Abstract
In type-2 diabetes, both insufficient insulin and excessive glucagon secretion contribute to hyperglycemia. We compared insulin, glucagon and somatostatin stores in pancreas obtained at autopsy of 20 lean and 19 obese non-diabetic (ND), and 18 type-2 diabetic (T2D) subjects. From concentrations and pancreas weight, total content of hormones was calculated. Insulin content was 35% lower in T2D than ND subjects (7.4 versus 11.3 mg), whereas glucagon content was similar (0.76 versus 0.81 mg). The higher ratio of glucagon/insulin contents in T2D was thus explained by the decrease in insulin. With increasing BMI of ND subjects, insulin and glucagon contents respectively tended to increase and decrease, resulting in a lower glucagon/insulin ratio in obesity. With aging, insulin and glucagon contents did not significantly change in ND subjects but declined in T2D subjects, without association with the duration of diabetes or type of treatment. The somatostatin content was lower in T2D than ND subjects (0.027 versus 0.038 mg), but ratios somatostatin/insulin and somatostatin/glucagon were not different. In conclusion, insulin stores are about 1/3 lower in T2D than ND subjects, whereas glucagon stores are unchanged. Abnormal secretion of each hormone in type-2 diabetes cannot be attributed to major alterations in their pancreatic reserves.
Introduction
Glucose homeostasis mainly relies on the opposite hypoglycemic and hyperglycemic properties of insulin and glucagon. In type-2 diabetic (T2D) subjects, hyperglycemia is largely the consequence of insufficient insulin secretion and excessive glucagon secretion in a context of insulin resistance1–3. These secretory abnormalities may result from isolated or combined alterations in the function and number of pancreatic β- and α-cells. Three pioneer groups used bioassays to compare insulin concentrations in extracts of the pancreas from non-diabetic (ND) and diabetic subjects4–6. Subsequent studies used radioimmunoassays to measure insulin and/or glucagon in the human pancreas7–17. However, those combining pancreatic insulin and glucagon measurements in ND subjects11–14, or comparing pancreatic insulin in ND and T2D subjects8, 9, 16, 17 remain infrequent. Pancreatic glucagon and somatostatin stores have not previously been measured in T2D subjects.
In this paper, we report measurements of insulin, glucagon and somatostatin in the pancreas of 18 T2D subjects and 39 ND subjects. The number of the latter also permitted assessment of the impact of obesity by comparing 20 lean non-diabetic subjects (LND) and 19 obese non-diabetic subjects (OND).
Results
Hormone concentrations in the pancreas
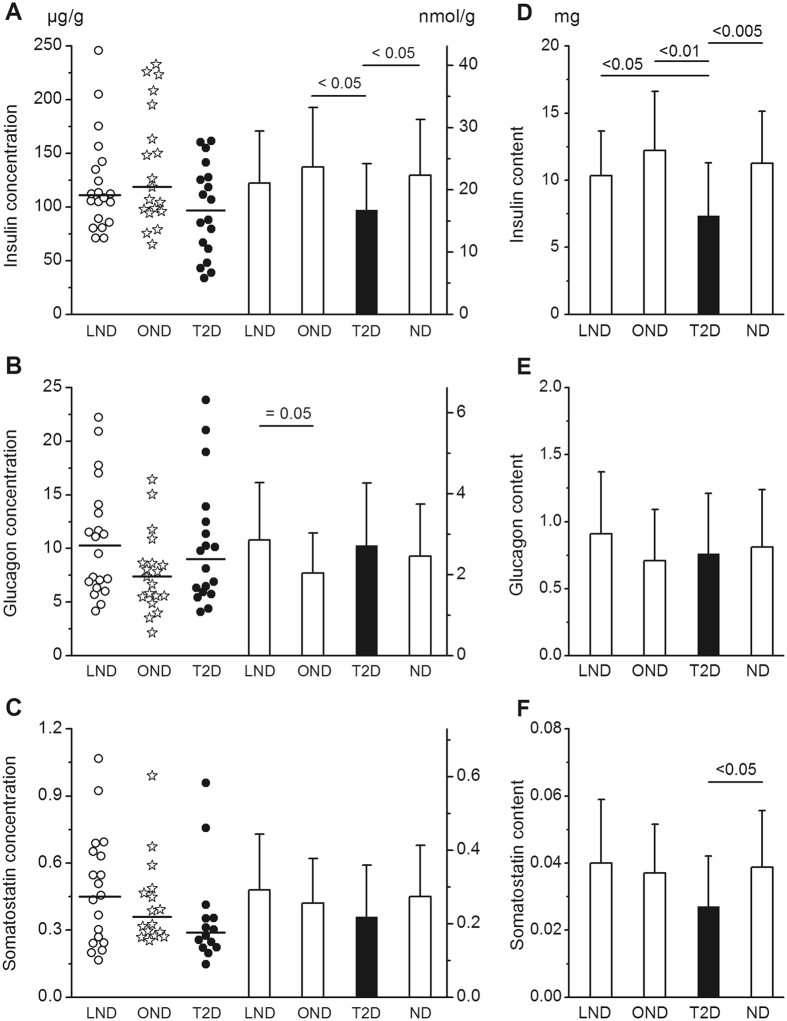
Pancreatic concentrations of insulin, glucagon and somatostatin were characterized by a great inter-subject variability (3.8- to 7.6-fold) in the three groups of subjects (Fig. 1A–C). Pancreatic insulin concentrations were not significantly different in LND and OND subjects (122 ± 48 and 138 ± 55 µg/g) (Fig. 1A). In T2D subjects, the mean concentration of insulin (97 ± 43 µg/g) was slightly lower than in the groups of OND or of all ND subjects. Notably, however, two thirds of T2D subjects had a pancreatic insulin concentration within the range of concentrations in ND subjects (Fig. 1A).
