Abstract
Early afterdepolarizations (EADs) have been attributed to two primary mechanisms: 1) recovery from inactivation of the L-type calcium (Ca) channel and/or 2) spontaneous Ca release, which depolarizes the membrane potential through the electrogenic sodium-calcium exchanger (NCX). The sodium (Na) current (INa), especially the late component of the Na current, has been recognized as an important player to set up the conditions for EADs by reducing repolarization reserve and increasing intracellular Na concentration, which leads to Ca overload. However, INa itself has not been considered as a direct initiator of EADs. A recent experimental study by Horvath et al. has shown that the amplitude of the late component of the Na current is as large as potassium (K) and Ca currents (∼1 pA/pF). This result suggests that INa by itself can exceeds the sum of outward currents and depolarize the membrane potential. In this study, we show that INa can also directly initiate EADs. Mathematical analysis reveals a fundamental dynamical origin of EADs arising directly from the Na channel reactivation. This system has three fixed points. The dynamics of the INa mediated EAD oscillation is different from that of the membrane voltage oscillation of the pacemaker cell, which has only one fixed point.
Keywords: Medicine, Applied mathematics, Systems biology, Cell biology, Physiology, Cardiology, Biophysics
1. Introduction
Cardiac arrhythmia is often triggered by premature ventricular contractions (PVCs), which have been linked to early afterdepolarizations (EADs) [1, 2, 3, 4, 5]. EADs have been thought to be caused by reactivation of the L-type calcium (Ca) channel or spontaneous Ca releases from the sarcoplasmic reticulum (SR), which depolarize the membrane potential (Vm) via the electrogenic sodium(Na)-Ca exchanger (NCX) [6, 7, 8, 9, 10, 11, 12, 13]. During the upstroke (phase 0) of the action potential (AP), Na channel opening gives large but extremely short (∼1 ms) inward current [14]. Then, immediately following the upstroke, the Na channel goes to the inactivated state. At the plateau phase of AP, the amplitude of Na current (INa) is thought to be much smaller than the other currents and the shape and the duration of AP are mainly determined by the other currents such as the L-type Ca current (ICaL), NCX, and potassium (K) currents [15]. Na channel mutations have been associated with long QT syndrome by increasing window current and non-inactivating current [16, 17, 18]. Recent experimental measurement by Horvath et al. has shown that the amplitude of INa at phases 2 and 3 of AP (‘late component of the Na current’ or simply ‘late Na current’) can be surprisingly large and of similar amplitude to outward K currents [19]. This implies that the inward current via INa may become larger than the sum of outward currents and Vm can be depolarized. In this study, using a physiologically detailed model of a cardiac ventricular myocyte, we show that INa not only sets up the conditions for EADs by reducing repolarization reserve and increasing intracellular Na concentration, which leads to Ca overload, but also can directly initiate EADs. Mathematical reduction of the detailed model was then performed to generate 2- and 3-variable models, whose variables are membrane potential, inactivation of the Na channel, and the K conductance (for the third variable of the 3-variable model). Analysis in the reduced models reveals a fundamental dynamical origin of EADs arising directly from the Na channel reactivation, as oscillations of Vm at phases 2 and/or 3 of AP. Oscillatory behavior has also been extensively investigated in neuron [20, 21, 22] and pacemaker cells [23, 24]. We show that these ventricular myocyte EADs have a different dynamical mechanism from those of pacemaker cell Vm oscillation.
2. Materials and methods
2.1. Mathematical formulation
We use a physiologically detailed mathematical model of the rabbit ventricular action potential by Mahajan et al. [25]. The membrane voltage is governed by
where V is the membrane voltage, Cm is the cell capacitance, I represents the transmembrane currents. The details of the mathematical model are described in the next section.
There are several proposed mechanisms of the late component of INa [16, 17, 18]. In this study we consider two mechanisms; (1) large window current mechanism and (2) non-inactivating current mechanism.
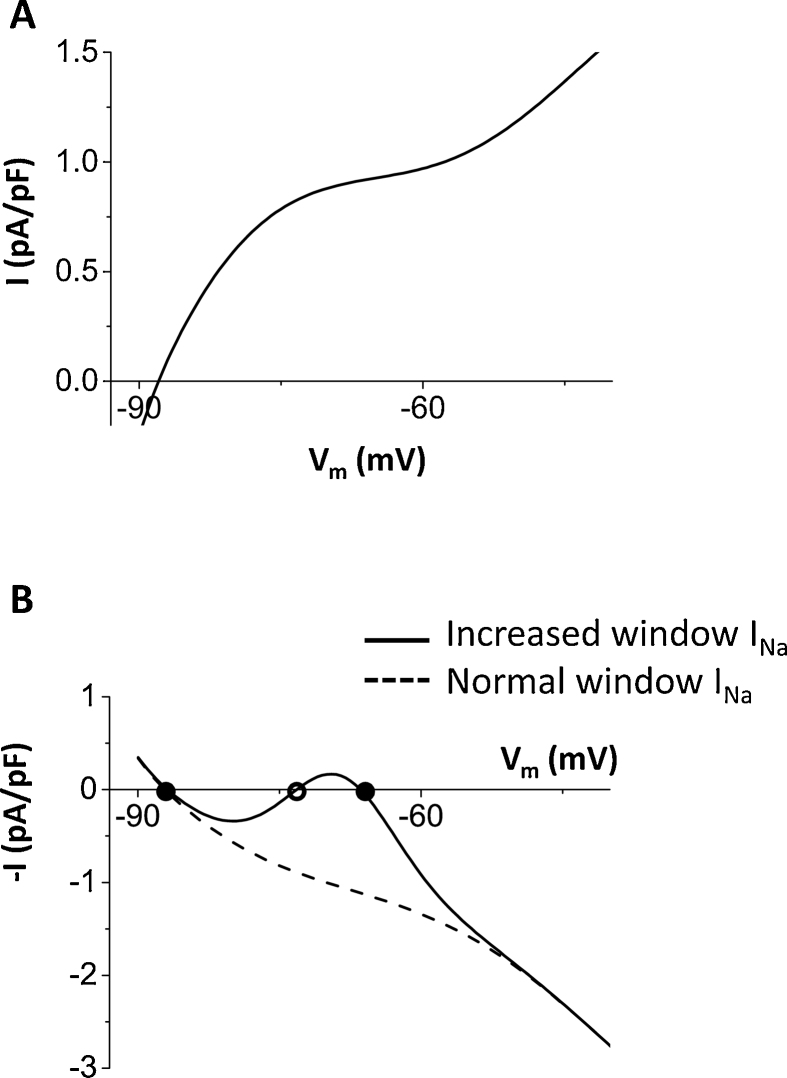
In order to increase the window current, activation and inactivation curves are shifted (Fig. 1A). The formula for INa is
where GNa is the maximum conductance, ENa is the reversal potential given by , where R is the gas constant, T is the temperature, F is the Faraday constant, [Na]o is the outside Na concentration, [Na]i is the cytosolic Na concentration. m is the Na activation and h and j are the fast and slow Na inactivation, respectively. The window current of INa was increased so that the peak of the ‘window’ becomes about 2–3 percent based on the experimental observations (Fig. 1A inset) [26]. Here we note that in the major mathematical models including Mahajan et al. model, Luo-Rudy passive model (LR1) [27], Luo-Rudy dynamic model (LRd) [28], and Shannon-Bers model [29], the peak Vm of the window is higher (−50 to −60 mV) than that in the experimental observations (−60 to –70 mV) [26]. Therefore, we shifted the curves so that the peak Vm of the window correspond reasonably well with the experimental measurements [26]. In order to shift the window, activation and inactivation are changed as follows.
