Abstract
The leukocyte immunoglobulin-like receptor (LILR) family comprises a set of paired immunomodulatory receptors expressed among human myeloid and lymphocyte cell populations. While six members of LILR subfamily A (LILRA) associate with membrane adaptors to signal via immunoreceptor tyrosine-based activating motifs (ITAM), LILR subfamily B (LILRB) members signal via multiple cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIM). Ligand specificity of some LILR family members has been studied in detail, but new perspective into the immunoregulatory aspects of this receptor family in human myeloid cells has been limited. LILRB receptors and the murine ortholog, paired immunoglobulin-like receptor B (PIRB), have been shown to negatively regulate maturation pathways in myeloid cells including mast cells, neutrophils, dendritic cells, as well as B cells. Our laboratory further demonstrated in mouse models that PIRB regulated functional development of myeloid-derived suppressor cell and the formation of a tumor-permissive microenvironment. Based on observations from the literature and our own studies, our laboratory is focusing on how LILRs modulate immune homeostasis of human myeloid cells and how these pathways may be targeted in disease states. Integrity of this pathway in tumor microenvironments, for example, permits a myeloid phenotype that suppresses antitumor adaptive immunity. This review presents the evidence supporting a role of LILRs as myeloid cell regulators and ongoing efforts to understand the functional immunology surrounding this family.
Keywords: LILRB, LILRA, ITIM, Macrophage, SHP, Regulatory myeloid suppressor cells
Introduction
The leukocyte immunoglobulin-like receptor (LILR, LIR, ILT, CD85) family can be divided into two classes: the inhibitory LILR subfamily B (LILRB1–5) and the activating LILR subfamily A (LILRA1–6), as shown in Table 1. Inhibitory LILRB receptors were first identified in 1997 [1]. Expression is enriched in myeloid cell populations and is primate specific, reflecting rapid gene duplication and evolution within the leukocyte receptor complex of chromosome 19 [2]. LILRs are in close linkage with the human killer cell inhibitory receptor (KIR) family, and both LILRs and KIRs share similar Ig-like structure and cytoplasmic signaling domains. Whereas KIR expression is restricted to natural killer (NK) cells, LILRs are expressed on various immune cells including NK, T, and B lymphocytes and myelomonocytic cells (monocytes, macrophages, DCs, and granulocytes) [3]. LILRB expression has also been reported in other cell types including osteoclasts [4], leukemia [2, 5], stromal and endothelial cells [6, 7], and various cancers. LILRB expression in cancer has been associated with enhanced tumor growth and correlates with poor patient outcomes. As the role of LILRB in tumor biology has been well reviewed in the literature [5, 8], we focus on the implications of LILRB immunomodulation on immune cell subsets. LILRB1 is broadly expressed on myeloid cells, as well as B cells and subsets of NK cells and T cells. LILRB2–5 is more restricted to cells of myeloid origin and DCs. LILRA receptors have a transmembrane domain containing a charged arginine or lysine residue that associated with the (YxxI/Lx6–12YxxI/L) ITAM-containing FcRγ [9]. ITAM activation recruits Syk/ZAP70 family kinases to drive downstream activation pathways important for immunity [10]. Conversely, LILRB receptors contain cytoplasmic (S/I/V/LxYxxI/V/L) ITIM domains to recruit Src homology 2 domain-containing phosphatases, SHP1/SHP2/SHIP, leading to inhibited immune signaling cascades. SHP/SHIP phosphatase activity is critical in maintaining immune homeostasis [11]. The present review focuses on ITIM-bearing inhibitory LILRB receptors that negatively regulate the activation of various immune cells, especially in myeloid cells, and summarizes current views on the mechanism of LILRB-mediated inhibition.
Table 1.
LILR family of receptors, signaling, reported ligands, and immune cell distribution
| Receptor | Signaling | Ligand | Cell distribution | Citation |
|---|---|---|---|---|
|
LILRB1 (ILT2, LIR-1, CD85j) |
ITIM (×4) |
β2m+ HLA-A/HLA-B/HLA-C/HLA-G UL18 S100A8/S100A9 |
B cells, NK, and T cell subsets Monocytes, macrophages, DCs, osteoclasts, granulocytes, placental stromal cells |
[4, 7, 13, 14, 21, 83–85] |
|
LILRB2 (ILT4, LIR-2, CD85d) |
ITIM (×3) |
HLA-A/HLA-B/HLA-C/HLA-G FHC ANGPTL β-amyloid Myelin (Nogo, MAG, OMgp) |
Monocytes, macrophages, DCs, osteoclasts, granulocytes, placental smooth muscle | [4, 7, 13, 14, 84, 85] |
|
LILRB3 (ILT5, LIR-3, CD85a) |