Figure 1.
Pancreatic insulin, glucagon and somatostatin concentrations and contents. Panels (A–C) show hormone concentrations (in µg/g or nmol/g) in the body of the pancreas of 20 lean non-diabetic (LND), 19 obese non-diabetic (OND) and 18 type-2 diabetic (T2D) subjects. Individual values are shown as scatter plots with medians. Columns show mean values ± SD. Right-hand columns show mean results for all (lean and obese) ND subjects. Panels (D–F) show mean insulin, glucagon and somatostatin contents (in mg) of the pancreas. In each subject hormone content was obtained by multiplying the concentration measured in the body by the weight of the whole pancreas. Significant differences between groups shown above columns were calculated by Anova and confirmed by non-parametric tests, except for insulin concentration in T2D versus ND subjects (P = 0.065).
The mean pancreatic glucagon concentration in T2D subjects (10.3 ± 5.8 µg/g) was not different from that in ND subjects (9.3 ± 4.8 µg/g) or in the two subgroups of controls (Fig. 1B). It was marginally lower in OND than LND subjects. The mean somatostatin concentration in T2D subjects (0.37 ± 0.25 µg/g) was not significantly different from that in ND subjects (0.46 ± 0.24 µg/g) or in the two subgroups of controls (Fig. 1C).
Hormone contents of the pancreas
In the whole group of ND subjects, the weight of the pancreas averaged 90.8 ± 22.6 g, and did not correlate with age (r = −0.301, P = 0.064) or BMI (r = 0.209, P = 0.210), but increased slightly with body weight (r = 0.366, P = 0.026). It was 16% lower (76.2 ± 20.0 g, P < 0.05) in T2D subjects. From the weight of each pancreas and measured hormone concentrations, the total content of each hormone could be calculated (Fig. 1D–F). In the group of 39 ND subjects the insulin content averaged 11.3 ± 3.9 mg and it was 35% lower in T2D subjects (7.4 ± 3.9 mg, P < 0.005). The decrease remained significant when compared with LND and OND subjects separately. Among controls, the insulin content was not significantly different between OND and LND subjects (Fig. 1D).
The pancreatic glucagon content averaged 0.81 ± 0.43 mg in ND subjects and 0.76 ± 0.45 mg in T2D subjects. There was no difference between glucagon contents in T2D subjects and any of the other groups (Fig. 1E). In ND subjects, the pancreatic somatostatin content averaged 0.038 ± 0.017 mg. It was 29% lower in T2D subjects (0.027 ± 0.015 mg, P < 0.05), but the difference was not significant when compared with the subgroups of LND and OND subjects (Fig. 1F).
Pancreatic hormone contents versus age
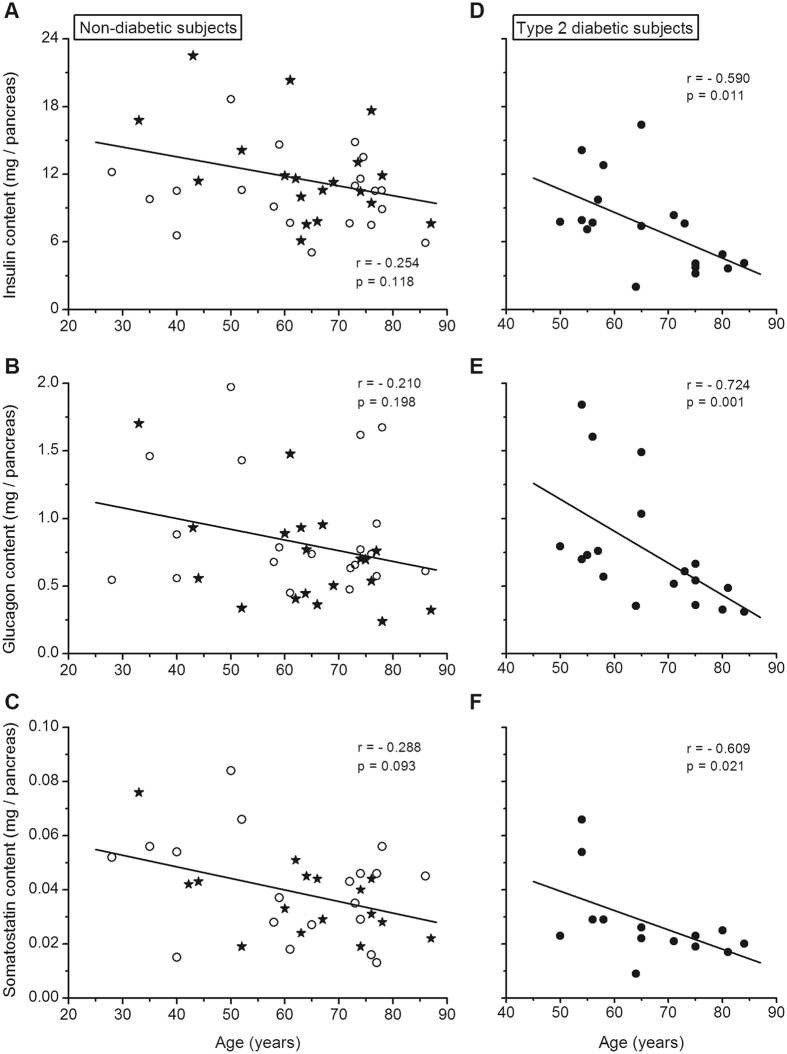
In the whole group of ND subjects, insulin, glucagon and somatostatin contents of the pancreas tended to decrease slightly with age, but the correlations did not reach statistical significance (Fig. 2A–C). There was also no significant correlation when the subgroups of LND and OND were tested separately. In addition, mean insulin, glucagon and somatostatin contents were not statistically different in ND subjects below and above 65 years. In contrast, aging was associated with a decrease in pancreatic content of the three hormones in T2D subjects (Fig. 2D–F). This was confirmed by a significant difference in mean insulin and glucagon contents in T2D subjects below and above 65 years (P ≤ 0.01).
