Abstract
Intestine waste generated from slaughterhouse (IWS) is difficult to degrade in anaerobic process due to the presence of high protein and lipid contents. However, anaerobic co-digestion helps to increase the degradation of IWS by the addition of carbon-rich food waste (FW). To increase the biogas yield, thermo-alkali pretreatment may be more viable method for the anaerobic digestion of protein and lipid rich wastes. In the present study, Thermo-alkali pretreatment of intestine waste from slaughterhouse and food waste alone and mixing of IWS and FW with different ratios (1:1–1:3) on VS basis have been studied. To study the effect of Thermo-alkali pretreatment on solubilization of substrate, the substrate was mixed with alkali solutions (NaOH and KOH) at different concentrations of 1, 2, 3, 4 and 5% solutions. The results revealed that the maximum solubilization was observed to be 94.7% and 90.1% at KOH (1:3 and 5%) and NaOH (1:3 and 5%), respectively. Based on the study, enhancement in biogas yield by 16% (IWS), 11.5% (FW), 12.2% (1:1), 18.11% (1:2) and 22.5% (1:3) in KOH pretreated waste when compared with NaOH pretreated waste.
Keywords: Thermo-alkali pretreatment, Solubilization, Intestine waste, Food waste, Slaughterhouse, Biogas
Introduction
Energy is one of the major concerns being focused by both developed and developing countries to find different sources. Especially in developing countries like India, considerable amount of waste is being generated from both domestic and industrial activities. To address these wastes, innovative research programmes are initiated to convert waste into energy or value-added products under various programmes. Anaerobic digestion (AD) process is one of the ancient proven solid waste treatments for recovery of energy (Lee et al. 2009a; Mata-Alvarez et al. 2000), especially for organic solid waste. AD is biological process involved by several microbial communities with sequence of reaction such as breakdown of macromolecules into simple monomers (hydrolysis), simple monomers to carbonic acid (acidogenesis), conversion of primer acetate from carbonic acids (acetogenesis) and conversion of methane and carbon di-oxide from acetate (methanogenisis) (Verma 2002; Ostrem 2004; Forster-Carneiro et al. 2008; Khalid et al. 2011; Gupta et al. 2012; Santiago et al. 2015; Veluchamy and Ajay 2017). However, AD is more sensitive process for several process parameters such as pH, temperature, volatile fatty acid (VFA), and alkalinity (Mata-Alvarez et al. 2014; Yuan and Zhu 2016).
Currently, all developing countries are facing the problem of disposal of mixed municipal solid waste generated from urban centres. To address the municipal solid waste management, various organic fractions generated from urban centers are identified and being tried to treat these organic fractions by anaerobic treatment for recovery of energy. Slaughterhouse waste from the urban center is identified as one of such wastes, which has high organic compounds and thus enables for considering as a substrate for the biogas recovery through the anaerobic process (Jhosané et al. 2014). Among the slaughterhouse waste, intestine waste (IWS) is found to cause problem in AD process due to the presence of high amount of protein and lipid. The higher protein and lipid contents of IWS significantly affect the anaerobic process in terms of free ammonia toxicity by degradation of protein (Braun et al. 2003; Ahring 2003; Gerardi 2003) and accumulation of long chain fatty acid (LCFA) through lipid degradation. The above problem affects the biological activity and reduces the reactor performance in terms of degradation efficiency and biogas yield by altering pH, VFA and ammonia concentration in the system (Mao et al. 2015). It is reported that higher ammonia and VFA concentrations inhibit the methanogenesis process by pH fluctuation, which leads to the “inhibited steady state” condition, i.e., the reactor runs stably, whereas biogas yield and VS reduction rates are observed to be quite low (Chen et al. 2008). The proposed co-digestion would alleviate the above issue by introducing suitable co-substrates (carbon-rich feed stock), which is a holistic approach to increase the C/N ratio and balance the nutrient inside the reactor, which helps to increase the biogas yield and treatment efficiency. To enhance the biogas yield, pretreatment is another better option for acceleration of rate limiting step, i.e., hydrolysis of protein and lipid in the solid waste (Carlsson et al. 2012; Val del Rio et al. 2011). In the current scenario, several pretreatment processes such as mechanical, thermal, thermo-chemical, chemical, biological and enzymes are being practiced (Javkhlan et al. 2014). Pretreatment helps to reduce the particle size, increase the surface area of the reactor, increase the rate of hydrolysis process and reduce the reaction time in the rate limiting step of hydrolysis (Guangxue et al. 2009; Javkhlan et al. 2014).
