Abstract
The aim of this study was to investigate the efficacy of postoperative scheduled intravenous acetaminophen to reduce the opioid use and enhance recovery after gastrectomy.
Opioid use is reportedly associated with delayed recovery of gastrointestinal (GI) peristalsis and postoperative nausea/vomiting (PONV) despite of acceptable efficacy for pain control.
Of 147 and 96 consecutive patients who underwent gastrectomy for gastric cancer before and after introduction of postoperative scheduled intravenous acetaminophen, propensity score matched population was created and short-term clinical outcomes were compared.
Significant defervescence was demonstrated in Acetaminophen group (A-group) compared with control group (C-group) during the perioperative period (P < 0.001), whereas no significant difference was observed in postoperative inflammatory parameters. The incidence of postoperative complications was similar between the groups. The number of patient-controlled analgesia (PCA) pushes was significantly reduced in the A-group (P = 0.007) and the frequency of use of other nonopioid analgesics was also significantly reduced in the A-group (P < 0.001). Both daily and cumulative opioid use was significantly reduced in the A-group (P < 0.001). The time to first flatus and defecation was decreased in the A-group (P < 0.001 and P = 0.038, respectively). The incidence of PONV was significantly reduced from 26.0% to 12.5% after introduction of intravenous acetaminophen (P = 0.017), and hospital stay tended to be decreased in the A-group (13.2 vs 14.7 days, P = 0.069)
Postoperative scheduled intravenous acetaminophen decreased opioid use and may be associated with enhanced recovery after gastrectomy.
Keywords: propensity score matched analysis, gastric cancer, intravenous acetaminophen, opioids use, postoperative nausea and vomiting
1. Introduction
Growing attention is being paid to perioperative management using the enhanced recovery after surgery (ERAS) protocol, which aims to reduce surgical invasiveness and postoperative complications and shorten hospital stay. Major limiting factors for the ERAS protocol include pain, gastrointestinal (GI) dysfunction, and immobility. Although these 3 factors interact with each other, it is particularly important to control postoperative pain for the improvement of GI dysfunction and immobility. The use of patient-controlled analgesia (PCA) using epidural anesthesia has recently been recommended as a postoperative pain management strategy for the ERAS protocol.[1–3] The combined use of a low-dose opioid with local anesthetics has been demonstrated to be very effective for postoperative pain control. Meanwhile, postoperative nausea and vomiting (PONV) and GI dysfunction caused by opioid use has been identified as a major limiting factor for the ERAS protocol. Recommended solutions to this problem include the concomitant use of nonopioid anti-inflammatory analgesics, such as Non-steroidal anti-inflammatory drugs (NSAIDs) and pentazocine hydrochloride.[4,5] Therefore, a postoperative pain control regimen that maintains GI peristalsis while minimizing the development of PONV is required for the facilitation of ERAS. In this study, we introduced the scheduled intravenous (IV) infusion of acetaminophen in combination with epidural anesthesia, with the aim of reducing the postoperative use of opioids, thereby reducing PONV, and evaluated changes in postoperative opioid use and effects on early postoperative recovery.
