Abstract
Identification of the active site is important in developing rational design strategies for solid catalysts but is seriously blocked by their structural complexity. Here, we use uniform Cu nanocrystals synthesized by a morphology-preserved reduction of corresponding uniform Cu2O nanocrystals in order to identify the most active Cu facet for low-temperature water gas shift (WGS) reaction. Cu cubes enclosed with {100} facets are very active in catalyzing the WGS reaction up to 548 K while Cu octahedra enclosed with {111} facets are inactive. The Cu–Cu suboxide (CuxO, x ≥ 10) interface of Cu(100) surface is the active site on which all elementary surface reactions within the catalytic cycle proceed smoothly. However, the formate intermediate was found stable at the Cu–CuxO interface of Cu(111) surface with consequent accumulation and poisoning of the surface at low temperatures. Thereafter, Cu cubes-supported ZnO catalysts are successfully developed with extremely high activity in low-temperature WGS reaction.
Nanocrystals display a variety of facets with different catalytic activity. Here the authors identify the most active facet of copper nanocrystals relevant to the low-temperature water gas shift reaction and further design zinc oxide-copper nanocubes with exceptionally high catalytic activity.
Introduction
Catalyst nanoparticles (NPs) generally expose a variety of surface sites (e.g., facets, steps, and corners), each with their distinct reactivity. Such structural complexity seriously impairs efforts to identify their active site for developing rational design strategies in heterogeneous catalysis. The complexity of catalyst NPs has been traditionally simplified by the use of single crystals with well-defined surface structures as model catalysts1. Recently uniform catalyst nanocrystals (NCs) with tunable structures have demonstrated great potential in heterogeneous catalysis either as a novel type of model catalysts that can be studied under the same conditions as powder catalysts or as excellent candidates as efficient catalysts2–5. However, capping ligands on catalyst NCs inherited from the wet chemistry synthesis strongly affect their applications in heterogeneous catalysis, particularly in gas-solid heterogeneous catalytic reactions6–8.
The water gas shift (WGS) reaction is commonly used in the chemical industry for the production of clean H2 9. It is mildly exothermic and thermodynamically favors low reaction temperatures. Cu-based catalysts are currently used industrially for low-temperature WGS reaction. Thus it is of great importance to fundamentally understand the structure-activity relation of Cu-based catalysts for optimizing the catalyst structure. The WGS reaction were studied over Cu (100), (110), and (111) single-crystal surfaces10, 11, and the results demonstrated its structure-sensitivity.
Here, we report the identification of the most active Cu facet for low-temperature WGS reaction up to 548 K employing uniform capping ligands-free Cu NCs synthesized by a morphology-preserved reduction of corresponding uniform Cu2O NCs. Cubic Cu NCs enclosed with {100} facets are more catalytically active than dodecahedral Cu NCs enclosed with {110} facets while octahedral Cu NCs enclosed with {111} facets are inactive. The Cu–Cu suboxide (CuxO, x ≥ 10) interface of Cu(100) surface is the active site on which all elementary surface reactions within the catalytic cycle proceed smoothly, but the Cu–CuxO interface of Cu(111) surface is poisoned by an accumulation of formate intermediate stable at low reaction temperatures. Thereafter, we successfully developed Cu cubes-supported ZnO catalysts with extremely high activity in low-temperature WGS reaction.
Results
Synthesis and structures of Cu NCs
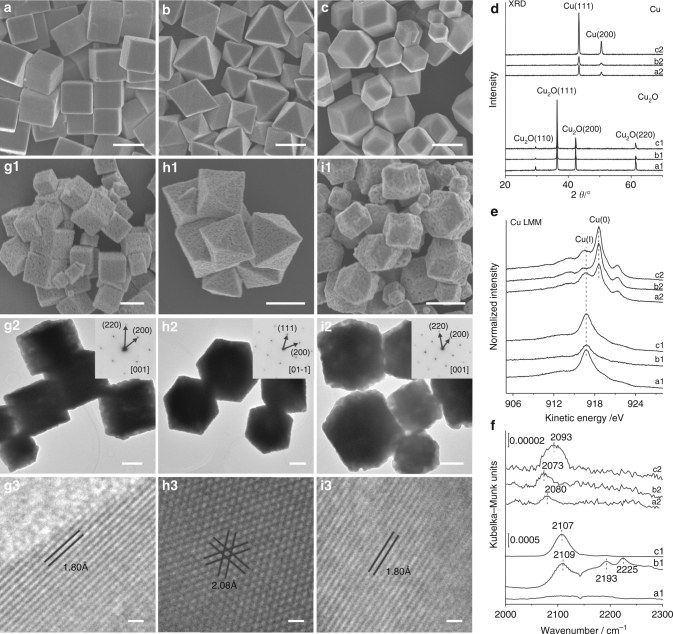
A morphology-preserved reduction strategy was firstly developed to synthesize uniform Cu NCs from corresponding Cu2O NCs. Uniform capping ligands-free Cu2O cubes (denoted as c-Cu2O), octahedra (denoted as o-Cu2O) and rhombic dodecahedra (denoted as d-Cu2O) respectively enclosed with {100}, {111} and {110} facets were synthesized following our previously established procedures12. Their structures were confirmed by both microscopic and spectroscopic characterizations (Fig. 1a–f, Supplementary Figs. 1 and 2). The size distribution of c-Cu2O, o-Cu2O, and d-Cu2O is 1000 ± 150, 1056 ± 207, and 595 ± 113 nm, respectively (Supplementary Fig. 3). According to the CO-temperature programmed reduction (CO-TPR) spectra (Supplementary Fig. 4), a reduction process in 5% CO/Ar at 548 K for 2 h was chosen to reduce Cu2O NCs. After CO reduction, the XRD patterns change from those of starting Cu2O (JCPDS card NO. 78-2076) completely into those of metallic Cu (JCPDS card NO. 89-2838) (Fig. 1d) while the scanning electron microscopy (SEM), transmission electron microscopy (TEM), and high-resolution transmission electron microscopy (HRTEM) images (Fig. 1g–i) show a well preservation of the morphologies of starting Cu2O NCs. The electron diffraction (ED) patterns confirm that all acquired Cu NCs are single crystals. Thus, uniform Cu2O cubes, octahedra, and rhombic dodecahedra can be reduced respectively into uniform Cu cubes (denoted as c-Cu), octahedra (denoted as o-Cu), and rhombic dodecahedra (denoted as d-Cu). Such a morphology-preserved reduction of Cu2O NCs into Cu NCs can be attributed to the cubic phase structures of both Cu2O and Cu, the not too large difference between the lattice constants of Cu2O (a = 426.7 pm) and Cu (a = 361.5 pm), the large sizes of Cu2O NCs, and the low reduction temperature. However, the acquired Cu NCs exhibit rough surfaces likely resulting from the lattice mismatch between Cu2O and Cu. The acquired Cu NCs are finer than the corresponding Cu2O NCs, and the size distribution of c-Cu, o-Cu, and d-Cu is 877 ± 157, 937 ± 216, and 497 ± 137 nm, respectively (Supplementary Fig. 3). Consequently, c-Cu, o-Cu, and d-Cu exhibit larger specific BET surface areas, respectively, of 1.17, 2.35, and 3.33 m2 g−1 than the corresponding c-Cu2O, o-Cu2O, and d-Cu2O, respectively, of 0.67, 1.58, and 1.98 m2 g−1. Although with a larger average edge length, the o-Cu2O and o-Cu NCs exhibit larger specific BET surface areas, respectively, than the c-Cu2O and c-Cu NCs due to the morphology and facet effects. The surface area-to-volume ratio of an octahedron and a cube can be geometrically calculated, respectively, as and 6/L (L being the edge length); meanwhile, the surface atom density of the {111} facets exposed on octahedral NCs is higher than that of the {100} facets exposed on cubic NCs for Cu2O and Cu with cubic phases.