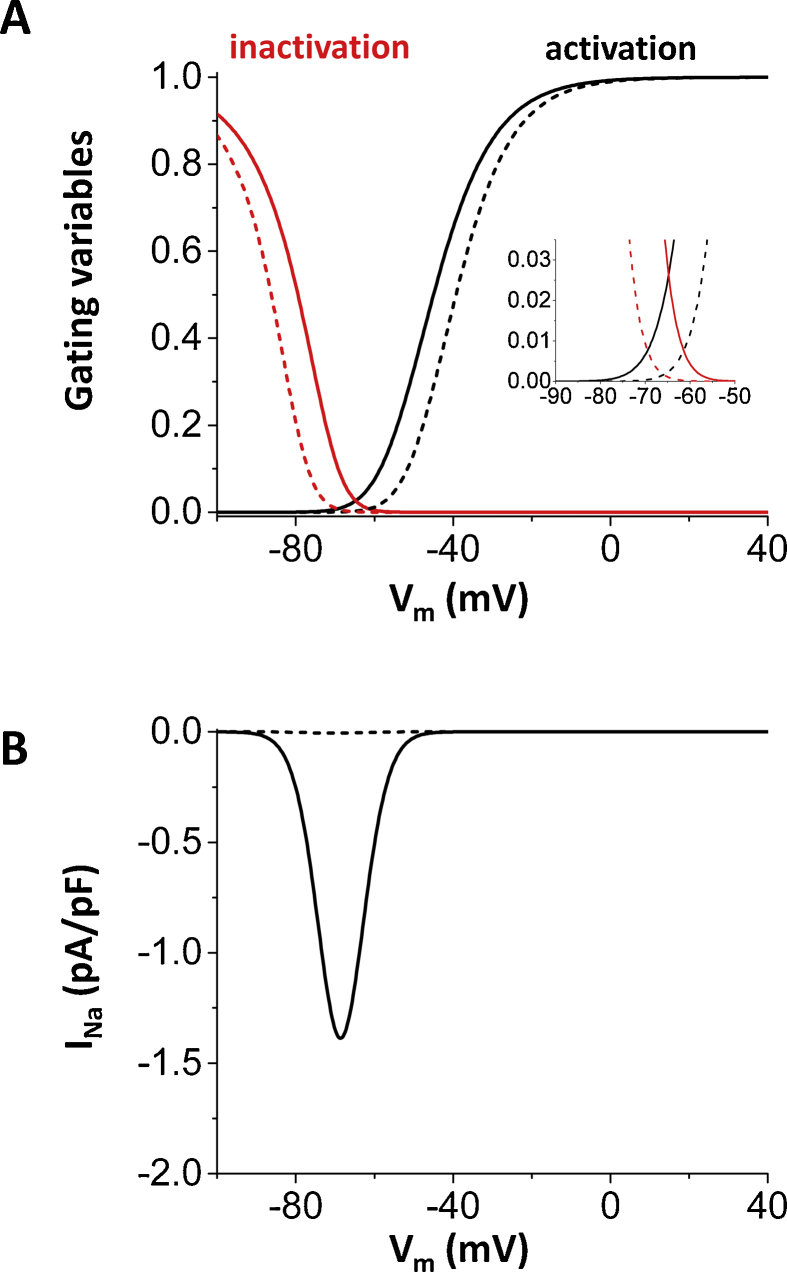
Fig. 1.
Normal Na current and pathological (increased late component of INa) Na current. (A) Activation and inactivation curves. Activation curve is m3 and inactivation curve is h × j. Solid lines: Increased window for the late component of INa. Dashed lines: normal Na channel. (B) Steady state current. Solid lines: Na current with the increased window. Dashed lines: normal Na current.
The difference between the h gate and the j gate is the time constant. The j gate is 20 times slower than the h gate.
In order to simulate non-inactivating current, we assumed 1% of channels are non-inactivating (smallest value of h (and j) is 0.1) gates are always open regardless of Vm). The activation curve and the inactivation curve are show in Fig. 6A.
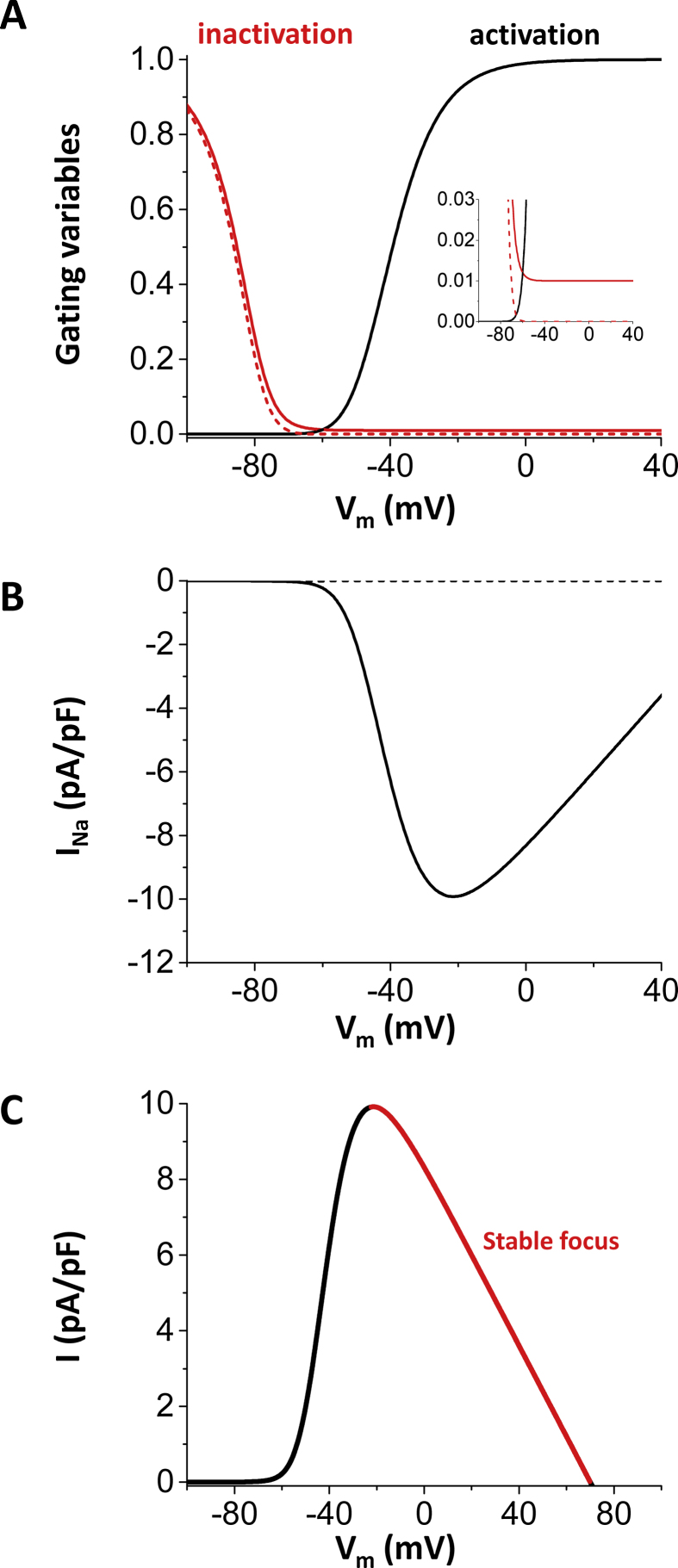
Fig. 6.
Non-inactivating INa does not cause EADs. (A) Activation and inactivation curves. Solid lines: non-inactivating Na channel. Dashed lines: normal Na channel. (B) Steady state current. Solid lines: non-inactivating Na current. Dashed lines: normal Na current. (C) Eigenvalues when the mechanism of the late component of INa is non-inactivation of the Na channel.
The physiologically detailed model has 26 variables. We reduce the number of variables in order to analyze the dynamical mechanism of EADs. We show the mechanism of oscillations of Vm i.e. EADs using the 2-variable model and the mechanism of termination of EADs using the 3-variable model.
2.2. Mathematical model (detailed description)
Our base model is an AP model by Mahajan et al. [25]. The ordinary differential equations are solved by the Euler method with adaptive time step of 0.01–0.1 ms. The program codes are written in C++. Parameters are shown in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 . Equations are as follows.
Table 1.
SR release parameters.
| Parameter | Definition | Value |
|---|---|---|
| τr | Spark lifetime | 30 ms |
| τa | NSR-JSR relaxation time | 100 ms |
| gRyR | Release current strength | 2.58 sparks cm2/mA |
| u | Release slope | 11.3 ms−1 |
| csr | Threshold for steep release function | 90 μM/l cytosol |
| s | Release function parameter | −977 μM/ms |
| τd | Submembrane-myoplasm diffusion time constant | 4 ms |
| τs | Dyadic junction-submembrane diffusion time constant | 0.5 ms |
Table 2.