2. Methods
This single-center intervention study was conducted to evaluate the efficacy of scheduled acetaminophen IV infusion for postoperative pain control and subsequent ability to reduce opioid use after gastrectomy for gastric cancer, as well as the usefulness of acetaminophen for the facilitation of ERAS. We have introduced a scheduled, postoperative IV acetaminophen regimen since February 2015. Two hundred fifty-four consecutive patients with gastric cancer were identified from the prospectively constructed database at the Department of Gastroenterological Surgery, Toranomon Hospital between January 2014 and December 2015. Of the 153 patients treated before the introduction of the acetaminophen regimen, 147 patients, after excluding 6 patients who did not receive epidural anesthesia, were allocated to the control (C-) group, while 96 of the 101 patients treated after the introduction of the regimen were allocated to the acetaminophen (A-) group, after excluding 5 patients not receiving epidural anesthesia. Patients in both groups were matched for demographic variables using propensity scores, and the following variables in the matched groups consisting of 96 patients each (Fig. 1) were compared: postoperative course (fever pattern, inflammatory responses); postoperative complications; postoperative pain assessment; and clinical efficacy (improvement in GI motility, incidence of PONV). Disease was staged according to the UICC TNM grading system, version 7.[6] Postoperative complications were graded according to the Clavien–Dindo classification system,[7] with grade ≥2 events recorded as complications. Postoperative liver dysfunction was graded according to the Common Terminology Criteria for Adverse Events, with grade ≥3 events (defined as ≥5 × the upper limit of normal) recorded as complications. Pain assessment was based on the number of PCA pushes for epidural anesthesia, postoperative opioid use, and the frequency of use of nonopioid analgesics, including NSAIDs and pentazocine hydrochloride. Improvement in GI motility was assessed on the basis of time to the first defecation/flatus after operation. Effects on PONV were assessed on the basis of the number of doses of metoclopramide after the operation and the rate of early withdrawal from epidural anesthesia. This study was carried out with approval from the Institutional Review Board of Toranomon Hospital.
Figure 1.

Patients tree. Patients in both groups were matched for demographic variables using propensity scores, and the following variables in the matched groups consisting of 96 patients each.
2.1. Operative procedure
According to the Japanese Gastric Cancer Treatment Guidelines,[8] we perform laparoscopic surgery in clinical stage (cStage) I cases and open surgery in cStage ≥II cases. The operative procedure was either total gastrectomy or distal gastrectomy, with D1+ or D2 lymphadenectomy depending on the degree of progression and surgical risk.[9–11] The reconstruction technique was either Billroth-I or Roux-en Y.
2.2. Epidural anesthesia
Epidural anesthesia was administered as a continuous infusion of a 300-mL mixture of Anapeine (288 mL) and fentanyl (6 ampules, 0.1 mg/2 mL) at a rate of 2 to 5 mL/h depending on pain intensity, starting immediately after the operation, with 1 to 3 mL PCA (i-Fuser, JMS Co., Ltd., Tokyo, Japan) as rescue analgesia.
2.3. Acetaminophen
An IV infusion of acetaminophen (Acelio Intravenous Injection; TERUMO Co. Ltd., Tokyo, Japan) was started on the day of the operation at 1000 mg/dose every 6 hurs for patients weighing ≥50 kg, or at 500 mg/dose every 6 hours for patients weighing <50 kg, at consistent times every day. The infusion continued until hospital day 3, when oral intake and oral medications were started.
2.4. Statistical analysis
Statistical analysis was performed using Statistic Package for Social Science (SPSS) version 19.0J for Windows (SPSS Inc., Chicago, IL). Intergroup comparisons were performed using the Pearson Chi-square test, McNemar test, Fisher exact test, or Mann–Whitney U test, as appropriate. In this study, we performed propensity score matching to minimize the selection bias between patients treated before and after the introduction of the acetaminophen regimen. The propensity score matching was calculated from a multivariate logistic regression model, including age, sex, body mass index (BMI), the American Society of Anesthesiologists (ASA) score, tumor staging, the extent of lymphadenectomy, operative approach, and operative procedure. With propensity score estimated, 96 pairs of patients before and after the introduction of the acetaminophen groups were matched using a 1 : 1 nearest neighbor matching algorithm. For all analyses, differences were considered statistically significant when P < 0.05.