Fig. 1.
Structural characterizations. The scale bars of a–c and (g1–i1) correspond to 1000 nm, that of (g2–i2) correspond to 500 nm, and that of (g3–h3) correspond to 2 nm. Representative SEM images of a Cu2O cubes, b Cu2O octahedral, and c Cu2O rhombic dodecahedra. d XRD patterns, e Cu LMM AES spectra measured without exposure to air, and f in situ DRIFTS spectra of CO adsorption at 123 K of Cu2O cubes (a1), octahedra (b1), rhombic dodecahedra (c1) and Cu cubes (a2), octahedra (b2) and rhombic dodecahedra (c2). Representative SEM, TEM and HRTEM images of (g1–g3) Cu cubes, (h1–h3) Cu octahedra and (i1–i3) Cu rhombic dodecahedra. The insets in the HRTEM images show the ED patterns of corresponding Cu nanocrystals. The lattice fringes of 1.80 and 2.08 Å, respectively, correspond to the spacing of Cu{200} and {111} crystal planes (JCPDS card NO. 89-2838)
The acquired Cu NCs were characterized by x-ray photoelectron spectroscopy (XPS) without exposure to air. The Cu LMM Augur electron spectra (AES) (Fig. 1e) and Cu 2p XPS spectra (Supplementary Fig. 5) demonstrate dominance of metallic Cu on surfaces of all acquired Cu NCs; however, the Cu(I) feature is still visible. In the corresponding O 1 s and C 1 s XPS spectra (Supplementary Fig. 5), all Cu NCs exhibit three O 1 s components at 529.5, 530.3, and 531.8 eV and three C 1 s components at 284.8, 289.0, and 287.7 eV while o-Cu NCs exhibit an additional minor C 1 s component at 286.6 eV. The O 1 s components at 529.5 and 531.8 eV could be respectively assigned to oxygen adatoms and hydroxyl groups/oxygenates species while the O 1 s component at 530.3 eV was observed to vary simultaneously with the Cu(I) feature for all acquired Cu NCs and, thus, could be assigned to Cu suboxide (CuxO, x ≥ 10)13, 14. The C 1 s components at 284.8, 289.0, 287.7, and 286.6 eV could be assigned respectively to adventitious carbon, carbonate, carboxylate and formate species15. In situ diffuse reflectance infrared Fourier transformed spectra (DRIFTS) of the reduction processes of Cu2O NCs into Cu NCs (Supplementary Fig. 6) show significant attenuations of Cu2O vibrational features (652, 794, and 1123 cm−1)16 and formations of carboxylate (1277 cm−1) and carbonate (1235, 1455 and 1506 cm−1) species on all Cu NCs and formate species (1588 cm−1)17, 18 exclusively on o-Cu NCs, in consistence with the above XPS results. All these observed surface species on acquired Cu NCs are common on metal powder catalysts.
Figure 1f compares in situ DRIFTS spectra of CO adsorption on Cu2O and Cu NCs at 123 K. Agreeing with previous reports12, 19–21, a vibrational band at ∼2108 cm−1 was observed on o-Cu2O and d-Cu2O and assigned to CO-adsorbed at the Cu(I) sites exposed on {111} and {110} facets while no feature was observed on c-Cu2O enclosed with O-terminated Cu2O (100) facets. Additional vibrational bands at ∼2193 and 2225 cm−1 were observed on o-Cu2O and assigned to CO2 adsorbed at the coordination-unsaturated Cu(I) sites of {111} facets formed by the reaction of CO with adsorbed oxygen species12, 19. Vibrational bands of CO adsorbed on Cu NCs are diffuse and weak, and depend on their morphologies. It is located at 2080 cm−1 on c-Cu, 2073 cm−1 on o-Cu, and 2093 cm−1 on d-Cu that respectively correspond well to that of CO adsorbed on Cu (100), (111), and (110) single-crystal surfaces22–25. No feature of CO adsorbed at the Cu(I) site could be observed for all Cu NCs, indicating that CuxO on Cu NCs is O-terminated. Absence of vibrational features above 2100 cm−1 corresponding to CO adsorbed at stepped sites and defects on Cu surfaces26 suggests low densities of stepped sites and defects on the surfaces of all acquired Cu NCs. These CO adsorption results demonstrate that although with rough surfaces, uniform c-Cu, o-Cu, and d-Cu NCs dominantly expose {100}, {111}, and {110} terrace Cu sites on the surfaces, respectively. Therefore, the above comprehensive characterization results demonstrate a successful synthesis of uniform c-Cu, o-Cu, and d-Cu NCs respectively with well-defined {100}, {111}, and {110} surfaces via a morphology-preserved reduction of corresponding c-Cu2O, o-Cu2O, and d-Cu2O NCs.
Effect of Cu facet on activity
Various types of Cu NCs exhibit morphology-dependent catalytic activity in the WGS reaction. CO conversion over the c-Cu and d-Cu NCs was observed, respectively, at 473 and 498 K but not for the o-Cu NCs up to 548 K (Supplementary Fig. 7). Catalytic activity of commercial Cu/ZnO/Al2O3 catalyst in the WGS reaction was also evaluated (Supplementary Fig. 8). On the basis of surface Cu atoms derived from the specific surface areas of c-Cu and d-Cu NCs and the Cu atom densities of Cu (100) and (110) surfaces, the calculated surface Cu atom-specific reaction rates of c-Cu NCs are much higher than those of d-Cu NCs (Fig. 2a). The c-Cu and d-Cu NCs are also catalytically stable at 548 K (Fig. 2b and Supplementary Fig. 7). SEM images (Supplementary Fig. 9) demonstrate that the Cu NCs preserve their morphologies after the activity evaluation up to 548 K. Results of a Weisz–Prater analysis and a Mears analysis27 (Supplementary Note 1, 2, and 3) show the absence of mass and heat transfer limitations under our reaction conditions. Thus the Arrhenius plots (Fig. 2c) were made to calculate the apparent activation energy (E a) of WGS reaction catalyzed by c-Cu, d-Cu, and Cu/ZnO/Al2O3, respectively, as 54.1 ± 3.1, 68.4 ± 8.0, and 51.6 ± 3.7 kJ mol−1. Therefore, employing various types of Cu NCs, we demonstrate an obvious facet effect of Cu particles in low-temperature WGS reaction with the Cu{100} facet as the most active facet.
Fig. 2.
