PubMed] [Google Scholar]
- 24.Hijiya N, Metzger ML, Pounds S, et al. Severe cardiopulmonary complications consistent with systemic inflammatory response syndrome caused by leukemia cell lysis in childhood acute myelomonocytic or monocytic leukemia. Pediatr Blood Cancer 2005; 44:63–69. [DOI] [PubMed] [Google Scholar]


