Abstract
In heart failure patients with preserved ejection fraction, their hemodynamic parameters usually change when they are from recumbent to passive leg raising. The authors designed this study to investigate the relationship between hemodynamic parameters measured by impedance cardiography (ICG) and 6-minute walk distance (6MWD) of heart failure with preserved ejection fraction (HFPEF). We recruited 49 subjects with HFPEF in the study, and all the subjects were separated into 2 groups: the patients whose hemodynamic parameters rose after passive leg raising were in group 1 (n = 26) and the patients whose hemodynamic parameters did not rise after passive leg raising were in group 2 (n = 23). Our study then compared the 6MWD, left ventricular ejection fraction, and plasma NT-pro-brain natriuretic peptide between the 2 groups. Group 1 had significantly longer 6MWD than group 2 (515.38 ± 24.97 vs 306.39 ± 20.20 m; P = 0.043). Hemodynamic parameters measured by ICG significantly correlated with 6MWD in both groups. Patients whose hemodynamic parameters rose in response to passive leg raising were more likely to have better exercise capacity. Hemodynamic variation in response to passive leg raising measured by ICG may be more sensitive in predicting exercise capacity of patients with HFPEF.
Keywords: 6-minute walk distance, exercise capacity, heart failure with preserved ejection fraction, impedance cardiography, passive leg raising
1. Introduction
Heart failure with preserved ejection fraction (HFPEF) has been regarded as a clinical entity distinct from other forms of heart failure and defined predominantly by symptoms of dyspnea and fluid retention in the absence of a significant reduction in left ventricular (LV) systolic function.[1,2] The prevalence of HFPEF has been increasing, and the morbidity, mortality, and healthcare costs has been equal to heart failure with reduced ejection fraction.[3] Knowledge of a patient's actual cardiac function is important for the treatment of HFPEF.[4] Predicting exercise capacity and severity of cardiac dysfunction of patients with HFPEF may contribute to better management of HFPEF.
Passive leg raising (PLR) may identify patients with impairment of diastolic functional reserve during exercise.[5] PLR is a reversible fluid-loading maneuver,[6] which may potentially increase intrathoracic blood volume, cardiac preload, and then cardiac output (CO), through circulating venous blood from the legs[7] towards the thorax.[8] CO increased in healthy persons in response to PLR.[9] For patients with impaired cardiac function, 1 of compensatory mechanisms to maintain normal CO is the Frank–Starling mechanism. The Frank–Starling mechanism states that an increase in diastolic filling causes an increase in peak systolic atrial pressure,[10] representing the intrinsic capability of the heart to respond to enhance preload with an increase in force development.[11] Cardiovascular responses to PLR is useful in assessing preload reserve, but it has seldom been studied longitudinally in predicting severity of cardiac dysfunction in HFPEF.
Impedance cardiography (ICG), a reliable and noninvasive technique, can be used to measure hemodynamic parameters continually.[12,13] The fundamental of ICG is Ohm law, which states that a constant current travels through a conductor as a result of voltage change directly proportional to variations in impedance.[12,14] A considerable proportion of previous data have confirmed the role of hemodynamic parameters measured by ICG in estimating cardiac function.[15–17] The hemodynamic parameters CO, cardiac index (CI), stroke volume (SV), stroke volume index (SVI), left stroke work (LSW), and left stroke work index (LSWI) correlated positively with cardiac function.[18] Stroke systemic vascular resistance (SSVR) represents the resistance of blood flow in the vascular system. Stroke systemic vascular resistance index (SSVRI) is the systemic vascular resistance normalized for body surface area. Both variables reflect the afterload of the heart and the degree of arteriosclerosis in the systemic artery.[19]
The present study will research the correlation among 6-minute walk distance (6MWD), left ventricular ejection fraction (LVEF), plasma NT-pro-brain natriuretic peptide (NT-proBNP), and hemodynamic parameters in patients with HFPEF. Our study will further explore the values of hemodynamic variation in response to PLR in predicting exercise capacity of patients with HFPEF.
2. Methods
2.1. Patients and controls
This observational study was approved by the Ethics Committee of Tongji Hospital, Wuhan, China. All the subjects had received informed consent and signed in informed consent before enrollment. The study was performed from January 2014 to June 2016. Our study recruited patients with HFPEF, and the inclusion criteria in our study were based on the following: typical symptoms of heart failure; representative signs of heart failure; the LVEF ≥50% (by echocardiography); evidence of diastolic dysfunction on echocardiography (mitral inflow E/A ratio, E′ measured at the mitral annulus, and E/E′ ratio).[20,21] Subjects with impaired cognition, atrial fibrillation, chronic obstructive pulmonary disease (COPD), asthma, severe hepatic disease, severe renal impairment, hyperthyroidism, arthritis, ankle, knee or hip injuries, and muscle wasting were excluded.[22,23] We even did not recruit patients with systolic blood pressure (SBP) of more than 180 mm Hg, or diastolic blood pressure (DBP) of more than 100 mm Hg or resting heart rate of more than 120, drugs and/or alcohol abuse, or any life-threatening disease.[22,23]Additionally, we excluded patients with recent myocardial infarction, unstable angina, pacemaker implantation, enlarged LV dimension, candidacy for revascularization, cardiomyopathy, left atrial enlargement, and valvular heart disease.
