Abstract
Purple acid phosphatases (PAPs) play important roles in phosphate (Pi) acquisition and utilization. These PAPs hydrolyze organic Phosphorus (P) containing compounds in rhizosphere as well as inside the plant cell. However, roles of PAPs in one of the most widely cultivated legumes, chickpea (Cicer arietnum L.), have not been unraveled so far. In the present study, we identified 25 putative PAPs in chickpea (CaPAPs) which possess functional PAP motifs and domains. Differential regulation of CaPAPs under different nutrient deficiencies revealed their roles under multiple nutrient stresses including Pi deficiency. Interestingly, most of the CaPAPs were prominently expressed in flowers and young pods indicating their roles in flower and seed development. Association mapping of SNPs underlying CaPAPs with seed traits revealed significant association of low Pi inducible CaPAP7 with seed weight and phytate content. Biochemical characterization of recombinant CaPAP7 established it to be a functional acid phosphatase with highest activity on most abundant organic-P substrate, phytate. Exogenous application of recombinant CaPAP7 enhanced biomass and Pi content of Arabidopsis seedlings supplemented with phytate as sole P source. Taken together, our results uncover the PAPs in chickpea and potential roles of CaPAP7 in seed phytate accumulation.
Introduction
Phosphorus (P) is essential for plant growth; however, its bio-availability is limited as the available inorganic phosphate (Pi) constitutes only 20–50% of total soil P and the rest remains fixed in the form of organic and inorganic complexes. Pi taken up by roots is subsequently translocated to vegetative and reproductive tissues including developing seeds. Mature plants store the majority of this Pi in the form of highly complex phytic acid and related salts (phytate) in seeds. Processes like seed germination require large amount of Pi where phytate is hydrolysed to release Pi. Few Acid Phosphatases (APases) like Purple Acid Phosphatases (PAPs) are involved in the hydrolysis of phytate complexes to release Pi for plant use in cell or rhizosphere1, 2. Secretory PAPs hydrolyse P in soil while non-secretory ones release Pi from cellular organic-P complexes.
PAPs belong to the metallophosphoesterases superfamily, members of which are cosmopolitan in animals, plants, bacteria and fungi3, 4. The active form of these enzymes has a binuclear metal center which consists of Fe(III)-Fe(II) in animals and either Fe(III)-Zn(II) or Fe(III)-Mn(II) in plants5. These chromophoric metal ions are critical for the functioning of PAPs as their replacement leads to the loss of enzymatic activity6. The five conserved motifs contain seven metal ligating residues (DxG–GDXXY–GNH(D/E)–VXXH–GHXH; bold letters indicate metal ligating residues) which form a bimetallic center and are responsible for their activity7. While most of the PAPs are active on wide range of substrate, some PAPs (NtPAP, AtPAP15, AtPAP23, GmPhy and OsPHY1) are reported to exhibit higher activity on phytate8–12. Nonspecific PAPs exhibit high activity against different phoshomonoesters which include ATP, phosphoenolpyruvate and phosphoproteins13–15. Functionally, PAPs play key roles in Pi acquisition and utilization. One common feature of PAPs is their inducibility by low Pi in plants. Many of them also conferred low Pi tolerance on overexpression11, 13–25. However, their other roles also include ROS metabolism, nitrogen fixation, cellulose and carbon metabolism22, 26, 27. Since, the functions of all known PAPs have not been elucidated, it is difficult to assign specific role for many PAPs. Further, PAPs often exist as multigene family members in plants7, 23, 28.
Chickpea is the world’s third-most important cultivated legume crop. Around 6.2 mha of arable land in India is under chickpea cultivation. However, majority of chickpea growing areas are either marginal or sub-marginal lands with sub-optimal Pi levels. Thus, Pi deficiency is a critical constraint for chickpea production in India29. Amidst this scenario, organic-P mobilizing PAPs can be an effective resource to improve Pi utilization efficiency of chickpea. However, information on chickpea PAPs (CaPAPs) is missing so far. Therefore, in the present study, we first identified 25 novel CaPAPs and investigated their potential roles in Pi homeostasis. Many of the CaPAPs were found to be differentially regulated by Pi deficiency. However, unlike some of the past studies, CaPAPs did not show specific induction to Pi deficiency. This intrigued us to explore broader roles of CaPAPs in chickpea. Interestingly, majority of the CaPAPs were highly expressed in reproductive tissues. Further, association studies revealed strong association of low Pi inducible CaPAP7 with seed weight and phytate content. Therefore, in the present study, we investigated CaPAP7 for its possible roles in low Pi tolerance and regulation of seed weight/phytate content.
Methods
Identification of PAPs in chickpea
The PAP sequences from 17 different organisms (Table S1) were identified from sequence databases like TAIR, TIGR, Plant GDB, Uniprot; using phrase “purple acid phosphatase”. The organisms selected were Arabidopsis thaliana, Oryza sativa, Solanum lycospersicum, Medicago trunculata, Hordeum vulgare, Physcomitrella patens, Vitis vinifera, Caenorhabditis elegans, Homo sapiens, Dictyostelum discordeum, Vigna radiata, Rhizobium galegae, Phaseolus vulgaris, Glycine max, Zea mays, Solanum tuberosum and Chlamydomonas reinharbtii. The sequences so obtained were searched for the presence of metallophos domain using SMART tool (http://smart.embl-heidelberg.de/). Total sequences retrieved with metallophos domain were 348 (Text S1). The sequences with metallophos domain were selected, aligned and used to generate a Hidden Markov Model (HMM). The HMM profile was used to identify PAPs in chickpea using HMMER search in chickpea (Kabuli) protein database (http://ceg.icrisat.org/gt-bt/ICGGC/GenomeManuscript.htm). Sequences so obtained were then screened for the presence of metallophos domain and five conserved motifs (DXG/GDXXY/GNH(D/E)/VXXH/GHXH), and finally 25 putative CaPAPs were identified. These PAPs were aligned with Arabidopsis PAPs (AtPAPs) using Clustal X2 and annotated in accordance to their homology with corresponding AtPAPs.
Analysis of CaPAPs sequence
For comprehensive nucleotide level structural annotation of CaPAPs, like chromosomal/pseudomolecule location, genomic DNA sequence and coding sequence (CDS) were obtained from kabuli database (http://ceg.icrisat.org/gt-bt/ICGGC/GenomeManuscript.htm). The number of exons and introns were identified by aligning cDNA and genomic DNA sequences in GSDS server (http://gsds.cbi.pku.edu.cn/). Number of amino acid, molecular weight and pI of the sequences were predicted using bioinformatics tool, Compute pI (http://web.expasy.org/compute_pi/). To analyse the homology between rice, chickpea, soybean and Arabidopsis PAPs, the amino acid sequences were aligned using Clustal X2 and an unrooted phylogenetic tree was generated using neighbour joining method with bootstrap value 1000. The phylogenetic trees were visualised in MEGA6. For cis-element analysis, 3 kb upstream region was selected and P1BS elements (PHR1 binding sites) (GNATATNC) were searched manually using Gene runner (http://www.generunner.net/). The presence or absence of signal peptide was predicted using Signal P4.1 (http://www.cbs.dtu.dk/services/SignalP/). Subcellular localization was predicted using CELLO v2.5 (http://cello.life.nctu.edu.tw/). Glycosylation sites were predicted using GlycoEP (http://www.imtech.res.in/cgibin/glycoep/glyechk). Peroxisome Targeted Signal (PTS) was predicted using PredPlantPTS1 as described previously30.
Expression analysis of CaPAPs in different plant tissues
We utilized the transcriptome data from CTDB (chickpea transcriptome database) (http://www.nipgr.res.in/ctdb.html) generated by Garg et al.31 in order to identify the expression pattern of CaPAPs in different tissues (root, shoot, flower bud and young pod). Heat map was generated on RPM (number of unique reads mapped to each transcript per million) values using MeV 4.6.0 tool (http://mev.tm4.org/#/welcome).
