Abstract
Globally, tuberculosis (Tb) notification data show a male-to-female ratio of 1.7 and higher, but the underlying reasons for the male bias remain elusive. Despite the well-known gender bias in human pulmonary Tb, a majority of experimental animal studies either do not separate and analyze data by sex or do not report the sex of their subjects at all. In the present study, we report increased male susceptibility in one of the most commonly used mouse models for Tb, C57BL/6 mice. Our study revealed that disease progression upon aerosol infection with Mycobacterium tuberculosis (Mtb) was accelerated in males resulting in increased morbidity and mortality compared to females. Elevated Mtb loads in males were associated with an early exaggerated pulmonary inflammatory response which likely was detrimental to the host, as reflected by exacerbated pathology and increased mortality. Our data emphasis the urgent need to include and separately analyze both sexes in future animal studies of Tb in order to appreciate the differences in immune responses and disease pathogenesis between males and females.
Introduction
Tuberculosis (Tb) is the most prevalent bacterial infectious disease in humans. The causative agent, Mycobacterium tuberculosis (Mtb), is carried by an estimated 2–3 billion people globally and claimed approximately 1.8 million lives in 20151. Tb rates are much higher in men than in women, as reflected by a global male/female ratio of 1.7 for case notifications in 20161. This excess of male pulmonary Tb cases can be observed in adult HIV-negative populations, but not in children and young adolescents2. It is widely believed that socioeconomic and cultural factors are responsible for the observed gender bias. Differences in access to health care and the quality of sputum samples are thought to result in under-notification of women and to bias case reporting2, 3. Furthermore, confounding factors such as smoking, alcohol or drug use are regarded responsible for higher Tb rates in men. However, several studies now provide evidence that sex differences in Tb are not merely due to differences in case reporting or other confounding factors. Studies in both developing and developed countries reported an excess of Tb cases in males even if confounding factors were taken into account4–6. Active case-finding in such surveys could rule out differences in health care access as underlying reason for the observed bias. A recent study on risk factors for Tb in Germany revealed that latently infected household contacts showed a balanced gender distribution but active Tb was more prominent in men7. These data suggest an increased risk for disease progression in men.
There is clearly a lack of information on the role of biological sex in Tb. Mouse infection models that reflect the epidemiological observations allow for the analysis of the molecular basis of sex-dependency in Tb disease outcome. Bini and colleagues reported male BALB/c mice to be more susceptible to Mtb infection, showing significantly higher bacilli burdens and mortality compared to females and castrated males8. The C57BL/6 mouse is the most widely used inbred strain to study experimental Tb. Therefore, it was of major interest to determine potential differences in resistance between the sexes in the C57BL/6 model of Tb. Our study revealed that disease progression upon aerosol Mtb infection was accelerated in males resulting in increased morbidity and mortality compared to females. In contrast to BALB/c, elevated Mtb loads in male C57BL/6 were associated with exaggerated inflammatory responses and worse pulmonary pathology which probably was detrimental to the host and responsible for the increased mortality.
Results
Decreased resistance of male C57BL/6 mice to Mtb infection
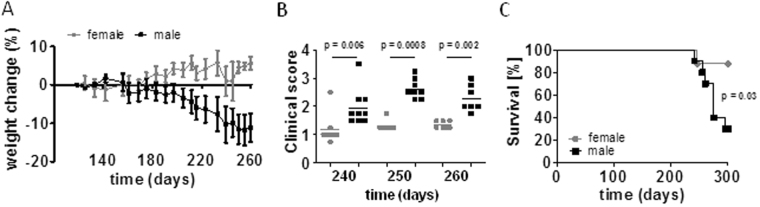
Female and male C57BL/6 mice were infected with Mtb H37Rv via the aerosol route and monitored for clinical signs of disease as described in materials and methods. Males started to lose weight around day 200 and developed symptoms as reflected by an increase in clinical score (Fig. 1A, B). Consequently, 70% of males succumbed to Mtb infection with the remaining animals showing high clinical scores whereas 90% of females survived the observation period of 300 days without overt symptoms (Fig. 1B, C).
Figure 1.