“Responds to PLR” in our study meant that CO calculated by ICG was changed in participants when they were from supine position to PLR. All the subjects were separated into 2 groups according to CO variation in response to PLR: the patients whose CO increased in response to PLR were in group 1 (n = 26), and the patients whose CO did not increase in response to PLR were in group 2 (n = 23). The 2 groups were matched for age, sex, height, weight, body mass index (BMI), underlying disease (chronic renal disease, coronary artery disease, diabetes mellitus, hypertension), and basic medicine [β-receptor blocker, digoxin, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB)].
2.2. Clinical evaluation
The machine to perform ICG was the Cheer Sails Medical (CSM3000 system). The basic principle of ICG was that specific waveform that can be used to calculate SV appeared as a result of the impedance changing with high-frequency (75 kHz) and low magnitude (1.8 mA) current across the thorax during cardiac ejection.[17,23] After 5 minutes’ rest, the technician put electrodes on the neck and hypochondriac regions of patients and performed ICG for 3 minutes when the subjects were in the supine position (Fig. 1A). After 30 minutes of rest, the technician performed ICG again for the same subjects for 3 minutes when they were raising legs at 45° (Fig. 1B).
Figure 1.
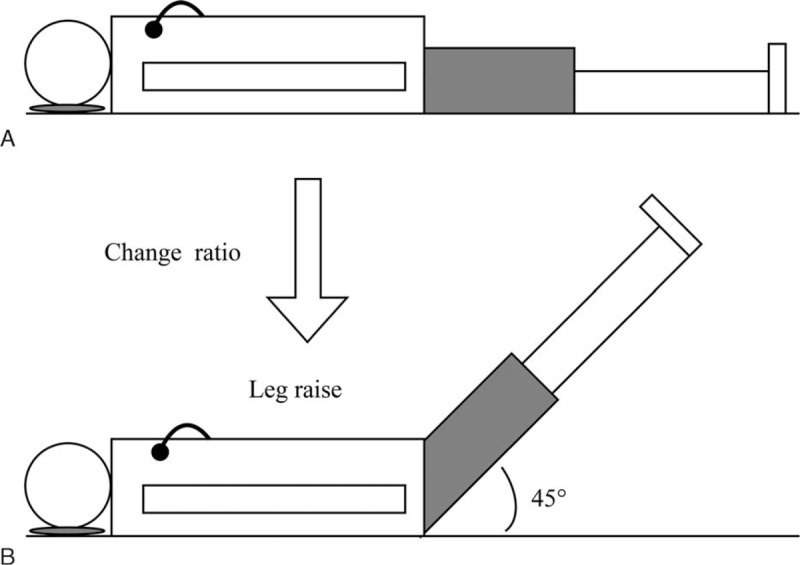
A, The subject was put electrodes on the neck and hypochondriac region and performed ICG when he was in the supine position. B, The subject was put electrodes on the neck and hypochondriac region and performed ICG when he was raising legs at 45°. ICG = impedance cardiography.
The 6MWT is used to measure the distance that a patient can quickly walk on a flat and hard surface in 6 minutes.[24] 6MWT was ordered by the physicians as an initial or follow-up assessment in cardiac function according to ATS standards.[13,14] We obtained the reference standards for the distance walk during the 6MWT from the study by Enright and Sherrill.[23] All distance of 6MWT were recorded as 6MWD accurately.
Weight and height were recorded to calculate BMI values. Our study also recorded underlying diseases (chronic renal disease, coronary artery disease, diabetes mellitus, and hypertension) or basic medicine (β-receptor blocker, digoxin, ACEI, and ARB). NT-proBNP was measured using an automatic biochemistry analyzer.[25] Our study measured LVEF and other parameters of cardiac structure by echocardiography, as previously reported.[26–29]
2.3. Statistical analysis
Analyses were carried out using IBM SPSS 16.0 for Windows (IBM, NY). Continuous variables were compared by independent-samples t test and dichotomous variables were compared by Pearson chi-square test in baseline subject characteristics [30]. The means ± standard error of continuous variable was considered as statistic value.[31] Dichotomous variables with fewer than 5 participants in a category were given continuity correction in chi-square test. Bivariate correlate analysis was used to compare the correlation among hemodynamic parameters, plasma NT-proBNP, and 6MWD. Sample size was calculated as per the following formula: n = 2 × [(uα + uβ) × σ/δ]2. The ratio of sample sizes was ≈1. ‘σ’ represented the population standard deviation of 6MWD. ‘δ’ was the difference in means of 6MWD in population between group 1 and group 2. Thus, the sample size in each group: n = 2 × [(uα + uβ) × σ/δ]2 = 21. All statistical tests were 2-sided. P < 0.05 was considered statistically significant.