Trait association analysis of CaPAPs
For association mapping, seed weight (100 seed weight in gm) and phytic acid content (in mg/100gm) data were retrieved from Kujur et al.32 and Joshi-Shah and Reddy33, respectively. CaPAPs based SNP genotyping information (MAF 5%) as well as kinship matrix (K), PCA (P) data and population structure ancestry coefficient (Q matrix) generated from 92 chickpea accessions (association panel) were analysed using TASSEL v5.0, as described32. The potential SNP loci in the diverse coding and non-coding sequence components of CaPAPs revealing significant association with seed weight and phytic acid traits at a highest R2 (degree of SNP marker-trait association) and lowest FDR adjusted P-values (threshold P ≥ 10−6) were selected.
Plant growth condition and nutrient deficiency treatments
Chickpea seeds of accession ICC 4958 were used in all the experiments. For generating nutrient deficiency conditions, seedlings were raised in Hoagland media according to the conditions and protocol as described34. Briefly, plant growth conditions were maintained at 12/12 h photoperiod, 23/18 °C, 200-300 µM photons/m2/s photon density and ~70% relative humidity. Tissues were harvested after 7 and 15 days of treatment and unless stated otherwise experiments were performed in three biological replicates.
qRT-PCR based analysis of gene expression
Gene expression analysis was done as described, previously34. Briefly, root samples were taken at 7 days and 15 days after treatment for each nutrient deficiency. Tissues were frozen in liquid nitrogen and stored at −80 °C. The TRIzol method was used to extract total RNA from root. Prior to cDNA synthesis, DNaseI treatment was given to get rid of genomic DNA contamination from RNA. cDNA was synthesised using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to manufacturer’s instructions.
The primers for quantitative real time PCR (qRT-PCR) were designed using PRIMER EXPRESS version 2.0 (PE Applied BiosystemsTM, USA) with default parameters from coding region of the genes (Table S2). Each primer pair was analysed for its specificity using NCBI BLAST. The reaction was performed in Applied Biosystems 7500 Fast Real Time PCR. The relative expression was calculated using the ΔΔCt method. The elongation factor 1-α (AJ004960) was used as endogenous control and three biological replicates were considered for the experiment. To test the level of significance, Student’s t-test was applied.
Cloning and subcellular localization of CaPAP7
The coding region of CaPAP7 was cloned in pSITE3CA vector to express it as N-terminal YFP fusion protein (YFP:CaPAP7). Plasmid carrying this construct was then transformed into onion epidermal cells as described25. Fluorescence was visualised under confocal microscope, AOBS TCS-SP2 (Leica, Germany).
Biochemical characterization of recombinant CaPAP7
For biochemical characterization, ORF sequence of CaPAP7 was amplified using gene-specific primers (Table S2). The PCR product of 1008 bp was cloned into pET28a vector and confirmed by sequencing. BL21 (DE3) pLysS cells were used for protein induction. The recombinant protein was induced with 0.3 mM IPTG at 16 °C for 12-16 h. The induced protein was purified using affinity chromatography (Ni-NTA). To confirm the identity of induced protein, coomassie-stained protein bands were digested with trypsin as per manufacturer’s protocol and then identified by 4000Q TRAP LC/MS/MS.
The phosphatase activity assays were performed to study optimum temperature, pH, cofactor and substrate for CaPAP7 as described25. The optimum temperature for the PAP activity was measured over a temperature range of 25 °C to 60 °C in 50 mM sodium acetate buffer (pH 4.9), MgCl2 (5 mM) using 10 mM para-nitrophenylphosphate (pNPP), as substrate for 30 min. To identify the optimum pH for CaPAP7 activity, a pH range of 4.0 to 8.0 was set using different buffers with varying pH and pNPP (10 mM) as substrate at 37 °C for 30 min. In order to determine the substrate specificity, CaPAP7 activity was assayed on different substrate namely, ATP, ADP, AMP, PPi, P-serine, P-threonine, glucose-6-P, fructose-6-P, pNPP, 2-deoxyriboseP and phytic acid at 37 °C for 30 min. In order to identify cofactors and inhibitors of CaPAP7, activity assays were performed with 5 mM of chloride salts of different metal ions and sodium salts of different cations as described earlier25.
Assessment of CaPAP7 phytase activity using Arabidopsis seedlings
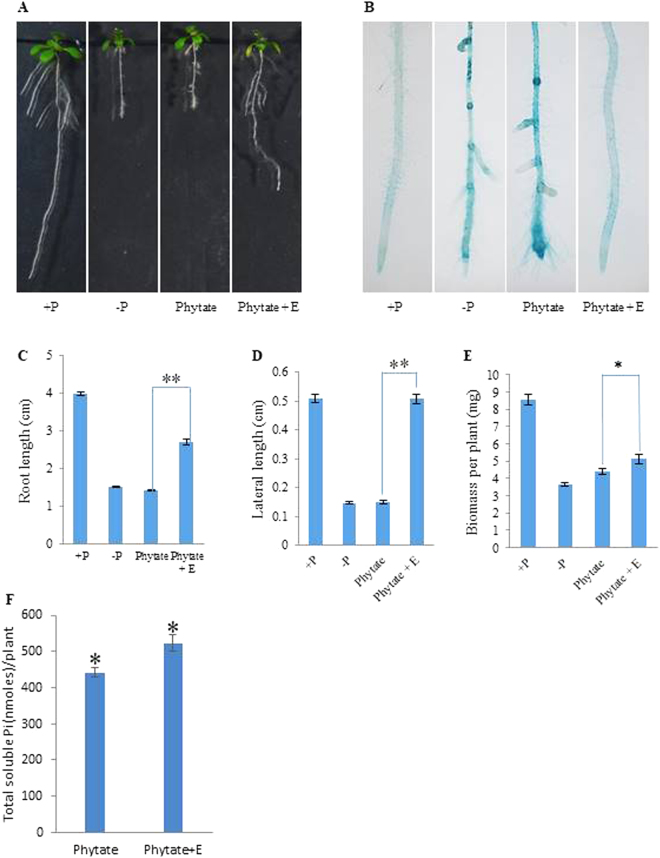
In order to assess the impact of recombinant CaPAP7 (phytase activity) on plant growth, Arabidopsis seeds were germinated on ½MS media in plates vertically. Four-days-old seedlings with uniform root growth were transferred to ½ MS plates containing 1 µM KH2PO4 (−Pi), 625 µM KH2PO4 (+Pi), 5 µM phytate (Phytate) and 5 µM phytate + 150 ng/ml enzyme (Phytate + E). CaPAP7 protein in Tris-Cl buffer was spread on 5 µM phytate plates before seedlings transfer. All the growth parameters (root length, biomass, and lateral root length) were analysed after 7 days of transfer. Soluble Pi estimation was done as described35. Root surface associated APase activity was visualized using 0.015% BCIP (5-bromo-4-chloro-3- indolyl-phosphate) as described25.
Gene expression profile of CaPAP7 in chickpea genotypes with contrasting seed weight
Four contrasting accessions for seed weight and phytate content were selected based on the information from previous reports32, 33. In order to reveal the role of CaPAP7 in phytate remobilisation, we analysed the expression of CaPAP7 in young pod of these contrasting accessions. Tissues for young pod were collected in three replicates pooled from multiple plants, frozen in liquid nitrogen and thereafter stored at −80 °C. RNA was isolated using TRIzol and cDNA was prepared as described above. The qRT-PCR was done and relative expression was calculated using 2−ΔCt method.