ITIM (×4) | ? | Monocytes, macrophages, DCs, osteoclasts, granulocytes | [4, 84, 85] |
|
LILRB4 (ILT3, LIR-5, CD85 k) |
ITIM (×3) | ? | Monocytes, macrophages, DCs, osteoclasts, endothelial cells | [4, 6] |
|
LILRB5 (LIR-8, CD85c) |
ITIM (×2) | HLA-B27 FHC | Macrophages, granulocytes | [4, 22, 23] |
|
LILRA1 (LIR-6, CD85i) |
Fcγ |
HLA-C FHC HLA-A/HLA-B/HLA-G FHC |
Monocytes, macrophages | [4, 13, 83] |
|
LILRA2 (ILT1, LIR-7, CD85 h) |
Fcγ | ? | Monocytes, macrophages, DCs, osteoclasts, granulocytes | [4, 85] |
|
LILRA3 (ILT6, LIR-4, CD85e) |
None (secreted) |
HLA-C FHC HLA-A/HLA-B/HLA-G FHC |
Activated monocytes | [13] |
|
LILRA4 (ILT7, CD85 g) |
Fcγ | BST2 | Plasmacytoid DCs | [25] |
|
LILRA5 (ILT11, LIR-9, CD85f) |
Fcγ | ? | Monocytes, macrophages | [4] |
|
LILRA6 (ILT8, CD85b) |
Fcγ and none (secreted) |
? | Monocytes, macrophages, osteoclasts | [4] |
Natural ligands for LILR receptors
Despite initial reports for major histocompatibility complex I (MHC-I) binding, the LILR family of receptors has emerged to bind multiple ligands. LILRB1 and LILRB2 were originally shown to broadly bind MHC-I molecules, also known as human leukocyte antigen (HLA) class I molecules (HLA-A, HLA-B, HLA-C) and non-classical HLA-class I (HLA-E, HLA-F, HLA-G, and HLA-H) [12, 13]. LILRB1 binds to β2m-associated HLA-I, whereas LILRB2 binds both β2m-associated and β2m-free heavy chain (FHC) forms of HLA-I [14]. Subsequent studies demonstrate LILRB binding to other ligands including the non-HLA angiopoietin-like (ANGPTL) protein family [15] and S100A8/S100A9 [16]. Structural analysis shows that two N-terminal Ig-like domains of LILRB1 and LILRB2 distinctly bind HLA as compared to the KIR–HLA interaction [17]. Binding of ANGPTL ligands is distinct from the LILRB2-HLA receptor–ligand interaction and appears to be of higher affinity binding various residues from all four Ig-like extracellular domains [18]. LILRB2/PIRB has also been shown to bind oligomeric β-amyloid and myelin component proteins Nogo, myelin-associated glycoprotein (MAG), and oligodendrocyte–myelin glycoprotein (OMgp) to affect neurite regeneration [19, 20]. The remaining LILRB receptors comprising LILRB3, LILRB4, and LILRB5 do not bind MHC-I or MHC-II and remain orphan receptors [12, 13, 21], although LILRA1 and LILRB5 were recently reported to bind HLA-B27 FHC [22, 23]. Like LILRB1 and LILRB2, LILRA1 and LILRA3 bind MHC-I but with reduced affinity and show preferential binding to HLA-C [13, 24]. LILRA4 has been shown to bind bone marrow stromal cell Ag 2 (BST2), an interaction important for plasmacytoid DC (pDC) maturation and function [25]. In all these instances, ligands for the LILR family have demonstrated acute effects on cell activation or maturation pathways.
ITIM-dependent inhibition of signaling cascades
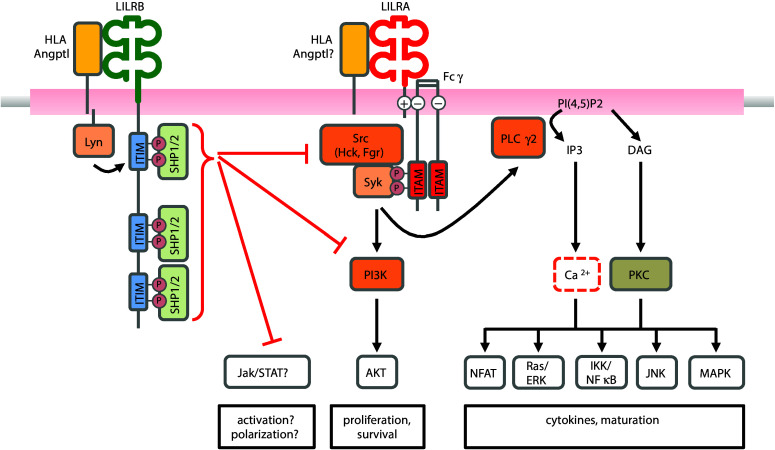
ITIM-bearing inhibitory LILRB receptors attenuate the crosslink-dependent activation of ITAM-bearing activating receptors, a process dependent on ITIM motif phosphorylation and the recruitment of SHP phosphatases [11], as shown in Fig. 1. The signal cascades mediated by ITAM-bearing receptor complexes, such as B cell receptor (BCR) activation, Fc-receptor aggregation, or T cell receptor (TCR) activation, activating KIR in NK cells or FcεRI-triggered mast cells activation, are shared activating pathways. Crosslink-dependent activation of the BCR, for example, triggers autophosphorylation of the Src-family protein, Lyn and activation of the cytoplasmic non-receptor tyrosine kinases, Bruton’s tyrosine kinase (Btk), and spleen tyrosine kinase (Syk) [26]. TCR activation triggers the Src-family kinases Lck and Fyn, leading to the recruitment of ZAP70 in T cells, an equivalent molecule to Syk in B cells [27]. These pathways converge on phospholipase C-mediated signaling to convert phosphatidyl bisphosphate to diacylglycerol (DAG) and inositol triphosphate (IP3). DAG induces the membrane translocation and activation of the serine/threonine kinase protein kinase C, while IP3 activates the release of intracellular Ca2+ stores leading to Ca2+ influx from the extracellular space. This signaling pathway subsequently activates mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), and c-JUN NH2-terminal kinase pathways and transcription factors including nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT) [28]. The PI3-kinase (PI3K) pathway is the second major downstream process involved in signaling mechanisms leading to immune cell proliferation, survival, and motility [26]. PI3K binds Ras to activate Ras/Raf signaling as well as binds pleckstrin homology domains to activate Btk and Akt at the cell membrane.
Fig. 1.