Figure 2.
Correlations between the insulin, glucagon and somatostatin content of the pancreas and the age of the subjects. Panels (A–C) show results for lean (open circles) and obese (filled stars) non-diabetic subjects by different symbols. Panels (D–F) show results for type-2 diabetic subjects. Correlation coefficients were calculated by the test of Spearman.
Pancreatic hormone contents versus BMI
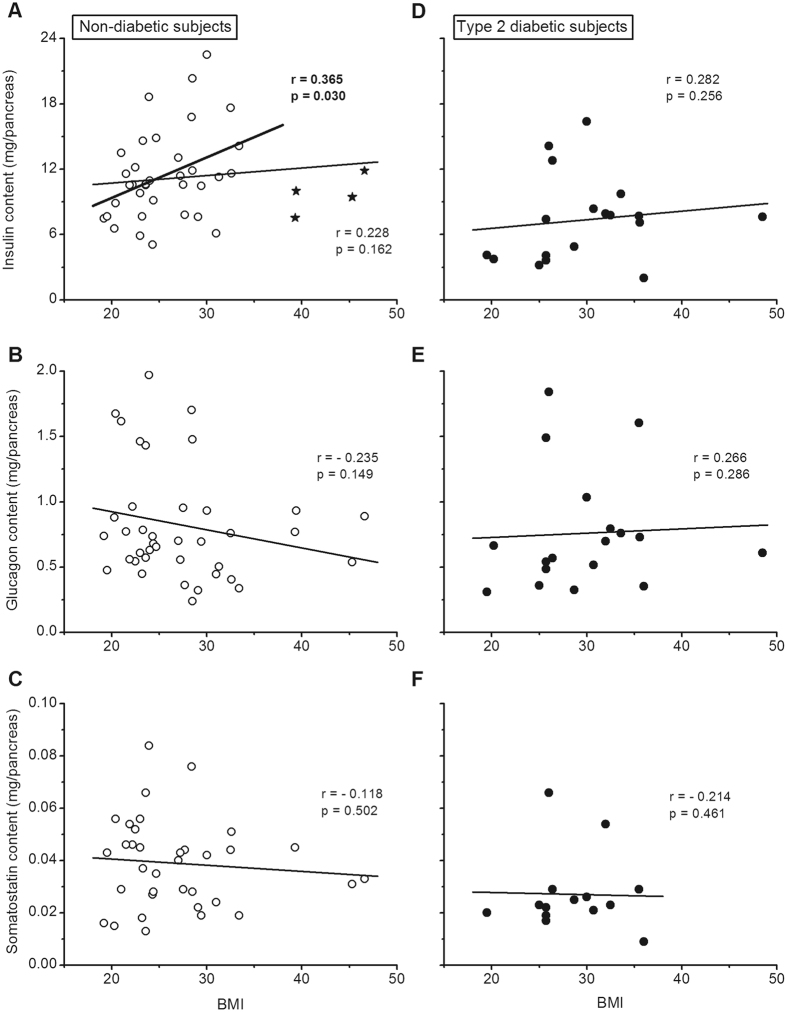
In ND subjects, no correlation was found between the insulin, glucagon or somatostatin content and BMI (Fig. 3A–C) or body weight. There was, however, a significant positive correlation between the insulin content and BMI when the 4 most obese subjects (BMI > 38) were excluded from the analysis (Fig. 3A). In T2D subjects, insulin, glucagon and somatostatin contents were also unrelated to BMI (Fig. 3D–F) or body weight.
Figure 3.
Correlations between the insulin, glucagon and somatostatin content of the pancreas and the BMI of the subjects. Panels (A–C) show results for non-diabetic subjects. (A) The correlation calculated for all subjects is shown by the thin line. The correlation calculated after exclusion of the 4 subjects with a BMI above 38 (stars) is shown by the thick line. Panels (D–F) show results for type-2 diabetic subjects. Correlation coefficients were calculated by the test of Spearman.