Among various pretreatment methods, thermo-alkali/thermo-chemical pretreatment was selected for solubilization as well as hydrolysis of organic macromolecule into simple monomers. In general, in the thermo-alkali method, different concentrations of alkali are used at selective temperature and time. Further, it is reported that alkalis such as sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)2) and sodium carbonate (Na2CO3) were used at temperature in the range of 60–140 °C and the retention time from 15 to 120 min. Guangxue et al. (2009) reported that VS reduction ratio is high in 131 °C when compared with 55 °C in meat bone meal pretreated with NaOH, but inhibition of methane production was observed to be less. Similarly, Ravindranath (2012) reported that, thermo-chemical pretreatment of fleshing at 120 °C with 1 bar pressure, the higher methane production occurred for the NaOH and KOH solutions. In addition it is stated that the Solubilization was found to be higher in KOH and NaOH then compared with Ca(OH)2 and Na2CO3. Kalambura and Kaje (2007) have reported that large protein molecules react with NaOH/KOH to form smaller molecules of volatile acids and Na/K-salts, which is more valuable raw material for AD. Penaud et al. (1999) depicted that the optimal conditions for COD solubilization were 140 °C and pH 5–12 for 30 min. Thermo-chemical pretreatment of poultry litter and chicken feathers with Ca(OH)2 and NaOH is reported to have a significant impact on the hydrolysis, which caused an accumulation of metabolites, such as VFA and ammonia and have inhibited methanogenesis (Costa et al. 2012). Thermo-chemical pretreatment with NaOH of cow dung and poultry litter is reported to have increased 46.3 and 26.1% of methane production and their respective controls (Animut et al. 2014). Tsukahara et al. (1999) reported that food waste (approximately 90% moisture content) was thermo-chemically liquefied at 175 °C for 1 h, the liquidized food waste was separated into a solid phase and a liquid phase and the diluted liquid phase was treated by AD. Thermo-chemical pretreatment results in the significant improvement in the rate of solubilization of the substrate but addition of alkali during the thermo-chemical pretreatment requires necessary pH adjustment prior to the anaerobic process. Hence, the present study is aimed to generate maximum biogas yield from individual IWS and FW and co-digestion of IWS with FW at different mixing ratios through the thermo-alkali pretreatment using NaOH and KOH at lower temperature and pressure. Further, the study has been concentrated to optimize the alkali concentration and mixing ratio of IWS and FW for maximum biogas yield.
Materials and methods
IWS and FW were collected from meat shop at Chennai and institute canteen, CLRI, Chennai, respectively. The anaerobic inoculum was obtained from municipal sewage treatment plant, Perungudi, Chennai. Primary characteristics of inoculum and substrates (pH, TS, VS, moisture content and total ammonia nitrogen) were estimated as per standards methods (APHA 1998). Volatile fatty acid (VFA) was estimated by the direct distillation method (Wagningan University). The free ammonia concentration (mg/L) was calculated based on Eqs. (1) and (2) (Cuetos et al. 2008; Belmonte et al. 2011):
| 1 |
| 2 |
where FA is the free ammonia concentration (mg/L), TAN is the total ammonia nitrogen (mg/L), pKa is the dissociation constant for the ammonium ion and T is the temperature in °C.
Effect of alkali concentration in thermo-alkali pretreatment
To investigate the effect of alkali concentration in thermo-alkali pretreatment, the experiments were performed with IWS, FW alone and mixture of IWS and FW in different ratios (1:1, 1:2 and 1:3) in terms of VS basis. 500 g of substrate was mixed with 500 ml of different alkali solutions (KOH and NaOH), at 1, 2, 3, 4 and 5% (w/v), respectively. The samples were subjected to 1 kg/cm2 pressure at temperature of 121 °C for 20 min. Solubilization of wastes was estimated by analyzing the COD and CODs before and after pretreatment. The percentage of solubilization in terms of CODs was calculated based on the Eq. (3) (Ravindranath 2012). Hydrolysate obtained after pretreatment were used as a substrate for batch anaerobic biogas yield experiments.
| 3 |
The percentage size reduction was calculated based on the following Eq. (4) by mass of waste retained in sieve 3.5 mm on weight basis.
| 4 |
Batch anaerobic biogas yield experiments
Hydrolysate obtained after thermo-alkali pretreatment of IWS, FW and mixed IWS and FW ratio were taken as substrates and anaerobic batch biogas tests were performed after adjusting its pH to neutral using 0.1 M Hydrochloric acid. Each experiment was carried out in triplicate with a reactor volume of 600 ml and the head space was maintained at 400 ml. The batch reactors were incubated at 35 °C temperature and inoculum to substrate ratio was maintained at 0.5. The pressure increase in the inside of the reactors was monitored at regular time intervals using manometer. The biogas yields were determined using the monitored pressure by the following Eq. (5):
| 5 |
where V 0 is the volume of dry gas at standard temperature and pressure (STP) in normal state (ml), V is the volume of the gas read off (milli bar), P is the pressure of the gas phase at the time of reading (hPa); P w is the vapor pressure of the water as a function of the temperature of the ambient space (hPa); T 0 is the normal temperature (273 K); P 0 is the normal pressure (1013 hPa); and T is the temperature of the fermented gas or of the ambient space (K).
Kinetic study
The kinetic studies on the degradation of organics in terms of VS were performed for the AD of thermo-alkali pretreated IWS, FW and mixed IWS and FW ratios using the following pseudo-first and second-order Eqs. (6) and (7).
| 6 |
| 7 |
where [C]0 is the concentration of VS at time t = 0; [C]t is the concentration of VS at time “t”; t is time in hours; k 1 and k 2 are the pseudo-first-order and second-order rate constants, respectively.
Kinetic evaluation of biogas yield from IWS, FW and mixed IWS and FW ratios was studied using the following Eq. (8):
| 8 |
where V e is the equilibrium concentration of biogas, V t is the volume of biogas at time “t”, t is time in hours, and k (d−1) is the rate constant.