Model of LILRB cis-activation and regulation of LILRA-/ITAM-dependent pathways. Autophosphorylation of ITIM domains in response to LILRB activation recruits SHP1/SHP2 phosphatases. Activated SHP1/SHP2 directly suppresses Syk and PI3K activation downstream of ITAM motif activation, which normally drives the downstream activation of NFAT, Ras/ERK, NF-κB, JNK, and MAPK pathways to promote effector function, maturation, and cytokine release. SHP1/SHP2 has been shown to inhibit JAK/STAT pathways, but not in the context of LILRB
The ITIM-dependent recruitment of SHP1/SHP2 to LILRB has broad implications for suppressing Syk/Src signal cascades associated with immune activation. Consistent with the biology of other receptors that signal via ITIMs, T cell lines transduced with LILRB1 recruit SHP1 to inhibit activation of the T cell receptor by attenuating CD3ζ and linker for activation of T cells (LAT) phosphorylation [29]. Antibody-based functional assays support the inhibitory role of LILRB1 on CD4 and CD8 T cell effector function and proliferation [30]. In B cells, LILRB1 activation suppresses maturation and proliferation associated with attenuated Akt signaling [31]. Similarly, another study showed LILRB1–HLA-G interactions cause G0/G1 cell cycle arrest resulting from dephosphorylation of Akt, GSK-3β, c-Raf, and Foxo proteins [31]. HLA-G functionally inhibited B cell proliferation and differentiation. Murine PIRB inhibits Syk and Btk downstream of B cell receptor signaling via SHP1 recruitment [32]. Similar signaling patterns hold true among myeloid cells. PIRB negatively regulates chemokine-induced activation in murine DCs and is dependent on the Src-family kinases Hck and Fgr [33]. In the THP-1 monocytic cell line, LILRB4 activation attenuates CD64-dependent activation of Lck, Syk, LAT, ERK, and c-Cbl [34]. Co-ligation of LILRB4 also appeared to disrupt FcγRI-dependent endocytosis/phagocytosis via SHP-dependent dephosphorylation of signaling components including Syk, clathrin, and E3 ubiquitin protein ligase Cbl, among others [35]. SHP1/SHP2 has also been reported to modulate the activation of JAK/STAT pathways [36] and is implicated to have roles in fate maturation and immune function.
LILRBs negatively regulate myeloid cell activation
Early experiments in DCs demonstrated that LILRB2 agonism inhibits Ca2+-flux in response to Fc-receptor engagement [12]. LILRB1 activation on DCs is associated with maintenance in an immature state with limited antigen-presenting cell function [37, 38]. In allogeneic settings, both LILRB2 and LILRB4 are up-regulated on monocyte and DC populations and correlate with transplantation tolerance [39]. Studies analyzing human immunodeficiency virus (HIV) mutational escape demonstrate that viral expression of an HLA-B variant directly activates LILRB2 to promote myeloid cell tolerance and downregulation of DC maturation and costimulatory molecules [40]. The result is viral clone escape from cytotoxic T cell lymphocytes. LILRs are also directly involved in myeloid cytokine release. In DCs, IL-10 directly up-regulates LILRB2 expression while it simultaneously down-regulates soluble LILR secretion [41]. Similarly, elevated IL-10 in HIV patients is associated with the compromised antigen-presenting ability among myeloid cells overexpressing LILRB2 [42]. In addition to modulating DC maturation and function, LILRB1 and LILRB2 compete with CD8 for ligand binding to HLA [14]. LILRBs can thus modulate antigen-presenting cell activation pathways directly in addition to indirectly by suppressing effector CD8 T cells. LILRB1 and LILRB2 competition for CD8 demonstrated how Langerhan cells and CD14+ dermal DCs differ in T cell priming. CD14+ dermal DCs, which express LILRB1 and LILRB2, stimulate type-2 CD8 T cell maturation while Langerhan cells, which lack LILRB1 and LILRB2, efficiently prime cytolytic CD8 T effector cells [43]. Soluble LILRB2 inhibited Langerhan cell priming of cytotoxic CD8 T cells while antibodies disrupting LILRB2 enhance cytotoxic CD8 T cell priming in dermal DCs.
Limited data are available regarding how LILRB on monocytes can alter macrophage maturation. Like LILRB2 on DCs, LILRB4 activation on monocytes and macrophages attenuates Ca2+-flux resulting from CD11b, HLA-DR, or FcγRIII acute activation [44]. Salmonella infection or LPS treatment is sufficient to enhance LILRB2 and LILRB4 expression on DCs and macrophages [45]. Under these conditions, activation of LILRB4 increases IL-10 while reducing IL-8, supporting a role of LILRBs in regulating innate immune inflammatory responses. In both macrophages and neutrophils, PIRB negatively regulates integrin signaling. PIRB deficiency in these cells results in excessive adhesion due to enhanced integrin signaling, increased activation, and effector function [46]. PIRB antibody blockade or PIRB deficiency further enhances macrophage IL-6 and TNFα inflammatory responses, while reducing IL-10 [47]. Unexpectedly, PIRB-deficient mice are more susceptible to Salmonella infection [48]. Although PIRB-deficient macrophages produce higher levels of TNFα and nitric oxide, defects in phagosomal oxidation prevent efficient Salmonella clearance. These findings highlight the multiple roles paired receptors appear to have in regulation and in microbial infection.