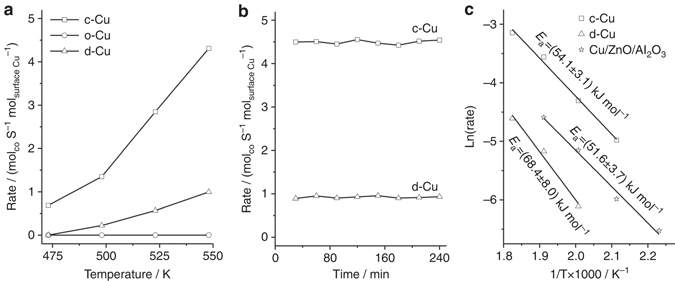
Catalytic performance. a Reaction rate (molCO s−1 molsurface Cu −1) of Cu cubes (c-Cu), octahedra (o-Cu), and rhombic dodecahedra (d-Cu) in the WGS reaction as a function of reaction temperature. b Reaction rate of Cu cubes (c-Cu) and rhombic dodecahedra (d-Cu) at 548 K in the WGS reaction as a function of reaction time. c Arrhenius plots of Cu cubes (c-Cu) and rhombic dodecahedra (d-Cu) and commercial Cu/ZnO/Al2O3 catalyst in the WGS reaction
It is noteworthy that the E a value of Cu/ZnO/Al2O3 is similar to the previously reported value (53 kJ mol−1)28 while the E a values of c-Cu and d-Cu NCs differ respectively from those of Cu (100) (63.5 kJ mol−1)11 and Cu (110) (41.8 kJ mol−1)10 single-crystal surfaces. Moreover, the Cu(111) single-crystal surface was reported to exhibit an E a of 71.1 kJ mol−1 in WGS reaction10 while o-Cu NCs are inactive under our reaction condition. Catalytic activity of Cu single-crystal surfaces were evaluated under the very different reaction conditions10, 11 from ours, for example, the adopted temperatures were between 573 and 673 K. We also evaluated catalytic activity of c-Cu and o-Cu in WGS reaction up to 673 K (Supplementary Fig. 10). o-Cu becomes active at 623 K, and c-Cu exhibits CO conversions that increase with the reaction temperature up to 623 K but then decrease. The results of recycling measurements of catalytic activity and SEM images of Cu NCs after the activity evaluation clearly demonstrate that both Cu NCs are not stable and undergo obvious morphological changes during the activity evaluation up to 673 K.
In situ restructuring of Cu2O NCs into Cu NCs
We found that, when directly used as catalysts for WGS reaction, various types of Cu2O NCs exhibited the steady-state catalytic activity and calculated apparent activation energy almost identical to those of corresponding Cu NCs (Supplementary Fig. 11). Structural characterization results (Supplementary Figs. 12–14) demonstrate that Cu2O NCs undergo an in situ morphology-preserved reduction into corresponding Cu NCs with the presence of both Cu(0) and Cu(I) on their surfaces during WGS reaction up to 548 K. These results demonstrate that Cu NCs with co-existing Cu(0) and Cu(I) species on the surfaces are active in catalyzing low-temperature WGS reaction and further support that the Cu{100} facet is the most active facet.
Reaction mechanism
Figure 3a presents temperature-programmed reaction spectra of WGS reaction over Cu NCs. The CO2 production was observed to occur prior to the H2 production, suggesting the H2 production as the rate-limiting step in the Cu-catalyzed WGS reaction. Both CO2 and H2 productions proceed most facilely over c-Cu, in consistence with its highest catalytic activity. o-Cu exhibits a higher initial CO2 production than d-Cu, but a much more difficult H2 production. With the WGS reaction proceeding at 548 K, c-Cu and d-Cu capable of catalyzing both CO2 and H2 productions exhibit stable activity, respectively, while o-Cu capable of catalyzing the CO2 production but few H2 production gets gradually poisoned. These observations suggest that the inactivity of o-Cu in WGS reaction up to 548 K should result from self-poisoning due to the formation and accumulation of stable hydrogen-containing intermediates on the surface.
Fig. 3.
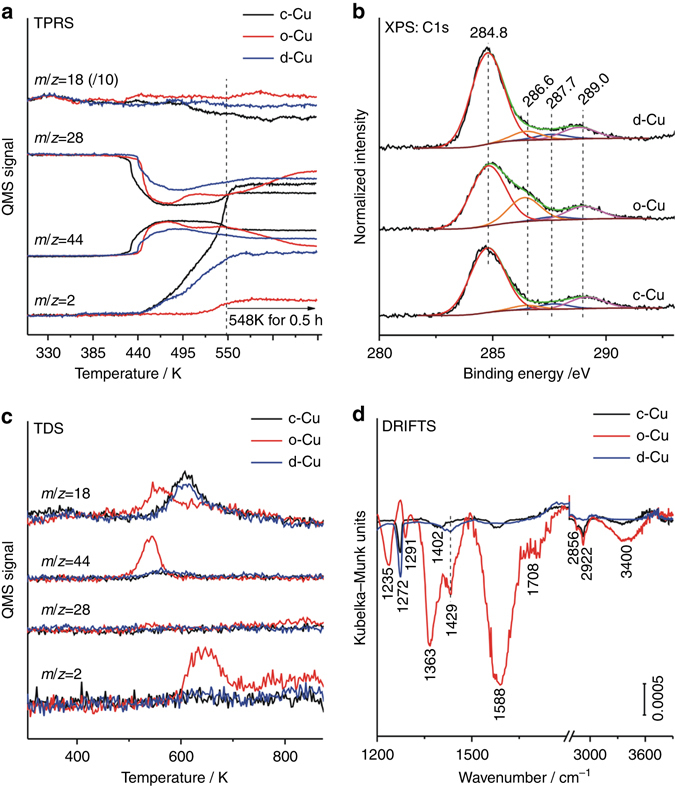
Reaction mechanism. a Temperature-programmed reaction spectra of WGS reaction over Cu cubes (c-Cu), octahedra (o-Cu), and rhombic dodecahedra (d-Cu). b C 1 s XPS spectra of Cu cubes (c-Cu), octahedra (o-Cu), and rhombic dodecahedra (d-Cu) after WGS reaction at 548 K measured without exposure to air. c Thermal desorption spectra of Cu cubes (c-Cu), octahedra (o-Cu), and rhombic dodecahedra (d-Cu) after WGS reaction at 548 K. d In situ diffuse reflectance infrared spectra of Cu cubes (c-Cu), octahedra (o-Cu), and rhombic dodecahedra (d-Cu) after WGS reaction at 548 K followed by heating in Ar to 723 K
Various used Cu NCs catalysts after WGS reaction at 548 K were characterized by XPS without exposure to air. As shown in the C 1 s XPS spectra (Fig. 3b), in addition to the C 1 s components of adventitious carbon (284.8 eV), carboxylate (287.7 eV), and carbonate (289.0 eV), the C 1 s component of formate species (286.6 eV) absent on the fresh c-Cu and d-Cu catalysts appears on the used c-Cu and d-Cu catalysts after the WGS reaction and its intensity greatly increases on the used o-Cu catalyst as compared to that on the fresh o-Cu catalyst. In the corresponding Cu LMM AES spectra, Cu 2p, and O 1 s XPS spectra (Supplementary Fig. 15), no Cu(II) XPS feature appears on all used Cu catalysts and the Cu LMM AES spectra and O 1 s XPS spectra of the used o-Cu catalyst change very slightly, but the metallic Cu LMM AES component increases at the expense of the Cu(I) LMM AES component on the used c-Cu and d-Cu catalysts, and accordingly, the O 1 s components of oxygen adatoms (529.5 eV) and hydroxyl groups/oxygenates species (531.8 eV) increase at the expense of the O 1 s component of the CuxO species (530.3 eV). Thus, during the WGS reaction up to 548 K, the copper speciation does not vary much on the inactive o-Cu catalyst surface but an accumulation of formate species obviously occurs, and the transformation of CuxO species into the Cu(0) and oxygen adatoms, together with the formation of hydroxyl groups and oxygenates species (including the formate species), occurs on the active c-Cu and d-Cu surfaces.