3. Results
Baseline characteristics of group 1 and group 2 are presented in Table 1. The subjects in the 2 groups had no statistical difference in age, sex, height, weight, BMI, SBP, DBP, and mean arterial pressure (MAP). There was no statistical difference in comorbidity such as chronic renal disease, coronary artery disease, diabetes mellitus, and hypertension. There were 5 (19.2%) individuals in group 1, and 1 (4.3%) individual in group 2 using β-receptor blocker. The number of characters taking digoxin was 1 (3.8%) in group 1, and 1 (4.3%) in group 2 in our study. There were 3 (11.5%) individuals in group 1 and 2 (8.7%) individuals in group 2 using ACEI/ARB. The basic medicine such as β-receptor blocker (P = 0.125), digoxin (P = 0.724), and ACEI/ARB (P = 0.560) in the 2 groups had no statistical difference.
Table 1.
Comparisons of the clinical data between two group 1 and group 2.
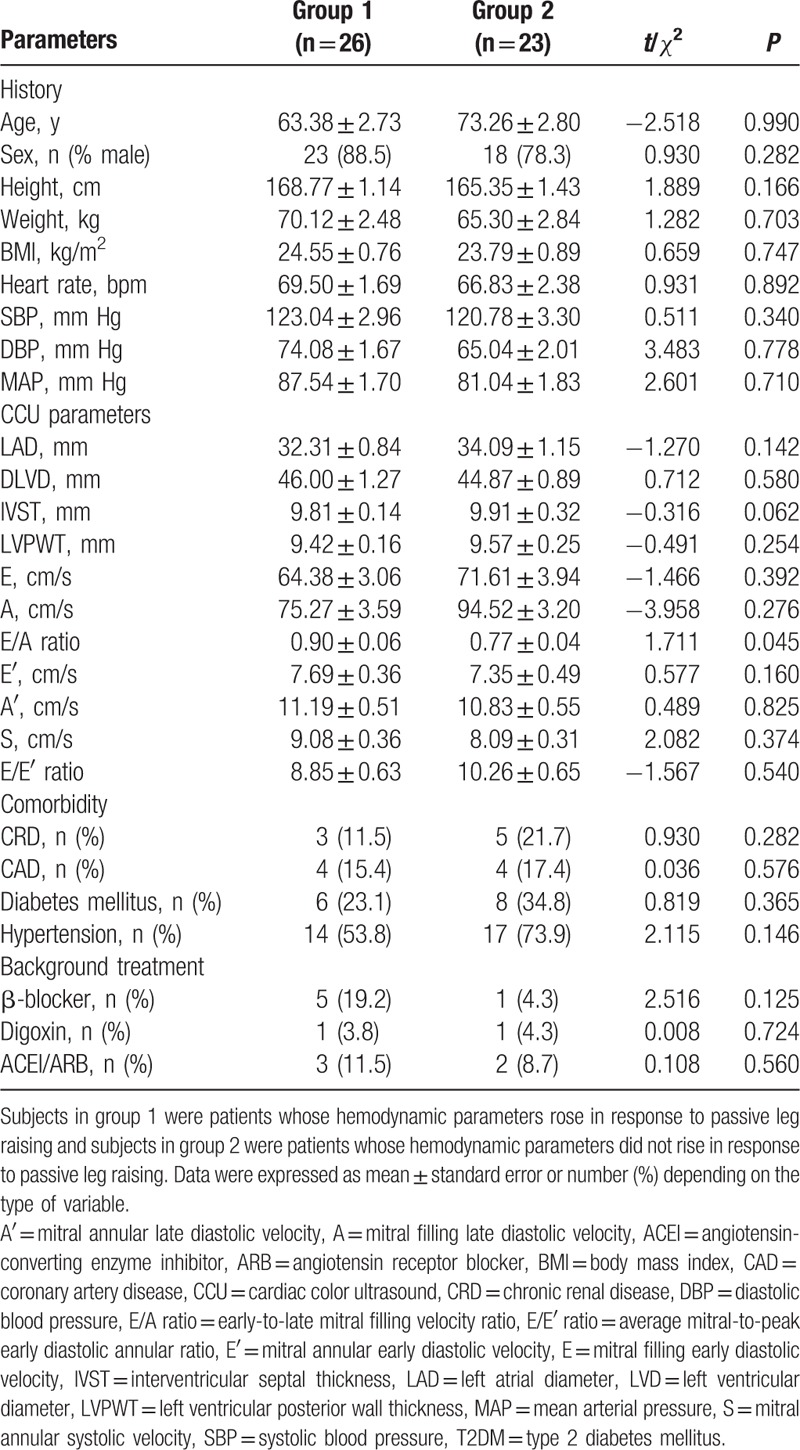
There were no significant differences in most cardiac color ultrasound parameters (Table 1), for instance, left atrial diameter (LAD), diastolic left ventricular diameter (DLVD), interventricular septal thickness (IVST), left ventricular posterior wall thickness (LVPWT), mitral filling early diastolic velocity (E), mitral annular early diastolic velocity (E′), mitral annular late diastolic velocity (A′), mitral annular systolic velocity (S), average mitral-to-peak early diastolic annular ratio (E/E′) (8.85 ± 0.63 vs 10.26 ± 0.65; P = 0.540). But the early-to-late mitral filling velocity ratio (E/A) (0.90 ± 0.06 vs 0.77 ± 0.04; P = 0.045) in 2 groups were statistically different.