Using a different disease model, PIRB-deficient macrophages similarly demonstrate exacerbated proinflammatory cytokine release and autoimmune colitis [49]. Consistent with the hypothesis that LILRB and PIRB expression on myeloid cells suppresses type-1 inflammatory responses, our laboratory demonstrated that PIRB is necessary to maintain the regulatory phenotype of tumor-infiltrating MDSCs [50]. MDSCs deficient in PIRB have inhibited maturation of an M2-like phenotype and favor activation of STAT1 and NF-κB pathways. PIRB loss in MDSCs was also associated with reduced tumor burden and fewer intra-tumoral regulatory T cells. RNAseq experiments from human macrophages demonstrated enhanced expression of LILRA family members under M1 inflammatory conditions and enhanced expression of LILRB family members under M2 inflammatory conditions [51]. These observations suggest that PIRB/LILRBs play important roles in maintaining regulatory macrophage phenotypes.
cis versus trans signaling of LILRs
Unique to a subset of MHC-I binding receptors, PIRB, LILRB1, and LILRB2 are sterically capable of recognizing ligand both in cis and in trans [52]. Because of ubiquitous HLA expression, the implication for LILRBs as tonic negative regulators of immune maturation and homeostasis in cis is significant. Constitutive phosphorylation of LILRB and PIRB receptors has been reported in the literature [4], and loss of HLA/β2m ligand in cis contributes to diminished tonic activation of PIRB [53]. Fluorescence resonance energy transfer experiments further demonstrate cis-interactions between LILRB1, LILRB2, and HLA as well between PIRB and H-2 [4, 54]. Osteoclasts, multinucleated cells derived from myeloid–macrophage precursors, broadly express LILRs similar to macrophages. Co-immunoprecipitation experiments showed constitutive activation of LILRB1–4 on monocyte-derived macrophages and osteoclast populations [4]. This suggests tonic signaling as a regulator for inflammation and/or maturation. PIRB loss up-regulates osteoclast differentiation and bone resorption activity. Similar findings have been observed in human and murine mast cells where LILRB2 and PIRB are constitutively expressed and activated. Loss of PIRB or MHC-I enhances mast cell cytokine response following IgE or LPS stimulation [54]. Surprisingly, trans availability of MHC-I does not appear to rescue PIRB-mediated regulation in the absence of cis signaling. Collectively, these studies suggest that cis-interactions of LILRB and PIRB with MHC-I may be the dominant mechanism for immunomodulation versus trans. In addition to acting as negative regulators of acute activation, tonic cis-activation is hypothesized to be responsible for maintaining immune homeostasis, preventing activation-induced cell stress/apoptosis, or enabling maturation of regulatory cell phenotypes as suggested by PIRB knockout mouse studies.
The role of LILRB-expressing myeloid cells in the tumor microenvironment
The crosstalk between malignant cells and myeloid cells, such as TAM, MDSC, and tolerogenic dendritic cells in the tumor microenvironment, is an emerging scientific theme critical for exploring novel cancer immunotherapies. LILRB4 is expressed on monocytic MDSC, polymorphonuclear MDSC, and classical monocyte subpopulations. NSCLC patients with an increased LILRB4high population had a shorter median survival than patients with a decreased LILRB4high subset [55]. LILRB4 expression on conventional DC and pDCs was increased in colorectal cancer patients when compared to healthy controls [56]. While LILRB4 is down-regulated following DC activation [57], its expression is up-regulated on tolerogenic DCs, leading to the induction of Treg cells [58]. CD68+ tumor-associated macrophages express high levels of LILRB4 in colorectal carcinomas, pancreatic carcinomas, and melanoma, found to reconstitute tumor-infiltrated lymph nodes at much higher frequencies versus non-invaded lymph nodes [59]. Furthermore, LILRB4 in either membrane-bound or secreted form could induce T cell anergy and promote the differentiation of CD8+ T suppressor cells within the tumor microenvironment or in sentinel lymph nodes [59, 60]. These findings support a pro-tumorigenic role of LILRB4 by altering immune activation.
The interaction between LILRB ligands and myeloid cells involved in antigen processing and presentation has been shown to exert regulatory functions and to be utilized by tumor cells to evade immune surveillance. Several studies have identified non-classical HLA (HLA-G, HLA-E, HLA-F) in various types of tumor cells/malignant tissues, e.g., lung cancer [61], colorectal [62], laryngeal [63], breast [64], and glioma [65]. HLA-F can bind to LILRB1 and LILRB2 and has been shown as a prognostic factor in lung cancer patients [61, 66] while the presence of serum soluble HLA-E has positive association with melanoma [67]. HLA-G/LILRB1 engagement results in expansion of CD11b+Gr-1+ MDSC populations in LILRB1 transgenic mice [68]. Consistent with regulatory function, HLA-G tetramer administration decreased IL-12, CD86, and MHC class II, but increased IL-10 and IL-6 in lymph node DC in LILRB2 transgenic mice. HLA-G tetramer further arrested the maturation/activation of LILRB2+ dendritic cells in a STAT3-dependent manner [69]. IL-4 and IL-13 expression downstream of HLA-G–LILRB1/LILRB2 engagement has been shown in several studies to not only suppress proinflammatory cytokine release, but promote secretion of IL-10 and TGFβ regulatory cytokines [70]. Other natural ligands for LILRB receptors include the ANGPTL family, which can stimulate ex vivo expansion of human cord blood hematopoietic stem cells [71, 72]. ANGPTL2–PIRB engagement can support leukemia development and the maintenance of stemness in the mouse [15]. The ANGPTL2–LILRB2 signaling axis in humans is important for the survival and migration of A549 lung cancer cells line [73]. ANGPTL2–LILRB2 engagement also plays a key role in sustaining epithelial mesenchymal transition during pancreatic ductal carcinogenesis [74]. Co-expression of ANGPTL2/ANGPTL5 and LILRB2 in human lung cancer tissue indicated a great level of lymph node metastasis and shorter overall survival rate in human lung cancer patients [75]. In other studies, the presence of LILRB ligands and ANGPTLs is reported to associate with tumor metastasis/progression in multiple cancer types [76, 77].