3. Results
3.1. Patient characteristics
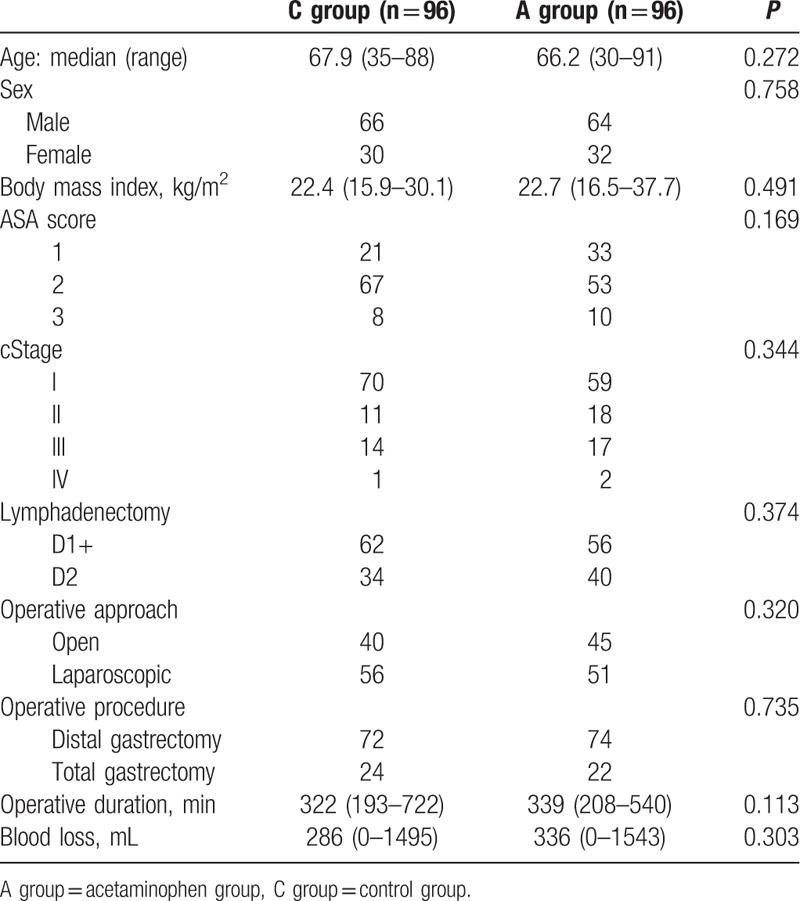
Table 1 summarizes the patient characteristics of A- and C-groups. Both groups were similar with respect to age, sex, BMI, ASA score, clinical stage, operative procedure, the extent of lymphadenectomy, operative approach and reconstruction technique, as well as operative outcomes, including operative duration and blood loss.
Table 1.
Clinicopathological characteristics of the 192 patients.
3.2. Anti-inflammatory effect
Table 2 compares the inflammatory measures between the 2 groups. Figure 2A shows the postoperative fever patterns in both groups. Significant defervescence was demonstrated in the acetaminophen group, with a peak temperature of 37.6°C in the acetaminophen group and 38.1°C in C-group (P < 0.001). Although no significant intergroup difference was observed in fever intensity immediately after operation, the A-group showed faster defervescence and significantly greater decreases in fever between postoperative day (POD) 1 and POD3. With regard to postoperative inflammatory responses, blood testing showed no significant difference in white blood cell (WBC) or C-reactive protein (CRP) values between the groups. The mean length of postoperative hospital stay in A-group (13.2 days) was one-and-a-half day more apt to shorten than in C-group (14.7 days), but the difference between the 2 groups was not significant (P = 0.062).
Table 2.
Effect of postoperative intravenous acetaminophen.
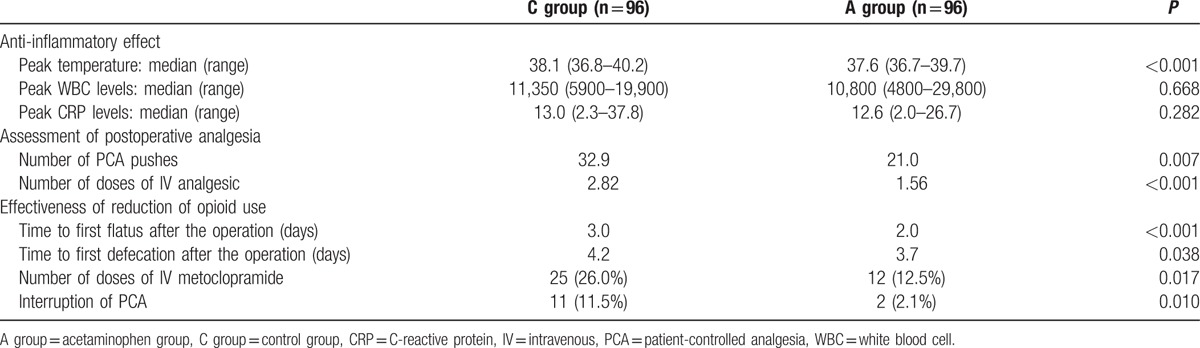
Figure 2.