Table 2 shows the comparisons of hemodynamic parameters in group 1 when subjects were recumbent and PLR. In group 1, the parameters CO (3.70 ± 0.25 vs 4.15 ± 0.28 L/min), CI (2.04 ± 0.13 vs 2.28 ± 0.15 L/min/m2), SV (54.38 ± 3.68 vs 60.14 ± 4.02 mL), SVI (29.92 ± 1.85 vs 33.04 ± 2.01 mL/m2), LSW (61.77 ± 4.52 vs 68.69 ± 5.16 gm-m/beat), and LSWI (33.87 ± 2.23 vs 37.62 ± 2.52 gm-m/m2/beat) in recumbent were lower than those parameters in PLR. Whereas the parameters SSVR (444.75 ± 30.22 vs 407.36 ± 29.17 dynes/cm5) and SSVRI (247.94 ± 17.78 vs 226.87 ± 16.68 dynes/cm5/m2) in recumbent were higher than those parameters in PLR.
Table 2.
Comparisons of hemodynamic parameters in group 1 (n = 26).
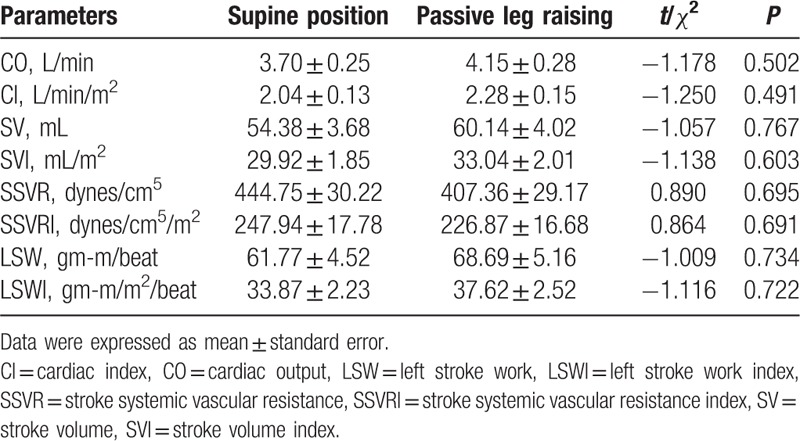
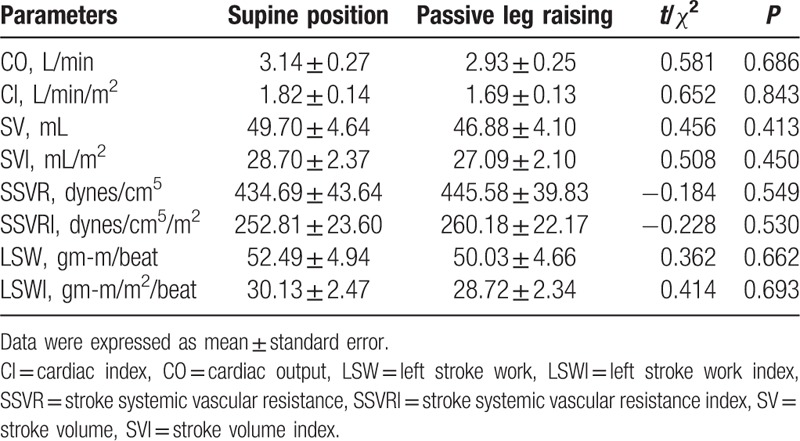
Table 3 shows the comparisons of hemodynamic parameters in group 2 when patients were recumbent and PLR. In group 2, the parameters CO (3.14 ± 0.27 vs 2.93 ± 0.25 L/min), CI (1.82 ± 0.14 vs 1.69 ± 0.13 L/min/m2), SV (49.70 ± 4.64 vs 46.88 ± 4.10 mL), SVI (28.70 ± 2.37 vs 27.09 ± 2.10 mL/m2), LSW (52.49 ± 4.94 vs 50.03 ± 4.66 gm-m/beat), and LSWI (30.13 ± 2.47 vs 28.72 ± 2.34 gm-m/m2/beat) in recumbent were not lower than those parameters in PLR. Whereas the parameters SSVR (434.69 ± 43.64 vs 445.58 ± 39.83 dynes/cm5) and SSVRI (252.81 ± 23.60 vs 260.18 ± 22.17 dynes/cm5/m2) in recumbent were lower than those parameters in PLR.
Table 3.
Comparisons of hemodynamic parameters in group 2 (n = 23).
The correlation among hemodynamic parameters, NT-proBNP, and 6MWD were showed in Table 4 and Table 5. When participants were at supine position, Pearson correlation analysis revealed that the parameters CO (R = 0.557, P < 0.001), CI (R = 0.493, P < 0.001), SV (R = 0.435, P = 0.002), SVI (R = 0.368, P = 0.009), LSW (R = 0.488, P < 0.001), and LSWI (R = 0.449, P < 0.001) significantly positively correlated with 6MWD. The parameters SSVR (R = −0.223, P = 0.124) and SSVRI (R = −0.312, P = 0.029) correlated inversely with 6MWD in the same group. The correlation among hemodynamic parameters and 6MWD was similar when patients were raising the leg. As shown in Table 5, NT-proBNP was statistically inversely correlated with 6MWD (R = −0.539, P < 0.001).