Our previous study suggested that monocytic MDSC from tumor-bearing PIRB knockout mice exhibited a diminished suppressive activity and an impaired Treg induction activity [50]. Tumor-infiltrating PIRB knockout macrophages have an M1-like phenotype that have lower levels of IL-10 and arginase activity, but higher levels of NO, IL-12, IL-1β, and TNFα following stimulation. PIRB knockout tumor-bearing mice have an attenuated tumor progression and prolonged survival period when compared to WT mice. Our study suggested that PIRB plays a pivotal role in regulation of myeloid differentiation and blockade of PIRB signaling could polarize MDSC to favor an environment conducive to antitumor immunity. In our research addressing LILRB2, we found LILRB2 antagonism can inhibit SHP1 phosphorylation and activate downstream ERK/NF-κB/STAT1 signaling, which can inhibit IL-10, enhance TNFα, and favor effector T cell activation (van der Touw et al., manuscript submitted). Furthermore, LILRB2 blockade can reprogram tumor-associated macrophages isolated from primary human lung cancer tissue into a proinflammatory phenotype, suggesting that blockade of LILRB2 as an immune checkpoint therapy may subvert the immune tolerance within the tumor microenvironments.
We hypothesized that in the tumor microenvironment, natural ligands expressed on or secreted by tumor cells/malignant tissues can engage members of the LILRB family, resulting in MDSC expansion, polarization favoring immunosuppressive activities in tumor tissues, and ultimately tumor progression and metastasis. Activation of LILRBs can restrict antigen presentation ability leading to antigen-specific unresponsiveness and immune tolerance. Tumor-associated macrophages and tumor-resident dendritic cells can express ligands and interact with LILRB1-expressing T lymphocytes or NK cells, resulting in the induction of regulatory T cells, suppression of T cell activation, and impairment of cytolytic CD8 T lymphocyte function.
Perspective and future studies
We hypothesize that the regulatory functions of LILRB and PIRB receptors are further fine-tuned by LILRA and PIRA-dependent signals, as has been suggested [78]. Like PIRB/LILRB, PIRA/LILRA is expressed on myeloid cells, but associates with ITAM-containing FcRγ chain [4, 9, 79]. LILRA family members share high homology with LILRB receptors and can compete for the same HLA ligands. LILRA1 and LILRA3 bind HLA but with reduced affinity compared to LILRB1 and LILRB2 and show preferential binding to HLA-C [13, 24]. Tonic levels of activation/inhibition by LILRA and LILRB depend on relative HLA ligand affinity, steady-state expression of LILRA/B, and potential regulation at the level of membrane co-localization to facilitate crosslink-dependent activation. These relative balances of LILRA/LILRB-mediated activation/inhibition in particular immune cells may play an important role in determining its cell fate and driving immune responses to counterbalance the microenvironment. In mice, we observed that monocytes expressed balanced levels of both PIRB and PIRA, but splenic macrophages and DC populations preferentially express PIRB over PIRA in tumor-bearing mice. These observations are consistent with reported findings of PIRB/PIRA expression [80].
Increased myeloid cell maturation capable of eliciting inflammatory responses is potentially accompanied by increased tonic regulation by PIRB/LILRB. This hypothesis resembles NK cell expression of NKG2A and KIR in response to HLA education/licensing: a maturation process associated with enhanced effector function potential [81, 82]. Like NK cells, similar dynamics may be occurring during monocyte differentiation into macrophage or DC subsets. Enhanced LILRB signaling during this process may educate differentiation toward regulatory phenotypes that favor tumor escape as we have demonstrated in PIRB-deficient tumor models. The important immunomodulatory characteristics of LILRA/B receptors represent unique targets on myeloid cells that can be manipulated through the use of agonistic or antagonistic antibodies for the treatment of various diseases, including cancers and autoimmune diseases. Development of therapeutics that modulate these pathways may tailor innate immune function to promote desired immunotherapy-driven outcomes.
Acknowledgements
The authors thank Ms. Marcia Meseck for editing the manuscript. This work was supported in part by grants from the National Cancer Institute to S.-H. Chen (R01CA109322, R01CA127483, and R01 CA 208703) and to P.-Y. Pan (R01CA140243 and R01 CA188610).