Result and discussion
Characteristics of substrates
Initial characteristics of IWS and FW were determined and are shown in Table 1. The pH of IWS and FW was observed to be 7.9 and 5.6, respectively. In AD, pH plays the important role as each of the microbial groups involved requires specific pH range for optimal growth (Eckenfelder 1999). Liu et al. (2008) reported that the most favorable pH for higher biogas yield is 6.5–7.5. From the initial pH of IWS and FW, it was not in the optimal range for AD indicating co-digestion of waste for better performance of the AD. Moisture content of the wastes was 74.5% (IWS) and 80.9% (FW) indicating its suitability for AD process (optimum range of 60–80% reported by Bouallagui et al. 2003). The volatile solids (VS) content of IWS and FW were 84.5 and 74.3% of the TS content, respectively, which is also found to be more suitable for AD. However, protein and lipids concentrations of IWS (35 and 11% w/w basis) were higher than FW (19 and 6% w/w basis). Similarly, C/N ratio of 3.5 and 38.2 was observed for IWS and FW, respectively, which is not in the Optimum range of 25–30 for AD (Lee et al. 2009b; Mata-Alvarez et al. 2014). Higher protein and lipid content and lesser C/N ratio in IWS could lead to ammonia toxicity and VFA accumulation during AD of IWS alone. The huge variations of the C/N ratio have been brought into the optimum range by mixing of IWS and FW in different ratios on VS basis.
Table 1.
Initial characteristics of inoculum, IWS, FW
| Parameters | Units | Inoculum | IWS | FW |
|---|---|---|---|---|
| pH | – | 7.3 | 7.9 | 5.6 |
| Moisture | % | 93.1 | 74.5 | 80.9 |
| TS | mg/g on dry weight basis | 62.8 | 272.8 | 192.5 |
| VS | mg/g on dry weight basis | 34.8 | 230.5 | 173.2 |
| C/N ratio | – | 8.5 | 3.5 | 38.9 |
Effect of alkali concentration during Thermo- alkali pretreatment
Effect of thermo-alkali pretreatment using KOH in terms of size reduction using 3.5 mm sieves and percentage solubilization in terms of CODs was observed and illustrated in Figs. 1a, b, respectively. The maximum percentage size reductions of 100% in 1:2 and 1:3 with 2% KOH, 91% in 1:1 with 4% KOH, 80% in FW with 5% KOH and 78% in IWS with 4% KOH were observed. Similarly, the maximum COD solubilization efficiency of 94.7% in 1:3 with 5% KOH, 94.4% in 1:2 with 5% KOH, 87.1% in FW with 5% KOH, 81% in 1:1 with 4% KOH and 52% in IWS with 4% KOH was observed. Based on the study, it is observed that the percentage size reduction increases with increase in percentage KOH for FW, mixed waste with 1:2 and 1:3. Whereas in IWS and 1:1 mixed ratio, the percentage size reduction declined after 4% KOH as the samples were solidified due to the addition of higher alkali concentration in the substrate, which was also reflected same in COD solubilization. Co-digestion of IWS: FW in 1:2 and 1:3 mixing ratios provided balanced nutrient and optimum C/N ratio of 26.4 and 29.7 suitable for AD which is also evident from higher solubilization efficiency of (94.4 and 94.7%).
Fig. 1.
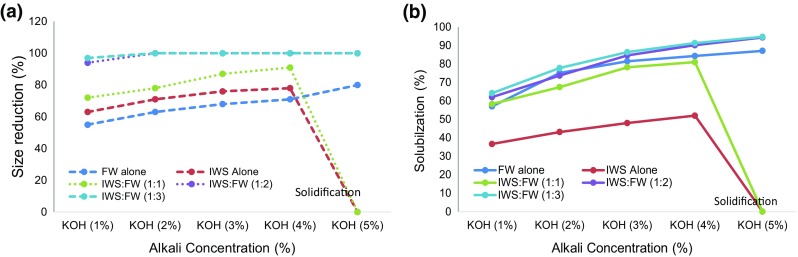
a Size reduction. b COD solubilization rate on KOH pretreatment of IWS, FW and mixing ratios (1:1, 1:2 and 1:3)
Similarly, the effect of NaOH pretreatment size reduction using 3.5 mm sieves and COD solubilization was studied and illustrated in Figs. 2a, b. The maximum size reduction of 100% was observed in 3% NaOH with mixing ratio of 1:2 and 1:3. The minimum size reduction was observed in IWS alone with 3% NaOH concentration, i.e., 64%. The maximum COD solubilization efficiency of 85.9% (FW alone; 5% NaOH), 46.6% (IWS alone; 3% NaOH), 75.8% (1:1; 3% NaOH), 89.9% (1:2; 5% NaOH), 90.1% (1:3; 5% NaOH) was obtained. Effect of NaOH concentration in terms of size reduction trend is similar to KOH pretreatment. Similarly, IWS alone and 1:1 mixing ratio showed declining trend after achieving the higher solubilization in 3% NaOH concentration. In 4 and 5% NaOH concentrations pretreatment with IWS alone and 1:1 mixing ratio were solidified due to dehydration of the protein from IWS during the higher Alkali concentration in 120 °C thermal treatment.
Fig. 2.