Table 4.
Correlation among hemodynamic parameters and 6MWD.
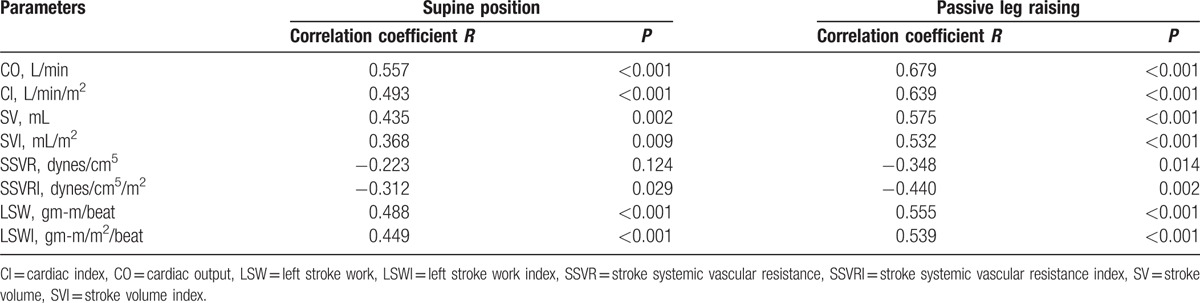
Table 5.
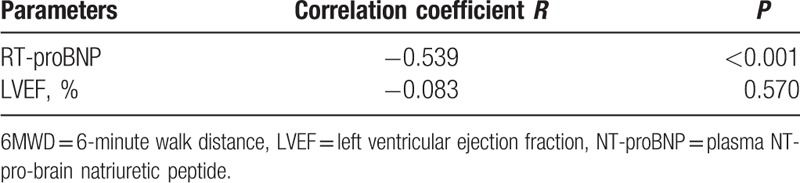
Correlation among RT-proBNP, LVEF, and 6MWD.
When participants were at supine position, the patients in group 1 had slightly higher CO (3.70 ± 0.25 vs 3.14 ± 0.27 L/min), CI (2.04 ± 0.13 vs 1.82 ± 0.14 L/min/m2), SV (54.38 ± 3.68 vs 49.70 ± 4.64 mL), SVI (29.92 ± 1.85 vs 28.70 ± 2.37 mL/m2), LSW (61.77 ± 4.52 vs 52.49 ± 4.94 gm-m/beat), and LSWI (33.87 ± 2.23 vs 30.13 ± 2.47 gm-m/m2/beat) than those parameters in group 2 (Table 6). Whereas SSVR (444.75 ± 30.22 vs 434.69 ± 43.64 dynes/cm5) and SSVRI (247.94 ± 17.78 vs 252.81 ± 23.60 dynes/cm5/m2) in group 1 were slightly higher than those parameters in group 2. NT-proBNP (172.92 ± 62.79 vs 431.13 ± 95.46 pg/mL; P = 0.059) and LVEF (64.04 ± 1.64 vs 64.30 ± 1.46%; P = 0.500) had no significant difference in the 2 groups (Fig. 2B and C, Table 6). Whereas patients in group 1 had significantly higher 6MWD than patients in group 2 (515.38 ± 24.97 vs 306.39 ± 20.20 m; P = 0.043) (Fig. 2A, Table 6).
Table 6.
Comparisons of parameters at supine position between group 1 and group 2.
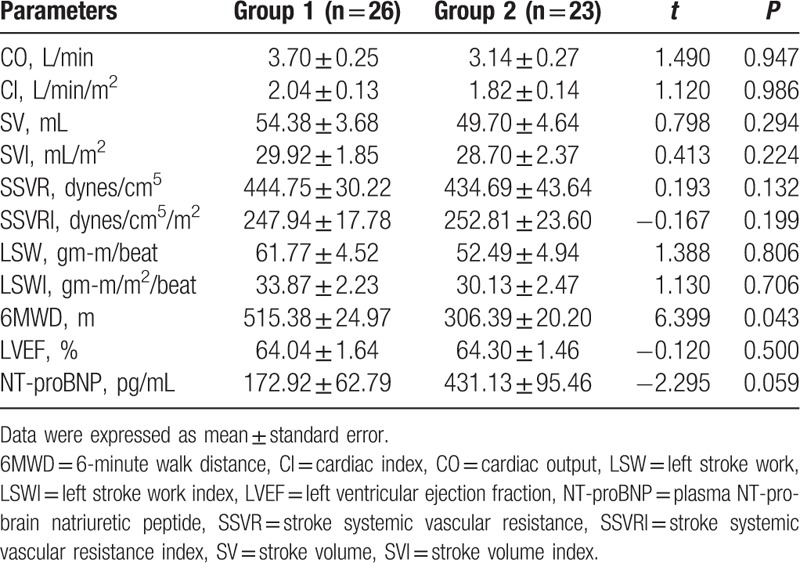
Figure 2.