Duration of diabetes, type of treatment and pancreatic hormone contents
Clinical duration of diabetes (time since diagnosis) was known in 14/18 T2D subjects (Supplementary Table 1). It did not correlate with pancreatic insulin content (r = −0.386, P = 0.172), glucagon content (r = −0.294, P = 0.308) or somatostatin content (r = −0.589, P = 0.081). Of the 18 T2D subjects, 8 received insulin alone or with an oral drug, and 8 received a sulfonylurea alone or with insulin (Supplementary Table 1). Treatment with insulin or sulfonylurea did not affect insulin, glucagon or somatostatin concentration or content of the pancreas (Supplementary Fig. 1).
Ratios of hormone concentrations in the pancreas
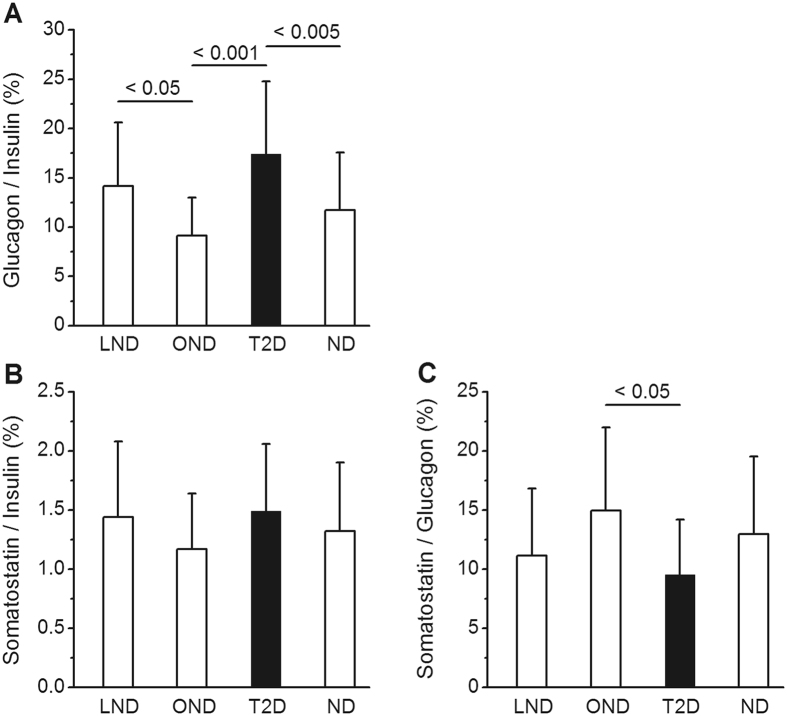
These ratios were calculated using molar concentrations of each hormone (right-hand scales in Fig. 1A–C) and were expressed as percentages. The same results would be obtained from calculations based on hormone content. In the group of 39 ND subjects the glucagon/insulin ratio averaged 11.7 ± 5.8%. It was higher (17.4 ± 7.7%) in T2D subjects (Fig. 4A). Among controls, the ratio was lower in OND than LND subjects (Fig. 4A) because glucagon and insulin concentrations in OND tended to be lower and higher, respectively (Fig. 1A and B). In ND subjects the somatostatin/insulin ratio averaged 1.32 ± 0.58% and it was similar in the two subgroups of LND and OND subjects and in T2D subjects (Fig. 4B). The somatostatin/glucagon ratio averaged 12.9 ± 6.6% in ND subjects. It was slightly lower in T2D than OND subjects (Fig. 4C). None of these ratios was significantly correlated with age or BMI.
Figure 4.
Molar ratios of hormone concentrations in the pancreas. Results are shown for lean non-diabetic subjects (LND), obese non-diabetic subjects (OND), all non-diabetic subjects (ND) and type-2 diabetic subjects (T2D). These ratios were calculated from hormone concentrations in nmol/g shown in Fig. 1 and are expressed as percentages. Values are means ± SD. Significant differences between groups shown above columns were calculated by Anova and confirmed by non-parametric tests.
Discussion
The present study, based on data obtained more than 25 years ago, suffers from one limitation that we were unable to rectify. The still accessible clinical records (autopsy notes) did not include primary results of blood glucose or HbA1c assays formally excluding diabetes in all ND subjects. Another potential problem is the heterogeneity of islet distribution, with a higher islet volume density in the tail than in the head and body of the pancreas17, 18. In two subsets of the studied subjects we also obtained samples from the head or the tail, and found that insulin concentrations were ~36% lower in the head than in the body and ~30% lower in the body than in the tail, in agreement with previous studies5, 12, 14. Glucagon concentrations were also higher in the body than the head but were not compared between body and tail. One consequence of these regional variations is that calculations of hormone content of the pancreas, based on the intermediate values measured in the body region, only provide reasonable estimates. Optimally, samples from the three regions of the pancreas should be extracted in parallel.