Results
PAPs form multigene family in chickpea
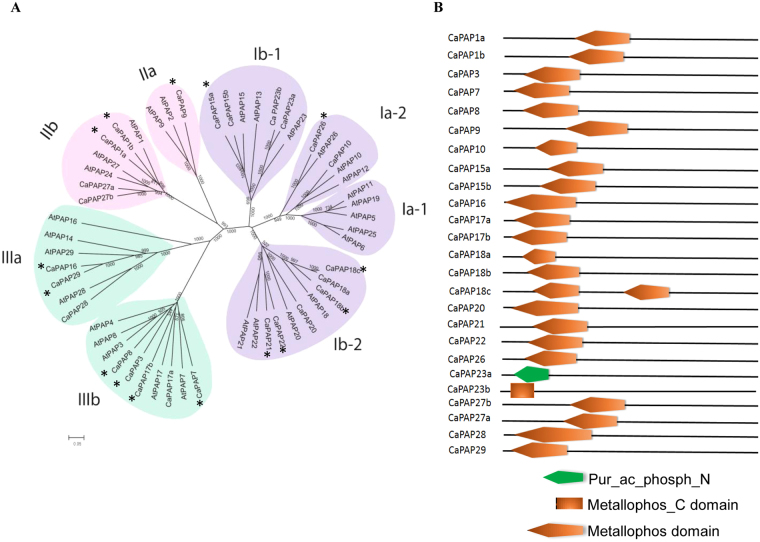
Using sequence homology approach and conserved domain analysis, we identified 25 putative PAPs in chickpea genome which were annotated according to their homology to Arabidopsis PAPs (Fig. 1A). Of these, complete metallophos domain was present in twenty-three while two (CaPAP23a and CaPAP23b) have partial domain. CaPAP23a had only pur_ac_phosphN terminal domain and CaPAP23b had only metallophos C domain which is essential for the phosphatase activity (Fig. 1B). The sequence analysis revealed that 21 CaPAPs have all the five blocks of conserved motifs (DXG/GDXXY/GNH(D/E)/VXXH/GHXH) and seven metal ligating residues which are characteristics of known PAPs. In CaPAP16, 28 and 29 “Y” residue is replaced by “F” in the second block (GDXXY). Among the remaining PAPs, CaPAP16, 23b lack the fourth block (VXXH), CaPAP18a lacks fifth block (GHXH), whereas CaPAP23a has two blocks missing; third (GNH(D/E) and fifth (GHXH) (Table S3 ). But both the sequences showed significant homology with known PAPs. So we considered them as putative PAPs.
Figure 1.
Phylogenetic relationship and domain architecture of CaPAPs. (A) Phylogenetic relationship of chickpea and Arabidopsis PAPs. The amino acid sequences of CaPAPs and AtPAPs were aligned using Clustal X2 and the phylogenetic tree was constructed using NJ method with bootstrap value 1000. Bootstrap value mentioned at each node. “*” indicates CaPAPs with signal peptide (B) Conserved domains present in CaPAPs. The amino acid sequence of CaPAPs were analysed in SMART (http://smart.embl-heidelberg.de/) tool. Pur_ac_phosph_N; N terminal domain of purple acid phosphatase, Metallophos_C domain; C terminal domain of metallophos domain.
Based on phylogenetic relationship between chickpea and Arabidopsis PAPs, CaPAPs were classified into three major (I, II and III) groups as per earlier classification7 (Fig. 1A ). The twenty- five CaPAPs were divided into seven subgroups (Ia-2, Ib-1, Ib-2, IIa, IIb, IIIa and IIIb) except subgroup Ia-1 where none of the CaPAPs was grouped. Further, phylogenetic analysis of CaPAPs with soybean, Arabidopsis and rice PAPs together suggested that CaPAPs are closer to dicots PAPs as only few of them were grouped with rice PAPs (Fig. S1).
Further, CaPAPs were found to be localised to different chromosomes, whereas five genes (CaPAP15a, 17a, 17b, 18c and 22) were present on scaffolds (scaffold 128, 88, 40, 6367 and 1710, respectively) (Table S4). Analysis of gene structure of CaPAPs showed that number of exons varies from 2 (CaPAP9) to 12 (CaPAP1a, 1b, 27a and 27b) (Table S4). Molecular mass of identified PAPs varies from 17.33 kDa (CaPAP23a) to 73.91 kDa (CaPAP9). On prediction of secretory or non-secretory PAPs, we found the presence of signal peptide in 15 CaPAPs namely, CaPAP1a, 1b, 3, 7, 8, 9, 15a, 16, 18b, 18c, 21, 22, 24, 26 and 29, whereas no signal peptide was identified in remaining 10 PAPs i.e. CaPAP10, 15b, 17a, 18a, 20, 23a, 23b, 27a, 27b and 28. In order to correlate the nature of signal peptide, we predicted the subcellular localization of CaPAPs with the help of CELLO tool (http://cello.life.nctu.edu.tw/). It turned out that most of the CaPAPs i.e. 22 out of 25 were either secretory or localised to lysosomes. CaPAP7 and 16 were predicted to be localised to cytoplasm while CaPAP28 to plasma membrane. Interestingly, CaPAP18b was predicted to be localised to nucleus and also has potential secretory nature (Table S5 ). Since, some of the PAPs have been reported to be localised in peroxisomes30, we determined presence of PTS1 (Peroxisome Targeted Signal) in CaPAPs. Interestingly, only low P responsive CaPAP17a showed presence of PTS in its C terminal region. Furthermore, glycosylation sites were present in all the CaPAPs varying in numbers 1 to 8 (Table S5).
As PAPs are largely reported as Phosphate Starvation Response (PSR) genes, we analysed CaPAPs promoters (3 kb upstream) for any putative cis-acting elements which can act as binding sites for transcription factors involved in Pi deficiency response. P1BS (PHR1 binding sites) elements are considered to be highly Pi responsive as AtPHR1 (master regulator of P deficiency response) binds to this region and regulate its downstream targets. Analysis of promoter regions of all chickpea PAPs for the presence of P1BS elements revealed that P1BS elements were present in the promoter region of 18 CaPAPs including CaPAP1a, 1b, 3, 8, 9, 10, 15a, 15b, 17a, 17b, 18c, 21, 22, 23a, 23b, 27b, 28 and 29 while we did not find P1BS elements in seven CaPAPs including CaPAP7, 16, 18a, 18b, 20, 26 and 27a (Table S5 ).
CaPAPs are differentially expressed under Pi deficiency
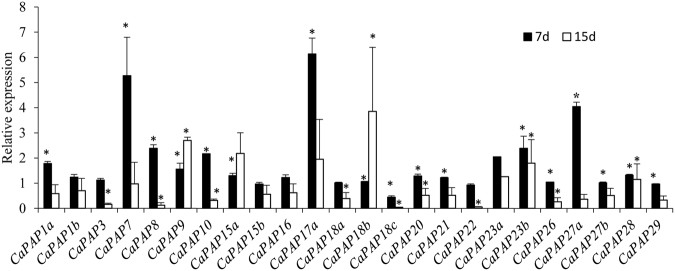
Since most of CaPAPs have P1BS elements in their promoter region, therefore, we studied their expression behaviour under Pi deficiency. Most of the CaPAPs (12 out of 25 genes which include CaPAP1a, 1b, 7, 8, 10, 15a, 17a, 20, 21, 23b, 27a and 28) showed significant upregulation except CaPAP18c which was downregulated after 7 days of deficiency (Fig. 2). CaPAP17b did not show detectable expression. The expression of ten genes (CaPAP3, 8, 10, 18a, 18c, 20, 22, 26, 27a and 29) found to be downregulated after 15 days of deficiency except two genes, CaPAP9 and CaPAP18b which were found upregulated. This indicated that most of the chickpea PAP genes are early Pi deficiency inducible, while CaPAP9b and CaPAP18b were late inducible to the Pi deficiency (Fig. 2). Moreover, four genes including CaPAP1b, 8, 15 and 27a were found exclusively upregulated under Pi deficiency showing their Pi specific behaviour (Figs S2 and S3). In addition to low Pi; CaPAPs also showed differential expression under low N, K, Fe and Zn indicating their roles in these deficiencies (Figs S2 and S3).
Figure 2.
Relative expression profile of CaPAPs under Pi deficiency after 7d (early response) and 15d (late response) of treatments. qRT-PCR was used for quantification of gene expression. The relative gene expression in stressed plants was calculated considering untreated plants as control. EF1α was used as endogenous control. Error bars represent SE of average of three replicates (n = 3). *p < 0.05.