Cytosolic buffering parameters.
| Parameter | Definition | Value |
|---|---|---|
| BT | Total concentration of Troponin C | 70 μmol/l cytosol |
| BSR | Total concentration of SR binding sites | 47 μmol/l cytosol |
| BCd | Total concentration of Calmodulin binding sites | 24 μmol/l cytosol |
| Bmem | Total concentration of membrane binding sites | 15.0 μmol/l cytosol |
| Bsar | Total concentration of sarcolemma binding sites | 42.0 μmol/l cytosol |
| konT | On rate for Troponin C binding | 0.0327 (μM)−1(ms)−1 |
| koffT | Off rate for Troponin C binding | 0.0196 ms−1 |
| KSR | Dissociation constant for SR binding sites | 0.6 μM |
| KCd | Dissociation constant for Calmodulin binding sites | 7 μM |
| K mem | Dissociation constant for membrane binding sites | 0.3 μM |
| K sar | Dissociation constant for sarcolemma binding sites | 13.0 μM |
Table 3.
Exchanger, uptake, and SR leak parameters.
| Parameter | Definition | Value |
|---|---|---|
| cup | Uptake threshold | 0.5 μM |
| vup | Strength of Uptake | 0.4 μM/ms |
| gNaCa | Strength of exchange current | 0.84 μM/s |
| ksat | constant | 0.2 |
| ξ | constant | 0.35 |
| Km,Nai | constant | 12.3 mM |
| Km,Nao | constant | 87.5 mM |
| Km,Cai | constant | 0.0036 mM |
| Km,Cao | constant | 1.3 mM |
| cnaca | constant | 0.3 μM |
| gl | Strength of leak current | 2.07 × 10−5 (ms)−1 |
| kj | Threshold for leak onset | 50 μM |
Table 4.
L-type Ca current parameters.
| Parameter | Definition | Value |
|---|---|---|
| PCa | Constant | 0.00054 cm/s |
| gCa | Strength of Ca current flux | 182 mmol/(cm C) |
| Strength of local Ca flux due to L-type Ca channels | 9000 mmol/(cm C) | |
| Strength of local Ca flux due to RyR channels | 26842 mmol/(cm C) | |
| kp° | Threshold for Ca-induced inactivation | 3.0 μM |
| Threshold for Ca dependence of transition rate k6 | 6.1 μM | |
| τpo | Time constant of activation | 1 ms |
| r1 | Opening rate | 0.3 ms−1 |
| r2 | Closing rate | 3 ms−1 |
| s1′ | Inactivation rate | 0.00195 ms−1 |
| k1′ | Inactivation rate | 0.00413 ms−1 |
| k2 | Inactivation rate | 0.0001 ms−1 |
| k21′ | Inactivation rate | 0.00224 ms−1 |
| TBa | Time constant | 450 ms |
Table 5.
Physical constants and ionic concentrations.
| Parameter | Definition | Value |
|---|---|---|
| Cm | Cell capacitance | 3.1 × 10−4 μF |
| vi | Cell volume | 2.58 × 10−5 μl |
| vs | Submembrane volume | 0.02 vi |
| F | Faraday constant | 96.5C/mmol |
| R | Universal gas constant | 8.315 J mol−1K−1 |
| T | Temperature | 308 K |
| [Na+]o | External sodium concentration | 136 mM |
| [K+]i | Internal potassium concentration | 140 mM |
| [K+]o | External potassium concentration | 5.4 mM |
| [Ca2+]o | External calcium concentration | 1800 μM |
Table 6.
Ion current conductances.
| Parameter | Definition | Value |
|---|---|---|
| gNa | Peak INa conductance | 12.0 mS/μF |
| gto,f | Peak Ito,f conductance | 0.11 mS/μF |
| gto,s | Peak Ito,s conductance | 0.04 mS/μF |
| gK1 | Peak IK1 conductance | 0.3 mS/μF |
| gKr | Peak IKr conductance | 0.0125 mS/μF |
| gKs | Peak IKs conductance | 0.1386 mS/μF |
| gNaK | Peak INaK conductance | 1.5 mS/μF |
2.3. Ionic currents
The membrane voltage (Vm) is given by
where Cm = 1 μF/cm2 is membrane capacitance, Iion is total ionic current density across the cell membrane, and Istim is the stimulus current. The total membrane current is given by
| Iion = INa + Ito,f + Ito,s + IKr + IKs + IK1 + INaK + ICa + INaCa |
2.4. The sodium current (INa)
We modified the original Mahajan formulation so that the peak Vm of the window corresponds reasonably well with the experimental measurements [26].
INa is given by
Na channel activation for the large window current is given by
Na channel inactivation for the large window current is given by
On the other hand, normal Na channel activation is given by
Normal Na channel inactivation is given by
2.5. Inward rectifier K+ current (IK1)
IK1 is given by
2.6. The rapid component of the delayed rectifier K+ current (IKr)
IKr is given by
2.7. The slow component of the delayed rectifier K+ current (IKs)
IKs is given by
2.8. The NaK exchanger current (INaK)
INaK is given by
2.9. The fast component of the rapid inward K+ current (Ito,f)
Ito,f is given by
2.10. The slow component of the rapid outward K+ current (Ito,s)
Ito,s is given by
2.11. Equations for Ca cycling
The equations for Ca cycling are:
where cs, ci, and cj are free [Ca] in the submembrane space, the cytosol, and the SR, with volumes vs, vi and vsrrespectively. The concentrations cs and ciare in units of μM, whereas cj and cj’ (for simplicity) are both in units of μMvsr/vi (μM/l cytosol). The current fluxes are: Jrel, the total release flux out of the SR via RyR channels; Jd, diffusion of Ca from the submembrane space to the bulk myoplasm; Jup, the uptake current via SERCA pumps in the SR; JCa, the current flux into the cell via L-type Ca channels; JNaCa, the current flux into the cell via the NaCa exchanger; Jleak, the leak current from the SR into the bulk myoplasm. All Ca fluxes are divided by vi and have units of μM/ms, which can be converted to units of μA/μF using the conversion factor nFvi/Cm, where n is the ionic charge of the current carrier, Cm is the cell membrane capacitance, and where F is Faraday's constant. Ionic fluxes can be converted to membrane currents using
where α = Fvi/Cm, and where the ion currents are in units of μA/μF.
The dependence of Ca release on SR Ca load is given by
where the parameter u controls the slope of the SR Ca release vs. SR Ca load relationship at high loads (cj’ > csr). The parameter s is chosen so that the function Q(cj’) is continuous at csr.
The number of sparks recruited over the whole cell in a time interval Δt is given by ΔNs, and the rate of spark recruitment is Ns’ = ΔNs/Δt. Since spark recruitment is initiated by the stochastic single channel opening of L-type Ca channels distributed throughout the cell, Ns’ follows a voltage dependence similar to the whole cell Ca entry. A phenomenological expression for spark rate is given by
where g(V) is the gain function, which controls the voltage dependence of Ca released into the SR in response to a trigger from the L-type Ca current. The voltage dependence is weak and has the form
2.12. The L-type Ca current flux
The Ca flux into the cell due to the L-type Ca current is given by
where α = VF/RT, and where cs is the submembrane concentration in units of mM.
2.13. Markov model of the L-type Ca current
The equations for the Markov states of L-type Ca channels are:
where the open probability satisfies
The rates are given by:
2.14. Diffusive flux
The flux of Ca from the submembrane space to the bulk myoplasm is given by:
where τd is the time constant for Ca diffusion from the submembrane space to the bulk myoplasm.
2.15. Nonlinear buffering
Buffering of Ca is modeled by incorporating instantaneous buffering to SR, calmodulin, membrane and sarcolemma binding sites.
Time dependent buffering to Troponin C is described by
2.16. NCX flux
The equation of the NCX is given by
where
and where
2.17. The SERCA (uptake) pump
The SERCA Ca pump is given by
where vup denotes the strength of uptake and cupis the pump threshold.
2.18. The SR leak flux
The leak flux from the SR is given by
where vsr/vi is the SR to cytoplasm volume ratio, and L(cj) is a threshold function of the form
2.19. Ca dynamics in the dyadic space
The average concentration in active dyadic clefts is given by
where,
2.20. Na dynamics
Intracellular Na dynamics is given by
where the factor 1/α’ converts membrane currents in μA/μF to Na fluxes in units of mM/ms. The conversion factor is given by α’ = 1000α, where α = Fvi/Cm.