Postoperative temperature. The postoperative fever patterns in both groups. The A-group showed faster defervescence and significantly greater decreases in fever between POD 1 and POD3. Number of PCA pushes. The change in the daily number of PCA pushes up to POD3. A significantly reduced frequency of PCA pushes in A-group was noted each day in the period between the day of operation (POD0) and POD3 (P < 0.05).
3.3. Postoperative complications
Table 3 summarizes the postoperative complications of both 2 groups. Postoperative complications were observed in 20 of 96 (20.8%) patients in C-group and 21 of 96 (21.9%) patients in A-group, with no significant difference between the groups (P = 0.860). No significant difference was also found in the incidence of individual complications. Concerning liver dysfunction, a known adverse reaction to acetaminophen, no significant increase was observed in its postoperative incidence, which demonstrates the safety of the current regimen. No significant difference was also observed in the incidence of CD grade ≥3 complications, which occurred in 6 (6.3%) patients in C-group and 4 (4.2%) patients in A-group (P = 0.516). No death was reported in both groups.
Table 3.
Postoperative complications.

3.4. Assessment of postoperative analgesia
The number of PCA pushes for postoperative epidural anesthesia, postoperative opioid use, and the number of bolus doses of other nonopioid analgesics were recorded for pain assessment (Table 2). The number of postoperative PCA pushes was significantly reduced in A-group compared with C-group (21.0 vs 32.9; P = 0.007). Figure 2B shows the change in the daily number of PCA pushes up to POD3. A significantly reduced frequency of PCA pushes in A-group was noted each day in the period between the day of operation (POD0) and POD3 (P < 0.05), indicating a significant reduction in pain. And the frequency of use of nonopioid analgesics outside the postoperative acetaminophen regimen was also significantly reduced in A-group (1.56 doses) compared with C-group (2.82 doses; P < 0.001). Figure 3 shows the changes in postoperative opioid uses. Each column denotes the daily opioid use, while the polygonal lines denote cumulative opioid uses. Daily opioid uses were significantly reduced in A-group each day between POD0 and POD2 (P < 0.001). Consistent with this, a significant reduction about 37% in the cumulative opioid use was also noted in A-group (P < 0.001).
Figure 3.
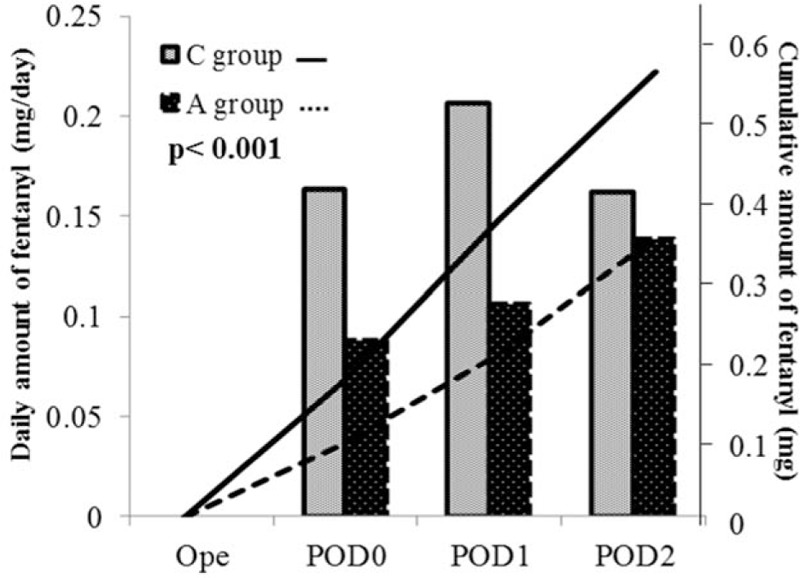
Opioid usage in PCA. The daily opioid use while the polygonal lines denote cumulative opioid uses. Daily opioid uses were significantly reduced in A-group each day between POD0 and POD2 (P < 0.001). Consistent with this, a significant reduction about 37% in the cumulative opioid use was also noted in A-group (P < 0.001).