CaPAPs showed preferential high expression in reproductive tissues
Spatial expression of CaPAPs in different tissues (root, shoot, mature leaf, flower bud and young pod) retrieved from CTDB database showed that most of the PAPs express in all the tissues but have exceptionally high expression in flower bud and young pod (Fig. 3). Further, except four CaPAPs, other root expressing ones did not express as high in roots as they did in reproductive tissues. Among all the tissues, CaPAPs showed least expression in shoot. This strong expression in flower bud and young pod indicates their important functions during reproductive development.
Figure 3.
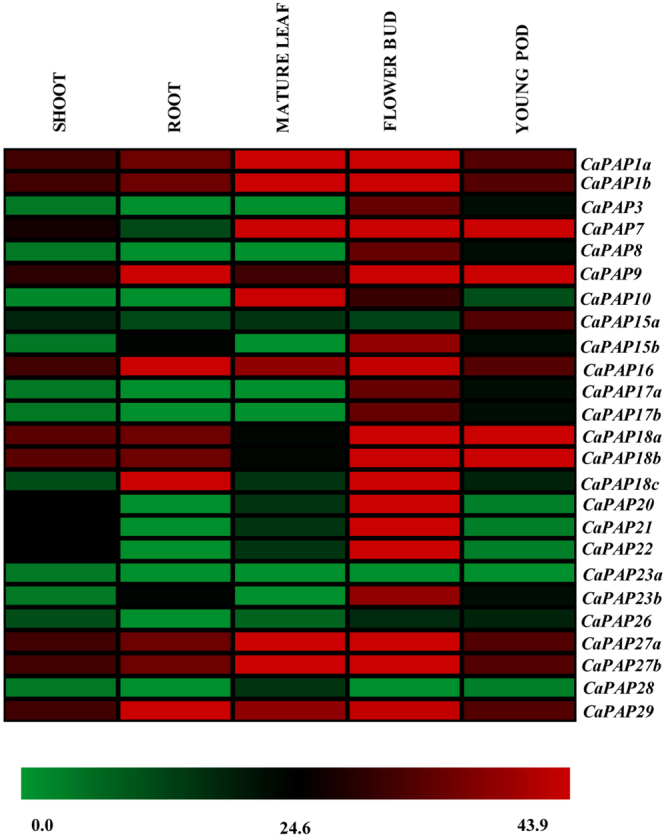
Gene expression profile of CaPAPs in shoot, root, mature leaf, flower bud and young pod of chickpea. Heat map was generated with RPM (Reads Per Million) values retrieved from CTDB (http://www.nipgr.res.in/ctdb.html). Scale bar represents RPM values.
CaPAPs showed strong association with seed phytate content and seed weight in diverse chickpea accessions
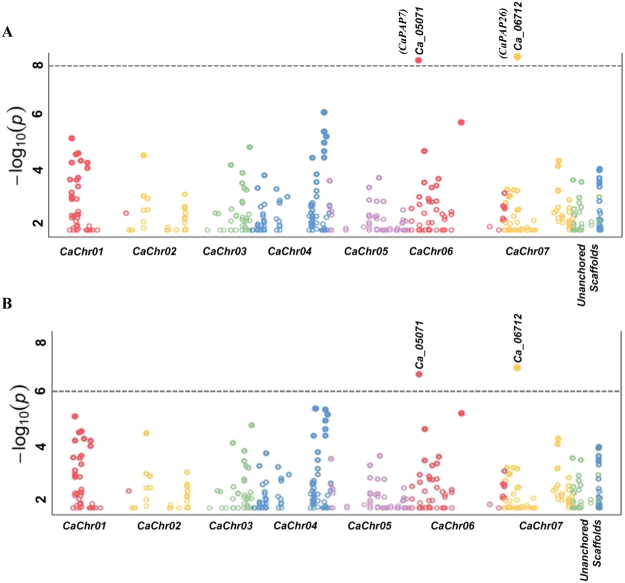
Since most of the CaPAPs showed high expression in reproductive tissues (flowering bud and young pod), we performed candidate gene based association mapping in diverse chickpea accessions for phytate content and seed weight. For this, genotyping data of SNPs from diverse coding and non-coding sequence components of CaPAPs along with phenotypic data (phytic acid and seed weight) of 92 accessions were collected. SNPs underlying CaPAPs were retrieved from 92 chickpea accessions and associated with seed weight data as described, previously32. Interestingly, we found strong association of a SNP (G/A) of Ca_05071 (CaPAP7) with seed weight (P value 1.8 × 10−9) and seed phytate content (P value 1.4 × 10−6). The proportion of phenotypic variation explained (PVE) for seed weight and phytate content by this SNP was found to be 25 and 19%, respectively (Table 1, Fig. 4). Additionally, another DRR (down-stream regulatory region) SNP, (A/C) in Ca_06712 (CaPAP26) was associated with seed weight trait (28% PVE with 2.4 × 10−10) and phytate content (21% PVE with P value 1.8 × 10−7). Closer analysis revealed that allele ‘G’ in URR of CaPAP7 mapped on chromosome 6 was associated with high seed weight and phytate, whereas allele ‘A’ is associated with low seed weight and low phytate content (Table 1). Similarly, allele ‘A’ in DRR of CaPAP26 mapped to chromosome 7 was associated with high seed weight and high phytate, whereas allele ‘C’ is associated with low seed weight and low phytate content.
Table 1.
PAP gene-derived SNP loci regulating seed weight and phytate content identified by association mapping.
| Chromosomes | SNP physical positions (bp) | SNPs | Gene accession IDs | Known/putative functions | Association analysis | Traits associated | |
|---|---|---|---|---|---|---|---|
| P | PVE (%) | ||||||
| Ca_Kabuli_Chr6 | 12978236 | [G/A] | Ca_05071 | CaPAP7 | 1.8 × 10−9 | 25 | 100-seed weight |
| Ca_Kabuli_Chr6 | 12978236 | [G/A] | Ca_05071 | CaPAP7 | 1.4 × 10−6 | 19 | Phytate content |
| Ca_Kabuli_Chr7 | 6395883 | [A/C] | Ca_06712 | CaPAP26 | 2.4 × 10−10 | 28 | 100-seed weight |
| Ca_Kabuli_Chr7 | 6395883 | [A/C] | Ca_06712 | CaPAP26 | 1.8 × 10−7 | 21 | Phytate content |
Figure 4.
Association of CaPAP7 and CaPAP26 with seed weight (A) and seed phytate content (B). The x-axis indicates the relative density of PAP gene based SNPs physically mapped on eight chromosomes and unannotated scaffold of Kabuli genome. The y-axis represents the −log10 p-value for significant association with traits. The SNPs with p-value ≤ 1 × 10−8 for seed weight (A) and 1 × 10−6 for seed phytate content (B) showing strong association are demarcated with dotted line.
CaPAP7 is differentially regulated in contrasting genotypes for seed weight and phytate content
Due to its induction in response to low Pi, conservation of all key residues and strong association with seed weight and seed phytate content traits, CaPAP7 was selected for further characterization. In order to validate association of URR SNP of CaPAP7 with seed weight and phytate content, we analysed the expression pattern of CaPAP7 in four accessions of chickpea varying in seed weight and phytate content (ICC16374, ICCV2, ICC12155 and ICC8151). Further, seed weight and phytate content were found strongly correlated with each other. Therefore, we analysed the expression pattern of CaPAP7 in young pod where phytate synthesis/accumulation is expected to be high. CaPAP7 showed higher expression in accessions with low seed weight and phytate content. While its expression was lower in accessions with higher seed weight and phytate content (Fig. 5). Therefore, a negative correlation of CaPAP7 expression was obtained with the seed weight (r = −0.82) and seed phytate content (r = −0.99). This data further strengthen the important role of CaPAP7 in chickpea seed phosphate/phytate accumulation.
Figure 5.