Decreased resistance of male C57BL/6 mice to Mtb infection. Male and female C57BL/6 were infected via aerosol with Mtb H37Rv and monitored for weight loss (A), development of clinical symptoms (B), and survival (C). Data pooled from two independent experiments are shown as mean ± SD (n = 8–10). Statistical analysis was performed by Mann-Whitney test (clinical score) or log rank test (survival).
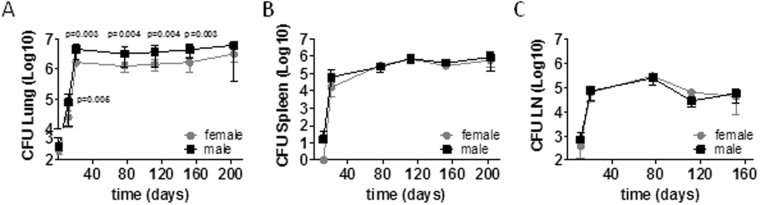
Mycobacterial loads in male lungs were significantly elevated compared to females but controlled at a steady level (Fig. 2A). We did not find differences in bacterial loads in the spleen and mediastinal lymph node between the sexes (Fig. 2B, C) indicating that bacterial dissemination from the lungs to other organs was not enhanced in males.
Figure 2.
Mycobacterial loads are increased in male lungs. Male and female C57BL/6 were infected via aerosol with Mtb H37Rv. At different time points, CFU were determined in organ homogenates. Data pooled from two independent experiments are shown as mean ± SD (n = 8–10). Statistical analysis was performed by Mann-Whitney test.
In conclusion, we found increased mycobacterial loads, accelerated disease progression and premature death upon aerosol Mtb infection in C57BL/6 males compared to females.
Pulmonary pathology develops differently in males and females
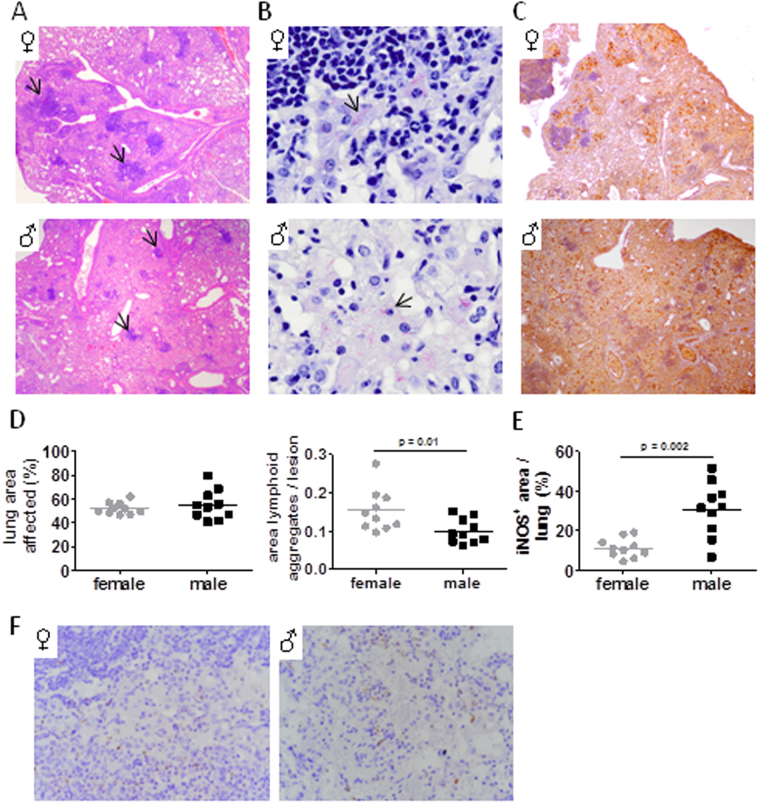
Histopathological examination of lungs five months after Mtb infection revealed progressive pathology with striking differences in the quality of the granulomatous lesions between the sexes. We observed typical lesions consisting of characteristic foamy macrophages surrounding a lymphocytic core structure in females (Fig. 3A, arrows). In contrast, these lymphoid aggregates appeared much smaller in size in males. Quantitative analysis indeed revealed that the overall lung area covered by lesions was comparable between the sexes, but the ratio of lymphocytic aggregate to lesion area was significantly reduced in males (Fig. 3D). Instead, surrounding myeloid infiltrates dominated in male lungs and covered greater regions compared to females, suggesting differences in immune cell recruitment and spatial organization. Mycobacteria were mainly associated with foamy macrophages in both sexes but observed in greater numbers in males (Fig. 3B, arrows), confirming CFU data. Increased mycobacterial burden in males prompted us to analyse iNOS expression which to our surprise was much more abundant and ubiquitously distributed in the male lung tissue while its expression was more discrete and localized to areas surrounding lymphoid infiltrates in females (Fig. 3C). Quantitative analysis of histological sections confirmed that iNOS expression was significantly increased in male compared to female lungs (Fig. 3E).These data indicate that in males, the immune response is dysregulated and generates an inflammatory response that leads to worse pulmonary pathology that is detrimental to the host, as reflected by increased mortality.