3.5. The effectiveness of the reduction of opioid use
With regard to improvement in postoperative GI motility, the median time to first flatus/defecation after the operation was 3.0/4.2 days in C-group versus 2.0/3.7 days in A-group, respectively, indicating significantly greater improvement in GI motility in A-group (P < 0.001 and P = 0.038, respectively; Table 2). As for the effects on PONV, significantly fewer patients used at least 1 dose of metoclopramide for postoperative nausea in A-group (12 patients, 12.5%) compared with C-group (25 patients, 26.0%) (P = 0.017). The number of patients who had to discontinue epidural anesthesia due to PONV was also significantly reduced in A-group (2, 2.1%) compared with C-group (11, 11.5%) (P = 0.010).
4. Discussion
PCA using epidural anesthesia has recently been recommended as a postoperative pain management strategy for the ERAS protocol.[1–3] The combined use of low-dose opioid with local anesthetics has been demonstrated to be very effective for postoperative pain control. Meanwhile, the use of opioids has been associated with the risk of PONV. Therefore, a postoperative pain control regimen that maintains GI peristalsis while minimizing the development of PONV is required for the facilitation of ERAS. At Toranomon Hospital, we have introduced scheduled postoperative IV infusion of nonopioid analgesic acetaminophen combined with epidural anesthesia, with the aim of reducing opioid use without increasing the intensity of postoperative pain to facilitate postoperative recovery.
There are 2 potential advantages of using the scheduled IV acetaminophen regimen after gastrectomy for gastric cancer. First, the acetaminophen regimen did not increase the frequency or severity of postoperative complications. Second, PONV was significantly reduced and motility of GI tract was improved compared with conventional pain management after gastrectomy probably due to decreased used of opioid, resulting in enhanced recovery and decreased duration of hospital stay after surgery.
Conventionally, acetaminophen has reportedly been associated with liver damage, as up to 90% of the absorbed dose of oral acetaminophen is metabolized in the liver.[12,13] However, Jahr and Lee[14] suggested that IV acetaminophen does not undergo first-pass metabolism in the liver and other studies reported that postoperative IV acetaminophen may not increase postoperative complications.[15,16] Current results were compatible with these reported outcomes. The A-group showed significant defervescence compared with the C-group. Defervescence leads to improvement of fever-related subjective symptoms, but there is a concern of masking postoperative complications. However, given that there was no significant difference in morbidity rates between the 2 groups, the postoperative scheduled IV acetaminophen may not be related to increased severity of complications.