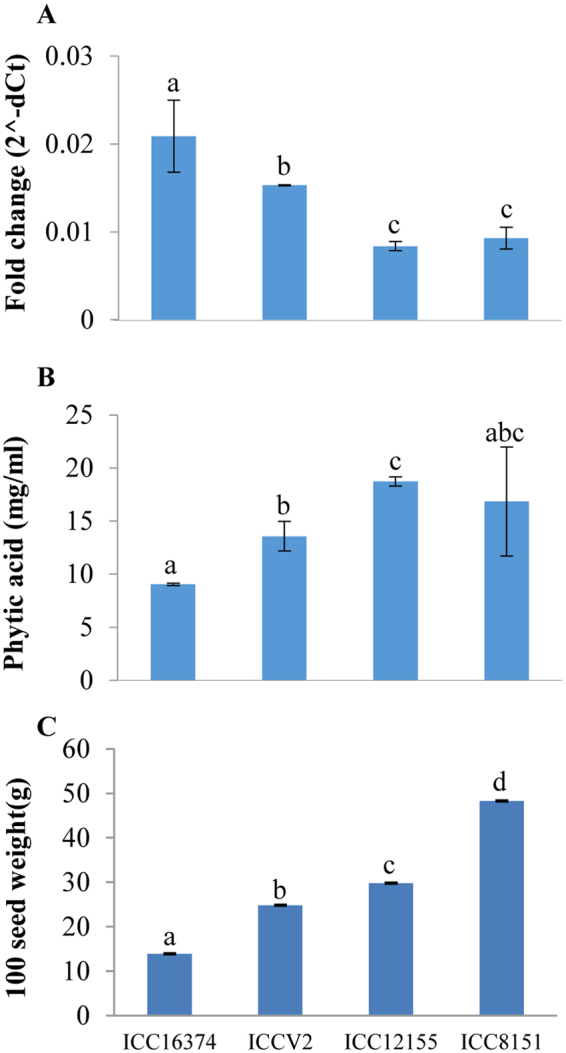
Expression profile of CaPAP7 in genotypes differing for seed weight and phytate content. (A) Expression pattern of CaPAP7 in young pod in contrasting chickpea accessions. Fold change was calculated using EF1-α as reference gene. Three biological replicates were considered for expression analysis. Each biological replicate comprise of pooled sample from multiple plants. Error bars indicate SE (n = 3). (B) Phytic acid content of contrasting chickpea accessions (Joshi-Saha et al.33). Error bars represent SE (n = 2) (C) Average seed weight (100 seed weight in g) of contrasting chickpea accessions (Kujur et al.32). Error bars represent SE (n = 3) CaPAP7 expression was in negative correlation with the seed weight (r = −0.82) and seed phytate content (r = −0.99). Different letters at the top of bar indicates different significant classes determined by one-way ANOVA followed by Duncan’s multiple range test.
CaPAP7 is localized in cytoplasm and encodes a functional acid phosphatase
In-silico tools predicted CaPAP7 to be localised in the cytoplasm. To confirm this in vivo, we cloned CaPAP7 CDS in pSITE3CA vector to produce an YFP fused protein and analysed its localisation in onion epidermal cells. YFP fluorescence analysis confirmed its cytoplasmic localisation as predicted (Fig. S4 ).
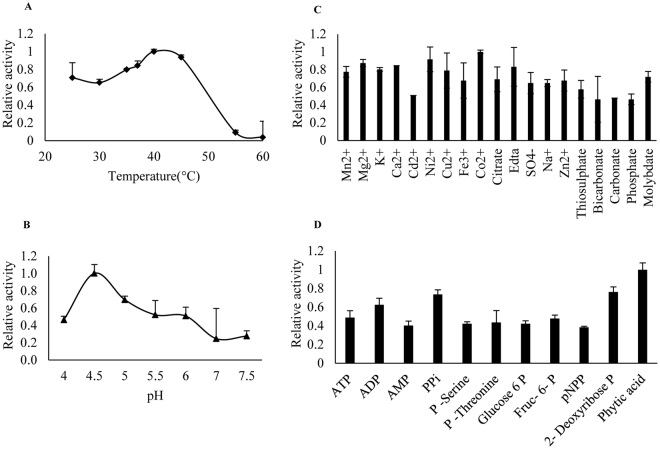
We next studied, whether CaPAP7 is a functional enzyme to further contemplate on its functions. Recombinant protein was produced in bacteria and purified. A band of expected size ~38 kDa was detected on SDS-page (Fig. S5). The identity of induced recombinant CaPAP7 was confirmed with LC-MS/MS (MASCOT score 120) (Fig. S6 ). CaPAP7 showed activity in the temperature range of 25–45 °C and pH range of 4–6 (Fig. 6). Its high phosphatase activity in acidic pH confirmed it as APase. Further, activity assays with different divalent cations and anions revealed no specific cofactor preference of CaPAP7. However, high phosphate and carbonate concentration seemed to be slightly inhibitory for phosphatase activity of CaPAP7 (Fig. 6C). The enzyme activity assay showed that CaPAP7 has a broad substrate specificity hydrolysing different Pi containing compounds namely, ATP, ADP, AMP, PPi, P-serine, P-threonine, glucose-6-P, fructose-6-P, pNPP, 2-deoxyribose-P and phytic acid. Intriguingly, it exhibited maximum catalytic activity for phytic acid (Fig. 6D ). The specific activity of recombinant CaPAP7 with pNPP and phytate was 0.3825 and 0.620 nmolug−1 min−1, respectively.
Figure 6.
Biochemical properties of recombinant CaPAP7. (A) The temperature profile of CaPAP7 on pNPP in sodium acetate buffer at various temperatures for 30 min. (B) pH profile. The CaPAP7 activity was assayed in various buffers (50 mM) with different pH at 37 °C for 30 min. (C) Effect of different cofactors on CaPAP7 activity. (D) CaPAP7 activity on different substrates (10 mM) at 37 °C for 30 min. Values represent average of two replicates with std. error. Experiment was repeated three times with similar results (n = 2).
Exogenous application of recombinant CaPAP7 protein improved Arabidopsis growth on phytate containing media
As CaPAP7 has highest activity for phytic acid which is an abundant non-available form of P in soil. So to validate its phytase activity and its impact on hydrolysis of phytate in plant, we grew Arabidopsis seedlings on phytate containing media. As expected, seedlings showed ~50% reduction in root elongation on media without Pi (−P) and phytate (as P source) as compared to +P media (Fig. 7). To further test whether phytate can induce low Pi stress in our set-up, we visualized root APase activity using BCIP and found increased APase activity under −P and phytate supplemented conditions. However, when seedlings were grown on phytate media overlaid with purified CaPAP7 enzyme; root length increased by 50% as compared to seedling on phytate without enzyme. Seedlings supplemented with CaPAP7 also showed increased lateral roots length (~233%) and higher biomass (~11%) than ones without enzyme (Fig. 7). Pi accumulation per plant was also increased (~22%) in seedlings with the application of CaPAP7 in phytate media in comparison to phytate only media. All these results strongly suggest that CaPAP7 was able to hydrolyze phytate to release Pi for better plant growth (Fig. 7 ). To this end, we conclude that CaPAP7 encodes a functional enzyme which is able to hydrolyze phytate and potentially influences seed phytate accumulation, and thereby seed weight.
Figure 7.
Effect of purified recombinant CaPAP7 on phytate hydrolysis and Arabidopsis growth parameters. (A) Phenotypic deference in P supplemented ( + P), P deficient (−P), P deficient + phytate (phytate) and P deficient + phytate + CaPAP7 (phytate + E) grown Arabidopsis seedlings. (B) Visualization of acid phosphatase activity by BCIP overlay on roots of Arabidopsis seedlings. (C) Average root length (n = 7 in six replicates). (D) Average lateral root length (n = 7 in six replicates). (E) Average biomass per plant (n = 7 in six replicates). (F) Total soluble Pi (nmoles per plant) (n = 6 in six replicates). All analysis was done after 7 days of growth on above said media. Error bars represent SE. Students t-test was performed for the statistical analysis **p < 0.001, *p < 0.05.