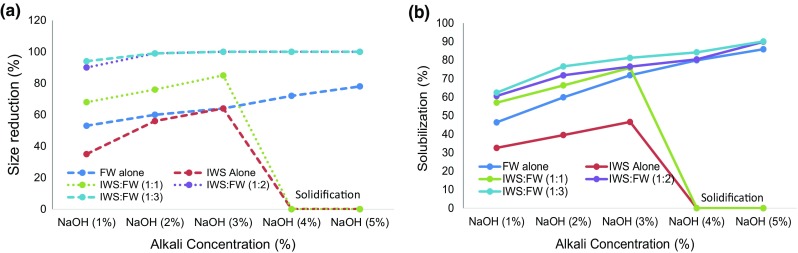
a Size reduction. b COD solubilization rate on NaOH pretreatment of IWS, FW and mixing ratios (1:1, 1:2 and 1:3)
Based on the above pretreatment studies with KOH, it is observed from Fig. 1a that the maximum size percentage size reduction and COD solubilization efficiency were obtained in IWS, FW and 1:1 with 4% KOH, 5% KOH and 4% KOH, respectively. Whereas in the case of 1:2 and 1:3 mixing ratio, the higher COD solubilization efficiency of 94.4 and 94.7 and 100% size reduction were observed with lesser KOH concentration of 2% indicating optimum condition. Similarly, it is observed from Fig. 2a, b with NaOH pretreatment that the maximum solubilization efficiency was obtained in IWS alone with 3%; FW alone with 5% and 1:1 mixing ratio with 3%. Higher solubilization efficiency could also be obtained in 1:2 and 1:3 mixing ratio with lesser NaOH concentration of 2% which followed similar trend with KOH pretreatment.
After KOH and NaOH solubilization study, the maximum solubilization at the lesser concentration of 3% NaOH was observed with IWS and 1:1 mixing ratio. Based on this pretreatment, batch biogas yield tests were carried out with 3% alkali concentration (NaOH and KOH) pretreated solubilized samples. The reactor details with different mixing ratio and 3% alkali concentration for biogas yield tests are given in Table 2. Before biogas yield test, pH of the solubilized samples was around 12, due to the addition of alkali, which affects anaerobic process severely. In addition, higher pH in the protein rich substrate produces higher free ammonia inside the reactor and it affects the acetate degrading microorganism. To address this issue, the pH of pretreated substrates was adjusted to neutral.
Table 2.
Reactor details for batch biogas yield test after thermo-alkali pretreatment
| Reactors | Substrate | Solubilization efficiency (%) | Size reduction (%) |
|---|---|---|---|
| R1 | IWS alone + 3% KOH pretreated | 48.0 | 76 |
| R2 | FW alone + 3% KOH pretreated | 81.5 | 68 |
| R3 | 1:1 + 3% KOH pretreated | 78.2 | 87 |
| R4 | 1:2 + 3% KOH pretreated | 84.6 | 100 |
| R5 | 1:3 + 3% KOH pretreated | 86.4 | 100 |
| R6 | IWS alone + 3% NaOH pretreated | 46.6 | 64 |
| R7 | FW alone + 3% NaOH pretreated | 71.8 | 64 |
| R8 | 1:1 + 3% NaOH pretreated | 75.8 | 85 |
| R9 | 1:2 + 3% NaOH pretreated | 76.5 | 100 |
| R10 | 1:3 + 3% NaOH pretreated | 81.2 | 100 |
Effect of pretreatment on biogas yield
The cumulative biogas yield, specific biogas yield and VS removal efficiency with 3% KOH pretreatment of IWS, FW, mixing ratios of 1:1, 1:2 and 1:3 (R1–R5) were shown in Figs. 3a, b. From the Fig. 3a, b, it is observed that R1 results in lower biogas yield of 0.21 L/g VS added due to lesser solubilization and percentage size reduction of substrate, i.e., 48 and 76%, respectively, which is due to the presence of higher protein and lipid content in IWS. Whereas in R2 and R3, it is observed that the biogas yield of 0.41 and 0.44 L/g VS added were moderate which is reflected by higher the solubilization and percentage size reduction. In R4 and R5 reactors with 1:2 and 1:3 mixing ratio, it is observed that the initial increased biogas yield was found to be faster up to 10th day due to balanced nutrient and optimum C/N ratio (29.5, 34.1) of the substrate and due to higher COD solubilization of 90.2 and 91.3%. After KOH pre-treatment, the maximum biogas yield was in the order of 0.55 (R4) > 0.51 (R5) > 0.44 (R3) > 0.41 (R2) > 0.21 (R1) L/g of VS added. The maximum biogas yield with KOH pretreatment was observed in R4 which is 61 and 25% higher than the R1 (IWS) and R2 (FW), respectively, whereas in R5 (1:3 mixing ratio), lower biogas yield was observed when compared to R4 due to the excess addition of FW with carbon source which is also reflected in C/N ratio (37.2 higher than optimum) of R4 reactor. The maximum VS removal efficiency was also achieved in R4 (1:2), i.e., 68%, whereas in R1 it is only 48%. The process parameters of all batch reactors such as pH, VFA/alkalinity ratio and free ammonia concentrations in reactor were monitored at regular intervals and are depicted in Figs. 3c, d. The pH of the all experimental reactors was within the range of 7–8 except R2 where it was 6.5–6.8 which is also reflected in the VFA/alkalinity ratio of 0.6 indicating unstable condition of the reactor performance and lower biogas yield. Similarly, in R1 reactor, the VFA/alkalinity ratio was found to be 0.46 (above 0.4) resulting in disturbed condition of reactor and its performance. However, in R3 and R5, i.e., mixing ratios of 1:1–1:3, the VFA/alkalinity ratio was within the limit (below 0.4) and it is more suitable for AD. The ammonia nitrogen concentration of 1700 mg/L was observed in R1 which is higher than the optimum value of 1500 mg/L and also reflected in the free ammonia concentration of 114 mg/L (based on the Eqs. (1) and 2), which affects AD process severely. R2 has very less free ammonia concentration, i.e., 3 mg/L, whereas the free ammonia concentration in R3 was found to be higher than R4 and R5 reactors which could be due to the higher amount of IWS in R3 when compared with R4 and R5 reactors. Based on the outcome of the results, higher biogas yield was obtained in R4 reactor with 1:2 mixing ration and KOH (3%) when compared to other experimental reactors, due to the double fold of carbon source added with IWS, optimum C/N ratio of 31.5 and higher solubilization with pretreatment.