Figure 3.
Histological characteristics of Mtb infected lungs. Male and female C57BL/6 were infected via aerosol with Mtb H37Rv and histologically evaluated after 152 days. (A) Granulomatous lesions in H&E stained lung sections (arrows: lymphoid aggregates). (B) Acid fast bacilli (arrows). (C) Immunohistochemical staining of iNOS. Representative micrographs from one mouse out of 10 mice per group are shown (original magnification × 40 in A and C; × 400 in B). (D) Quantitative assessment of total lung area affected by lesions (left graph) and the ratio of lymphoid aggregates to lesion area (right graph). (E) Quantitative evaluation of iNOS expression shown in (C. F) Immunohistochemical staining of neutrophils (Ly6B.2). Representative micrographs from one mouse out of 10 mice per group are shown (original magnification × 100).
Pulmonary inflammatory responses to Mtb are enhanced in males
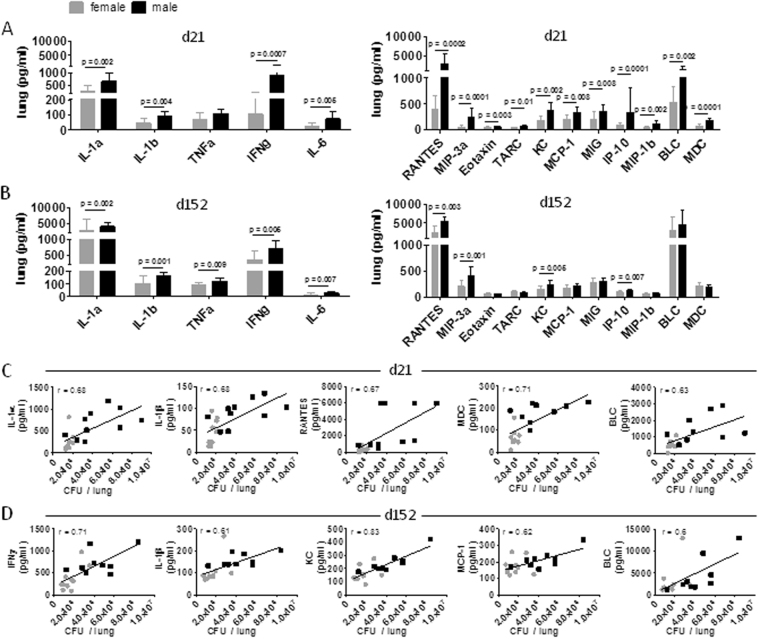
In order to identify immune differences in female and male lungs that might explain differences in pathology observed we next analyzed the production of a number of relevant cytokines and chemokines early and late during Mtb infection. Among those tested the pro-inflammatory mediators IL1α, IL1β, IFNγ and IL-6 and numerous chemokines were substantially elevated in male lungs as early as 21 days post infection (Fig. 4A). All cytokines, RANTES, MIP-3α, KC and IP-10 and in addition TNFα were still elevated in males after 152 days (Fig. 4B), indicating sustained inflammation. Correlation analysis including all mice revealed that certain cyto- and chemokines were positively correlated with bacterial burden in lungs on day 21 and 152 of Mtb infection, respectively (Fig. 4C and D). When separating individual mice by sex it became obvious that those mice that showed high CFU and high cyto- or chemokine levels were predominantly males. Since the strongest positive correlation in males was observed for KC in late infection, we stained lung sections with the anti-neutrophil antibody 7/4 (recognizing Ly-6B.2) to see whether increased neutrophil recruitment was apparent in males. However, we only found few neutrophils in both sexes and no apparent difference or increase in male lungs (Fig. 3F).