Discussion
Chickpea is one of the most important legume crops cultivated in the semiarid regions of the world. Chickpea being a leguminous crop has higher ability to mobilise the soil P36. Along with phosphate, legumes are known to restore other nutrients to the soil such as nitrogen through symbiotic N2 fixation which makes them highly important for the sustainable agriculture37. Legumes demand more Pi than non nodulating nitrogen-fixing plants as it is critically important for legume- rhizobia symbiosis38–40. Therefore, Pi deficiency affects chickpea development in dual manner; limits both growth and nitrogen fixation. One of the adaptive strategies evolved in diverse plants to increase labile Pi pool is secretion of APases41, 42. PAPs are APases with broad substrate specificity and are reported to hydrolyse various organic-P compounds in different plants (Arabidopsis, rice, soybean, kidney bean)4, 8, 11, 12. Here we identified 25 novel PAPs in chickpea which form three main groups (I, II, III) and seven subgroups (Ia-2, Ib-1, Ib-2, IIa, IIb, IIIa and IIIb) as described for Arabidopsis7. This indicates similarity in their function and behaviour; except clade Ia-1 which includes only AtPAPs. CaPAPs exhibited more similarity to AtPAPs and GmPAPs than to OsPAPs, suggesting that their behaviour and function are similar to other dicots. While all CaPAPs had an Arabidopsis homolog, a lower number of PAPs were retrieved in chickpea as compared to other legumes and Arabidopsis7, 23. This could be due to either incomplete genome sequence in chickpea or peculiar genetic constitution of chickpea, and raise the possibility of identification of more PAPs in future.
PAPs are known to be induced by Pi deficiency to help plants utilize organic sources of P13, 17, 25, 43, 44. CaPAPs also had P1BS cis-elements in their promoter which is involved in low Pi inducibility. Although CaPAPs were induced under Pi deficiency; however, level of induction was not very high. Moreover, low Pi inducible CaPAP7 didn’t possess any P1BS element. Further, a direct correlation wasn’t seen between existence of P1BS element and low Pi inducibility of PAPs in other plants28, 45 indicating role of other unknown regulators. Notably, many of the low Pi inducible genes possess other low Pi responsive cis-elements [such as W box (TTGACY), TC element (TCTCTCT) or NIT 2-like elements (AAATATCT)] instead of P1BS 46. This indicates that P1BS elements are not obligatory for low Pi responsiveness of many such genes. Analysis of low Pi inducible CaPAP7 revealed presence of two TC elements in its promoter which might be involved in its low Pi dependent transcriptional regulation.
Most of the CaPAPs were also upregulated by nitrogen deficiency, which can be correlated with the large amount of Pi requirement during nitrogen fixation by the nitrogenase complex. Their differential expression in other nutrient deficiencies hinted towards other roles of CaPAPs. This deviation from their well-established roles in Pi deficiency is in quite agreement with other observations. For instance, GmPAP21 is involved in P metabolism in root nodules. AtPAP17 is involved in the metabolism of reactive oxygen species while AtPAP26 plays a role in senescence driven Pi remobilization. Similarly, GmPAP3 participates in ROS response while AtPAP2 plays key roles in carbon metabolism16, 47–49. In rice PAPs have also been reported to play role in grain filling50. Heat inducible PgPAP18 was also reported to play defensive role against several environmental stresses51. Some PAPs such as NtPAP12 are involved in root system modulation by degradation of xyloglucan oligosaccharides and cello-oligosaccharides in the cell walls52. These evidences suggest diverse roles of PAPs in plants. On such similar lines CaPAPs may also be playing other roles besides Pi deficiency response.
To contemplate on these roles, we studied expression patterns of CaPAPs in different tissues. Interestingly, they showed least expression in root. Majority of them showed high expression in flower buds and young pods suggesting their important roles in reproductive development. Incidentally, several AtPAPs are also reported to be expressed during reproductive development9. High expression of PAPs in Arabidopsis pollens has been correlated with increased hydrolysis of phytate and germination of pollen11. Function of these enzymes therefore, could also be intracellular; in remobilizing Pi towards seed as seed development requires more P in comparision to normal cellular processes. Seeds also accumulate more P than other organs53 and increasing seed P content has been suggested to improve seedling vigour54. Interestingly, among 25 identified CaPAPs, two PAPs, CaPAP7 and CaPAP26 showed strong association with both seed weight and phytate content. Chickpea seeds are high in phytate content, and seed size directly correlates with the phytate content of the seed33. It is noteworthy here that CaPAP26 is a close homologue of AtPAP26 which is known to be involved in Pi remobilization and senescence48. CaPAP7 showed high low Pi inducibility, localizes to cytoplasm and is of non-secretory nature. Recently, the Arabidopsis homologue of CaPAP7 (AtPAP7) was found to localise in peroxisomes30. However, we did not find any PTS in CaPAP7. Further, particle bombardment assay revealed no co-localisation of CaPAP7 with peroxisome marker in onion epidermal cells (data not shown). Therefore, CaPAP7 may not be a true homolog of AtPAP7. Further studies revealed that CaPAP7 to be enzymatically active at pH range (4–6) with optima at 4.5, validating its function as acid phosphatase. Intriguingly, while CaPAP7 showed broad substrate specificity, highest activity was observed on phytate, concluding CaPAP7 as PAP with phytase activity. PAPs are known to be glycosylated which affect their stability, targeting and enzyme kinetics2. Therefore, a high phytase activity of CaPAP7 needs further investigation using plant purified native CaPAP7. Nevertheless, PAPs with phytase activity do exist in plants8, 11. Few purple acid phosphatases (PAPs) have also been reported to possess phytase activity in soybean, Arabidopsis, rice and tobacco8, 9, 11, 12, 19. Recently, OsHAD1, an APase has also been reported to possess both acid phosphatase and phytase activity55. Further, improvement of Arabidopsis growth on phytate, when supplemented with CaPAP7 enzyme validated CaPAP7 efficacy to release Pi from phytate by its hydrolysis. Phytases from different sources have also been earlier reported to enhance the plant growth by increasing Pi acquisition and biomass accumulation10, 56–58.
In plants, P used by vegetative tissues is ultimately mobilized to developing seeds, where nearly 75% of it is stored in the form of phytic acid59. During germination and seedling growth, this phytic acid is broken down by the phytases for utilization by seedlings60. Phytate content of chickpea seed is quite high which again corresponds to the need of these phytases during seed development and seed germination. Also, expression of few CaPAPs was quite high in mature leaf tissue, where their role can be correlated with the Pi recycling from old tissues to the new ones. Phytate binds with other minerals like Fe, Mg, Zn and therefore, reduces their bioavailability61, 62. High consumption of phytate rich food is considered as one reason for mineral deficiency in humans in developing world63. Further, almost 50% of applied P fertilizers are removed from farmers field in the form of phytate in seeds every year59. Monogasteric animals cannot digest phytate which is excreted out and led to the eutrophication of water bodies. It is further shown that phytate accumulation is not absolutely necessary for seed functions. Rather, plants store excess of Pi in the form of phytate which seeds use during germination53. Therefore, low phytate food crops are a necessity and such crops can be engineered with new emerging resources.
Different approaches have been used for reducing seed phytate content by targeting phytate biosynthesis enzymes63, its transporter in tissue-specific manner64 or by blocking the translocation of Pi to developing seed65. One of the problems coupled with lowering phytate content by reducing its biosynthesis in seed is corresponding increase in Pi which inhibits starch biosynthesis66. Consequently, low starch accumulation leads to reduced seed weight and crop yield. However, lowering of seed total P by reducing Pi transporter activity does not lead to any significant yield loss while reducing the seed phytate by 50% in rice64. Therefore, while reducing phytate content may affect seed fitness in the wild, it may not be a problem for cultivated crops in fertilized farmers’ fields63. Here, CaPAP7 emerged as potential new genetic/genomic regulator for seed phytate content in chickpea. CaPAP7, besides phytate, can also hydrolyse other organic Pi compounds abundant in cell cytoplasm. It showed high expression in tissues with high phytate content i.e. flower bud (pollens) and young pod, its expression levels correlate negatively with seed phytate and weight. Therefore, it is very likely that CaPAP7 mediated phytate hydrolysis in these tissues results in high soluble Pi content which inhibits starch biosynthesis and ultimately leads to low phytate and seed weight. How precisely CaPAP7 regulates this process, needs further investigations. Nevertheless, our data revealed that CaPAPs have functional diversification in chickpea while some of them may be involved in Pi acquisition, CaPAP7 may play important roles in seed phytate accumulation and seed yield.