The surface species on various used Cu NCs catalysts after WGS reaction at 548 K were further probed by temperature-programmed desorption spectra (TDS) in Ar and in situ DRIFTS spectra. In the TDS spectra in Ar (Fig. 3c), the used c-Cu and d-Cu catalysts exhibit a weak and broad CO2 desorption peak at 560 K and a strong H2O desorption peak at 610 K while the used o-Cu catalyst exhibits strong CO2 and H2O desorption peaks simultaneously at 540 K and H2, and H2O desorption peaks simultaneously at 640 K. In the corresponding in situ DRIFTS spectra (Fig. 3d), the used c-Cu and d-Cu catalysts exhibit weak attenuation of vibrational peaks of carboxylate (1272–1291 cm−1), carbonate (1235 and 1402–1429 cm−1), formate (1363, 1588, 2856, and 2922 cm−1), and hydroxyl (1708 and 3400 cm−1) species17, 18 while the used o-Cu catalyst exhibits strong loss of vibrational peaks of formate and hydroxyl species and weak attenuation of vibrational peaks of carboxylate and carbonate species. Therefore, upon heating, the reactions of carboxylate, carbonate and formate species on the used c-Cu and d-Cu catalysts produce minor CO2 at 560 K and the hydroxyl groups react each other to predominantly produce water at 610 K while the reaction between co-adsorbed formate species and hydroxyl group on the used o-Cu catalyst produce much CO2 and H2O at 540 K29 and the hydroxyl groups react each other to simultaneously produce H2 and H2O desorption peaks at 640 K.
The above comprehensive characterization results demonstrate accumulations of formate and hydroxyl intermediates during the WGS reaction up to 548 K on the o-Cu surface but not on the c-Cu and d-Cu surfaces that should lead to the self-poisoning and subsequent inactivity of o-Cu NCs. It is noteworthy that the surface reaction between co-adsorbed formate and hydroxyl to produce CO2 and H2O at 540 K observed on the used o-Cu catalyst should be thermodynamically quenched during WGS reaction with H2O as a reactant. Meanwhile, the accumulation of formate and hydroxyl intermediates on the o-Cu surface could be alleviated at higher reaction temperatures, as the o-Cu NCs are active in catalyzing the WGS reaction at 623 K and above.
Activation of H2O on various Cu NCs, a key step in WGS reaction, was studied by XPS without exposure to air (Fig. 4, Supplementary Figs. 16, 17, and Supplementary Table 1). H2O dissociation was observed to occur on the c-Cu and d-Cu surfaces at 423 K but barely on the o-Cu surface, resulting in the growth of both hydroxyl group and oxygen adatoms at the expense of CuxO. This suggests the Cu–CuxO interface as the active site for the H2O dissociation into the hydroxyl group accompanied by the decomposition of CuxO into oxygen adatoms. In the corresponding Cu LMM AES spectra, the decomposition of CuxO on the c-Cu and d-Cu surfaces at 423 K is also evidenced by the growth of Cu(0) at the expense of Cu(I), and this process is more extensive on the c-Cu surface than on the d-Cu surface. H2O dissociates on all Cu NCs at 523 K, leading to the growth of oxygen adatoms and CuxO at the expense of hydroxyl group, indicating the decomposition of hydroxyl group into oxygen adatom that can further transform into CuxO.
Fig. 4.
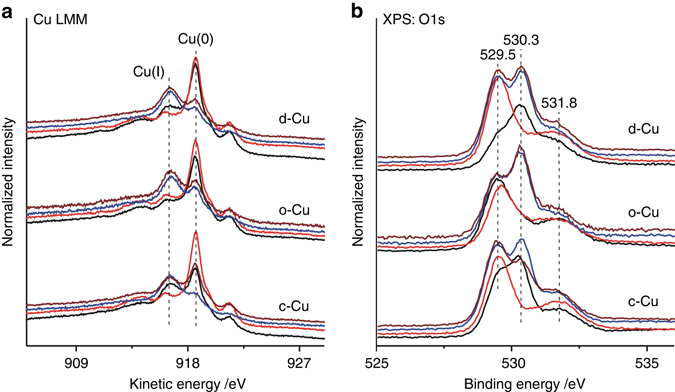
Water activation. a Cu LMM AES and b O 1 s XPS spectra of various Cu NCs (black lines), Cu NCs exposed to H2O at 423 K (red lines), Cu NCs exposed to H2O at 523 K (blue lines) and Cu NCs exposed to H2O at 523 K and then to CO at 473 K (brown lines). All spectra were measured without exposure to air
The Cu NCs subjected to H2O dissociation at 523 K were then exposed to CO at 473 K. Both Cu LMM AES spectra and O 1 s XPS spectra (Fig. 4, Supplementary Fig. 17 and Supplementary Table 1) demonstrate a reaction of CuxO with CO on the c-Cu surface, but not on the d-Cu and o-Cu surfaces. Thus CuxO on the Cu surface is an active oxygen species for the CO2 production during WGS reaction while the co-existing oxygen adatoms serve as an oxygen reservoir for the formation and decomposition of CuxO. Since CO adsorbs on the metallic Cu surface instead of the CuxO surface (Fig. 1f), the reaction of CuxO with CO on the c-Cu surface should proceed at the Cu–CuxO interface. The reactivity of CuxO at the Cu–CuxO interface toward CO is higher on the c-Cu surface than on the d-Cu and o-Cu surfaces.
Therefore, the Cu–CuxO interface on the Cu surface capable of both dissociating water and reacting with CO should be the active site to catalyze the WGS reaction. The Cu–CuxO interface on the c-Cu surface is more active in both water dissociation and CO2 production than that on the d-Cu and o-Cu surfaces, consequently, the c-Cu NCs is more catalytic active in the WGS reaction than the d-Cu and o-Cu Cu NCs.
The mechanisms of morphology-dependent catalytic performance of Cu NCs in WGS reaction were further explored by DFT calculations employing Cu(100) and Cu(111) surfaces with Cu2O islands to respectively model the c-Cu and o-Cu surfaces with CuxO (Fig. 5a, b). According to previous results30–33, ring-like structures were adopted for Cu2O islands on both Cu(100) and Cu(111) surfaces. This gives a zig-zag chain structure of Cu2O at the Cu–Cu2O interface that was used during simulations of reactions at the Cu–Cu2O interface. In consistence with previous reports34, 35, the dissociation of water on Cu(100) and Cu(111) into OH group and H adatom needs to overcome barriers respectively of 1.23 and 1.36 eV, and the further dissociation of OH group into O and H adatoms on Cu(100) and Cu(111) needs to overcome barriers respectively of 1.68 and 1.70 eV (Fig. 5c, Supplementary Figs. 18, 19 and Supplementary Table 2); however, the dissociation of water at the Cu–Cu2O interface on Cu(100) and Cu(111) into a OH group on Cu surface (OHCu) and a H adatom at the O of Cu2O (OCu2OH) proceeds facilely with barriers respectively of 0.40 and 0.58 eV, and the H of OCu2OH can migrate to the neighboring Cu atom to form a H adatom on the Cu surface (OCu2OH → OCu2O + HCu) with activation energies of 1.09 and 0.98 eV at the Cu–Cu2O interface respectively on Cu(100) and Cu(111) surfaces. Thus the water activation is much more facile at the Cu2O–Cu interface than at the Cu surface, and the water dissociation at the Cu–Cu2O interface into OHCu and OCu2OH should proceed more facilely on Cu(100) than on Cu(111). These agree with the above experimental observations (Fig. 4).