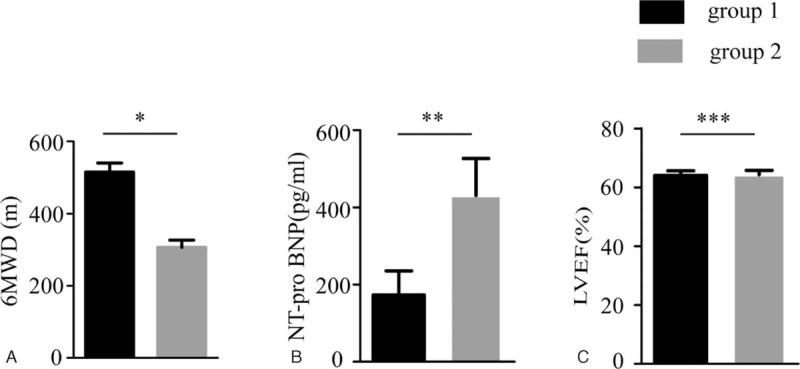
Comparisons of 6MWD, NT-proBNP, and LVEF when patients were supine in position. A, The comparison of the 6MWD between group 1 and group 2 (∗P = 0.043). B, The comparison of the NT-proBNP between group 1 and group 2 (∗∗P = 0.059). C, The comparison of the LVEF between group 1 and group 2 (∗∗∗P = 0.500). 6MWD = 6-minute walk distance, LVEF = left ventricular ejection fraction, NT-proBNP = plasma NT-pro-brain natriuretic peptide.
4. Discussion
In previous study, ICG has been used to reveal hemodynamic characteristics in heart failure.[32] However, few studies have been published on the hemodynamic changes in response to PLR in patients with HFPEF. Our study found that after PLR, the hemodynamic parameters of some patients rose and others did not rise. We separated the subjects into 2 groups: the patients whose hemodynamic parameters rose after PLR were in group 1 (n = 26) and the patients whose hemodynamic parameters did not rise after PLR were in group 2 (n = 23).
The 2 groups had similar cardiac structure according to echocardiography parameters LAD, DLVD, IVST, and LVPWT (Table 1). The parameter E/E′ (8.85 ± 0.63 vs 10.26 ± 0.65; P = 0.540) in echocardiography was not significantly different between the 2 groups. Early-to-late mitral filling velocity ratio (E/A) (0.90 ± 0.06 vs 0.77 ± 0.04; P = 0.045) was significantly different between the 2 groups. E/A ratio usually decreased to be less than 1 in patients with diastolic dysfunction.[33,34] A higher E/A ratio in group 1 likely represents a less degree of diastolic stiffness, and therefore a better cardiac preload reserve,[35] which in itself would predict a better response to 6MWT. The present study showed that group 1 had significantly higher 6MWD than group 2 (515.38 ± 24.97 vs 306.39 ± 20.20 m; P = 0.043). As the role of 6MWD in assessing exercise tolerance and functional capacity in patients with impaired cardiac function,[36] our study indicated that the patients in group 1 had better cardiac function and exercise capacity than patients in group 2.
Our study found that CO, CI, SV, SVI, LSW, and LSWI increased, and SSVR and SSVRI decreased after PLR in group 1; CO, CI, SV, SVI, LSW, and LSWI did not increase, and SSVR and SSVRI increased in group 2 (Tables 2 and 3). According to the curvilinearity of the Frank–Starling relationship, if the heart is operating on the initial and steep part of the curve, it should have some preload reserve, and an increase in cardiac preload results in an increase in SV.[18,37] Previous studies of hemodynamic effects of PLR indicated that hemodynamic changes related to PLR were only induced by increased cardiac preload.[38] CO in response to PLR was according to the central volume status and the degree of cardiac preload reserve.[39] Our study indicated that patients in group 1 had better central volume status and larger cardiac preload reserve than patients in group 2.
Previous studies showed that LVEF and NT-proBNP levels were correlated with cardiac function.[40,41] Even though NT-proBNP (172.92 ± 62.79 vs 431.13 ± 95.46 pg/ml; P = 0.059) had no significant difference between the 2 groups, NT-proBNP significantly inversely correlated with 6MWD (R = −0.539, P < 0.001). The present study showed that there was no significant difference in the hemodynamic parameters measured by ICG between the 2 groups. Consistent with the hemodynamic parameters, our study found that LVEF (64.04 ± 1.64 vs 64.30 ± 1.46%; P = 0.500) was not significantly different between the 2 groups. Our results also showed that LVEF was not correlated with 6MWD (R = −0.083, P = 0.570).