Electronic supplementary material
Acknowledgements
Our research is supported by core grant from NIPGR, DBT India. JB, LV and APS acknowledge CSIR, DBT and UGC, respectively for junior and senior research fellowships.
Author Contributions
Study concept and design: J.G. and S.K.P. Experiments: J.B., A.P.S., P.M., L.V. and R.S. Analysis and interpretation of data: J.B., A.P.S., P.M., L.V., R.S., S.K.P. and J.G. Manuscript writing: J.B., A.P.S., P.M., S.K.P. and J.G.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11490-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olczak M, Morawiecka B, Watorek W. Plant purple acid phosphatases-genes, structures and biological function. Acta Biochim. Pol. 2003;50:1245–1256. [PubMed] [Google Scholar]
- 2.Tran HT, Hurley BA, Plaxton WC. Feeding hungry plants: the role of purple acid phosphatases in phosphate nutrition. Plant Sci. 2010;179:14–27. doi: 10.1016/j.plantsci.2010.04.005. [DOI] [Google Scholar]
- 3.Duff SMG, Gautam S, Plaxton WC. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994;90:791–800. doi: 10.1111/j.1399-3054.1994.tb02539.x. [DOI] [Google Scholar]
- 4.Klabunde T, Sträter N, Fröhlich R, Witzel H, Krebs B. Mechanism of Fe (III)–Zn (II) purple acid phosphatase based on crystal structures. J. Mol. Biol. 1996;259:737–748. doi: 10.1006/jmbi.1996.0354. [DOI] [PubMed] [Google Scholar]
- 5.Schenk G, et al. Binuclear metal centers in plant purple acid phosphatases: Fe–Mn in sweet potato and Fe–Zn in soybean. Arch. Biochem. Biophys. 1999;370:183–189. doi: 10.1006/abbi.1999.1407. [DOI] [PubMed] [Google Scholar]
- 6.Mitić N, Noble CJ, Gahan LR, Hanson GR, Schenk G. Metal-ion mutagenesis: conversion of a purple acid phosphatase from sweet potato to a neutral phosphatase with the formation of an unprecedented catalytically competent MnII-MnII active site. J. Am. Chem. Soc. 2009;131:8173–8179. doi: 10.1021/ja900797u. [DOI] [PubMed] [Google Scholar]
- 7.Li D, et al. Purple acid phosphatases of Arabidopsis thaliana comparative analysis and differential regulation by phosphate deprivation. J. Biol. Chem. 2002;277:27772–27781. doi: 10.1074/jbc.M204183200. [DOI] [PubMed] [Google Scholar]
- 8.Hegeman CE, Grabau EA. A novel phytase with sequence similarity to purple acid phosphatases is expressed in cotyledons of germinating soybean seedlings. Plant Physiol. 2001;126:1598–1608. doi: 10.1104/pp.126.4.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, et al. Expression patterns of purple acid phosphatase genes in Arabidopsis organs and functional analysis of AtPAP23 predominantly transcribed in flower. Plant Mol. Biol. 2005;59:581–594. doi: 10.1007/s11103-005-0183-0. [DOI] [PubMed] [Google Scholar]
- 10.Lung SC, et al. Secretion of beta-propeller phytase from tobacco and Arabidopsis roots enhances phosphorus utilization. Plant Sci. 2005;169:341–349. doi: 10.1016/j.plantsci.2005.03.006. [DOI] [Google Scholar]
- 11.Kuang R, Chan KH, Yeung E, Lim BL. Molecular and biochemical characterization of AtPAP15, a purple acid phosphatase with phytase activity, in Arabidopsis. Plant Physiol. 2009;151:199–209. doi: 10.1104/pp.109.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li RJ, et al. Molecular characterization and functional analysis of OsPHY1, a purple acid Phosphatase (PAP)–type phytase gene in rice (Oryza sativa L.) J. Integr. Agric. 2012;11:1217–1226. doi: 10.1016/S2095-3119(12)60118-X. [DOI] [Google Scholar]
- 13.Cashikar AG, Kumaresan R, Rao NM. Biochemical characterization and subcellular localization of the red kidney bean purple acid phosphatase. Plant Physiol. 1997;114:907–915. doi: 10.1104/pp.114.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang C, et al. Biochemical and molecular characterization of PvPAP3, a novel purple acid phosphatase isolated from common bean enhancing extracellular ATP utilization. Plant Physiol. 2010;152:854–865. doi: 10.1104/pp.109.147918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, et al. The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol. 2011;157:1283–1299. doi: 10.1104/pp.111.183723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Pozo JC, et al. A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilising/oxidative stress conditions. Plant J. 1999;19:579–589. doi: 10.1046/j.1365-313X.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 17.Bozzo GG, Raghothama KG, Plaxton WC. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate‐starved tomato (Lycopersicon esculentum) cell cultures. Eur. J. Biochem. 2002;269:6278–6286. doi: 10.1046/j.1432-1033.2002.03347.x. [DOI] [PubMed] [Google Scholar]
- 18.Veljanovski V, Vanderbeld B, Knowles VL, Snedden WA, Plaxton WC. Biochemical and molecular characterization of AtPAP26, a vacuolar purple acid phosphatase up-regulated in phosphate-deprived Arabidopsis suspension cells and seedlings. Plant Physiol. 2006;142:1282–1293. doi: 10.1104/pp.106.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lung SC, et al. Phytase activity in tobacco (Nicotiana tabacum) root exudates is exhibited by a purple acid phosphatase. Phytochemistry. 2008;69:365–373. doi: 10.1016/j.phytochem.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Gruszewski HA, Chevone BI, Nessler CL. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008;146:431–440. doi: 10.1104/pp.107.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hur YJ, Yi YB, Kim TH, Kim DH. Biochemical and molecular analysis of OsPAP1: A phosphate starvation induced purple acid phosphatase gene from rice. Kor. J. Breed. Sci. 2010;42:455–462. [Google Scholar]
- 22.Kaida R, et al. Activation of β-glucan synthases by wall-bound purple acid phosphatase in tobacco cells. Plant Physiol. 2009;150:1822–1830. doi: 10.1104/pp.109.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, et al. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann. Bot. 2012;109:275–285. doi: 10.1093/aob/mcr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Vecchio HA, et al. The cell wall-targeted purple acid phosphatase AtPAP25 is critical for acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. Plant J. 2014;80:569–581. doi: 10.1111/tpj.12663. [DOI] [PubMed] [Google Scholar]
- 25.Mehra P, Pandey BK, Giri J. Improvement in phosphate acquisition and utilization by a secretory purple acid phosphatase (OsPAP21b) in rice. Plant Biotechnol. J. 2017;15:1054–1067. doi: 10.1111/pbi.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao H, et al. GmPAP3, a novel purple acid phosphatase-like gene in soybean induced by NaCl stress but not phosphorus deficiency. Gene. 2003;318:103–111. doi: 10.1016/S0378-1119(03)00764-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Heterologous expression of AtPAP2 in transgenic potato influences carbon metabolism and tuber development. FEBS Lett. 2014;588:3726–3731. doi: 10.1016/j.febslet.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Wang C, Tian J, Li K, Shou H. Identification of rice purple acid phosphatases related to phosphate starvation signalling. Plant Biol. 2011;13:7–15. doi: 10.1111/j.1438-8677.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasarao C, Ganeshamurthy AN, Ali M, Venkateswarlu B. Phosphorus and micronutrient nutrition of chickpea genotypes in a multi-nutrient-deficient typic ustochrept. J. Plant Nutri. 2006;29:747–763. doi: 10.1080/01904160600567082. [DOI] [Google Scholar]
- 30.Kataya AR, Schei E, Lillo C. Towards understanding peroxisomal phosphoregulation in Arabidopsis thaliana. Planta. 2016;243:699–717. doi: 10.1007/s00425-015-2439-5. [DOI] [PubMed] [Google Scholar]
- 31.Garg R, Patel RK, Tyagi AK, Jain M. De novo assembly of chickpea transcriptome using short reads for gene discovery and marker identification. DNA Res. 2011;18:53–63. doi: 10.1093/dnares/dsq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kujur A, et al. A genome-wide SNP scan accelerates trait-regulatory genomic loci identification in chickpea. Sci. Rep. 2015;5:11166. doi: 10.1038/srep11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi-Saha A, Reddy KS. Repeat length variation in the 5ʹUTR of myo-inositol monophosphatase gene is related to phytic acid content and contributes to drought tolerance in chickpea (Cicer arietinum L.) J. Exp. Bot. 2015;6619:5683–5690. doi: 10.1093/jxb/erv156. [DOI] [PubMed] [Google Scholar]
- 34.Singh AP, et al. JAZ repressors: potential involvement in nutrients deficiency response in rice and chickpea. Front. Plant Sci. 2015;6:975. doi: 10.3389/fpls.2015.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehra P, Pandey BK, Giri J. Comparative morphophysiological analyses and molecular profiling reveal Pi-efficient strategies of a traditional rice genotype. Front. Plant Sci. 2015;6:1184. doi: 10.3389/fpls.2015.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamh M, Horst WJ, Amer F, Mostafa H, Maier P. Mobilization of soil and fertilizer phosphate by cover crops. Plant Soil. 1999;211:19–27. doi: 10.1023/A:1004543716488. [DOI] [Google Scholar]
- 37.Nuruzzaman M, Lambers H, Bolland MD, Veneklaas EJ. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil. 2005;271:175–187. doi: 10.1007/s11104-004-2386-6. [DOI] [Google Scholar]
- 38.Sulieman S, Tran LSP. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015;239:36–43. doi: 10.1016/j.plantsci.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Graham PH, Vance CP. Nitrogen fixation in perspective: an overview of research and extension needs. Field Crops Res. 2000;65:93–106. doi: 10.1016/S0378-4290(99)00080-5. [DOI] [Google Scholar]
- 40.Nandwa, S. M. et al. Inter and intra-specific variation of legumes and mechanisms to access and adapt to less available soil phosphorus and rock phosphate in Fighting poverty in sub-Saharan Africa: the multiple roles of legumes in integrated soil fertility management (eds. Bationo, A. et al.) 47–66 (Springer, 2011).