3. Results
3.1. EADs directly initiated by INa
One possible mechanism of the increased late component of INa is the increased window current mechanism [26, 30, 31]. The peak of the window defined as the cross point of the activation and inactivation curves, which is observed when about 0.16% of Na channels are activated and 0.16% of channels are not inactivated (observed as the value 0.0016 on the ordinal axis in Fig. 1A dashed lines). The conductance at this point is only 0.000256% of the maximum conductance (GNa), and thus the maximum steady state INa (conductance × (Vm − ENa)) is about 0.0057 pA/pF (Fig. 1B dashed line). Note here that the time scale of Na channel inactivation is much shorter (a few ms ∼25 ms) than the time scale of AP (>100 ms). Therefore, the steady state approximation is close to the value of the late component of INa measured during APs.
Next, based on published experimental results for Na channel mutations in the congenital long QT syndrome [26], we shifted the activation curve to lower Vm and inactivation curve to higher Vm so that the peak of the window becomes between 2 to 3 percent (Fig. 1A solid lines). This window of the Na channel is sufficient to increase the amplitude of INa to ∼1.5 pA/pF which is similar to the other K currents at phases 2 and 3 (Fig. 1B solid line).
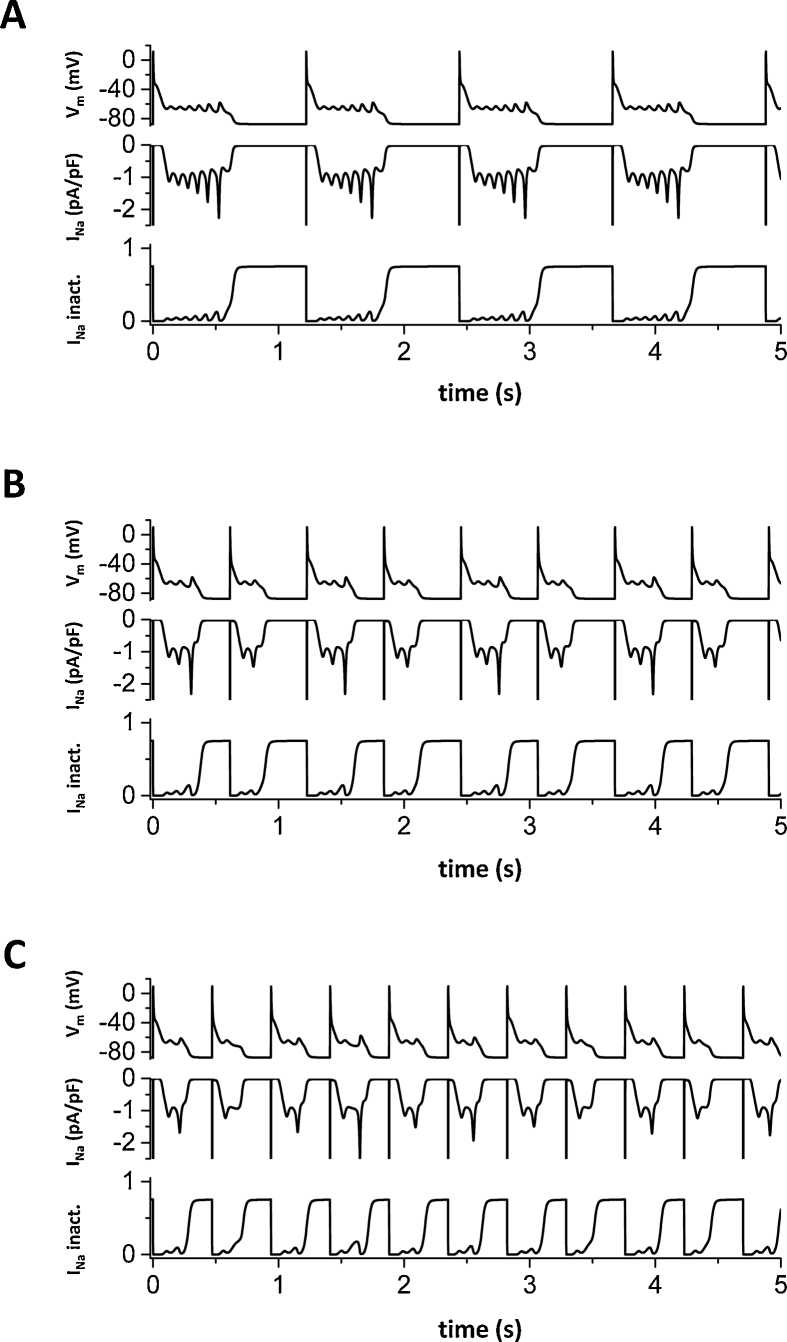
Using the increased window of INa, we show examples of EADs (Fig. 2). Fig. 2A shows periodic EADs. Note that action potentials are slightly different from what we usually observe in experiments because the ICaL was completely blocked (GCaL = 0). The main purpose of this figure is to show INa by itself can generate EADs. Blocking ICaL prevents both EADs due to reactivation of the L-type Ca channel and spontaneous Ca releases from the SR. Therefore, these EADs are solely due to reactivation of the Na channel as it enters the window current range of Vm. Lower panels in each figure show INa and inactivation (h × j). These panels show that the Na channel recovers and reactivates along with EADs. These EADs occur around −70 mV, which is lower than the voltage range (−20 ∼ 10 mV) of EADs due to reactivation of the L-type Ca channel (ICaL-mediated EADs).
Fig. 2.
INa mediated EADs. Typical EADs due to reactivation of INa in the physiologically detailed model. ICaL was blocked (GCaL = 0) to show explicitly these EADs are due to reactivation of INa. (A) EADs are periodic when pacing cycle length (PCL) = 1220 ms. (B) EADs show period 2 when PCL = 613 ms. (C) EADs are irregular when PCL is 470 ms. Irregular EADs shown here are sensitive to the initial conditions (see Fig. 3).
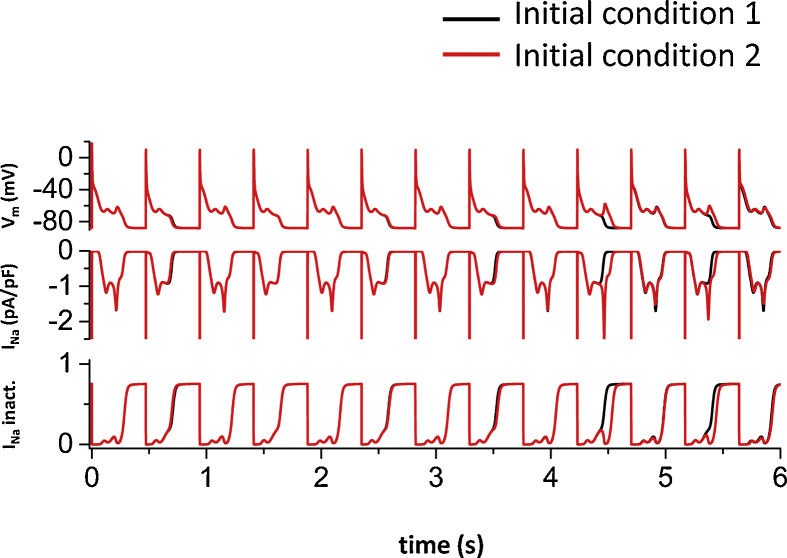
Fig. 2B shows period two EADs, that is AP with three EADs and with two EADs appear alternately. Fig. 2C shows irregular EADs. These irregular EADs are probably chaotic since these EADs are sensitive to the initial conditions, which is a hallmark of chaotic systems. (Fig. 3). When two simulations are performed with slightly different initial conditions, both APs are initially very similar. However, after a couple of beats (about 10 beats in Fig. 3), APs became completely different.
Fig. 3.
Sensitivity to initial conditions. Irregular EADs with slightly different initial conditions (initial Vm in the second simulation (Red line) is 1 mV higher (−86.9 mV) than the first simulation (−87.9 mV) (Black line).
In this study, we used the ventricular cell model. The cell remains excitable around −86 mV and Vm stays at the resting potential if there is no external stimulus. This dynamical behavior is clearly different from that of the pacemaker cell, which is oscillatory without stimuli [23, 24].