Abbreviations
- ANGPTL
Angiopoietin-like
- BCR
B cell receptor
- BST2
Bone marrow stromal cell Ag 2
- Btk
Bruton’s tyrosine kinase
- DAG
Diacylglycerol
- DCs
Dendritic cells
- ERK
Extracellular signal-regulated kinase
- FHC
Free heavy chain
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- IP3
Inositol triphosphate
- ITAM
Immunoreceptor tyrosine-based activating motif
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- KIR
Killer cell inhibitory receptor
- LAT
Linker for activation of T cells
- LILR
Leukocyte immunoglobulin-like receptor
- LILRA
Leukocyte immunoglobulin-like receptor subfamily A
- LILRB
Leukocyte immunoglobulin-like receptor subfamily B
- MAG
Myelin-associated glycoprotein
- MAPK
Mitogen-activated protein kinase
- MDSC
Myeloid-derived suppressor cell
- MHC-I
Major histocompatibility complex I
- NF-κB
Nuclear factor-κB
- NFAT
Nuclear factor of activated T cells
- NK
Natural killer
- OMgp
Oligodendrocyte–myelin glycoprotein
- PI3K
PI3-kinase
- Syk
Spleen tyrosine kinase
- TCR
T cell receptor
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th–19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
References
- 1.Samaridis J, Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol. 1997;27:660–665. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- 2.Volz A, Wende H, Laun K, Ziegler A. Genesis of the ILT/LIR/MIR clusters within the human leukocyte receptor complex. Immunol Rev. 2001;181:39–51. doi: 10.1034/j.1600-065X.2001.1810103.x. [DOI] [PubMed] [Google Scholar]
- 3.Katz HR. Inhibition of inflammatory responses by leukocyte Ig-like receptors. Adv Immunol. 2006;91:251–272. doi: 10.1016/S0065-2776(06)91007-4. [DOI] [PubMed] [Google Scholar]
- 4.Mori Y, Tsuji S, Inui M, et al. Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J Immunol. 2008;181:4742–4751. doi: 10.4049/jimmunol.181.7.4742. [DOI] [PubMed] [Google Scholar]
- 5.Kang X, Kim J, Deng M, John S, Chen H, Wu G, Phan H, Zhang CC. Inhibitory leukocyte immunoglobulin-like receptors: immune checkpoint proteins and tumor sustaining factors. Cell Cycle. 2016;15:25–40. doi: 10.1080/15384101.2015.1121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim-Schulze S, Seki T, Vlad G, Scotto L, Fan J, Colombo PC, Liu J, Cortesini R, Suciu-Foca N. Regulation of ILT3 gene expression by processing of precursor transcripts in human endothelial cells. Am J Transplant. 2006;6:76–82. doi: 10.1111/j.1600-6143.2005.01162.x. [DOI] [PubMed] [Google Scholar]
- 7.McIntire RH, Sifers T, Platt JS, Ganacias KG, Langat DK, Hunt JS. Novel HLA-G-binding leukocyte immunoglobulin-like receptor (LILR) expression patterns in human placentas and umbilical cords. Placenta. 2008;29:631–638. doi: 10.1016/j.placenta.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Zheng J, Kang X, Deng M, Lu Z, Kim J, Zhang C. Inhibitory leukocyte immunoglobulin-like receptors in cancer development. Sci China Life Sci. 2015;58:1216–1225. doi: 10.1007/s11427-015-4925-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima H, Samaridis J, Angman L, Colonna M. Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor gamma-chain. J Immunol. 1999;162:5–8. [PubMed] [Google Scholar]
- 10.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 12.Colonna M, Samaridis J, Cella M, et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol. 1998;160:3096–3100. [PubMed] [Google Scholar]
- 13.Jones DC, Kosmoliaptsis V, Apps R, et al. HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J Immunol. 2011;186:2990–2997. doi: 10.4049/jimmunol.1003078. [DOI] [PubMed] [Google Scholar]
- 14.Shiroishi M, Tsumoto K, Amano K, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J, Umikawa M, Cui C, et al. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485:656–660. doi: 10.1038/nature11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold V, Cummings JS, Moreno-Nieves UY, Didier C, Gilbert A, Barre-Sinoussi F, Scott-Algara D. S100A9 protein is a novel ligand for the CD85j receptor and its interaction is implicated in the control of HIV-1 replication by NK cells. Retrovirology. 2013;10:122. doi: 10.1186/1742-4690-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroki K, Furukawa A, Maenaka K. Molecular recognition of paired receptors in the immune system. Front Microbiol. 2012;3:429. doi: 10.3389/fmicb.2012.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng M, Lu Z, Zheng J, et al. A motif in LILRB2 critical for Angptl2 binding and activation. Blood. 2014;124:924–935. doi: 10.1182/blood-2014-01-549162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 is a beta-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 21.Willcox BE, Thomas LM, Bjorkman PJ. Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat Immunol. 2003;4:913–919. doi: 10.1038/ni961. [DOI] [PubMed] [Google Scholar]
- 22.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001;167:5543–5547. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Hatano H, Shaw J, Olde Nordkamp M, Jiang G, Li D, Kollnberger S. The leukocyte immunoglobulin-like receptor family member LILRB5 binds to HLA-Class I heavy chains. PLoS One. 2015;10:e0129063. doi: 10.1371/journal.pone.0129063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu M, Chen Y, Qi J, Liu J, Fan Z, Nam G, Shi Y, Cheng H, Gao GF. LILRA3 binds both classical and non-classical HLA class I molecules but with reduced affinities compared to LILRB1/LILRB2: structural evidence. PLoS One. 2011;6:e19245. doi: 10.1371/journal.pone.0019245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavano B, Galao RP, Graham DR, Neil SJ, Aquino VN, Fuchs D, Boasso A. Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1.24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood leukocytes. J Immunol. 2013;190:2622–2630. doi: 10.4049/jimmunol.1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/S0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 27.Tan SL, Liao C, Lucas MC, Stevenson C, DeMartino JA. Targeting the SYK-BTK axis for the treatment of immunological and hematological disorders: recent progress and therapeutic perspectives. Pharmacol Ther. 2013;138:294–309. doi: 10.1016/j.pharmthera.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol. 2001;166:2514–2521. doi: 10.4049/jimmunol.166.4.2514. [DOI] [PubMed] [Google Scholar]