Our study found that CO, CI, SV, SVI, LSWI, and LSW correlated positively with 6MWD, whereas SSVR and SSVRI correlated negatively with 6MWD in 2 groups (Table 4). With higher CO, CI, SV, SVI, LSWI, and LSW, patients would have stronger functional capacity, exercise tolerance, and longer 6MWD. Our study may indicate that the patients in group 1 whose hemodynamic parameters (CO, CI, SV, SVI, LSWI, and LSW) rose after PLR, had better exercise capacity and cardiac function. The hemodynamic variation after PLR could screen patients with cardiac dysfunction, which would contribute to better management of HFPEF. Digoxin therapy could increase the CO, improve exercise capacity, and reduce symptoms in patients with HFPEF.[42]
Our study has limitations. The subjects in our study were relatively older and their parameters maybe could not represent the state of the whole population. The sample of the study was also small, which limited the statistical power of group analyses.
5. Conclusions
Measuring hemodynamic parameters by ICG, the patients whose CO, CI, SV, SVI, LSW, and LSWI increased, whereas SSVR and SSVRI decreased in response to PLR, were more likely to have better exercise capacity. Hemodynamic variation in response to PLR measured by ICG may be sensitive in predicting exercise capacity of patients with HFPEF.
Acknowledgments
The authors are thankful for the vital contribution of the participating volunteer patients in this study. The authors would like to be grateful to the local research ethics committee and the individual researchers, clinicians, and nurses who participated in the study.
Footnotes
Abbreviations: 6MWD = 6-minute walk distance, 6MWT = 6-minute walk test, A = mitral filling late diastolic velocity, A′ = mitral annular late diastolic velocity, ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor blocker, BMI = body mass index, CAD = coronary artery disease, CCU = cardiac color ultrasound, CI = cardiac index, CO = cardiac output, COPD = chronic obstructive pulmonary disease, CRD = chronic renal disease, DBP = diastolic blood pressure, E = mitral filling early diastolic velocity, E/A ratio = early-to-late mitral filling velocity ratio, E/E′ ratio = average mitral-to-peak early diastolic annular ratio, E′ = mitral annular early diastolic velocity, HFPEF = heart failure with preserved ejection fraction, ICG = impedance cardiography, IVST = interventricular septal thickness, LAD = left atrial diameter, LSW = left stroke work, LSWI = left stroke work index, LV = left ventricular, LVD = left ventricular diameter, LVEF = left ventricular ejection fraction, LVPWT = left ventricular posterior wall thickness, MAP = mean arterial pressure, NT-proBNP = plasma NT-pro-brain natriuretic peptide, PLR = passive leg raising, S = mitral annular systolic velocity, SBP = systolic blood pressure, SSVR = stroke systemic vascular resistance, SSVRI = stroke systemic vascular resistance index, SV = stroke volume, SVI = stroke volume index, T2DM = type 2 diabetes mellitus.
H-LZ and LD contributed equally to this study.
Funding: This work is supported by the grant from the National Natural Science Foundation of China (81300141).
The authors have no conflicts of interest to disclose.
References
- 1.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126:65–75. [DOI] [PubMed] [Google Scholar]
- 2.He B, Zhao Y, Xu L, et al. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J Pineal Res 2016; 60:313–326. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 68:1476–1488. [DOI] [PubMed] [Google Scholar]
- 4.Tschope C, Pieske B. “One size does not fit all”: how to individualize decongestive therapy strategies in heart failure. JACC Heart Fail 2016; 4:460–463. [DOI] [PubMed] [Google Scholar]
- 5.Choi EY, Shim CY, Kim SA, et al. Passive leg-raise is helpful to identify impaired diastolic functional reserve during exercise in patients with abnormal myocardial relaxation. J Am Soc Echocardiogr 2010; 23:523–530. [DOI] [PubMed] [Google Scholar]
- 6.Gaffney FA, Bastian BC, Thal ER, et al. Passive leg raising does not produce a significant or sustained autotransfusion effect. J Trauma 1982; 22:190–193. [DOI] [PubMed] [Google Scholar]
- 7.Rutlen DL, Wackers FJ, Zaret BL. Radionuclide assessment of peripheral intravascular capacity: a technique to measure intravascular volume changes in the capacitance circulation in man. Circulation 1981; 64:146–152. [DOI] [PubMed] [Google Scholar]
- 8.Reich DL, Konstadt SN, Raissi S, et al. Trendelenburg position and passive leg raising do not significantly improve cardiopulmonary performance in the anesthetized patient with coronary artery disease. Crit Care Med 1989; 17:313–317. [DOI] [PubMed] [Google Scholar]
- 9.Wong DH, Tremper KK, Zaccari J, et al. Acute cardiovascular response to passive leg raising. Crit Care Med 1988; 16:123–125. [DOI] [PubMed] [Google Scholar]
- 10.Kayhan N, Bodem JP, Vahl CF, et al. The positive staircase (force-frequency relationship) and the Frank-Starling mechanism are altered in atrial myocardium of patients in end-stage heart failure transplanted for dilative cardiomyopathy. Transplant Proc 2002; 34:2185–2191. [DOI] [PubMed] [Google Scholar]
- 11.Weil J, Eschenhagen T, Hirt S, et al. Preserved Frank-Starling mechanism in human end stage heart failure. Cardiovasc Res 1998; 37:541–548. [DOI] [PubMed] [Google Scholar]
- 12.Sodolski T, Kutarski A. Impedance cardiography: a valuable method of evaluating haemodynamic parameters. Cardiol J 2007; 14:115–126. [PubMed] [Google Scholar]