Comparing biochemical measurements of pancreatic hormones with quantitative morphological studies of the endocrine pancreas is not straightforward. The major pitfall is that immunohistochemical techniques detect the presence but do not usually measure the concentration of hormones in cells. Yet, relative changes in the volume density of a given cell type can be compared with relative changes in the concentration of the corresponding hormone. Theoretically, comparisons with the hormone content of isolated islets should also be instructive. However, the pitfall is that the cell composition varies with islet size19–21. Selected isolated islets may not always be representative of the whole islet population.
Pancreatic insulin and glucagon concentrations displayed a marked variability in ND subjects, in keeping with the known inter-subject variations in islet volume density17. In the whole group of 39 ND subjects, mean pancreatic insulin concentration (130 µg/g) was slightly above the average of previously published values (~100 µg/g, range 53–199)4–6, 8–14, 16, 17. The glucagon concentration (9.3 µg/g) was within the range of reported values (7–18 µg/g)7, 11–15. From these measurements we calculated that the pancreas of ND subjects contains about 11.3 mg insulin (~325 IU) and 0.81 mg glucagon. In seven immunohistochemical studies of the pancreas from ND subjects, the ratio of α-cell/β-cell volume densities averaged 31% (range 19–44)18, 20, 22–26. This ratio is higher than the molar ratio of glucagon to insulin contents which averaged 11.7% (7.6% in µg/g). The discrepancy is even greater with isolated islets in which a glucagon/insulin ratio of only 3% has been measured27. The obvious implication is that α-cells contain less glucagon than β-cells contain insulin. That is indeed the case28, but quantitative comparisons would be hazardous because α-cells are smaller than β-cells and the proportion of α/β cells increases with islet size20–22. Further studies are necessary to resolve the issue.
Aging is associated with deterioration in glucose tolerance29. Both β- and α-cell masses have been found to decrease slightly with aging in ND subjects17, 20, 30 although one study measured no change in the β-cell mass31. In partial contrast with these morphological observations, pancreatic insulin and glucagon contents did not significantly decline with age and mean contents were not significantly different between ND subjects below or above 65 years of age. In a study based on bioassays, a small non-significant decline of pancreatic insulin stores was measured in ND subjects between the second and eighth decades5. No correlation was found between the insulin content of isolated islets and age between 19 and 64 years32, and a slight increase (20%) was measured in islets isolated from donors >60 years compared with donors <50 years33. Overall these observations indicate that the decrease in insulin secretion occurring in older subjects34 is not attributable to insufficient insulin reserves.
Whereas isolated obesity notoriously increases basal and stimulated insulin secretion35, 36, its influence on glucagon secretion is uncertain37, 38. The α-cell mass does not change with BMI20, 39. Compared with LND subjects, the β-cell mass is ~50% larger in very obese subjects (mean BMI of 35)31 but is much less increased (~20%) or even unchanged in cohorts with milder obesity (mean BMI of 29.9 and 28.5)17, 39. Islets isolated from lean and obese donors contain similar amounts of insulin32, 33. The impact of obesity on pancreatic insulin stores has not been examined in previous studies. We found that pancreatic insulin concentration and content were similar in OND and LND subjects (BMI 32.5 vs 22.4). The slight difference in insulin content (19%) was of the same magnitude as the increase in β-cell mass in OND, but failed to reach significance perhaps because the sample size was too small. Intriguingly, however, a positive correlation between pancreatic insulin content and BMI was disclosed when the 4/19 OND subjects with the highest BMI (>38) were excluded from the analysis. It is possible that insulin synthesis fails to compensate adequately for the high secretory demands in extreme obesity. In contrast to insulin, pancreatic glucagon concentrations were slightly lower in OND than LND subjects, which resulted in a lower glucagon/insulin ratio in the pancreas of OND subjects. Overall, insulin and glucagon stores, and β-cell and α-cell masses change relatively little with increasing BMI in ND subjects.
Insufficient insulin secretion is a key factor in the pathogenesis of type-2 diabetes1, 40, but the underlying causes remain unclear. The β-cell volume density is lower in T2D than ND subjects17, 18, 23–26, 41, 42, but the magnitude of the reported decrease varies between 24 and 60%, and the overlap between ND and T2D values is important17. In the present cohort of T2D subjects, mean pancreatic insulin concentration was 25% lower than in ND subjects, also with an important overlap between the two groups. Total insulin content was 35% lower owing to the slightly smaller size of the pancreas in T2D subjects. These differences are similar to those found in our previous report17 but smaller than the average 54% decrease in others series4–6, 8, 16. Notably, the greatest differences were found in the oldest studies, in which the characteristics of diabetic subjects were uncertain. For example, in the large cohort of Wrenshall5, the pancreatic insulin concentration was much lower in diabetic subjects aged 20–40 years than in those above 55 years. Several groups have isolated pancreatic islets from diabetic organ donors, but only few independent studies compared the insulin content of islets from significant numbers of T2D and ND donors. The decrease measured in T2D islets averaged 26% (14–35%)27, 43, 44. In our group of T2D subjects, pancreatic insulin stores did not correlate with BMI but decreased with age, which suggests that a progressive lowering of insulin reserves might contribute to age-dependent aggravation of type-2 diabetes29. We did not find links between insulin stores and duration of clinical diabetes (true duration of the disease cannot be determined) or type of treatment. Studies of larger cohorts would however be useful to ascertain these findings.