Figure 4.
Increased inflammatory responses upon Mtb infection in male lungs. Cytokine and chemokine levels were measured in lung lysates of female and male C57BL/6 mice on day 21 (A) and 152 (B) after Mtb H37Rv aerosol infection. Data pooled from two independent experiments are shown as mean ± SD (n = 9–10). Statistical analysis was performed by Mann-Whitney test. Correlations between cyto- or chemokines plotted versus bacterial burden in in lungs for day 21 (C) or d152 (D) in males and females. Each dot represents an individual sample (n = 9–10; females gray; males black).
Our results suggest that differences in early cytokine and chemokine signaling during pulmonary Tb contribute to the magnitude and quality of the granulomatous response, which participate in differences in disease development and progression between males and females.
Discussion
Many studies in humans and experimental animals have established clear links between sex-specific factors and the differential susceptibility of males and females to a number of infectious and noninfectious diseases9, 10. Generally, females mount higher innate and adaptive immune responses than males, which enables faster clearance of pathogens but increases susceptibility to inflammatory and autoimmune diseases10. A few years ago the NIH announced new policies to ensure that preclinical research funded by the NIH considers both males and females11 however many experimental studies continue to neglect sex-based considerations and analyses of data by sex. Likewise, despite the well-known gender bias in human pulmonary Tb, a majority of experimental animal studies either do not separate and analyze data by sex or do not report the sex of their subjects at all. Importantly, sex differences in mycobacterial infections are not only observed in humans but also in wildlife. M. bovis infection is commonly reported in deer where males seem to be more affected than females12, 13. Moreover, an important reservoir and source of bovine tuberculosis in cattle is the European badger, where females are more resilient to established M. bovis infection than male badgers and present with longer survival times following the detection of bacterial excretion14. Therefore, there is an urgent need to investigate the molecular basis of sex-dependency in Tb disease outcome. We show here for the first time that the widely used C57BL/6 model of experimental Tb reflects the epidemiological findings of increased male susceptibility. Like in BALB/c8, aerosol Mtb infection resulted in increased morbidity and mortality in C57BL/6 males compared to females, indicating that increased male susceptibility is independent of the genetic background. In clear contrast to BALB/c, male C57BL/6 mice responded with augmented and sustained pulmonary inflammation to Mtb infection. Profound differences between the sexes were already observed after three weeks with overproduction of hallmark pro-inflammatory cytokines IL1α, IL1β and IFNγ and a broad range of chemokines being indicative of excessive inflammation in response to Mtb infection in males. Despite a robust pro-inflammatory immune response, mycobacterial loads in male lungs were significantly increased, indicating that Mtb induces a sustained non-protective inflammatory reaction in the male lung that is detrimental to the host, as reflected by increased mortality. Our results are in line with the overall conception that cytokines which are critically required for resistance to Mtb infection become potentially destructive when produced uncontrolled. For instance, IL1β production needs to be controlled at the inflammasome level in order to prevent detrimental innate inflammatory responses during Mtb infection15 and a polymorphism in the human IL1B promoter region associated with increased production of IL1β is associated with active Tb, severity of disease and poor treatment outcome16. Recently, Sakai and colleagues elegantly demonstrated that increasing pulmonary IFNγ production exacerbated Mtb infection and that negative regulation of IFNγ production is required to prevent lethal immune-mediated pathology17.