3.2. Mathematical analysis
The condition that the sum of the inward currents is greater than the sum of the outward currents is necessary for depolarization. However, this does not mean that the system always shows oscillatory behavior [32]. In order to elucidate the mechanisms of INa mediated EADs, we reduced the model to 3 variables, which are the membrane potential (v), the inactivation gate of the Na channel (h), and the total conductivity of K currents (gk). The set of ordinary differential equations is
.
The first equation shows Vm change due to the simplified currents of INa and IK. Inactivation gates h and j are almost identical except for their time constants. Here we reduce them as simply h2. The smaller τh gives faster oscillations. However, the fixed points remain the same. The third equation represents the fact that K currents (IKs, IKr etc) increase with time and bring Vm back to the resting Vm. This generic K current was adopted from the simplified model of the cardiac action potential by Echebarria and Karma [33].
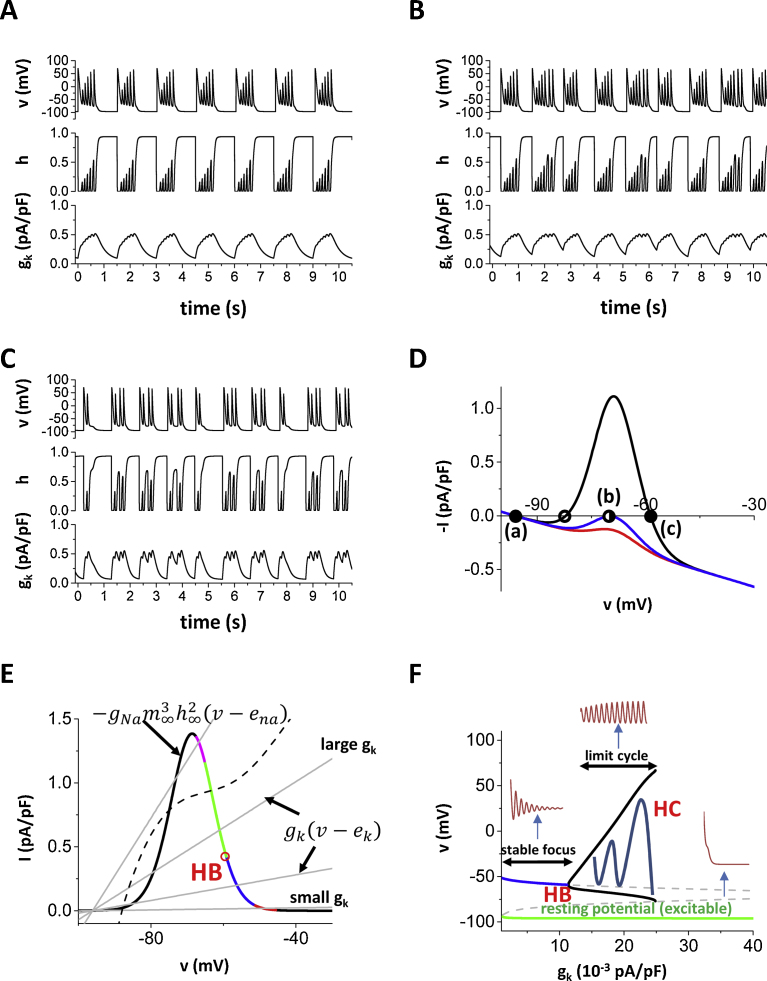
This reduced model shows both excitability (i.e. action potential) and oscillatory (i.e. EADs) (Fig. 4A). EADs can be periodic (Fig. 4A), period 2, period 3 (Fig. 4B) and even chaotic (Fig. 4C). Steady state (−I) vs. V curves are shown in Fig. 4D. Here we chose (−I) instead of I according to standard nonlinear dynamics notation (in contrast to standard electrophysiology nomenclature). If the inward window current is small (red curve), there is only one fixed point (a, filled circle), which is the resting potential of the ventricular cell. This system shows only excitability at the resting potential. As the window current is increased, another fixed point (b, half-filled circle) appears (blue curve) and then, at higher window current, a third fixed point (c, filled circle) appears.
Fig. 4.
Eigenvalues and dynamical behaviors. (A) EADs in the simplified 3-variable model. PCL is 1500 ms. (B) Period 3 EADs. (C) Irregular (probably chaotic) EADs. (D) Fixed points. With the normal INa, the system has only one fixed point (red). As INa becomes larger (red → blue → black), three fixed points appear. (E) Eigenvalues change as IK is increased. Red: negative real (e.g. −0.0242 at gk = 0.001), Blue: negative complex (e.g. −0.005545 ± 0.081 at gk = 0.01), Green: positive complex (e.g 0.0293 ± 0.09 at gk = 0.02), Magenta: positive real (e.g. 0.1405 at gk = 0.04). HB: Hopf bifurcation. Dashed line shows sum of K currents of the physiologically detailed model. (F) Bifurcation diagram. Green line: resting state (stable). The system is always excitable from here. Blue line: stable state (stable focus). Black brunches: maximum and minimum v of the limit cycle. Dashed line: unstable steady state. HB: Hopf bifurcation. HC: homoclinic bifurcation.
In order to understand the oscillation around the upper fixed point, we consider the two variable system of v and h. Since gk is the slowest variable of this system, we can identify the behavior of the v-h system for each gk value using eigenvalues of the v-h system described by the following matrix.
where
If the eigenvalues of the system are complex, the system of v and h is oscillatory. In other words, having complex eigenvalues is the necessary condition for EADs. In the simplified model, IK is shown as a straight line (no rectification) in Fig. 4E. At the cross point of this line and −INa is the stable fixed point (dv/dt = 0, the system does not move from this point). In other words, the outward current is equal to the inward current (|INa| = |IK|). We compute eigenvalues, which determine the stability of the system, for the fixed point. Depending on the value of gk, eigenvalues can be real positive, real negative, complex positive, or complex negative. Corresponding biological phenomena are
-
1)
real negative → prolongation of AP without oscillation
-
2)
complex negative → decaying EADs
-
3)
complex positive → growing EADs
-
4)
real positive → repolarization to the resting potential (no EAD)
In Fig. 4E eigenvalues are shown in different colors. If IK, which is the conductance (gk) times the driving force (v − ek), is very small, the larger eigenvalue is real negative. In this case, the fixed point is an attractor without oscillations. Since this requires very small IK, this may not occur physiologically. If IK is slightly larger (red part), then the eigenvalues are complex negative and the fixed point is an attractor with Vm oscillations (damped EADs) (stable focus). When gk is included as a third variable, IK increases as time goes. The eigenvalues become complex positive (green part) from complex negative. At this point (red circle in Fig. 4E and ‘HB’ in Fig. 4F), Hopf bifurcation occurs and the EAD amplitude grows. As IK becomes sufficiently large, homoclinic bifurcation occurs (‘HC’ in Fig. 4F) and Vm goes back to the resting potential.
The I–V curve of the simplified K current is slightly different from the I–V curve of the total K current in the physiological model (Fig. 5). This simplification may affect the results quantitatively but will not qualitatively as far as the INa exceeds the sum of K currents and Hopf bifurcation occurs. Fig. 5A shows the total K current in the physiologically detailed model. With the normal window current, there is only one fixed point (Fig. 5B dashed line). However, when the window current is increased, three fixed points appear (Fig. 5B solid line). EADs in the physiologically detailed model are oscillation around the rightmost fixed point.
Fig. 5.
Fixed points of the physiologically detailed model. (A) The generic K current in the simplified model represents the total K current in the physiological model. Total K current vs voltage. Total K current = IKs + IKr + IK1 + Ito + INaK. (B) Total current vs voltage. Solid line: total current with the increased window INa. Dashed line: total current with the normal window INa.
Non-inactivation also increases the late component of INa. However, when the late component of INa is due to non-inactivating current, although three fixed points can appear, the eigenvalues are always real negative, which indicates no oscillation of Vm (Fig. 6). For example, when 1% of channels are non-inactivating (Fig. 6A), steady state INa becomes extremely large (Fig. 6B). However, this will not cause EADs since eigenvalues are always real negative (stable focus) although it prolongs the action potential (Fig. 6C).