Because our insulin assay cross-reacted with proinsulin, reported concentrations of insulin are overestimated. However, the error is likely to be small. Previous studies have shown that the proinsulin/insulin ratio is only 2–3% in human normal pancreas11 and purified β-cells45. Whether this proportion is modified in type-2 diabetes and obesity is not known. We are aware of only one report succinctly stating that there is no significant difference in proinsulin proportion between pancreatic extracts from diabetic and non-diabetic subjects9.
The mechanisms causing excessive glucagon secretion in type-2 diabetes remain unclear2, 3, 46, 47. Owing to the decrease in β-cell volume density, an increase in the relative proportion of α-cells/β-cells has consistently been observed in the pancreas of T2D subjects. However, the absolute α-cell mass was variably found to be decreased21, increased25 or unchanged20, 26. Our unprecedented measurements of pancreatic glucagon in T2D subjects showed a high inter-subject variability, no relation to BMI and a decrease with aging, similar to that of insulin. Most importantly, pancreatic glucagon content was similar in T2D and ND subjects, which indicates that the higher glucagon/insulin ratio was caused by the decrease in insulin content. In one study of islets isolated from 6 T2D donors, the glucagon content was three-fold higher than in islets from ND donors27. The discrepancy between measurements in whole pancreas and isolated islets is intriguing and cannot be resolved without further investigations. In summary, based on the present measurements and previous morphological studies20, we propose that the excessive secretion of glucagon in T2D results from a defective control of α-cell function rather than from changes in α-cell mass and glucagon stores.
In ND subjects, pancreatic somatostatin concentration averaged 0.46 µg/g, a value that is close to the 0.60 µg/g found in infants48, but 5-fold lower than in previous measurements in adult pancreas14. It did not correlate with age or BMI. Concentrations measured in T2D subjects overlapped those in ND subjects. Although the total content was 29% lower in T2D subjects, the ratio between somatostatin and insulin or glucagon concentrations was unchanged. No differences have been observed in the volume density or mass of δ-cells between T2D and ND subjects23, 41, 49 except in one study reporting a 27% decrease in T2D subjects18. Although these biochemical and morphological approaches do not suggest major alterations in δ-cell mass and somatostatin stores, further studies are needed to determine whether altered somatostatin secretion plays a significant (paracrine) role in the dysfunction of α- and β-cells in type-2 diabetes.
Conclusion
Pancreatic insulin stores are reduced by 30–35% in T2D compared to ND subjects, whereas glucagon stores are unchanged. In general, these biochemical findings agree with reported changes of β- and α-cell mass in the pancreas. Together, they point to the importance of functional defects in the insufficient secretion of insulin and excessive secretion of glucagon in type-2 diabetes. Not all comparisons between hormone contents in the pancreas and isolated islets are in agreement, indicating that caution must be exercised before extrapolating data obtained in selected islets to the whole islet population. Ideal experiments should compare cell proportions and hormone concentrations in parallel samples of whole pancreas and isolated islets.
Methods
Subjects
The study was conducted according to the regulations of the Commission d’Ethique Biomédicale of the Faculty of Medicine of the University of Louvain in Brussels. Hormone concentrations were measured in the pancreas of 57 European subjects, who underwent an autopsy within 12 h of death at the University Hospital Saint-Luc in Brussels between 1985 and 1989. All extractions and assays were completed in 1989–1990, but the results were inexplicably consigned to oblivion until very recently. Subjects were assigned to the T2D group on the basis of their clinical history and use of an antidiabetic treatment for several months or years. The group of ND subjects was composed of individuals without a clinical history of diabetes. Unfortunately, for a number of these ND subjects, no definitive proof of the absence of diabetes (such as normal blood glucose or HBA1c levels) could be traced owing to the long delay between acquisition of the data and their recent analysis. Ten subjects of the present series of 57 were also included in the cases whose pancreatic insulin concentration was reported in a previous publication that did not analyze glucagon or somatostatin concentrations17.
The whole group of 39 ND subjects was composed of two subgroups of 20 lean subjects (LND, BMI < 25) and 19 obese subjects (OND, BMI ≥ 27). The group of LND subjects (15 M/5 F) had a mean age of 62.4 years (range 28–86) and a mean BMI of 22.5 (19.2–24.7). The group of OND subjects (13 M/6 F) had a mean age of 63.8 years (range 33–87) and a mean BMI of 32.4 (27.0–46.6). The group of T2D subjects (12 M/6 F) had a mean age of 66.2 years (range 54–84) and a mean BMI of 29.9 (19.5–48.5). Gender proportions and ages were not different between groups. The BMI of T2D subjects was higher than that of LND subjects (P < 0.001) but not different from that of OND subjects or the whole group of ND subjects. The time between diagnosis of T2D and death was known in 14/18 cases and averaged 10.5 y (0.5–22). Eight of the T2D subjects received insulin (combined with an oral drug in 4 cases) and 10 were on oral drug only (2 on metformin and 8 on sulfonylureas). Clinical characteristics of the studied subjects are given in Supplementary Table 1.