- 13.Xu GJ, Gan TY, Tang BP, et al. Predictive factors and clinical effect of optimized cardiac resynchronization therapy. Exp Ther Med 2013; 5:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Shen Y, Wang Y, et al. Increased expression of hypoxia-inducible factor-1alpha in proliferating neointimal lesions in a rat model of pulmonary arterial hypertension. Am J Med Sci 2013; 345:121–128. [DOI] [PubMed] [Google Scholar]
- 16.Han F, Sun R, Ni Y, et al. Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. Am J Med Sci 2015; 349:199–205. [DOI] [PubMed] [Google Scholar]
- 17.Ding L, Quan XQ, Zhang S, et al. Correlation between impedance cardiography and 6 min walk distance in atrial fibrillation patients. BMC Cardiovasc Disord 2016; 16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Ao X, Liu Q, et al. Intermittent low-dose digoxin may be effective and safe in patients with chronic heart failure undergoing maintenance hemodialysis. Exp Ther Med 2014; 8:1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiajuan Z, Ding D, Yanyan H, et al. Impedance cardiographic hemodynamic variables and hypertension in elderly Han residents. Ups J Med Sci 2013; 118:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mcmurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 21.Pal N, Sivaswamy N, Mahmod M, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 2015; 132:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cody RJ, Covit AB, Schaer GL, et al. Sodium and water balance in chronic congestive heart failure. J Clin Invest 1986; 77:1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158 (5 Pt 1):1384–1387. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Dai HP, Kang J, et al. Double-blind randomized trial of pirfenidone in Chinese idiopathic pulmonary fibrosis patients. Medicine (Baltimore) 2015; 94:e1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilal S, Chai YL, Ikram MK, et al. Markers of cardiac dysfunction in cognitive impairment and dementia. Medicine (Baltimore) 2015; 94:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudaverdi M, Hamilton-Craig C, Platts D, et al. Echocardiography for the clinician: a practical update. Intern Med J 2010; 40:476–485. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Na S, Kim N, et al. The effects of dexmedetomidine on myocardial function assessed by tissue Doppler echocardiography during general anesthesia in patients with diastolic dysfunction: a CONSORT-prospective, randomized, controlled trial. Medicine (Baltimore) 2016; 95:e2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res 2014; 57:228–238. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Liang H, Lu Z, et al. Membrane receptor-dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: in vivo and in vitro studies. J Pineal Res 2015; 59:420–433. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Guo Y, Yuan Q, et al. Melatonin prevents neural tube defects in the offspring of diabetic pregnancy. J Pineal Res 2015; 59:508–517. [DOI] [PubMed] [Google Scholar]
- 31.Pu J, Mintz GS, Brilakis ES, et al. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J 2012; 33:372–383. [DOI] [PubMed] [Google Scholar]
- 32.Tang WH, Tong W. Measuring impedance in congestive heart failure: current options and clinical applications. Am Heart J 2009; 157:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morka A, Szydlowski L, Moric-Janiszewska E, et al. Left ventricular diastolic dysfunction assessed by conventional echocardiography and spectral tissue Doppler imaging in adolescents with arterial hypertension. Medicine (Baltimore) 2016; 95:e2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satpathy C, Mishra TK, Satpathy R, et al. Diagnosis and management of diastolic dysfunction and heart failure. Am Fam Physician 2006; 73:841–846. [PubMed] [Google Scholar]
- 35.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29:277–314. [DOI] [PubMed] [Google Scholar]
- 36.Cahalin LP, Arena R, Guazzi M. Comparison of heart rate recovery after the six-minute walk test to cardiopulmonary exercise testing in patients with heart failure and reduced and preserved ejection fraction. Am J Cardiol 2012; 110:467–468. [DOI] [PubMed] [Google Scholar]
- 37.Monnet X, Teboul JL. Passive leg raising. Intensive Care Med 2008; 34:659–663. [DOI] [PubMed] [Google Scholar]
- 38.Caille V, Jabot J, Belliard G, et al. Hemodynamic effects of passive leg raising: an echocardiographic study in patients with shock. Intensive Care Med 2008; 34:1239–1245. [DOI] [PubMed] [Google Scholar]
- 39.Wong DH, O’connor D, Tremper KK, et al. Changes in cardiac output after acute blood loss and position change in man. Crit Care Med 1989; 17:979–983. [DOI] [PubMed] [Google Scholar]
- 40.Koukoui F, Desmoulin F, Lairy G, et al. Benefits of cardiac rehabilitation in heart failure patients according to etiology: INCARD French study. Medicine (Baltimore) 2015; 94:e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feola M, Testa M, Leto L, et al. Role of galectin-3 and plasma B type-natriuretic peptide in predicting prognosis in discharged chronic heart failure patients. Medicine (Baltimore) 2016; 95:e4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocco G, Jerie P. Comparison between ivabradine and low-dose digoxin in the therapy of diastolic heart failure with preserved left ventricular systolic function. Clin Pract 2013; 3:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]