The concomitant use of IV acetaminophen significantly decreased the use of opioid and the other analgesics. With reduced used of opioid, significant reduction in the incidence of PONV and enhanced motility of GI tract was confirmed in the A-group. These factors may contribute to enhanced recovery and shortened hospital stay, though the statistical difference was marginal between the 2 groups in this study. Several recent studies have shown that the concomitant use of acetaminophen reduces the postoperative use of opioids, such as morphine and fentanyl, and that postoperative acetaminophen is also clinically effective in reducing opioid use and consequently reducing the incidence of PONV.[6,7,13,17–19] The incidence of PONV is generally reported to be 20% to 40%, affecting more females than males, and increases up to 70% to 80% in high-risk patients.[5,20] Early PONV, defined as occurring within the first 2 hours after the operation, is primarily attributable to anesthetics used during the operation, whereas those occurring thereafter are considered to be predominantly due to postoperative opioid use.[21–24] We have introduced the scheduled acetaminophen regimen, which resulted in reduced incidence of PONV by indirectly reducing opioid use. Acetaminophen has also been shown to have a direct antiemetic effect. Decreased brain concentration of anandamide, a CB1/CB2 cannabinoid receptor, is associated with aggravation of nausea/vomiting.[25–27] In fact, the present study showed that, although 11.5% of patients had to discontinue epidural anesthesia due to PONV before introduction of the acetaminophen regimen, the percentage significantly decreased to 2.1% after introduction of the regimen (P = 0.010). The percentage of patients who used at least 1 dose of metoclopramide for PONV after the operation also significantly decreased from 26.0% before introduction to 12.5% after introduction of the regimen (P = 0.017).
Limitations of this study may include its retrospective nature and relatively limited number of patients after propensity score-matching. However, perioperative management was similar except for the pain management during the study period and the current data are based on a prospectively collected database for consecutive patients. In addition, significant reduction in opioid use and PONV seem to have strong association with enhanced recovery and decreased duration of hospital stay after gastrectomy. External validation study using sufficient number of patients would be needed to confirm the current observations.
5. Conclusions
Scheduled postoperative IV acetaminophen after gastrectomy may reduce the use of opioids and might be associated with enhanced GI motility and decreased incidence of PONV after gastrectomy. Routine use of IV acetaminophen may be a feasible pain management option in ERAS protocol.
Footnotes
Abbreviations: GI = gastrointestinal, PONV = postoperative nausea/vomiting, ERAS = enhanced recovery after surgery, NSAIDs = Non-steroidal anti-inflammatory drugs, PCA = patient-controlled analgesia, BMI = body mass index, ASA = the American Society of Anesthesiologists.
Authorship: YO, SH, and JS designed the study and wrote the paper; YO, SH, JS, and TT drafted the article, revised it critically for important intellectual content, and gave final approval for the content; YO, SH, JS, TT, MU, and HU created study materials or recruited patients.
The authors have no conflicts of interest.
References
- 1.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005; 24:466–477. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008; 248:189–198. [DOI] [PubMed] [Google Scholar]
- 3.Jensen K, Kehlet H, Lund CM. Post-operative recovery profile after laparoscopic cholecystectomy: a prospective, observational study of a multimodal anaesthetic regime. Acta Anaesthesiol Scand 2007; 51:464–471. [DOI] [PubMed] [Google Scholar]
- 4.Carli F, Halliday D. Continuous epidural blockade arrests the postoperative decrease in muscle protein fractional synthetic rate in surgical patients. Anesthesiology 1997; 86:1033–1040. [DOI] [PubMed] [Google Scholar]
- 5.American Society of PeriAnesthesia Nurses PONV/PDNV Strategic Work Team. ASPAN'S evidence-based clinical practice guideline for the prevention and/or management of PONV/PDNV. J Perianesth Nurs 2006; 21:230–250. [DOI] [PubMed] [Google Scholar]