Therefore, EADs due to reactivation of INa will not occur in this case although this late component of INa may set up the conditions for ICaL-mediated EADs by reducing repolarization reserve and EADs due to spontaneous Ca releases by increasing [Na]i, which leads to Ca overload.
3.3. Interplay of INa and ICaL mediated EADs
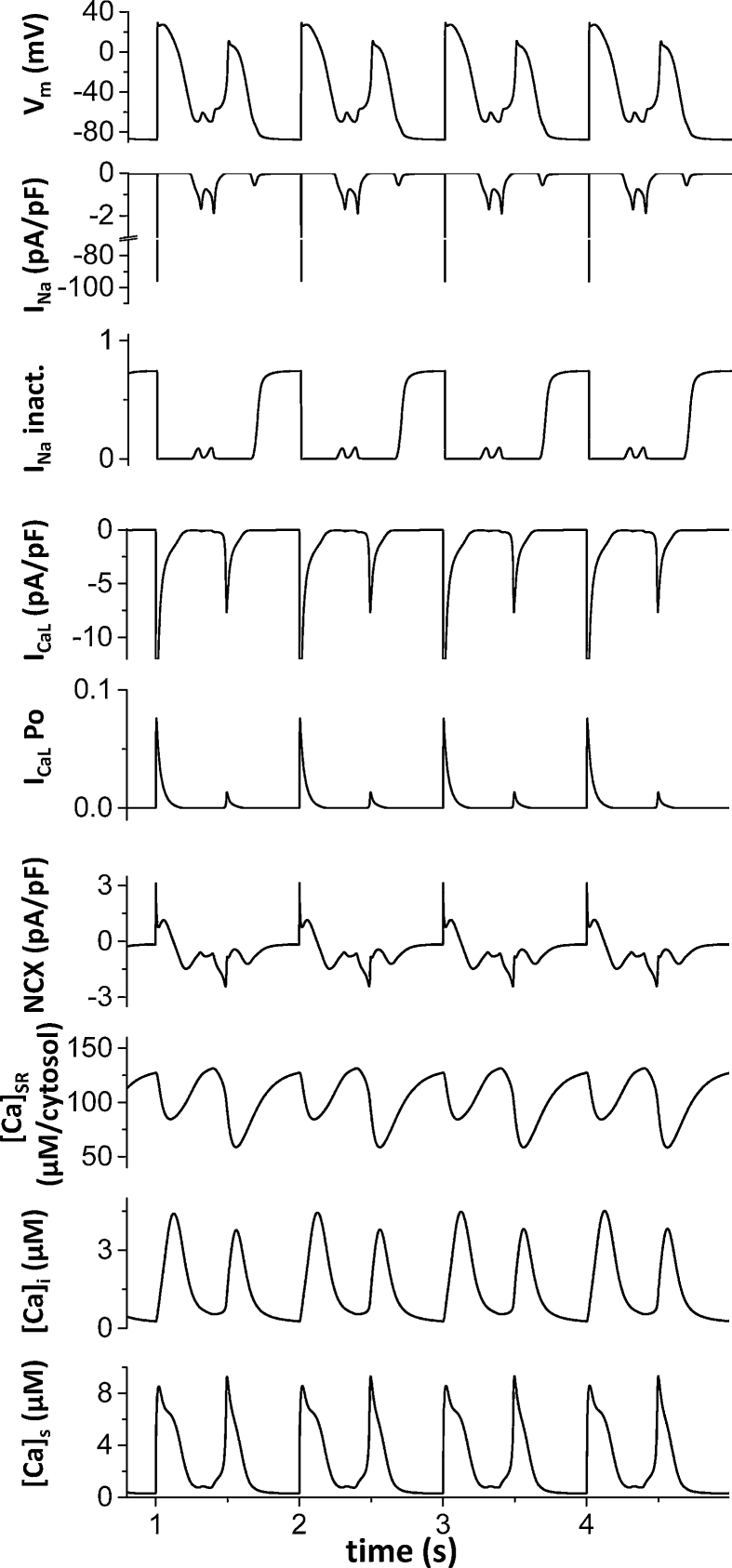
In Fig. 2, in order to explore the possibility of INa mediated EADs, ICaL was blocked. Fig. 7 shows how INa mediated EADs directly promote ICaL mediated EADs in the presence of INa and ICaL. Although we cannot say which the cause of EADs is since these are nonlinearly coupled in the system, Fig. 7 clearly shows that reopening of the Na channel precedes reopening of the L-type Ca channel. In this simulation, we used the normal (healthy) L-type Ca channel [25], which is much more difficult to generate EADs than the L-type Ca channel under administration of ISO[32] or H2O2 [6]. When Vm became around −70 mV, the Na channel was reactivated first. This caused elevation of Vm, which promoted reactivation of the healthy L-type Ca channel. Ca entry through the L-type Ca channels also helps depolarization via NCX and further promotes EADs.
Fig. 7.
Interplay of INa and ICaL mediated EADs. Reactivation of the Na channel promotes reactivation of the L-type Ca channel. Example of EADs when both ICaL and INa present. The membrane potential, Na current, Na inactivation, L-type Ca current, L-type Ca channel open probability, NCX, SR [Ca], cytosolic [Ca], and submembrane [Ca] are shown. The INa mediated EADs occurred prior to the ICaL mediated EADs. Ca entry via ICaL also activates NCX and further promotes EADs. Here μM/cytosol means μM vsr/vi, where vsr is the SR volume and vi is the cell volume.
4. Discussion
In this study, we showed that INa by itself is able to generate EADs. The dynamical mechanism is oscillation in the INa-IK system around the higher Vm fixed point, which is distinguished from the oscillation in the pacemaker cell (oscillation around the single fixed point). INa, especially the late component of INa has been recognized as an important player to set up the conditions for EADs by reducing repolarization reserve and increasing intracellular Na concentration, which leads to Ca overload. However, INa itself has not been considered as a direct initiator of EADs. Under normal conditions, the late component of INa is so small (Fig. 1B dashed line) that the amplitude of INa cannot be larger than the sum of K currents at phases 2 and 3, and therefore, INa itself cannot initiate EADs. However, under pathological conditions such as heart failure [34, 35, 36] and myocardial ischemia [37, 38], large late INa has been observed. Recent experimental study by Horvath et al. showed that INa is as large (∼1 pA/pF) as the other Ca and K currents. We reconstructed INa based on activation and inactivation curves (Fig. 1A) measured by Wang et al. and the amplitude of INa predicted by the model gives similar amplitude (Fig. 1B solid line). This implies that INa may overcome the sum of IK and depolarize Vm during AP without the help of the other inward currents such as ICaL, NCX, non-specific Ca-activated cation current, especially, when IK become small under pathological conditions and/or administration of K channel blockers. Using the physiologically detailed model of the ventricular action potential, we showed INa mediated EADs (Fig. 2). As we have shown in ICaL-mediated EADs [6], INa-mediated EADs can be periodic (Fig. 2A), period-2 (Fig. 2B), and even chaotic (Fig. 2C).
We have shown the mechanisms of ICaL-mediated EADs [32, 39]. Also, bursting behaviors are widely observed and studied in many biological systems [20, 21, 22]. In this study, we reduced the physiologically detailed model to the 3-variable model and analyzed the dynamical mechanism of INa mediated EADs. This methodology has been used in theoretical neuroscience to understand underlying mechanisms [22, 40]. We have also used this type of analysis for ICaL-mediated EADs [32, 39]. For the formation of EADs, positive feedback processes such as ICaL or Ca induced Ca release from the SR are necessary. In addition to them, INa has a positive feedback process since more Na channels open as Vm elevates. In this study, we showed INa-mediated EADs due to the Na channel reactivation. The EAD, namely the oscillation of Vm at phases 2 and/or 3, in this case is distinguished from the Vm oscillation in the pacemaker cell [23, 24]. In the model of the voltage clock of the pacemaker cell, the system has only one fixed point, which is unstable, gives Vm oscillation. On the other hand, the model shown in this study has three fixed points as the window current is increased. INa mediated EADs are due to oscillation around the higher Vm fixed point. The lower fixed point (the resting potential) is still stable and the system shows excitability.