Fig. 3.
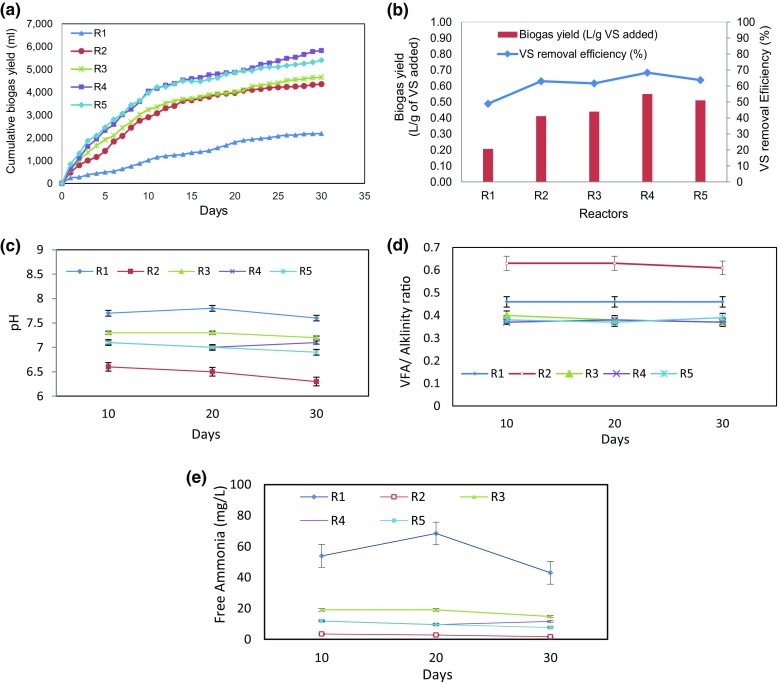
a Cumulative biogas yield, b biogas yield and VS removal efficiency, c pH, d VFA/alkalinity ratio and e free ammonia concentration in KOH pre-treated anaerobic digestion
Similarly, biogas yield tests were carried out for solubilized substrate obtained after NaOH pretreatment and pH adjustment. The cumulative biogas yield of IWS (R6), FW (R7), 1:1 (R8), 1:2 (R9) and 1:3 (R10) is shown in Figs. 4a–e. In R6 reactor (IWS), the lowest biogas yield was obtained when compared all experimental reactors which is mainly due to the lesser solubilization efficiency of 46.6%. In all reactors with NaOH pretreated during initial 10 days, the biogas production was found to be faster comparatively and after 10 days, the biogas production showed gradual increase. The maximum biogas yields at the end of 30th day in all reactors were found to be 0.17 (R6), 0.36 (R7), 0.39 (R8), 0.45 (R9) and 0.40 (R10) L/g VS added. The maximum biogas yield of 0.45 L/g VS added was observed in R9 (1:2, 3% NaOH) which is 18% lower than the maximum biogas yield of 0.55 L/g VS in R4 (1:2, 3% KOH). Similar lower biogas yields were observed in all other reactors with NaOH pretreatment when compared with the corresponding reactors with KOH pretreatment. It is reported that sodium has more effect on propionic acid utilizing micro-organism than potassium in anaerobic process, which is reflected in the biogas yield (Soto et al. 1993). In addition, the VS removal efficiency was also got slightly affected the acetogenic process due to the presence of sodium ions. From Fig. 4b, the maximum VS removal efficiency is in the following order 67.8% (R9) > 62.8% (R7) > 62.3% (R10) > 61.5% (R8) > 48.1% (R7). In IWS (R7) reactor, the pH is almost alkaline (8.1–8.3) in nature, which reflects in ammonia nitrogen and free ammonia concentration of 1017 and 154 mg/L, respectively. The free ammonia concentration in the alkali medium affects the methanogens population in the reactors and leads to VFA accumulation. While in R8 (FW) reactor, VFA production is high and the pH in the reactor reached up to 6.5. Due to the lower pH, the buffering capacity of reactor was affected and it is reflected in VFA/alkalinity ratio of 0.62, which in turn affects the biogas yield severely. Whereas, in all other reactors with different mixing ratios, the pH and VFA/alkalinity ratio was found to be within the limits, resulting in enhanced biogas yield than the individual substrates.
Fig. 4.