Fig. 5.
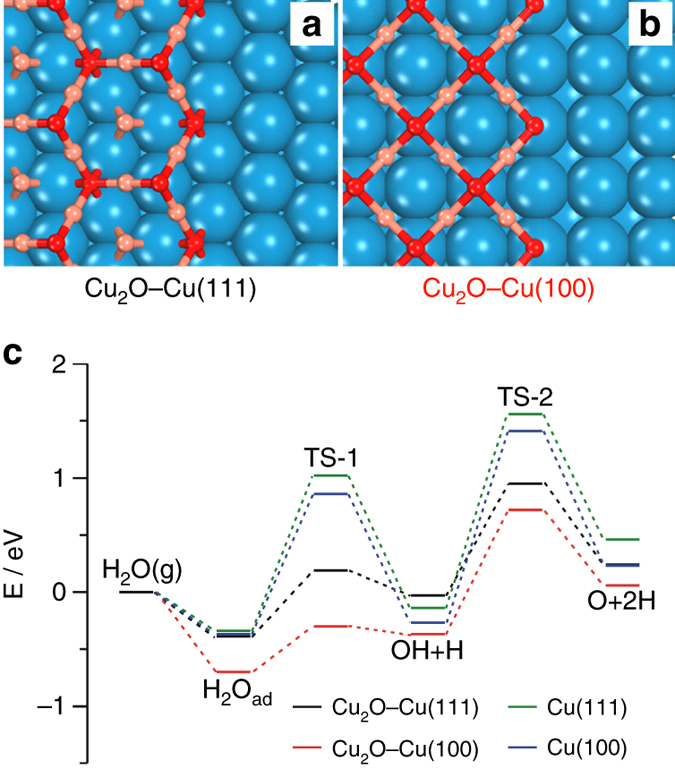
DFT calculations. Schematic a Cu2O–Cu(111) and b Cu2O–Cu(100) interface structures. Blue, orange, and red balls represent Cu, Cu of Cu2O, and O atoms, respectively. c Calculated energy diagram of water adsorption and decomposition on Cu2O–Cu(111), Cu2O–Cu(100), Cu(111), and Cu(100) surfaces
Following the water dissociation, CO adsorbed at the Cu site (COCu) can react with O of Cu2O at the Cu–Cu2O interface to complete the WGS reaction cycle via the redox mechanism (Fig. 6a, Supplementary Figs. 18, 20 and Supplementary Table 3); COCu can also react either with OCu2OH to produce COOHCu2O (Fig. 6b, Supplementary Figs. 18, 21 and Supplementary Table 4) or with OHCu to produce COOHCu (Fig. 6c, Supplementary Figs. 18, 22 and Supplementary Table 5) to complete the WGS reaction cycle via the associative mechanism. All three pathways can proceed at the Cu–Cu2O interface of Cu(100) and Cu(111) surfaces with similar largest activation energies of around 1 eV. In all three pathways the elementary surface reaction with the largest activation energy is the reaction of OCu2OH → OCu2O + HCu involved for the H2 production. This agrees with the experimental observations that the H2 production is the rate-limiting step in the Cu-catalyzed WGS reaction (Fig. 3a).
Fig. 6.
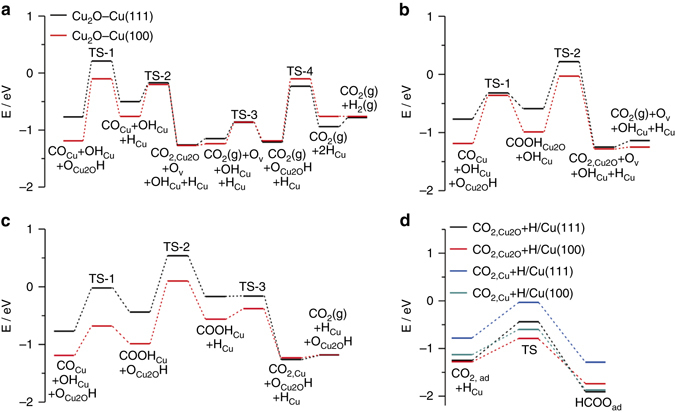
Calculated energy diagram of WGS reaction. a The reaction for CO at the Cu site (COCu) and O of Cu2O at Cu2O–Cu(111) (black line) and Cu2O–Cu(100) (red line) interfaces. Ov represents an oxygen vacancy in Cu2O. b The reaction for COCu and OCu2OH at the Cu2O–Cu(111) (black line) and Cu2O–Cu(100) (red line) interfaces. c The reaction for COCu and OHCu at the Cu2O–Cu(111) (black line) and Cu2O–Cu(100) (red line) interfaces; the subsequent elementary reactions following the formation of CO2(g) + Ov + OHCu + HCu are identical to those in a. d The elementary reaction between CO2,ad and HCu to form HCOOad at the Cu2O–Cu(111) and Cu2O–Cu(100) interfaces
Besides producing H2 and CO2, the formed HCu and CO2,ad at the Cu–Cu2O interface can facilely react to form the formate species on both Cu(111) and Cu(100) surfaces (Fig. 6d, Supplementary Figs. 18, 23 and Supplementary Tables 3–5), in consistence with previous reports34. The barriers of the decomposition of HCOOCu species into CO2,Cu + HCu at the Cu–Cu2O interfaces of Cu(111) and Cu(100) surfaces are similar, suggesting their similar stabilities; however, the barrier of the decomposition of HCOOCu2O species into CO2,Cu2O + HCu is respectively 1.47 and 0.95 eV at the Cu–Cu2O interfaces of Cu(111) and Cu(100) surfaces, suggesting a much higher stability of HCOOCu2O species on Cu(111) than on Cu(100). Thus, it can be expected that the formate species will accumulate at the Cu–Cu2O interface of Cu(111) surface due to the large barrier for HCOOCu2O decomposition during low-temperature WGS reaction but not at the Cu–Cu2O interface of Cu(100) surface, which will eventually block the active Cu–Cu2O interface of Cu(111) surface. This is consistent with the experimental observations that the c-Cu catalyst is active in catalyzing the low-temperature WGS reaction up to 548 K while the o-Cu catalyst is inactive and with accumulated formate species on the surface (Figs. 2 and 3).
Therefore, our comprehensive experimental and theoretical calculation results clearly demonstrate a facet-dependent catalytic performance of Cu catalysts in low-temperature WGS reaction in which Cu{100} is the most active facet with the Cu–Cu suboxide interface as the active site. Interestingly, such a facet-dependent catalytic performance results mainly from a facet-dependent surface poisoning of the active site instead of from a facet-dependent intrinsic activity. All elementary surface reactions within the catalytic cycle can proceed smoothly at the Cu–CuxO interface of active Cu{100} facets during low-temperature WGS reaction; however, the Cu–CuxO interface of Cu{111} facets initially is also active, but will be soon self-poisoned by the accumulation of stable formate intermediate. These results nicely demonstrate a key feature of an active site of solid catalysts that an active site must be able to recycle during catalytic reactions.