Pancreas processing
Only pancreases without macroscopic or microscopic signs of autolysis were studied. After resection, the pancreas was trimmed of adherent fat and mesenchymal tissue and weighed. A fragment was then taken from the body, weighed and frozen until hormone extraction. These fragments of about 1 g were finely minced, homogenized in acid-ethanol and sonicated three times after 24-h periods of storage at −20 °C. Hormone concentrations in extracts were measured by radioimmunoassays using a) human mono-component insulin (Novo Biolabs, Bagsvaerd, Denmark) and insulin antiserum L619 that cross-reacted with proinsulin (obtained from P.H. Wright, Indianapolis, U.S.A.), (b) porcine glucagon (Novo) and pancreatic glucagon antiserum 30 K (obtained from R.H. Unger, Dallas, U.S.A.), and (c) synthetic somatostatin-14 and an antiserum against it (U.C.B., Braine-L’Alleud, Belgium).
Presentation of results
Results are presented as scatter plots of individual values or as means ± SD. The number of measurements sometimes slightly differed from the total number of subjects because somatostatin was not measured in 1/20 LND, 3/19 OND and 4/18 T2D cases. The statistical significance of differences between groups was assessed by Student’s t test when only two groups were compared and by Anova followed by a Newman-Keuls test for multiple comparisons. Results were also analyzed using non-parametric tests: Mann-Whitney test to compare two groups and Kruskal-Wallis test followed by a Dunn test for multiple comparisons. The few discrepancies between parametric and non-parametric analyses are mentioned in Figure legends. Correlations between pancreas weight or hormone content and age or BMI were assessed by the test of Spearman.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Table and Figure.
Electronic supplementary material
Acknowledgements
We are grateful to A.M. Pottier for extraction of the pancreases. J.C.H. thanks Y. Guiot and R.M. Goebbels for their help with the retrieval of old data and M. Nenquin for preparing the figures.
Author Contributions
J.C.H. designed the study, obtained, analyzed and interpreted results, and wrote the manuscript. M.M.I. collected pancreatic samples and revised the manuscript. J.R. designed the study and collected pancreatic samples.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jacques Rahier is deceased.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10296-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrannini E, et al. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J. Clin. Endocrinol. Metab. 2005;90:493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 2.Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr. Rev. 2007;28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes. Metab. 2011;13(Suppl 1):126–132. doi: 10.1111/j.1463-1326.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 4.Scott DA, Fischer AM. The insulin and zinc content of normal and diabetic pancreas. J. Clin. Invest. 1938;17:727–731. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrenshall GA, Bogoch A, Ritchie RC. Extractable insulin of pancreas; correlation with pathological and clinical findings in diabetic and nondiabetic cases. Diabetes. 1952;1:87–107. doi: 10.2337/diab.1.2.87. [DOI] [PubMed] [Google Scholar]
- 6.Steinke J, Soeldner JS, Renold AE. Measurement of small quantities of insulin-like activity with rat adipose tissue. IV Serum insulin-like activity and tumor insulin content in patients with functioning islet-cell tumors. J. Clin. Invest. 1963;42:1322–1329. doi: 10.1172/JCI104816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samols E, Tyler J, Megyesi C, Marks V. Immunochemical glucagon in human pancreas, gut and plasma. Lancet. 1966;2:727–729. doi: 10.1016/S0140-6736(66)92982-5. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel JR, Pollock HG. Studies of human insulin from nondiabetic and diabetic pancreas. Diabetes. 1967;16:687–694. doi: 10.2337/diab.16.10.687. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi GK, Sinha MK, Dash RJ. Insulin and proinsulin content of pancreases from diabetic and nondiabetic subjects. Diabetes. 1973;22:804–807. doi: 10.2337/diab.22.11.804. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland DE, Matas AJ, Steffes MW, Najarian JS. Infant human pancreas. A potential source of islet tissue for transplantation. Diabetes. 1976;25:1123–1128. doi: 10.2337/diab.25.12.1123. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Floyd JC, Pek S, Fajans SS. Insulin, proinsulin, glucagon and gastrin in pancreatic tumors and in plasma of patients with organic hyperinsulinism. J. Clin. Endocrinol. Metab. 1977;44:681–694. doi: 10.1210/jcem-44-4-681. [DOI] [PubMed] [Google Scholar]
- 12.Gersell DJ, Gingerich RL, Greider MH. Regional distribution and concentration of pancreatic polypeptide in the human and canine pancreas. Diabetes. 1979;28:11–15. doi: 10.2337/diabetes.28.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Tasaka, Y. et al. Time course of content of insulin, glucagon and pancreatic polypeptide in human pancreatic tissue obtained by surgery. Endocrinol. Jpn.28, 261–264 (1981). [DOI] [PubMed]
- 14.Tomita, T., Kimmel, J. R., Friesen, S. R., Doull, V. & Pollock, H. G. Pancreatic polypeptide in islet cell tumors. Morphologic and functional correlations. Cancer56, 1649–1657 (1985). [DOI] [PubMed]
- 15.Uttenthal LO, et al. Molecular forms of glucagon-like peptide-1 in human pancreas and glucagonomas. J. Clin. Endocrinol. Metab. 1985;61:472–479. doi: 10.1210/jcem-61-3-472. [DOI] [PubMed] [Google Scholar]