In line with elevated pro-inflammatory mediators was the increased pulmonary pathology characterized by reduced lymphoid aggregates but large areas of myeloid cell infiltrates in male lungs. Despite increased levels of KC in males, neutrophils were hardly detected in lungs of both sexes and thus were unlikely to contribute to pathology in males. In contrast to more susceptible mouse models such as C3H, DBA or I/St, the C57BL/6 mouse model of Tb is not associated with neutrophil accumulation and neutrophil-driven pathology18–21 and neutrophil depletion in C57BL/6 mice did not increase mycobacterial loads in different studies22, 23. Increased IFNγ in males might explain why despite the high levels of KC neutrophils were not found to accumulate in male lung tissue. Nandi and Behar have demonstrated that IFNγ impairs neutrophil survival and inhibits pathogenic neutrophil accumulation in the infected lung24 presumably by suppressing the expression of E- and P-selectin on endothelial cells which are important for neutrophil trafficking into inflamed tissue25, 26 and by the induction of iNOS expression. NO prevents neutrophil recruitment15, 27 while its absence results in increased neutrophil recruitment and tissue necrosis at the site of Mtb infection15, 27–30. Thus, increased IFNγ together with IL1 likely induced the strong and ubiquitous iNOS expression in male lung tissue and thereby prevented the accumulation of neutrophils. Moreover, the ratio of local to systemic chemokine concentrations seems to be critical for local neutrophil recruitment31. We did not determine systemic cyto- or chemokine levels and therefore can only speculate that systemic levels of KC might even exceed those of local concentrations determined in infected lung tissue.
Instead of neutrophils, the most prominent myeloid cell in Mtb infected C57BL/6 lungs is the macrophage20 and in particular foamy macrophages are key players in sustaining persistent bacteria and tissue pathology32. Reports by the group of Sher and Ramakrishnan demonstrated that the recruitment of a permissive monocyte/macrophage population in a CCL2/CCR2 dependent manner is detrimental in Mtb infection33, 34. We believe that more permissive macrophages are recruited to male compared to female lungs and less lymphocytes as reflected by smaller lymphoid cores, and ultimately promote disease progression and premature death.
It has become increasingly clear that early events in Mtb infection are of major importance in dictating clinical outcome. Data from nonhuman primate models suggest that between 3–6 weeks post infection, one can predict whether the animal will progress to active Tb or remain latent until 6–9 month later35. The numbers of initial granulomas and increasing inflammation in the first 6 weeks were associated with development of active Tb. Moreover, early greater IFNγ production was observed among macaques that would later develop active Tb than for those that developed latent infection36. Likewise, in a panel of genetically heterogeneous mice, Tb progression correlated with increased lung expression of inflammation-related factors37. The identification of biomarkers or transcriptional signatures that prospectively predict progression of Mtb infection to active disease is a great area of research. However, lung or BAL samples are not available for routine analysis and thus cannot be used to predict disease progression. Instead whole blood transcriptional profiling offers great prognostic potential and several groups have identified transcripts that are associated with active Tb in humans38–40. Together the different studies suggest an inflammatory manifestation of Tb and a predictive signature identified by Zak et al. suggests that peripheral activation of an IFN-inducible transcriptional signature which was also associated with active Tb in other studies precedes the onset of active disease41. Peripheral responses might differ from those observed locally at the site of infection and differences in Mtb specific T cell responses between BAL fluid and PBMCs were demonstrated among active Tb patients42, 43. Nevertheless, human blood transcriptional signatures in active Tb are supported by data from experimental Mtb infection models which consistently show that excess inflammation in the lung is associated with progressive Tb. In a macaque model, Gideon and colleagues could demonstrate that early blood transcript activity was highly correlated with lung inflammation44, indicating that indeed peripheral responses reflect events at the site of infection. Moreover, analysis of preinfection signatures of macaques revealed that IFN signatures could influence eventual clinical outcomes even before infection, reflecting the observations in humans. In order to better complement epidemiological studies it will be important to analyse blood levels of immune mediators in our mouse model of experimental Mtb infection in the future. This is of particular interest in light of preinfection signatures predictive for the risk to develop active Tb disease which may differ between the sexes. Inherent functional differences in innate and adaptive immune mechanisms involved in early host-pathogen interaction likely influence the overall outcome of Mtb infection. It is well known that innate recognition of pathogens and the induction of inflammatory immune responses differ between the sexes, one reason being the influence of sex steroid hormones10, 45. Alveolar macrophages are the first cells that encounter Mtb in the host lung and initiate a local inflammatory immune response18. In addition, macrophages, together with DCs, serve as antigen-presenting cells that initiate and regulate effector T-cell responses. Scotland and colleagues reported on fundamental sex differences in resident immune cell phenotype in the peritoneal cavity of mice which was reflected by greater TLR expression and enhanced phagocytosis and bacterial killing in females compared to males46. On the other hand, macrophage-derived cytokine production was diminished in females because of proportionally more immunomodulatory CD4+ T-cells. Likewise, Yang and colleagues observed superior bacterial killing by female alveolar macrophages47 accompanied by diminished lung inflammation and better survival. Thus, while females show superior anti-bacterial killing over males, they seem to counterbalance excess inflammation which results in better survival. We observed increased mycobacterial loads in male lungs as early as 12 days after infection. Thus, an important question to answer in the future is whether mycobacterial killing is impaired in alveolar macrophages of males. Overproduction of pro-inflammatory mediators secondary to elevated bacterial loads may perpetuate exaggerated inflammatory responses leading to inappropriate immune cell trafficking and activation in the male lung which ultimately leads to increased mortality of males compared to females. Alternatively, the ability to regulate inflammatory responses induced by Mtb might be impaired in males.