Design and realization of highly efficient ZnO/c-Cu catalyst
The WGS reaction is mildly exothermic and thermodynamically favors low reaction temperatures. The above fundamental understandings of facet-dependent catalytic performance of Cu catalysts reveal a strategy of designing efficient copper-based catalysts for low-temperature WGS reaction using c-Cu NCs. Cu–ZnO-based catalysts are commercial catalysts for WGS reaction. A c-Cu2O NCs-supported ZnO catalyst with a ZnO weight ratio of 1% (1%wt-ZnO/c-Cu2O) was thus synthesized and then reduced to acquire a c-Cu NCs-supported ZnO catalyst with a ZnO weight ratio of 1.67% (1.67%wt-ZnO/c-Cu) (Fig. 7a–d and Supplementary Fig. 24). Similarly, an o-Cu2O NCs-supported ZnO catalyst (1%wt-ZnO/o-Cu2O) was also synthesized and then reduced to acquire an o-Cu NCs-supported ZnO catalyst (1.67%wt-ZnO/o-Cu) (Supplementary Figs. 24 and 25). It can be seen that both Cu(0) and Cu(I) exist on the surface of Cu NCs in ZnO/Cu catalysts. The ZnO/Cu NCs catalysts are much more active than the corresponding Cu NCs catalysts in catalyzing the WGS reaction (Fig. 7e and Supplementary Fig. 25e), and the calculated apparent activation energy of ZnO/c-Cu (32.4 ± 0.8 kJ mol−1) (Fig. 7f) and ZnO/o-Cu (55.9 ± 3.9 kJ mol−1) (Supplementary Fig. 25f) catalysts is much smaller than that of the corresponding Cu NCs catalysts. These results suggest that the copper-ZnO interface in ZnO/Cu NCs catalysts exhibits much higher intrinsic activity than the Cu–CuxO interface in Cu NCs catalysts. However, the detailed mechanism needs further study. Meanwhile, the ZnO/c-Cu catalyst is stable (Supplementary Fig. 26).
Fig. 7.
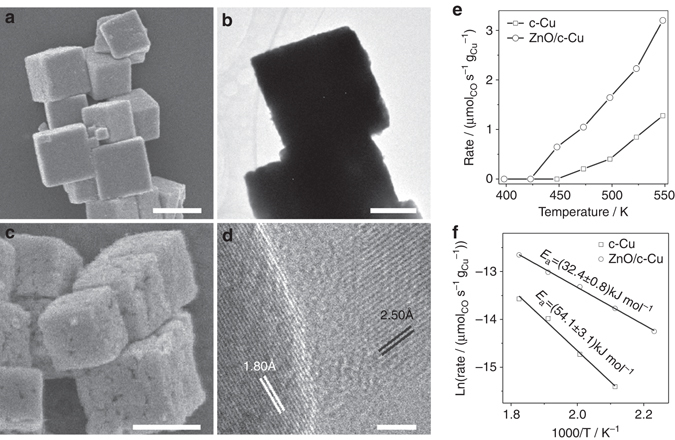
ZnO/c-Cu catalyst. The scale bars of a, b correspond to 1000 nm, that of c correspond to 500 nm, and that of d correspond to 2 nm. a Representative SEM images of 1%wt-ZnO/c-Cu2O; b representative SEM, c TEM, d HRTEM images of 1.67%wt-ZnO/c-Cu catalysts; e Reaction rate (μmolCO s−1 gCu −1) of 1.67%wt-ZnO/c-Cu catalyst in the WGS reaction as a function of reaction temperature and f Arrhenius plot of 1.67%wt-ZnO/c-Cu in the WGS reaction. The data of c-Cu NCs are included for comparisons. The data of c-Cu NCs are included for comparisons. The lattice fringes of 1.80 and 2.50 Å, respectively, correspond to the spacing of Cu{200} (JCPDS card NO. 89-2838) and hexagonal ZnO{101} (JCPDS card NO 89-1397) crystal planes
The apparent activation energy of ZnO/c-Cu catalyst is smaller than those of commercial Cu/ZnO/Al2O3 catalyst, Cu/ZnO(0001) (52 kJ mol−1)11, and ZnO/Cu (46 kJ mol−1)36 model catalysts, and is similar to those acquired on CeOx/Cu(111) (30 kJ mol−1)37 and Cu/CeO2(111) (37 kJ mol−1)11 model catalysts. It is noteworthy that the activity of CeOx/Cu(111) model catalyst was evaluated between 573 and 673 K37 under which the Cu(111) surface might restructure. Meanwhile, no Cu–CeO2 and Cu–ZnO powder catalysts was reported to exhibit Ea in WGS reaction as low as the CeOx/Cu(111) and Cu/CeO2(111) model catalysts, suggesting the presence of so-called materials gap. The ZnO/o-Cu catalyst exhibits a similar apparent activation energy to the commercial Cu/ZnO/Al2O3 catalyst but much larger than that of ZnO/c-Cu catalyst. Therefore, the active structure of ZnO/c-Cu catalyst should be intrinsically more active than those of ZnO/o-Cu and commercial Cu/ZnO/Al2O3 catalysts. This demonstrates a key role of the Cu structure in the Cu–ZnO-based catalysts in determining the catalytic activity in WGS reaction, in which the ZnO/c-Cu catalyst with the Cu{100} structures is highly efficient. Meanwhile, it could also be inferred that the copper structure of commercial Cu/ZnO/Al2O3 catalyst should be dominated by the Cu{111} structures and its catalytic activity can be improved by engineering the Cu structure from the dominant Cu{111} structure into the Cu{100} structure. These findings demonstrate a successful experimental strategy of using catalyst NCs that realizes an all-chain investigation of heterogeneous catalysis from the fundamental understanding of active site and reaction mechanism to the structural design and realization of highly efficient catalysts.
Methods
Materials
All chemicals were purchased from Sinopharm Chemical Reagent Co. Ltd. and used without further purification. Commercial Cu/ZnO/Al2O3 WGS catalyst was purchased from Alfa Aesar Chemical Co. Ltd. 5% CO/Ar, 0.432% CO/Ar, Ar (99.999%), CO (99.99%), C3H6 (99.95%), O2 (99.999%), and N2 (99.999%) were purchased from Nanjing Shangyuan Industrial Factory and used without further purification. ultrapure water (>18.5 MΩ) was used.