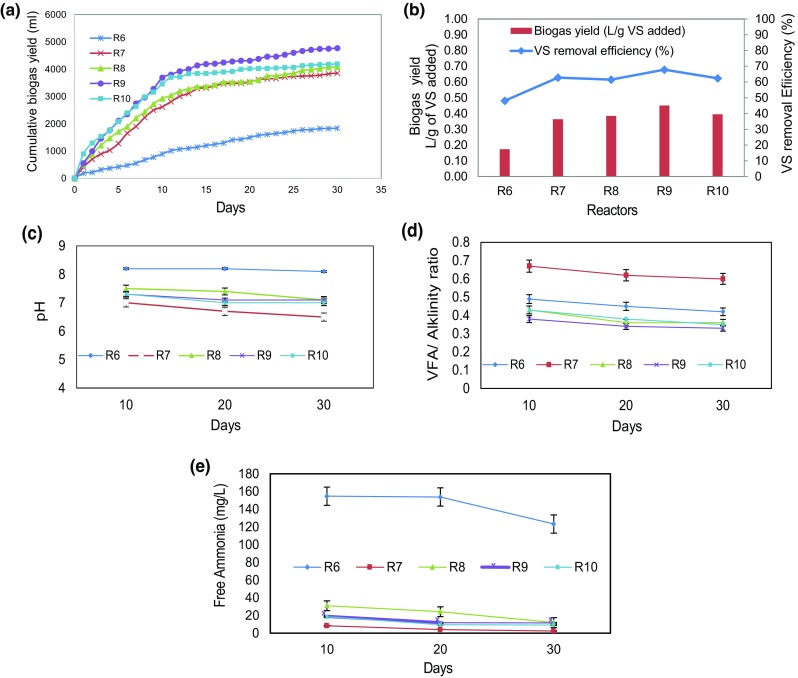
a Cumulative biogas yield, b biogas yield and VS removal efficiency, c pH, d VFA/alkalinity ratio and e free ammonia concentration in NaOH pre-treated anaerobic digestion
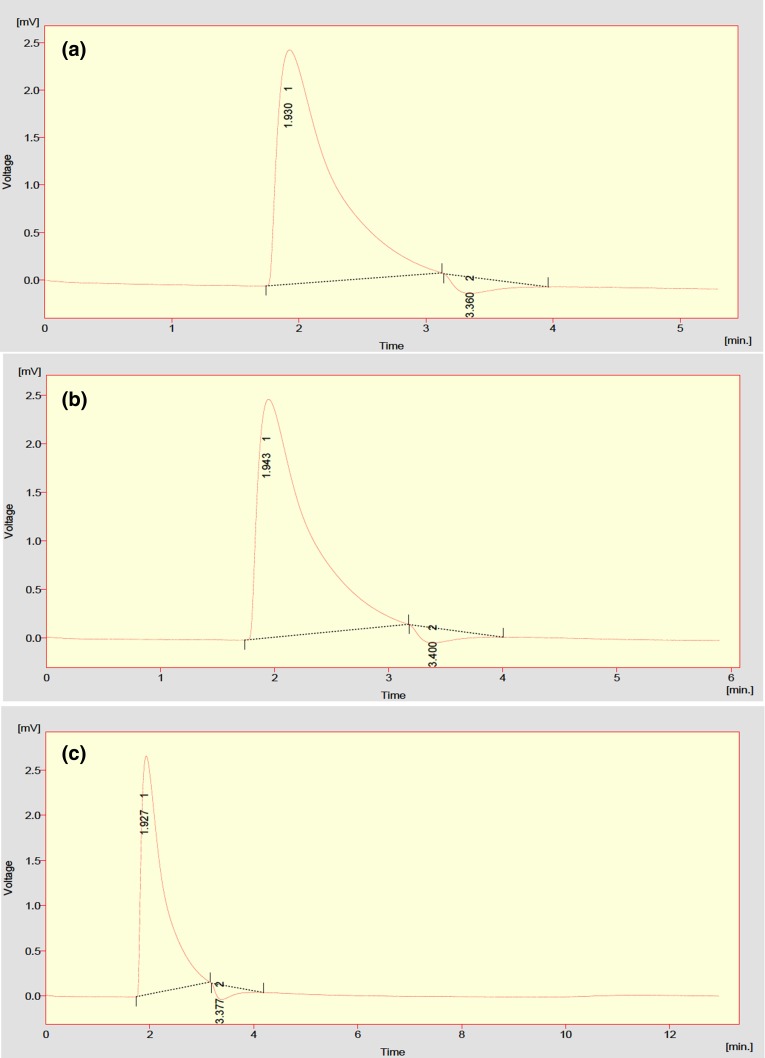
The methane content of the biogas generated from IWS, FW and 1:2 was determined by Gas Chromatography and the results are depicted in Fig. 5a–c. Based on the higher biogas yield from the batch scale reactor studies, methane composition of IWS (3% KOH), FW (3% KOH) and 1:2 (3% KOH) was found to be 57.01%, 58.08 and 60.28%, respectively. The higher methane composition of 60.28 reflects the increase in performance of the reactor by mixing of IWS:FW in 1:2 ratio which is found to be optimum condition.
Fig. 5.
a–c Methane content of biogas produced from IWS, FW and 1:2 by gas chromatography
Kinetics study
The pseudo-first and second-order kinetics were evaluated to fit the degradation of VS during biogas yield test of thermo-alkali pretreated such as IWS alone, FW alone and mixing ratios of 1:1–1:3 (IWS:FW) and presented in Table 3. Based on the regression coefficient, it is observed that the pseudo-second order was best fitted the experimental data than first order for both the KOH and NaOH pretreatment reactors. In the mixing ratios 1:1, 1:2 and 1:3 reactors, the second-order kinetics were observed the best rate constant (0.0042 in KOH and 0.0041 in NaOH) and R 2 values (0.9194 in KOH and 0.9158 in NaOH). Based on the kinetic data, it is observed that the best rate constant and higher R 2 values were observed in 1:2 mixing ratio with KOH and NaOH.