- 16.Tasaka Y, Marumo K, Inoue Y, Hirata Y. C-peptide immunoreactivity and insulin content in the diabetic human pancreas and the relation to the stability of diabetic serum glucose level. Acta Endocrinol. (Copenh) 1986;113:355–362. doi: 10.1530/acta.0.1130355. [DOI] [PubMed] [Google Scholar]
- 17.Rahier, J. et al. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab.10(Suppl 4), 32–42 (2008). [DOI] [PubMed]
- 18.Clark A, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: Quantitative changes in pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 19.Bosco D, et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilimnik G, et al. Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PLoS One. 2011;6:e27445. doi: 10.1371/journal.pone.0027445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan Y, et al. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. doi: 10.2337/diab.31.8.694. [DOI] [PubMed] [Google Scholar]
- 23.Sakuraba H, et al. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 24.Yoon KH, et al. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 25.Mizukami H, et al. Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of β-cell mass in Japanese type 2 diabetic patients. Diabetes Care. 2014;37:1966–1974. doi: 10.2337/dc13-2018. [DOI] [PubMed] [Google Scholar]
- 26.Inaishi. J, et al. Effects of obesity and diabetes on α- and β-cell mass in surgically resected human pancreas. J. Clin. Endocrinol. Metab. 2016;101:2874–2882. doi: 10.1210/jc.2016-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker JN, et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab. 2011;13(Suppl 1):95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 28.Motté E, et al. Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am. J. Physiol. Endocrinol. Metab. 2014;307:E838–E846. doi: 10.1152/ajpendo.00219.2014. [DOI] [PubMed] [Google Scholar]
- 29.Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444–452. doi: 10.2337/dc16-1732. [DOI] [PubMed] [Google Scholar]
- 30.Mizukami H, et al. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J. Diabetes Investig. 2014;5:38–47. doi: 10.1111/jdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saisho Y, et al. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregg T, et al. Pancreatic β-cells from mice offset age-associated mitochondrial deficiency with reduced KATP channel activity. Diabetes. 2016;65:2700–2710. doi: 10.2337/db16-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon J, et al. Research-focused isolation of human islets from donors with and without diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology. 2016;157:560–569. doi: 10.1210/en.2015-1562. [DOI] [PubMed] [Google Scholar]
- 34.Chang AM. & Halter, J.B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2003;284:E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 35.Polonsky, K. S. et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J. Clin. Invest.81, 435–441 (1988). [DOI] [PMC free article] [PubMed]
- 36.Ferrannini E, et al. β-cell function in obesity: effects of weight loss. Diabetes. 2004;53(Suppl 3):S26–S33. doi: 10.2337/diabetes.53.suppl_3.S26. [DOI] [PubMed] [Google Scholar]
- 37.Reaven GM, et al. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 38.Schmid R, Schusdziarra V, Schulte-Frohlinde E, Maier V, Classen M. Circulating amino acids and pancreatic endocrine function after ingestion of a protein-rich meal in obese subjects. J. Clin. Endocrinol. Metab. 1989;68:1106–1110. doi: 10.1210/jcem-68-6-1106. [DOI] [PubMed] [Google Scholar]
- 39.Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J. Clin. Endocrinol. Metab. 2013;98:3724–3730. doi: 10.1210/jc.2013-1373. [DOI] [PubMed] [Google Scholar]
- 40.Cerasi E. Insulin deficiency and insulin resistance in the pathogenesis of NIDDM: is a divorce possible? Diabetologia. 1995;38:992–997. doi: 10.1007/BF00400591. [DOI] [PubMed] [Google Scholar]
- 41.Saito K, Yaginuma N, Takahashi T. Differential volumetry of A, B and D cells in the pancreatic islets of diabetic and nondiabetic subjects. Tohoku J. exp. Med. 1979;129:273–283. doi: 10.1620/tjem.129.273. [DOI] [PubMed] [Google Scholar]
- 42.Butler AE, et al. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 43.Marchetti P, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J. Clin. Endocrinol. Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 44.Taneera J, et al. γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia. 2012;55:1985–1994. doi: 10.1007/s00125-012-2548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hostens K, Ling Z, Van Schravendijk C, Pipeleers D. Prolonged exposure of human β-cells to high glucose increases their release of proinsulin during acute stimulation with glucose or arginine. J. Clin. Endocrinol. Metab. 1999;84:1386–1390. doi: 10.1210/jcem.84.4.5621. [DOI] [PubMed] [Google Scholar]
- 46.Marroquí L, et al. Nutrient regulation of glucagon secretion: involvement in metabolism and diabetes. Nutr. Res. Rev. 2014;27:48–62. doi: 10.1017/S0954422414000031. [DOI] [PubMed] [Google Scholar]
- 47.Rorsman P, Ramracheya R, Rorsman NJ, Zhang Q. ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion. Diabetologia. 2014;57:1749–1761. doi: 10.1007/s00125-014-3279-8. [DOI] [PubMed] [Google Scholar]
- 48.Bishop AE, et al. Decrease of pancreatic somatostatin in neonatal nesidioblastosis. Diabetes. 1981;30:122–126. doi: 10.2337/diab.30.2.122. [DOI] [PubMed] [Google Scholar]
- 49.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Table and Figure.