- 30.Saverino D, Fabbi M, Ghiotto F, et al. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J Immunol. 2000;165:3742–3755. doi: 10.4049/jimmunol.165.7.3742. [DOI] [PubMed] [Google Scholar]
- 31.Naji A, Menier C, Morandi F, et al. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J Immunol. 2014;192:1536–1546. doi: 10.4049/jimmunol.1300438. [DOI] [PubMed] [Google Scholar]
- 32.Maeda A, Scharenberg AM, Tsukada S, Bolen JB, Kinet JP, Kurosaki T. Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1. Oncogene. 1999;18:2291–2297. doi: 10.1038/sj.onc.1202552. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Meng F, Chu CL, Takai T, Lowell CA. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity. 2005;22:235–246. doi: 10.1016/j.immuni.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Lu HK, Rentero C, Raftery MJ, Borges L, Bryant K, Tedla N. Leukocyte Ig-like receptor B4 (LILRB4) is a potent inhibitor of FcgammaRI-mediated monocyte activation via dephosphorylation of multiple kinases. J Biol Chem. 2009;284:34839–34848. doi: 10.1074/jbc.M109.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park M, Raftery MJ, Thomas PS, Geczy CL, Bryant K, Tedla N. Leukocyte immunoglobulin-like receptor B4 regulates key signalling molecules involved in FcgammaRI-mediated clathrin-dependent endocytosis and phagocytosis. Sci Rep. 2016;6:35085. doi: 10.1038/srep35085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu D, Qu CK. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–4932. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young NT, Waller EC, Patel R, Roghanian A, Austyn JM, Trowsdale J. The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood. 2008;111:3090–3096. doi: 10.1182/blood-2007-05-089771. [DOI] [PubMed] [Google Scholar]
- 38.Tenca C, Merlo A, Merck E, et al. CD85j (leukocyte Ig-like receptor-1/Ig-like transcript 2) inhibits human osteoclast-associated receptor-mediated activation of human dendritic cells. J Immunol. 2005;174:6757–6763. doi: 10.4049/jimmunol.174.11.6757. [DOI] [PubMed] [Google Scholar]
- 39.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 40.Lichterfeld M, Kavanagh DG, Williams KL, et al. A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J Exp Med. 2007;204:2813–2824. doi: 10.1084/jem.20061865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beinhauer BG, McBride JM, Graf P, et al. Interleukin 10 regulates cell surface and soluble LIR-2 (CD85d) expression on dendritic cells resulting in T cell hyporesponsiveness in vitro. Eur J Immunol. 2004;34:74–80. doi: 10.1002/eji.200324550. [DOI] [PubMed] [Google Scholar]
- 42.Vlad G, Piazza F, Colovai A, Cortesini R, Della Pietra F, Suciu-Foca N, Manavalan JS. Interleukin-10 induces the upregulation of the inhibitory receptor ILT4 in monocytes from HIV positive individuals. Hum Immunol. 2003;64:483–489. doi: 10.1016/S0198-8859(03)00040-5. [DOI] [PubMed] [Google Scholar]
- 43.Banchereau J, Zurawski S, Thompson-Snipes L, et al. Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming. Proc Natl Acad Sci USA. 2012;109:18885–18890. doi: 10.1073/pnas.1205785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cella M, Dohring C, Samaridis J, Dessing M, Brockhaus M, Lanzavecchia A, Colonna M. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med. 1997;185:1743–1751. doi: 10.1084/jem.185.10.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown DP, Jones DC, Anderson KJ, Lapaque N, Buerki RA, Trowsdale J, Allen RL. The inhibitory receptor LILRB4 (ILT3) modulates antigen presenting cell phenotype and along with LILRB2 (ILT4), is upregulated in response to Salmonella infection. BMC Immunol. 2009;10:56. doi: 10.1186/1471-2172-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira S, Zhang H, Takai T, Lowell CA. The inhibitory receptor PIR-B negatively regulates neutrophil and macrophage integrin signaling. J Immunol. 2004;173:5757–5765. doi: 10.4049/jimmunol.173.9.5757. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama M, Underhill DM, Petersen TW, Li B, Kitamura T, Takai T, Aderem A. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J Immunol. 2007;178:4250–4259. doi: 10.4049/jimmunol.178.7.4250. [DOI] [PubMed] [Google Scholar]
- 48.Torii I, Oka S, Hotomi M, Benjamin WH, Jr, Takai T, Kearney JF, Briles DE, Kubagawa H. PIR-B-deficient mice are susceptible to Salmonella infection. J Immunol. 2008;181:4229–4239. doi: 10.4049/jimmunol.181.6.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munitz A, Cole ET, Beichler A, et al. Paired immunoglobulin-like receptor B (PIR-B) negatively regulates macrophage activation in experimental colitis. Gastroenterology. 2010;139:530–541. doi: 10.1053/j.gastro.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beyer M, Mallmann MR, Xue J, et al. High-resolution transcriptome of human macrophages. PLoS One. 2012;7:e45466. doi: 10.1371/journal.pone.0045466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho LH, Uehara T, Chen CC, Kubagawa H, Cooper MD. Constitutive tyrosine phosphorylation of the inhibitory paired Ig-like receptor PIR-B. Proc Natl Acad Sci USA. 1999;96:15086–15090. doi: 10.1073/pnas.96.26.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masuda A, Nakamura A, Maeda T, Sakamoto Y, Takai T. Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J Exp Med. 2007;204:907–920. doi: 10.1084/jem.20060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Goeje PL, Bezemer K, Heuvers ME, et al. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. Oncoimmunology. 2015;4:e1014242. doi: 10.1080/2162402X.2015.1014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orsini G, Legitimo A, Failli A, Ferrari P, Nicolini A, Spisni R, Miccoli P, Consolini R. Quantification of blood dendritic cells in colorectal cancer patients during the course of disease. Pathol Oncol Res. 2014;20:267–276. doi: 10.1007/s12253-013-9691-4. [DOI] [PubMed] [Google Scholar]