- 6.Arici S, Gurbet A, Türker G, et al. Preemptive analgesic effects of intravenous paracetamol in total abdominal hysterectomy. Agri 2009; 21:54–61. [PubMed] [Google Scholar]
- 7.Wininger SJ, Miller H, Minkiwitz HS, et al. A randomized, double-blind, placebo-controlled, multicenter, repeat-dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clin Ther 2010; 32:2348–2369. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2010 (ver.3). Gastric Cancer 2011; 14:113–123. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara H, Haruta S, Ohkura Y, et al. Tracing dissectable layers of mesenteries overcomes embryologic restrictions when performing infrapyloric lymphadenectomy in laparoscopic gastric cancer surgery. J Am Coll Surg 2015; 220:e81–e87. [DOI] [PubMed] [Google Scholar]
- 10.Haruta S, Shinohara H, Ueno M, et al. Anatomical considerations of the infrapyloric artery and its associated lymph nodes during laparoscopic gastric cancer surgery. Gastric Cancer 2015; 18:876–880. [DOI] [PubMed] [Google Scholar]
- 11.Ohkura Y, Shinohara H, Shindoh J, et al. A new scoring using preoperative factors and contour mapping for predicting postoperative complications of laparoscopic gastrectomy. Dig Surg 2016; 33:74–81. [DOI] [PubMed] [Google Scholar]
- 12.Bertolini A, Ferrari A, Ottani A, et al. Paracetamol: new vistas of an old drug. CNS Drug Rev 2006; 12:250–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unal SS, Aksoy M, Ahiskalioglu A, et al. The effect of intravenous preemptive paracetamol on postoperative fentanyl consumption in patients undergoing open nephrectomy: a prospective randomized study. Niger J Clin Pract 2015; 18:68–74. [DOI] [PubMed] [Google Scholar]
- 14.Jahr JS, Lee VK. Intravenous acetaminophen. Anesthesiol Clin 2010; 28:619–645. [DOI] [PubMed] [Google Scholar]
- 15.Scott G, Susan F. Use of intravenous acetaminophen in the treatment of postoperative pain. J Peri Anesthesia Nurs 2011; 26:74–80. [DOI] [PubMed] [Google Scholar]
- 16.Zafar N, Davies R, Greenslade GL, et al. The evolution of analgesia in an ‘accelerated’ recovery programme for resectional laparoscopic colorectal surgery with anastomosis. Colorect Dis 2010; 12:119–124. [DOI] [PubMed] [Google Scholar]
- 17.Singla NK, Parulan C, Samson R, et al. Plasma and cerebro spinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract 2012; 12:523–532. [DOI] [PubMed] [Google Scholar]
- 18.Moon YE, Lee YK, Lee J, et al. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet 2011; 284:1455–1460. [DOI] [PubMed] [Google Scholar]
- 19.Uvarov DN, Orlov MM, Levin AV, et al. Role of paracetamol in a balanced postoperative analgesia scheme after thoracotomy. Anesteziol Reanimatol 2008; 4:46–49. [PubMed] [Google Scholar]
- 20.Morino R, Ozaki M, Nagata O, et al. Incidence of and risk factors for postoperative nausea and vomiting at a Japanese Cancer Center: first large-scale study in Japan. J Anesth 2013; 27:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003; 97:62–71. [DOI] [PubMed] [Google Scholar]
- 22.Ferna’ndez-Guisasola J, Go’mez-Arnau JI, Cabrera Y, et al. Association between nitrous oxide and the incidence of postoperative nausea and vomiting in adults: a systematic review and meta-analysis. Anaesthesia 2010; 65:379–387. [DOI] [PubMed] [Google Scholar]
- 23.Apfel CC, Meyer A, Orhan-Sungur M, et al. Supplemental intravenous crystalloids for the prevention of postoperative nausea and vomiting: quantitative review. Br J Anaesth 2012; 108:893–902. [DOI] [PubMed] [Google Scholar]
- 24.Masumeh S, Atefeh G, Ramin N, et al. A Comparison between postoperative nausea and vomiting in general anesthesia with isoflurane-remifentanil or isoflurane in cholecystectomy laparoscopic patients. J Perianesth Nurs 2015; 30:418–422. [DOI] [PubMed] [Google Scholar]
- 25.Roberts GW, Bekker TB, Carlsen HH, et al. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg 2005; 101:1343–1348. [DOI] [PubMed] [Google Scholar]
- 26.Chouker A, Kaufmann I, Kreth S, et al. Motion sickness, stress and the endocannabinoid system. PLoS One 2010; 5:e10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apfela CC, Turanc A, Souza K, et al. Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain 2013; 154:677–689. [DOI] [PubMed] [Google Scholar]