- 41.Tran HT, et al. Biochemical and molecular characterization of AtPAP12 and AtPAP26: the predominant purple acid phosphatase isozymes secreted by phosphate-starved Arabidopsis thaliana. Plant Cell Environ. 2010;33:1789–1803. doi: 10.1111/j.1365-3040.2010.02184.x. [DOI] [PubMed] [Google Scholar]
- 42.Nuruzzaman M, Lambers H, Bolland MD, Veneklaas EJ. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil. 2006;281:109–120. doi: 10.1007/s11104-005-3936-2. [DOI] [Google Scholar]
- 43.Liang, C., Sun, L., Yao, Z., Liao, H. & Tian, J. Comparative analysis of PvPAP gene family and their functions in response to phosphorus deficiency in common bean. PloS One7, (2012). [DOI] [PMC free article] [PubMed]
- 44.Zhang Y, Wang X, Lu S, Liu D. A major root-associated acid phosphatase in Arabidopsis, AtPAP10, is regulated by both local and systemic signals under phosphate starvation. J. Exp. Bot. 2014;65:6577–6588. doi: 10.1093/jxb/eru377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Munoz E, et al. The maize (Zea mays ssp. mays var. B73) genome encodes 33 members of the purple acid phosphatase family. Front. Plant Sci. 2015;6:341. doi: 10.3389/fpls.2015.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehra P, Giri J. Rice and chickpea GDPDs are preferentially influenced by low phosphate and CaGDPD1 encodes an active glycerophosphodiester phosphodiesterase enzyme. Plant Cell Rep. 2016;35:1699–1717. doi: 10.1007/s00299-016-1984-0. [DOI] [PubMed] [Google Scholar]
- 47.Li WYF, Shao G, Lam HM. Ectopic expression of GmPAP3 alleviates oxidative damage caused by salinity and osmotic stresses. New Phytol. 2008;178:80–91. doi: 10.1111/j.1469-8137.2007.02356.x. [DOI] [PubMed] [Google Scholar]
- 48.Robinson WD, Carson I, Ying S, Ellis K, Plaxton WC. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 2012;196:1024–1029. doi: 10.1111/nph.12006. [DOI] [PubMed] [Google Scholar]
- 49.Sun F, et al. AtPAP2 is a tail-anchored protein in the outer membrane of chloroplasts and mitochondria. Plant Signal. Behav. 2012;7:927–932. doi: 10.4161/psb.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong K, et al. Phosphorus remobilization from rice flag leaves during grain filling: an RNA‐seq study. Plant biotechnology journal. 2017;15:15–26. doi: 10.1111/pbi.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy CS, Kim KM, James D, Varakumar P, Reddy MK. PgPAP18, a heat-inducible novel purple acid phosphatase 18-like gene (PgPAP18-like) from Pennisetum glaucum, may play a crucial role in environmental stress adaptation. Acta Physiologiae Plantarum. 2017;39:54. doi: 10.1007/s11738-017-2348-2. [DOI] [Google Scholar]
- 52.Kaida R, et al. Activation of β-glucan synthases by wall-bound purple acid phosphatase in tobacco cells. Plant physiology. 2009;150:1822–1830. doi: 10.1104/pp.109.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raboy V. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 2009;177:281–296. doi: 10.1016/j.plantsci.2009.06.012. [DOI] [Google Scholar]
- 54.White PJ, Veneklaas EJ. Nature and nurture: the importance of seed phosphorus content. Plant Soil. 2012;357:1–8. doi: 10.1007/s11104-012-1128-4. [DOI] [Google Scholar]
- 55.Pandey BK, Mehra P, Verma L, Bhadouria J, Giri J. OsHAD1, a haloacid dehalogenase-like APase enhances phosphate accumulation. Plant Physiol. 2017 doi: 10.1104/pp.17.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yip W, et al. The introduction of a phytase gene from Bacillus subtilis improved the growth performance of transgenic tobacco. Biochem. Biophys. Res. Commun. 2003;310:1148–1154. doi: 10.1016/j.bbrc.2003.09.136. [DOI] [PubMed] [Google Scholar]
- 57.Richardson AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001;28:897–906. doi: 10.1071/PP01093. [DOI] [Google Scholar]
- 58.Wang X, et al. Overexpressing AtPAP15 enhances phosphorus efficiency in soybean. Plant Physiol. 2009;151:233–240. doi: 10.1104/pp.109.138891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lott JNA, Ockenden I, Raboy V. & Batten, G. D. Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci. Res. 2000;10:11–33. [Google Scholar]
- 60.Loewus FA, Murthy PP. Myo-Inositol metabolism in plants. Plant Sci. 2000;150:1–19. doi: 10.1016/S0168-9452(99)00150-8. [DOI] [Google Scholar]
- 61.Sandberg AS. Bioavailability of minerals in legumes. Br. J. Nutr. 2002;88:281–285. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- 62.Weaver, C. M. & Kannan, S. Phytate and mineral bioavailability in Food phytates (eds Reddy, N. R. & Sathe, S. K.) 211–23 (CRC Press, 2002).
- 63.Raboy V. Seeds for a better future:‘low phytate’grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001;6:458–462. doi: 10.1016/S1360-1385(01)02104-5. [DOI] [PubMed] [Google Scholar]
- 64.Shi J, et al. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nature Biotechnol. 2007;25:930–937. doi: 10.1038/nbt1322. [DOI] [PubMed] [Google Scholar]
- 65.Yamaji N, et al. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature. 2017;541:92–95. doi: 10.1038/nature20610. [DOI] [PubMed] [Google Scholar]
- 66.Plaxton WC, Preiss J. Purification and properties of nonproteolytic degraded ADP glucose pyrophosphorylase from maize endosperm. Plant Physiol. 1987;83:105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.