To the best of our knowledge, this is the first report showing sex differences in the commonly used C57BL/6 model of aerosol Mtb infection. Although the increased susceptibility of males only becomes apparent during late-stage infection with regard to clinical score and survival, this phenotype clearly is associated with an augmented early inflammatory immune response which presumably contributes significantly to the accelerated disease development and progression in males. Therefore, our data emphasis the need to include and separately analyze both sexes in future animal studies of Tb in order to appreciate the differences in immune responses and disease pathogenesis between males and females. A comprehensive understanding of the sex disparity in Tb will help to develop prognostic tools or host-directed therapies and may be important when evaluating the effectiveness of vaccines and other immunological interventions.
Methods
Ethics Statement
Animal experiments were in accordance with the German Animal Protection Law and approved by the Ethics Committee for Animal Experiments of the Ministry of Energy, Agriculture, Environment, and Rural Areas of the State of Schleswig-Holstein under the license 103–9/14.
Mice, bacterial infection and colony forming units (CFU)
C57BL/6 mice were bred under specific-pathogen-free conditions at the Research Center Borstel. Female and male C57BL/6 mice aged between 8–12 weeks were used and maintained under specific barrier conditions in BSL 3 facilities.
Mtb H37Rv was grown in Middlebrook 7H9 broth (BD Biosciences) supplemented with 10% v/v OADC (Oleic acid, Albumin, Dextrose, Catalase) enrichment medium (BD Bioscience). Bacterial aliquots were frozen at −80 °C. Viable cell counts in thawed aliquots were determined by plating serial dilutions onto Middlebrook 7H10 agar plates supplemented with 10% v/v heat-inactivated bovine serum followed by incubation at 37 °C.
For infection of experimental animals, Mtb stocks were diluted in sterile distilled water at a concentration providing an uptake of 200 viable bacilli per lung. Infection was performed via the respiratory route by using an aerosol chamber as described previously48. The inoculum size was quantified 24 h after infection by determining CFU in the lungs of infected mice. CFU in lung, mediastinal lymph nodes and spleen were evaluated at different time points after aerosol infection by mechanical disruption of the organs in 0.05% v/v Tween 20 in PBS containing a proteinase inhibitor cocktail (Roche) prepared according to the manufacturer’s instructions. Tenfold serial dilutions of organ homogenates in sterile water/1% v/v Tween 80/1% w/v albumin were plated onto Middlebrook 7H11 agar plates supplemented with 10% v/v heat-inactivated bovine serum and incubated at 37 °C for 3–4 weeks.
Evaluation of disease
A clinical score was used to indicate severity of disease progression. Animals were scored in terms of general behavior, activity, feeding habits, and weight gain or loss. Animals with severe symptoms (reaching a clinical score of ≥ 3.5) were euthanized to avoid unnecessary suffering, and the time point that followed was denoted the time of death.
Multiplex cytokine assay
The concentrations of various cytokines and chemokines in lung homogenates were determined by LEGENDplexTM (Mouse Inflammation panel and Mouse Proinflammatory Chemokine Panel, BioLegend) according to the manufacturer’s protocol.