Table 3.
Pseudo-first and second-order kinetic study for the degradation of VS
| Samples | KOH | NaOH | ||||||
|---|---|---|---|---|---|---|---|---|
| Pseudo 1st order | 2nd order | Pseudo 1st order | 2nd order | |||||
| Rate constant (k 1) | R 2 | Rate constant (k 2) | R 2 | Rate constant (k 1) | R 2 | Rate constant (k 2) | R 2 | |
| IWS | 0.0242 | 0.9713 | 0.0021 | 0.9909 | 0.237 | 0.9685 | 0.002 | 0.9887 |
| FW | 0.0391 | 0.7664 | 0.0035 | 0.9095 | 0.383 | 0.7808 | 0.0034 | 0.9213 |
| IWS:FW (1:1) | 0.0382 | 0.6647 | 0.0032 | 0.8324 | 0.0378 | 0.682 | 0.0031 | 0.8463 |
| IWS:FW (1:2) | 0.0435 | 0.7712 | 0.0042 | 0.9194 | 0.0428 | 0.7698 | 0.0041 | 0.9158 |
| IWS:FW (1:3) | 0.0403 | 0.7170 | 0.0035 | 0.8772 | 0.0386 | 0.7157 | 0.0033 | 0.8745 |
Based on the biogas yield from IWS, FW and different mixing ratios, the following first-order kinetics equation was used to fit the biogas yield data with time. A straight line should be obtained with ln (V e − V t/V e) versus ‘t’ and slope equal to k and intercept zero. The value of V e has been considered equal to the volume of biogas accumulated at the end of each experiment. Representation of the experimental data in the above equation gives straight lines with intercept practically zero and slope equal to k (d−1). The value of “k” was obtained from a linear regression analysis. The values of first-order rate constant (k) of KOH pretreated samples were in the order of 0.1277(FW) > 0.1175 (1:3) > 0.1101 (1:1) > 0.1003 (1:2) > 0.0948 (IWS) d−1, and NaOH pretreated samples were in the order of 0.1533(1:3) > 0.1304 (1:2) > 0.13(FW) > 0.1119 (1:1) > 0.0939 (IWS), respectively.
Conclusion
Based on the thermo-alkali study, the maximum solubilization of 94.7 and 90.1% were obtained in 1:3 with 5% KOH and NaOH, respectively. Based on the biogas yield tests, it is concluded that the higher biogas yield of 0.55 L/g of VS added was observed in 3% KOH with 1:2 mixing ratio, whereas in NaOH-pretreated samples, only 0.45 L/g of VS added for similar condition. It is also concluded that the biogas production with KOH pretreatment for various experimental runs has resulted in better biogas yield by 16% (IWS), 11.5% (FW), 12.2% (1:1), 18.11% (1:2) and 22.5% (1:3) when compared with NaOH pretreatment.
Acknowledgements
The authors thank the Director, CLRI, Chennai for his support in research work. Authors wish to thank the staff and research scholars in Environmental Science and Engineering Division, CLRI, Chennai. Sincere and Special thanks Prime minster PhD fellowship and our industry mentor M/s Envian Engineers Pvt. Ltd., Chennai.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no potential conflict of interest.
References
- Ahring BK (2003) Perspectives for Anaerobic Digestion. In: Biomethanation I. Part of the advances in biochemical engineering/biotechnology book series (ABE), vol 81, pp 1–30 [DOI] [PubMed]
- Animut A, Meseret C, Egigu AK. Thermal and chemical pre-treatments of cow dung and poultry litter enhance biogas production in batch fermentation. Int J Sci Technol Res. 2014;3(11):165–170. [Google Scholar]
- APHA . Standard Methods for the examination of water and wastewater. 20. Washington, DC: Am Publ Health Assoc; 1998. [Google Scholar]
- Belmonte M, Hsieh CF, Figueroa C, Campos JL, Vidal G. Effect of free ammonia nitrogen on the methanogenic activity of swine wastewater. Electron J Biotechnol. 2011;14(1):2. [Google Scholar]
- Bouallagui H, Cheikh RB, Marouani L, Hamdi M. Mesophilic biogas production from fruit and vegetable waste in tubular digester. Bioresour Technol. 2003;86:85–89. doi: 10.1016/S0960-8524(02)00097-4. [DOI] [PubMed] [Google Scholar]
- Braun R, Brachtl E, Grasmug M. Codigestion of proteinaceous industrial waste. Appl Biochem Biotechnol. 2003;109:139–153. doi: 10.1385/ABAB:109:1-3:139. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Lagerkvist A, Morgan-Sagastume F. The effects of substrate pretreatment on anaerobic digestion: a review. Waste Manage. 2012;32:1634–1650. doi: 10.1016/j.wasman.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cheng JJ, Creamer KS. Inhibition of anaerobic digestion process: a review. Bioresour Technol. 2008;99:4044–4064. doi: 10.1016/j.biortech.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Costa JC, Barbosa SG, Alves MM, Sousa DZ. Thermochemical pre- and biological co-treatments to improve hydrolysis and methane production from poultry wastes. Biores Technol. 2012;111:141–147. doi: 10.1016/j.biortech.2012.02.047. [DOI] [PubMed] [Google Scholar]
- Cuetos MJ, Gomez X, Otero M, Antonio M. Anaerobic digestion of solid slaughterhouse waste (SHW) at laboratory scale: influence of co-digestion with the organic fraction of municipal solid waste (OFMSW) Biochem Eng J. 2008;40:99–106. doi: 10.1016/j.bej.2007.11.019. [DOI] [Google Scholar]
- Eckenfelder WW. Industrial water pollution control. Boston: McGraw-Hill Inc; 1999. [Google Scholar]