Synthesis
Synthesis of cubic and octahedral Cu2O NCs followed the procedure reported by Zhang et al38. Typically, NaOH aqueous solution (2.0 mol L−1; 10 mL) was added dropwise into CuCl2 aqueous solution (0.01 mol L−1; 100 mL) at 328 K (c-Cu2O without poly(vinylpyrrolidone) (PVP), o-Cu2O containing 4.44 g PVP (M w = 30 000)). After stirring for 0.5 h, ascorbic acid solution (0.6 mol L−1; 10 mL) was added dropwise. The resulting solution was stirred at 328 K for 5 and 3 h to synthesize cubic and octahedra Cu2O NCs, respectively. The resulting precipitate was collected by centrifugation, decanted by repeated washing with distilled water and absolute ethanol, and finally dried under vacuum at RT for 12 h. Synthesis of rhombic dodecahedral Cu2O followed the procedure by Liang et al.39. Typically, 4 mL oleic acid (OA) and 20 mL absolute ethanol were added successively into 40 mL CuSO4 aqueous solution (0.025 mol L−1) under vigorous stirring. The solution was heated to 373 K and then 10 mL NaOH aqueous solution (0.8 mol L−1) was added. After stirring for 5 min, 30 mL d-(+)-glucose aqueous solution (0.63 mol L−1) was added. The resulting mixture was stirred at 373 K for additional 1 h and gradually turned into a brick-red color. The resulting precipitate was collected by centrifugation, decanted by repeated washing with distilled water and absolute ethanol, and finally dried in vacuum at room temperature (RT) for 12 h. The acquired cubic, octahedral, and rhombic dodecahedral Cu2O NCs were denoted as c-Cu2O, o-Cu2O-PVP, and d-Cu2O-OA, respectively.
Capping ligands on as-synthesized o-Cu2O-PVP and d-Cu2O-OA NCs were removed following a controlled oxidation procedure developed by Hua et al.12. Typically, Cu2O NCs (0.2 g) were placed in a U-shaped quartz microreactor and purged in the stream of C3H6 + O2 + N2 gas mixture (C3H6:O2:N2 = 2:1:22, flow rate: 50 mL min−1) at room temperature for 0.5 h, and then heated to the desirable temperature (o-Cu2O-PVP: 473 K; d-Cu2O-OA: 488 K) at a rate of 5 K min−1 and kept for 0.5 h, then the stream was switched to pure Ar (flow rate: 30 mL min−1) in which the sample was cooled down to room temperature. The acquired octahedral and rhombic dodecahedral Cu2O NCs without capping ligands were denoted as o-Cu2O and d-Cu2O, respectively.
Cu NCs with various morphologies were synthesized via a morphology-preserved reduction method. Cu2O NCs (0.1 g) were placed in a quartz microreactor and purged in the stream of 5% CO balanced with Ar (flow rate: 30 mL min−1) at room temperature for 0.5 h, and then heated to 548 K at a rate of 1 K min−1 and kept for 2 h, then cooled down to room temperature. The acquired cubic, octahedral and rhombic dodecahedral Cu NCs were denoted as c-Cu, o-Cu, and d-Cu, respectively.
Cu2O NCs-supported ZnO catalysts (ZnO/Cu2O) were synthesized by incipient wetness impregnation method. Typically, Cu2O NCs (0.2 g) were adequately incipient wetness impregnated with 200 μL aqueous solution containing calculated amounts of zinc nitrate (Zn(NO3)2·6H2O), and the resulting mixture was dried under vacuum at room temperature overnight and then heated at 623 K for 2 h in high-pure Ar with a flow of 30 mL min−1. The acquired ZnO/Cu2O catalysts were then reduced in 5% CO/Ar with a flow of 30 mL min−1 to 423 K at a rate of 1 K min−1 and kept for 2 h to prepare corresponding ZnO/Cu catalysts that were stored in vacuum oven.
Details on structural characterizations, catalytic activity evaluation, and DFT calculations can be found in the Supplementary Methods.
Data availability
All data are available from the authors on reasonable request.
Electronic supplementary material
Acknowledgements
This work was financially supported by National Basic Research Program of Ministry of Science and Technology of China (2013CB933104, 2013CB834603), National Natural Science Foundation of China (21525313, 21173204, U1332113, 91645202), Fundamental Research Funds for the Central Universities of Ministry of Education of China (WK2060030017), Hefei Science Center of Chinese Academy of Sciences (2015HSC-UP014), and Collaborative Innovation Center of Suzhou Nano Science and Technology.
Author contributions
W.H. designed and supervised the project. W.-X.L. supervised the DFT calculations. Z.Z. carried out all experiments. S.-S.W. carried out all DFT calculations. R.S., T.C., L.L., Y.G., X.C., and J.L. assisted with the experiments. W.H. and Z.Z. analyzed all the results. S.-S.W. and W.-X.L. analyzed the DFT calculation results. W.H., Z.Z., S.-S.W. and W.-X.L. prepared the manuscript and other authors commented on the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Zhenhua Zhang and Sha-Sha Wang contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-00620-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ertl G. Reactions at surfaces: from atoms to complexity (Nobel lecture) Angew. Chem. Int. Ed. 2008;47:3524–3535. doi: 10.1002/anie.200800480. [DOI] [PubMed] [Google Scholar]
- 2.Bell AT. The impact of nanoscience on heterogeneous catalysis. Science. 2003;299:1688–1691. doi: 10.1126/science.1083671. [DOI] [PubMed] [Google Scholar]
- 3.Somorjai GA, Frei H, Park HY. Advancing the frontiers in nanocatalysis, biointerfaces, and renewable energy conversion by innovations of surface techniques. J. Am. Chem. Soc. 2009;131:16589–16605. doi: 10.1021/ja9061954. [DOI] [PubMed] [Google Scholar]
- 4.Zhou K, Li Y. Catalysis based on nanocrystals with well-defined facets. Angew. Chem. Int. Ed. 2012;51:602–613. doi: 10.1002/anie.201102619. [DOI] [PubMed] [Google Scholar]
- 5.Huang W. Oxide nanocrystal model catalysts. Acc. Chem. Res. 2016;49:520–527. doi: 10.1021/acs.accounts.5b00537. [DOI] [PubMed] [Google Scholar]
- 6.Niu Z, Li Y. Removal and utilization of capping agents in nanocatalysis. Chem. Mater. 2014;26:72–83. doi: 10.1021/cm4022479. [DOI] [Google Scholar]
- 7.Huang W, Hua Q, Cao T. Influence and removal of capping ligands on catalytic colloidal nanoparticles. Catal. Lett. 2014;144:1355–1369. doi: 10.1007/s10562-014-1306-5. [DOI] [Google Scholar]
- 8.Wu Z, et al. Thiolate ligands as a double-edged sword for CO oxidation on CeO2 supported Au25(SCH2CH2Ph)18 nanoclusters. J. Am. Chem. Soc. 2014;136:6111–6122. doi: 10.1021/ja5018706. [DOI] [PubMed] [Google Scholar]
- 9.Ratnasamy C, Wagner JP. Water gas shift catalysis. Catal. Rev. 2009;51:325–440. doi: 10.1080/01614940903048661. [DOI] [Google Scholar]
- 10.Nakamura J, Campbell JM, Campbell CT. Kinetics and mechanism of the water-gas shift reaction catalyzed by the clean and Cs-promoted Cu (110) surface: a comparison with Cu (111) J. Chem. Soc. Faraday Trans. 1990;86:2725–2734. doi: 10.1039/ft9908602725. [DOI] [Google Scholar]