Histology
Superior lung lobes from infected mice were fixed with 4% w/v paraformaldehyde for 24 h, embedded in paraffin, and sectioned (4 µm). Sections were stained with hematoxylin and eosin (H&E), carbol fuchsin (Merck) followed by decolorization with acid-alcohol to visualize mycobacteria in the lungs. iNOS or neutrophils were detected by a polyclonal rabbit anti inducible nitric oxid synthase (iNOS; Merck Millipore) or monoclonal rat anti neutrophils (clone 7/4; Cedarlane), respectively, followed by secondary antibody (biotinylated goat anti rabbit; Dianova), amplification (avidin-HRP) and color reaction (DAB solution; Vectastain). Slides were imaged with a BX41 light microscope and cell^B software. The quantitative analysis of lesion size and lymphoid aggregates was conducted by determination of whole lung area (5–8 images per mouse) as well as the lesion area of H&E stained slides with cell^B. The same images were analyzed by a second person to determine the area of lymphocytic aggregates per lung using the software Fiji ImageJ (freeware). The analyses were performed in a blinded manner. Quantification of iNOS expression was analyzed by ImageJ (1–2 representative images per mouse). Hematoxylin and DAB color were deconvolved (pre-existing plug-in by ImageJ) and the threshold was set for the DAB component to exclude unspecific signals. Lung area of the representative images was determined and the proportion of iNOS signal was calculated. iNOS signals of blood vessels were excluded.
Statistical Analysis
Statistical analysis was performed by Mann-Whitney test or by log rank test as described in the figure legends. Correlation between variables was determined by calculating Pearson’s coefficient using a 2-tailed analysis. A correlation was taken into account as of r ≥ 0.60 (defined as strong correlation). Significance was defined as P ≤ 0.05. All data were analysed using GraphPad Prism 5 (GraphPad Software, Inc.).
Acknowledgements
We thank Doreen Beyer for culturing of Mtb H37Rv, Imke Storm for technical assistance, Johanna Volz for helpful discussions regarding immunohistochemistry and Jochen Behrends and Hannelore Lotter for critical discussions and helpful comments on the manuscript. This work was supported by in-house funding from the Research Center Borstel.
Author Contributions
E.S. conceived and designed the experiments; J.D. and L.E. performed the experimental work; J.D. and B.E.S. analyzed the data; B.E.S. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-24598-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Global tuberculosis report 2016. (2016).
- 2.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS medicine. 2009;6:e1000199. doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhines AS. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis. 2013;93:104–107. doi: 10.1016/j.tube.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2000;4:123–132. [PubMed] [Google Scholar]
- 5.Martinez AN, Rhee JT, Small PM, Behr MA. Sex differences in the epidemiology of tuberculosis in San Francisco. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2000;4:26–31. [PubMed] [Google Scholar]
- 6.Boum Y, 2nd, et al. Male Gender is independently associated with pulmonary tuberculosis among sputum and non-sputum producers people with presumptive tuberculosis in Southwestern Uganda. BMC infectious diseases. 2014;14:638. doi: 10.1186/s12879-014-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzmann, C. et al. Risk for latent and active tuberculosis in Germany. Infection, doi:10.1007/s15010-016-0963-2 (2016). [DOI] [PMC free article] [PubMed]
- 8.Bini EI, et al. The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PloS one. 2014;9:e93831. doi: 10.1371/journal.pone.0093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markle JG, Fish EN. SeXX matters in immunity. Trends in immunology. 2014;35:97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Olvera JR, et al. Sex-related differences in body condition and serum biochemical parameters in red deer (Cervus elaphus) naturally infected with Mycobacterium bovis. Veterinary journal. 2013;198:702–706. doi: 10.1016/j.tvjl.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Shury TK, Bergeson DL. Distribution and Epidemiology of Mycobacterium bovis in Elk and White-Tailed Deer in South-Western Manitoba, Canada. Veterinary medicine international. 2011;2011:591980. doi: 10.4061/2011/591980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson AJ, Chambers MA, Wilson GJ, McDonald RA, Delahay R. J. Sex-related heterogeneity in the life-history correlates of Mycobacterium bovis infection in European badgers (Meles meles). Transboundary and emerging diseases. 2013;60(Suppl 1):37–45. doi: 10.1111/tbed.12097. [DOI] [PubMed] [Google Scholar]
- 15.Mishra BB, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nature immunology. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G, et al. Allele-specific induction of IL-1beta expression by C/EBPbeta and PU.1 contributes to increased tuberculosis susceptibility. PLoS pathogens. 2014;10:e1004426. doi: 10.1371/journal.ppat.1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai S, et al. CD4 T Cell-Derived IFN-gamma Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease. PLoS pathogens. 2016;12:e1005667. doi: 10.1371/journal.ppat.1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10:995–1004. doi: 10.1016/j.micinf.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Dallenga, T. & Schaible, U. E. Neutrophils in tuberculosis–first line of defence or booster of disease and targets for host-directed therapy? Pathogens and disease74, doi:10.1093/femspd/ftw012 (2016). [DOI] [PubMed]