- Forster-Carneiro T, Pérez M, Romero LI. Thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Biores Technol. 2008;99:6763–6770. doi: 10.1016/j.biortech.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Gerardi MH. The microbiology of anaerobic digesters. Hoboken: Wiley; 2003. [Google Scholar]
- Guangxue WU, Zhenhu HU, Mark GH, Xinmin Z. Thermochemical pretreatment of meat and bone meal and its effect on methane production. Front Environ Sci Eng China. 2009;3(3):300–306. doi: 10.1007/s11783-009-0031-6. [DOI] [Google Scholar]
- Gupta P, Shekhar SR, Sachan A, Vidyarthi AS, Gupta A. A reappraisal on intensification of biogas production. Renew Sustain Energy Rev. 2012;16:4908–4916. doi: 10.1016/j.rser.2012.05.005. [DOI] [Google Scholar]
- Javkhlan A, Antonio P, Giovanni E, Francesco P, Piet NLL. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl Energy. 2014;23:143–156. [Google Scholar]
- Jhosané PD, Ileana PR, Mohammad JT, Ilona SH, Magnus L. Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: synergistic and antagonistic interactions determined in batch digestion assays. Chem Eng J. 2014;245:89–98. doi: 10.1016/j.cej.2014.02.008. [DOI] [Google Scholar]
- Kalambura S, Kaje GI (2007) Thermo-alkaline hydrolysis of animal waste. In: Eleventh international waste management and landfill symposium, 1–5 October 2007, S. Margherita di Pula-Cagliari, Sardinia, Italy
- Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L. The anaerobic digestion of solid organic waste. Waste Manage. 2011;31:1737–1744. doi: 10.1016/j.wasman.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Lee M, Hidaka T, Hagiwara W, Tsuno H. Comparative performance and microbial diversity of hyperthermophilic and thermophilic co-digestion of kitchen garbage and excess sludge. Biores Technol. 2009;100:578–585. doi: 10.1016/j.biortech.2008.06.063. [DOI] [PubMed] [Google Scholar]
- Lee J, Song J, Hwang S. Effects of acid pre-treatment on bio hydrogen production and microbial communities during dark fermentation. Bioresour Technol. 2009;100:1491–1493. doi: 10.1016/j.biortech.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Liu C, Yuan X, Zeng G, Li W, Li J. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour Technol. 2008;99:882–888. doi: 10.1016/j.biortech.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Mao C, Feng Y, Wang X, Ren G. Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev. 2015;45:540–555. doi: 10.1016/j.rser.2015.02.032. [DOI] [Google Scholar]
- Mata-Alvarez J, Mace S, Llabres P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Biores Technol. 2000;74:3–6. doi: 10.1016/S0960-8524(00)00023-7. [DOI] [Google Scholar]
- Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sust Energ Rev. 2014;36:412–427. doi: 10.1016/j.rser.2014.04.039. [DOI] [Google Scholar]
- Ostrem K (2004) Greening waste: Anaerobic digestion for treating the organic fraction of municipal solid wastes, Earth Engineering Center Columbia University
- Penaud V, Delgenes JP, Moletta R. Thermo-chemical pretreatment of a microbial biomass: influence of sodium hydroxide addition on solubilization and anaerobic biodegradability. Enzyme Microbial Technol. 1999;25(3–5):258–263. doi: 10.1016/S0141-0229(99)00037-X. [DOI] [Google Scholar]
- Ravindranath E (2012) Studies on liquefaction of limed fleshings and enhancement of biomethanization from tannery waste, Ph.D. dissertation, Anna University, Chennai
- Santiago GG, et al. Kinetic modelling of anaerobic hydrolysis of solid wastes, including disintegration processes. Waste Manage. 2015;35:96–104. doi: 10.1016/j.wasman.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Soto M, Mendez R, Kema JM. Sodium inhibition and sulphate reduction in the anaerobic treatment of mussel processing wastewaters. J Chem Technol Biotechnol. 1993;58:1–7. [Google Scholar]
- Tsukahara K, Yaguishita T, Ogi T, Sawayama S. Treatment of liquid fraction separated from liquidized food waste in an upflow anaerobic sludge blanket reactor. J Biosci Bioeng. 1999;4:554–556. doi: 10.1016/S1389-1723(99)80112-1. [DOI] [PubMed] [Google Scholar]
- Val del Rio A, et al. Thermal pretreatment of aerobic granular sludge: impact on anaerobic biodegradability. Water Res. 2011;45:6011–6020. doi: 10.1016/j.watres.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Veluchamy C, Ajay SK. Enhanced methane production and its kinetics model of thermally pretreated lignocellulose waste material. Biores Technol. 2017;241:1–9. doi: 10.1016/j.biortech.2017.05.068. [DOI] [PubMed] [Google Scholar]
- Verma S (2002) Anaerobic digestion of biodegradable organics in municipal solid wastes. Fu Foundation School of Engineering and Applied Science Columbia University
- Yuan H, Zhu N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew Sust Energ Rev. 2016;58:429–438. doi: 10.1016/j.rser.2015.12.261. [DOI] [Google Scholar]