- 11.Rodriguez JA, Liu P, Hrbek J, Evans J, Pérez M. Water gas shift reaction on Cu and Au nanoparticles supported on CeO2 (111) and ZnO (000-1): intrinsic activity and importance of support interactions. Angew. Chem. Int. Ed. 2007;46:1329–1332. doi: 10.1002/anie.200603931. [DOI] [PubMed] [Google Scholar]
- 12.Hua Q, et al. Crystal-plane-controlled selectivity of Cu2O catalysts in propylene oxidation with molecular oxygen. Angew. Chem. Int. Ed. 2014;53:4856–4861. doi: 10.1002/anie.201402374. [DOI] [PubMed] [Google Scholar]
- 13.Niedrig TS, Hävecker M, Gericke AK, Schlögl R. Partial methanol oxidation over copper: active sites observed by means of in situ X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2000;2:3473–3481. doi: 10.1039/b002308f. [DOI] [Google Scholar]
- 14.Bukhtiyarov VI, Prosvirin IP, et al. In situ study of selective oxidation of methanol to formaldehyde over copper. React. Kinet. Catal. Lett. 2003;79:181–188. doi: 10.1023/A:1024184423361. [DOI] [Google Scholar]
- 15.Mudiyanselage K, et al. Importance of the metal-oxide interface in catalysis: in situ studies of the water-gas shift reaction by ambient-pressure X-ray photoelectron spectroscopy. Angew. Chem. Int. Ed. 2013;52:5101–5105. doi: 10.1002/anie.201210077. [DOI] [PubMed] [Google Scholar]
- 16.Nagase K, Zheng Y, Kodama Y, Kakuta J. Dynamic study of the oxidation state of copper in the course of carbon monoxide oxidation over powdered CuO and Cu2O. J. Catal. 1999;187:123–130. doi: 10.1006/jcat.1999.2611. [DOI] [Google Scholar]
- 17.Graciani J, et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science. 2014;345:546–550. doi: 10.1126/science.1253057. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. In situ studies of the active sites for the water gas shift reaction over Cu–CeO2 catalysts: complex interaction between metallic copper and oxygen vacancies of ceria. J. Phys. Chem. B. 2006;110:428–434. doi: 10.1021/jp055467g. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, et al. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C. 2016;120:21472–21485. doi: 10.1021/acs.jpcc.6b06158. [DOI] [Google Scholar]
- 20.Martínez-Arias A, Fernández-García M, Soria J, Conesa JC. Spectroscopic study of a Cu/CeO2 catalyst subjected to redox treatments in carbon monoxide and oxygen. J. Catal. 1999;182:367–377. doi: 10.1006/jcat.1998.2361. [DOI] [Google Scholar]
- 21.Xu F, et al. Redox-mediated reconstruction of copper during carbon monoxide oxidation. J. Phys. Chem. C. 2014;118:15902–15909. doi: 10.1021/jp5050496. [DOI] [Google Scholar]
- 22.Wadayama T, Kubo K, Yamashita T, Tanabe T, Hatta A. Infrared reflection absorption study of carbon monoxide adsorbed on submonolayer Fe-covered Cu (100), (110), and (111) bimetallic surfaces. J. Phys. Chem. B. 2003;107:3768–3773. doi: 10.1021/jp026212v. [DOI] [Google Scholar]
- 23.Horn K, Hussain M, Pritchard J. The adsorption of CO on Cu (110) Surf. Sci. 1977;63:244–253. doi: 10.1016/0039-6028(77)90341-7. [DOI] [Google Scholar]
- 24.Horn K, Pritchard J. Infrared spectrum of CO chemisorbed on Cu (100) Surf. Sci. 1976;55:701–704. doi: 10.1016/0039-6028(76)90267-3. [DOI] [Google Scholar]
- 25.Hayden BE, Kretzschmar K, Bradshaw AM. An infrared spectroscopic study of CO on Cu (111): the linear, bridging and physisorbed species. Surf. Sci. 1985;155:553–566. doi: 10.1016/0039-6028(85)90013-5. [DOI] [Google Scholar]
- 26.Dulaurent O, Courtois X, Perrichon V, Bianchi D. Heats of adsorption of CO on a Cu/Al2O3 catalyst using FTIR spectroscopy at high temperatures and under adsorption equilibrium conditions. J. Phys. Chem. B. 2000;104:6001–6011. doi: 10.1021/jp9943629. [DOI] [Google Scholar]
- 27.Oyama ST, Zhang X, Lu J, Gu Y, Fujitani T. Epoxidation of propylene with H2 and O2 in the explosive regime in a packed-bed catalytic membrane reactor. J. Catal. 2008;257:1–4. doi: 10.1016/j.jcat.2008.04.023. [DOI] [Google Scholar]
- 28.Sakurai, H., Ueda, A., Kobayashi, T. & Haruta, M. Low-temperature water-gas shift reaction over gold deposited on TiO2. Chem. Commun. 271-272 (1997).
- 29.Stone P, et al. TPD and XPS investigation of formic acid adsorption on the oxygen-precovered c(6×2) surface of Cu (110) Surf. Sci. 1998;418:71–83. doi: 10.1016/S0039-6028(98)00683-9. [DOI] [Google Scholar]
- 30.Besenbacher F, Nørskov JK. Oxygen chemisorption on metal surfaces: general trends for Cu, Ni and Ag. Prog. Surf. Sci. 1993;44:5–66. doi: 10.1016/0079-6816(93)90006-H. [DOI] [Google Scholar]
- 31.Matsumoto T, et al. Scanning tunneling microscopy studies of oxygen adsorption on Cu(111) Surf. Sci. 2001;471:225–245. doi: 10.1016/S0039-6028(00)00918-3. [DOI] [Google Scholar]
- 32.Soon A, Todorova M, Delley B, Stampfl C. Oxygen adsorption and stability of surface oxides on Cu(111): a first-principles investigation. Phys. Rev. B. 2006;73:165424–165434. doi: 10.1103/PhysRevB.73.165424. [DOI] [Google Scholar]
- 33.Yang F, Choi Y, Liu P, Hrbek J, Rodriguez JA. Autocatalytic reduction of a Cu2O/Cu(111) surface by CO: STM, XPS, and DFT studies. J. Phys. Chem. C. 2010;114:17042–17050. doi: 10.1021/jp1029079. [DOI] [Google Scholar]
- 34.Gokhale AA, Dumesic JA, Mavrikakis M. On the mechanism of low-temperature water gas shift reaction on copper. J. Am. Chem. Soc. 2008;130:1402–1414. doi: 10.1021/ja0768237. [DOI] [PubMed] [Google Scholar]
- 35.Wang G-C, Nakamura J. Structure sensitivity for forward and reverse water-gas shift reactions on copper surfaces: a DFT study. J. Phys. Chem. Lett. 2010;1:3053–3057. doi: 10.1021/jz101150w. [DOI] [Google Scholar]
- 36.Bu Y, Weststrate CJ, Niemantsverdriet JW, Fredriksson HOA. Role of ZnO and CeOx in Cu-based model catalysts in activation of H2O and CO2 dynamics studied by in situ ultraviolet-visible and X-ray photoelectron spectroscopy. ACS Catal. 2016;6:7994–8003. doi: 10.1021/acscatal.6b02242. [DOI] [Google Scholar]
- 37.Rodriguez JA, et al. Water-gas shift reaction on a highly active inverse CeOx/Cu(111) catalyst: unique role of ceria nanoparticles. Angew. Chem. Int. Ed. 2009;48:8047–8050. doi: 10.1002/anie.200903918. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, et al. Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorption ability. J. Mater. Chem. 2009;19:5220. doi: 10.1039/b816349a. [DOI] [Google Scholar]
- 39.Liang X, Gao L, Yang BS, Sun J. Facile synthesis and shape evolution of single-crystal cuprous oxide. Adv. Mater. 2009;21:2068. doi: 10.1002/adma.200802783. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the authors on reasonable request.