- 20.Tsai MC, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cellular microbiology. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Yeremeev V, Linge I, Kondratieva T, Apt A. Neutrophils exacerbate tuberculosis infection in genetically susceptible mice. Tuberculosis. 2015;95:447–451. doi: 10.1016/j.tube.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Keller C, et al. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infection and immunity. 2006;74:4295–4309. doi: 10.1128/IAI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiler P, et al. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. The Journal of infectious diseases. 2000;181:671–680. doi: 10.1086/315278. [DOI] [PubMed] [Google Scholar]
- 24.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. The Journal of experimental medicine. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melrose J, Tsurushita N, Liu G, Berg EL. IFN-gamma inhibits activation-induced expression of E- and P-selectin on endothelial cells. Journal of immunology. 1998;161:2457–2464. [PubMed] [Google Scholar]
- 27.Mishra BB, et al. Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nature microbiology. 2017;2:17072. doi: 10.1038/nmicrobiol.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beisiegel M, et al. Combination of host susceptibility and virulence of Mycobacterium tuberculosis determines dual role of nitric oxide in the protection and control of inflammation. J Infect Dis. 2009;199:1222–1232. doi: 10.1086/597421. [DOI] [PubMed] [Google Scholar]
- 29.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper AM, Pearl JE, Brooks JV, Ehlers S, Orme IM. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect Immun. 2000;68:6879–6882. doi: 10.1128/IAI.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Call DR, et al. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. The American journal of pathology. 2001;158:715–721. doi: 10.1016/S0002-9440(10)64014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature immunology. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonelli LR, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. The Journal of clinical investigation. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cambier CJ, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman MT, et al. Early Changes by (18)Fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after Mycobacterium tuberculosis infection in cynomolgus macaques. Infection and immunity. 2014;82:2400–2404. doi: 10.1128/IAI.01599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin PL, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infection and immunity. 2006;74:3790–3803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyadova IV, et al. In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs. PloS one. 2010;5:e10469. doi: 10.1371/journal.pone.0010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry MP, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maertzdorf J, et al. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PloS one. 2011;6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maertzdorf J, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes and immunity. 2011;12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 41.Zak DE, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387:2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barry SM, Lipman MC, Bannister B, Johnson MA, Janossy G. Purified protein derivative-activated type 1 cytokine-producing CD4 + T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. The Journal of infectious diseases. 2003;187:243–250. doi: 10.1086/346112. [DOI] [PubMed] [Google Scholar]
- 43.Jafari C, et al. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. The European respiratory journal. 2008;31:261–265. doi: 10.1183/09031936.00096707. [DOI] [PubMed] [Google Scholar]
- 44.Gideon HP, Skinner JA, Baldwin N, Flynn JL, Lin PL. Early Whole Blood Transcriptional Signatures Are Associated with Severity of Lung Inflammation in Cynomolgus Macaques with Mycobacterium tuberculosis Infection. Journal of immunology. 2016;197:4817–4828. doi: 10.4049/jimmunol.1601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein SL, Flanagan KL. Sex differences in immune responses. Nature reviews. Immunology. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 46.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, Z. et al. Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target. eLife3, doi:10.7554/eLife.03711 (2014). [DOI] [PMC free article] [PubMed]
- 48.Mueller, A. K., Behrends, J., Blank, J., Schaible, U. E. & Schneider, B. E. An Experimental Model to Study Tuberculosis-Malaria Coinfection upon Natural Transmission of Mycobacterium tuberculosis and Plasmodium berghei. Journal of visualized experiments: JoVE, doi:10.3791/50829 (2014). [DOI] [PMC free article] [PubMed]