The Na channel can reactivate without large window current if there is another positive feedback mechanism, which helps reactivation of the Na channel. For example, Edward et al. have shown that reactivation of Na channel when SR Ca release occurs [41, 42]. In these cases, the higher Vm fixed point is not necessary. Stimulation such as spontaneous SR Ca release via NCX activates the Na channel from the lower Vm fixed point.
The sustained Na current can also be due to non-inactivation of the Na channel. However, our analysis shows that AP will be simply prolonged and EADs will not occur (Fig. 6) in this case. The prolongation of AP without EADs is also observed with late component of the Na current experimentally [19]. In this case, although the Na channel will not reactivate, prolongation can promote ICaL-mediated EADs and Ca overload.
In order to increase the window current, activation and inactivation curves are shifted. The amplitude of the upstroke of AP becomes larger since activation occurs at the lower potential and inactivation requires higher potential. Zhao et al. have shown that smaller amplitude of the upstroke promotes EADs [43]. In our case, the amplitude is smaller with the normal INa. However, there is only one fixed point with the normal INa (Fig. 4D red curve, Fig. 5 dashed curve). Therefore, even if the amplitude of the upstroke is smaller, EADs will not occur with the normal INa unless K currents are unphysiologically small.
In the case of ICaL-mediated EADs, ICaL is responsible only for oscillation at phases 2 and/or 3. On the other hand, in the case of INa-mediated EADs, INa is responsible for both excitation and oscillation. In addition, ICaL-mediated EADs occur around −20 ∼ 10 mV. In some experiments, EADs are observed at more negative Vm than the reactivation Vm of the L-type Ca channel [42, 44]. Damiano and Rosen observed more EADs as the PCL becomes longer [44]. As the PCL becomes longer, SR Ca load becomes smaller and EADs due to spontaneous Ca releases occur less in large mammalian ventricular cells. Therefore, if the mechanism of EADs is due to spontaneous Ca releases, EADs should occur less as the PCL becomes longer. In addition, if the mechanism of EADs is due to reactivation of the L-type Ca channel, EADs should occur at more positive voltage. This experimental observation suggests that these EADs are due to Na channel reactivation and consistent with our results (Fig. 2, more EADs with longer PCLs). Our mechanism may explain these EADs although we need additional experiments to differentiate them more explicitly from EADs caused by the other mechanisms.
Recently, the current through the Nav1.8 channel has been considered to be a possible mechanism of INaL in cardiac cells [45, 46]. This channel also has a positive feedback process like Nav1.5 and would similarly cause INa mediated EADs. However, EADs would occur a much higher Vm range, because for Nav1.8 the window current Vm range is closer to that of the L-type Ca channel [45].
Declarations
Author contribution statement
Daisuke Sato: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Colleen E. Clancy, Donald M. Bers: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by National Institutes of Health grant K99/R00-HL111334, American Heart Association Grant-in-Aid 16GRNT31300018, and Amazon AWS Cloud Credits for Research (D.S.), National Institutes of Health grants U01-HL126273 and R01-HL128170 (C.E.C.), and National Institutes of Health grants R37-HL30077 and R01-HL105242 (D.M.B.)
Additional Information
No additional information is available for this paper.
References
- 1.Frazier D.W., Wolf P.D., Wharton J.M., Tang A.S., Smith W.M., Ideker R.E. Stimulus-induced critical point. Mechanism for electrical initiation of reentry in normal canine myocardium. J. Clin. Invest. 1989;83(3):1039–1052. doi: 10.1172/JCI113945. PubMed PMID: 2921316; PMCID: 303781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez B., Li L., Eason J.C., Efimov I.R., Trayanova N.A. Differences between left and right ventricular chamber geometry affect cardiac vulnerability to electric shocks. Circ. Res. 2005;97(2):168–175. doi: 10.1161/01.RES.0000174429.00987.17. PubMedPMID: 15976315; PMCID: 2925187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akar F.G., Rosenbaum D.S. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ. Res. 2003;93(7):638–645. doi: 10.1161/01.RES.0000092248.59479.AE. PubMed PMID: 12933704. [DOI] [PubMed] [Google Scholar]
- 4.Patterson E., Jackman W.M., Beckman K.J., Lazzara R., Lockwood D., Scherlag B.J., Wu R., Po S. Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J. Cardiovasc. Electrophysiol. 2007;18(10):1067–1075. doi: 10.1111/j.1540-8167.2007.00909.x. PubMed PMID: 17655663. [DOI] [PubMed] [Google Scholar]
- 5.Burashnikov A., Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107(18):2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. PubMed PMID: 12695296. [DOI] [PubMed] [Google Scholar]
- 6.Sato D., Xie L.H., Sovari A.A., Tran D.X., Morita N., Xie F., Karagueuzian H., Garfinkel A., Weiss J.N., Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc. Natl. Acad. Sci. U. S. A. 2009;106(9):2983–2988. doi: 10.1073/pnas.0809148106. PubMed PMID: 19218447; PMCID: 2651322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.January C.T., Moscucci A. Cellular mechanisms of early afterdepolarizations. Ann. N.Y. Acad. Sci. 1992;644:23–32. doi: 10.1111/j.1749-6632.1992.tb30999.x. PubMed PMID: 1562117. [DOI] [PubMed] [Google Scholar]
- 8.Clusin W.T. Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit. Rev. Clin. Lab. Sci. 2003;40(3):337–375. doi: 10.1080/713609356. PubMed PMID: 12892319. [DOI] [PubMed] [Google Scholar]
- 9.Volders P.G., Vos M.A., Szabo B., Sipido K.R., de Groot S.H., Gorgels A.P., Wellens H.J., Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc. Res. 2000;46(3):376–392. doi: 10.1016/s0008-6363(00)00022-5. PubMed PMID: 10912449. [DOI] [PubMed] [Google Scholar]
- 10.Zeng J., Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys. J. 1995;68(3):949–964. doi: 10.1016/S0006-3495(95)80271-7. PubMed PMID: 7538806; PMCID: 1281819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi B.R., Burton F., Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J. Physiol. 2002;543(Pt 2):615–631. doi: 10.1113/jphysiol.2002.024570. PubMed PMID: 12205194; PMCID: 2290501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo D., Zhao X., Wu Y., Liu T., Kowey P.R., Yan G.X. L-type calcium current reactivation contributes to arrhythmogenesis associated with action potential triangulation. J. Cardiovasc. Electrophysiol. 2007;18(2):196–203. doi: 10.1111/j.1540-8167.2006.00698.x. PubMed PMID: 17212595. [DOI] [PubMed] [Google Scholar]
- 13.Spencer C.I., Sham J.S. Effects of Na+/Ca2+ exchange induced by SR Ca2+ release on action potentials and afterdepolarizations in guinea pig ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2003;285(6):H2552–62. doi: 10.1152/ajpheart.00274.2003. PubMed PMID: 12933341. [DOI] [PubMed] [Google Scholar]
- 14.Fozzard H.A., Hanck D.A. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol. Rev. 1996;76(3):887–926. doi: 10.1152/physrev.1996.76.3.887. PubMed PMID: 8757791. [DOI] [PubMed] [Google Scholar]
- 15.Nerbonne J.M., Kass R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 2005;85(4):1205–1253. doi: 10.1152/physrev.00002.2005. PubMed PMID: 16183911. [DOI] [PubMed] [Google Scholar]
- 16.Song W., Shou W. Cardiac sodium channel Nav1.5 mutations and cardiac srrhythmia. Pediatr. Cardiol. 2012;33(6):943–949. doi: 10.1007/s00246-012-0303-y. PubMed PMID: PMC3393812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehrens X.H., Rossenbacker T., Jongbloed R.J., Gewillig M., Heidbuchel H., Doevendans P.A., Vos M.A., Wellens H.J., Kass R.S. A novel mutation L619F in the cardiac Na+ channel SCN5A associated with long-QT syndrome (LQT3): a role for the I-II linker in inactivation gating. Hum. Mutat. 2003;21(5):552. doi: 10.1002/humu.9136. Epub 2003/04/04. PubMed PMID: 12673799. [DOI] [PubMed] [Google Scholar]