- 57.Vlad G, Suciu-Foca N. Induction of antigen-specific human T suppressor cells by membrane and soluble ILT3. Exp Mol Pathol. 2012;93:294–301. doi: 10.1016/j.yexmp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Hum Immunol. 2008;69:681–686. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 59.Suciu-Foca N, Feirt N, Zhang QY, et al. Soluble Ig-like transcript 3 inhibits tumor allograft rejection in humanized SCID mice and T cell responses in cancer patients. J Immunol. 2007;178:7432–7441. doi: 10.4049/jimmunol.178.11.7432. [DOI] [PubMed] [Google Scholar]
- 60.Kim-Schulze S, Scotto L, Vlad G, Piazza F, Lin H, Liu Z, Cortesini R, Suciu-Foca N. Recombinant Ig-like transcript 3-Fc modulates T cell responses via induction of Th anergy and differentiation of CD8+ T suppressor cells. J Immunol. 2006;176:2790–2798. doi: 10.4049/jimmunol.176.5.2790. [DOI] [PubMed] [Google Scholar]
- 61.Lin A, Zhang X, Ruan YY, Wang Q, Zhou WJ, Yan WH. HLA-F expression is a prognostic factor in patients with non-small-cell lung cancer. Lung Cancer. 2011;74:504–509. doi: 10.1016/j.lungcan.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Benevolo M, Mottolese M, Tremante E, et al. High expression of HLA-E in colorectal carcinoma is associated with a favorable prognosis. J Transl Med. 2011;9:184. doi: 10.1186/1479-5876-9-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva TG, Crispim JC, Miranda FA, et al. Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol Histopathol. 2011;26:1487–1497. doi: 10.14670/HH-26.1487. [DOI] [PubMed] [Google Scholar]
- 64.de Kruijf EM, Sajet A, van Nes JG, et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185:7452–7459. doi: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- 65.Fan X, Wang Y, Zhang C, Liu X, Qian Z, Jiang T. Human leukocyte antigen-G overexpression predicts poor clinical outcomes in low-grade gliomas. J Neuroimmunol. 2016;294:27–31. doi: 10.1016/j.jneuroim.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Lepin EJ, Bastin JM, Allan DS, et al. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol. 2000;30:3552–3561. doi: 10.1002/1521-4141(200012)30:12<3552::AID-IMMU3552>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 67.Allard M, Oger R, Vignard V, et al. Serum soluble HLA-E in melanoma: a new potential immune-related marker in cancer. PLoS One. 2011;6:e21118. doi: 10.1371/journal.pone.0021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+ Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86:1125–1134. doi: 10.1097/TP.0b013e318186fccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shakhawat A, Shaikly V, Elzatma E, Mavrakos E, Jabeen A, Fernandez N. Interaction between HLA-G and monocyte/macrophages in human pregnancy. J Reprod Immunol. 2010;85:40–46. doi: 10.1016/j.jri.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, Lodish HF. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Yu X, Xie J, et al. ANGPTL2/LILRB2 signaling promotes the propagation of lung cancer cells. Oncotarget. 2015;6:21004–21015. doi: 10.18632/oncotarget.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carbone C, Piro G, Fassan M, et al. An angiopoietin-like protein 2 autocrine signaling promotes EMT during pancreatic ductal carcinogenesis. Oncotarget. 2015;6:13822–13834. doi: 10.18632/oncotarget.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Geng T, Guo X, Liu J, Zhang P, Yang D, Li J, Yu S, Sun Y. Co-expression of immunoglobulin-like transcript 4 and angiopoietin-like proteins in human non-small cell lung cancer. Mol Med Rep. 2015;11:2789–2796. doi: 10.3892/mmr.2014.3029. [DOI] [PubMed] [Google Scholar]
- 76.Gao L, Ge C, Fang T, Zhao F, Chen T, Yao M, Li J, Li H. ANGPTL2 promotes tumor metastasis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:396–404. doi: 10.1111/jgh.12702. [DOI] [PubMed] [Google Scholar]
- 77.Wang PF, Li HL, Qi X, Yao K, Han S, Liu N, Yang YK, Li SW, Yan CX. Clinical significance of angiopoietin-like protein 3 expression in patients with glioblastoma. Neoplasma. 2016;63:93–98. doi: 10.4149/neo_2016_011. [DOI] [PubMed] [Google Scholar]
- 78.Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeda A, Kurosaki M, Kurosaki T. Paired immunoglobulin-like receptor (PIR)-A is involved in activating mast cells through its association with Fc receptor gamma chain. J Exp Med. 1998;188:991–995. doi: 10.1084/jem.188.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ujike A, Takeda K, Nakamura A, Ebihara S, Akiyama K, Takai T. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(-/-) mice. Nat Immunol. 2002;3:542–548. doi: 10.1038/ni801. [DOI] [PubMed] [Google Scholar]
- 81.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 82.Anfossi N, Andre P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Davidson CL, Li NL, Burshtyn DN. LILRB1 polymorphism and surface phenotypes of natural killer cells. Hum Immunol. 2010;71:942–949. doi: 10.1016/j.humimm.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 84.Sloane DE, Tedla N, Awoniyi M, Macglashan DW, Jr, Borges L, Austen KF, Arm JP. Leukocyte immunoglobulin-like receptors: novel innate receptors for human basophil activation and inhibition. Blood. 2004;104:2832–2839. doi: 10.1182/blood-2004-01-0268. [DOI] [PubMed] [Google Scholar]
- 85.Tedla N, Bandeira-Melo C, Tassinari P, Sloane DE, Samplaski M, Cosman D, Borges L, Weller PF, Arm JP. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci USA. 2003;100:1174–1179. doi: 10.1073/pnas.0337567100. [DOI] [PMC free article] [PubMed] [Google Scholar]