- 18.Horne A.J., Eldstrom J., Sanatani S., Fedida D. A novel mechanism for LQT3 with 2:1 block: a pore-lining mutation in Nav1.5 significantly affects voltage-dependence of activation. Heart Rhythm. 2011;8(5):770–777. doi: 10.1016/j.hrthm.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Horvath B., Banyasz T., Jian Z., Hegyi B., Kistamas K., Nanasi P.P., Izu L.T., Chen-Izu Y. Dynamics of the late Na(+) current during cardiac action potential and its contribution to afterdepolarizations. J. Mol. Cell Cardiol. 2013;64:59–68. doi: 10.1016/j.yjmcc.2013.08.010. PubMed PMID: 24012538; PMCID: 3856763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X.-J., Rinzel J. Oscillatory and bursting properties of neurons. Michael A.A.; The handbook of brain theory and neural networks: MIT Press; 1998. pp. 686–691. [Google Scholar]
- 21.Rinzel J. Springer; 1985. Bursting Oscillations in an Excitable Membrane Model. Ordinary and Partial Differential Equations; pp. 304–316. [Google Scholar]
- 22.Izhikevich E.M. MIT press; 2007. Dynamical Systems in Neuroscience. [Google Scholar]
- 23.Kurata Y., Matsuda H., Hisatome I., Shibamoto T. Regional difference in dynamical property of sinoatrial node pacemaking: role of na+ channel current. Biophys. J. 2008;95(2):951–977. doi: 10.1529/biophysj.107.112854. PubMed PMID: 18390617; PMCID: 2440451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Pol B., van der Mark J.M., XXII The heartbeat considered as a relaxation oscillation, and an electrical model of the heart. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1928;6(38):763–775. [Google Scholar]
- 25.Mahajan A., Shiferaw Y., Sato D., Baher A., Olcese R., Xie L.H., Yang M.J., Chen P.S., Restrepo J.G., Karma A., Garfinkel A., Qu Z., Weiss J.N. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys. J. 2008;94(2):392–410. doi: 10.1529/biophysj.106.98160. PubMedPMID: 18160660; PMCID: 2157228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D.W., Yazawa K., George A.L., Jr., Bennett P.B. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc. Natl. Acad. Scie. U. S. A. 1996;93(23):13200–13205. doi: 10.1073/pnas.93.23.13200. PubMed PMID: 8917568; PMCID: 24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo C.H., Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ. Res. 1991;68(6):1501–1526. doi: 10.1161/01.res.68.6.1501. PubMed PMID: 1709839. [DOI] [PubMed] [Google Scholar]
- 28.Luo C.H., Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ. Res. 1994;74(6):1071–1096. doi: 10.1161/01.res.74.6.1071. PubMed PMID: 7514509. [DOI] [PubMed] [Google Scholar]
- 29.Shannon T.R., Wang F., Puglisi J., Weber C., Bers D.M. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys. J. 2004;87(5):3351–3371. doi: 10.1529/biophysj.104.047449. PubMed PMID: 15347581; PMCID: 1304803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaza A., Rocchetti M. The late Na+ current-origin and pathophysiological relevance. Cardiovasc. Drugs Ther. 2013;27(1):61–68. doi: 10.1007/s10557-012-6430-0. PubMed PMID: 23274937 ; PMCID: 3555240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltsev V.A., Sabbah H.N., Higgins R.S., Silverman N., Lesch M., Undrovinas A.I. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98(23):2545–2552. doi: 10.1161/01.cir.98.23.2545. PubMed PMID: 9843461. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y., Izu L.T., Bers D.M., Sato D. Arrhythmogenic transient dynamics in cardiac myocytes. Biophys. J. 2014;106(6):1391–1397. doi: 10.1016/j.bpj.2013.12.050. PubMed PMID: 24655514; PMCID: 3984988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Echebarria B., Karma A. Mechanisms for initiation of cardiac discordant alternans. Eur. Phys. J. Spec. Top. 2007;146(1):217–231. [Google Scholar]
- 34.Coppini R., Ferrantini C., Yao L., Fan P., Del Lungo M., Stillitano F., Sartiani L., Tosi B., Suffredini S., Tesi C., Yacoub M., Olivotto I., Belardinelli L., Poggesi C., Cerbai E., Mugelli A. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127(5):575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. PubMed PMID: 23271797. [DOI] [PubMed] [Google Scholar]
- 35.Pourrier M., Williams S., McAfee D., Belardinelli L., Fedida D. CrossTalk proposal: the late sodium current is an important player in the development of diastolic heart failure (heart failure with a preserved ejection fraction) J. Physiol. 2014;592(Pt 3):411–414. doi: 10.1113/jphysiol.2013.262261. PubMed PMID: 24488066; PMCID: 3930422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Undrovinas A.I., Belardinelli L., Undrovinas N.A., Sabbah H.N. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J. Cardiovasc. Electrophysiol. 2006;17(Suppl. 1):S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. PubMed PMID: 16686675; PMCID: 1482456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier L.S., Sossalla S. The late Na current as a therapeutic target: where are we? J. Mol. Cell. Cardiol. 2013;61:44–50. doi: 10.1016/j.yjmcc.2013.03.001. PubMed PMID: 23500390. [DOI] [PubMed] [Google Scholar]
- 38.Belardinelli L., Shryock J.C., Fraser H. The mechanism of ranolazine action to reduce ischemia-induced diastolic dysfunction. Eur. Heart J. Suppl. 2006;8(Suppl. A):A10–A13. 2006-02-01. [Google Scholar]
- 39.Tran D.X., Sato D., Yochelis A., Weiss J.N., Garfinkel A., Qu Z. Bifurcation and chaos in a model of cardiac early afterdepolarizations. Phys. Rev. Lett. 2009;102(25):258103. doi: 10.1103/PhysRevLett.102.258103. PubMed PMID: 19659123; PMCID: 2726623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krouchev N.I., Rattay F., Sawan M., Vinet A. From squid to mammals with the HH model through the nav channels' half-activation-voltage parameter. PloS One. 2015;10(12):e0143570. doi: 10.1371/journal.pone.0143570. Eub 2015/12/03. PubMed PMID: 26629692; PMCID: PMC4667926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morotti S., McCulloch A.D., Bers D.M., Edwards A.G., Grandi E. Atrial-selective targeting of arrhythmogenic phase-3 early afterdepolarizations in human myocytes. J. Mol. Cell. Cardiol. 2016;96:63–71. doi: 10.1016/j.yjmcc.2015.07.030. Epub 2015/08/05. PubMed PMID: 26241847; PMCID: PMC4734906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards A.G., Grandi E., Hake J.E., Patel S., Li P., Miyamoto S., Omens J.H., Heller Brown J., Bers D.M., McCulloch A.D. Nonequilibrium reactivation of Na+ current drives early afterdepolarizations in mouse ventricle. Circ. Arrhythm. Electrophysiol. 2014;7(6):1205–1213. doi: 10.1161/CIRCEP.113.001666. PubMedPMID: 25236710; PMCID: 4301603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z., Xie Y., Wen H., Xiao D., Allen C., Fefelova N., Dun W., Boyden P.A., Qu Z., Xie L.H. Role of the transient outward potassium current in the genesis of early afterdepolarizations in cardiac cells. Cardiovasc. Res. 2012;95(3):308–316. doi: 10.1093/cvr/cvs183. Epub 2012/06/05. PubMed PMID: 22660482; PMCID: PMC3400356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damiano B.P., Rosen M.R. Effects of pacing on triggered activity induced by early afterdepolarizations. Circulation. 1984;69(5):1013–1025. doi: 10.1161/01.cir.69.5.1013. PubMed PMID: 6705157. [DOI] [PubMed] [Google Scholar]
- 45.Yang T., Atack T.C., Stroud D.M., Zhang W., Hall L., Roden D.M. Blocking Scn10a channels in heart reduces late sodium current and is antiarrhythmic. Circ. Res. 2012;111(3):322–332. doi: 10.1161/CIRCRESAHA.112.265173. PubMed PMID: 22723299; PMCID: 3412150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savio-Galimberti E., Weeke P., Muhammad R., Blair M., Ansari S., Short L., Atack T.C., Kor K., Vanoye C.G., Olesen M.S., LuCamp Yang T, George A.L., Jr., Roden D.M., Darbar D. SCN10A/Nav1.8 modulation of peak and late sodium currents in patients with early onset atrial fibrillation. Cardiovasc. Res. 2014 doi: 10.1093/cvr/cvu170. PubMed PMID: 25053638. [DOI] [PMC free article] [PubMed] [Google Scholar]