Abstract
Background
How plants adapt their developmental patterns to regular seasonal changes is an important question in biology. The annual growth cycle in perennial long-lived trees is yet another example of how plants can adapt to seasonal changes. The two main signals that plants rely on to respond to seasonal changes are photoperiod and temperature, and these signals have critical roles in the temporal regulation of the annual growth cycle of trees.
Scope
This review presents the latest findings to provide insight into the molecular mechanisms that underlie how photoperiodic and temperature signals regulate seasonal growth in trees.
Conclusion
The results point to a high level of conservation in the signalling pathways that mediate photoperiodic control of seasonal growth in trees and flowering in annual plants such as arabidopsis. Furthermore, the data indicate that symplastic communication may mediate certain aspects of seasonal growth. Although considerable insight into the control of phenology in model plants such as poplar and spruce has been obtained, the future challenge is extending these studies to other, non-model trees.
Keywords: Hybrid aspen (Populus tremula × P. tremuloides), growth cessation, dormancy, seasonal growth, phenology, ecodormant, endodormant
INTRODUCTION
Throughout their life cycle, plants are continuously exposed to varying environmental conditions. Since plants are sessile, they have developed a multitude of strategies – termed developmental adaptations – to survive under changing environmental conditions. A particularly striking example of one such developmental adaptation is the cycling of vegetative (post-embryonic) shoot apical meristem (SAM) between active growth and dormancy in the long-lived perennial trees of boreal and temperate regions (Rohde and Bhalerao, 2007; Cooke et al., 2012; Petterle et al., 2013; Singh et al., 2017) and also in herbaceous perennials (Gillespie and Volaire, 2017). Although the activity–dormancy cycle is well studied physiologically, the signalling pathways that control this cycle have only recently been revealed, and are the focus of this review.
Perennial trees in the boreal and temperate regions experience regular temperature fluctuations due to changing seasons. These fluctuations can range from +30 to –40°C. As a result, these plants have developed unique strategies to cope with, and protect their meristems from, such temperature extremes. The survival of these trees depends on the termination of growth, establishment of dormancy and acquisition of cold hardiness prior to the advent of winter, along with an activation of growth following dormancy release in the spring. A key question, and one that has received considerable interest in the field of tree biology, is what signals and signalling pathways control growth cessation and dormancy establishment, as well as the subsequent release from dormancy and reactivation of growth. In this review, we discuss various aspects of growth cessation and dormancy including signal perception and downstream signalling components, primarily relying on recent findings made in the tree models poplar and spruce in which most of the molecular studies have been performed.
INDUCTION OF GROWTH CESSATION AND THE ESTABLISHMENT OF DORMANCY
As described above, trees undergo growth cessation and establish dormancy prior to the advent of winter. Induction of growth cessation in many, if not all, trees in the boreal and temperate regions is controlled photoperiodically. The shorter days of autumn herald the approach of winter. In response to this reduction in daylength [short days (SDs)], elongation growth and the formation of new leaves is terminated in the SAM (Nitsch, 1957; Weiser, 1970; Cooke et al., 2012; Singh et al., 2017). In angiosperm trees such as poplar, a specialized structure, the apical bud, encloses the arrested leaf primordia within the bud scales that are derived from morphogenetic transformation of stipules (Goffinet and Larson, 1981; Rohde et al., 2002; El-Kayal et al., 2011). Simultaneously, metabolism shifts towards the generation of storage compounds such as vegetative storage protein and lipids (Druart et al., 2007). The SAM and arrested leaf primordia in the bud develop dormancy. A distinguishing feature of dormancy is that while a plant is in a dormant state, the reactivation of growth becomes insensitive to growth-promotive signals. As a result, once dormancy is established, growth will not start under favourable conditions unless dormancy is terminated by exposure to dormancy-breaking signals, which, in many boreal and temperate trees, include prolonged exposure to low temperature (Saure, 1985; Hannerz et al., 2003; Brunner et al., 2014). Once dormancy is broken, relatively warm temperatures will induce reactivation of growth (Fu et al., 2015). Thus, in trees such as poplar, photoperiod and temperature are the two main signals that control key developmental transitions in the activity–dormancy cycle. In recent years, several reviews (e.g. Cooke et al., 2012) have described the global transcriptomic and metabolic changes that accompany distinct stages of the activity–dormancy cycle. This review will instead focus on the signalling pathways that control the key developmental stages of the activity–dormancy cycle (Fig. 1).
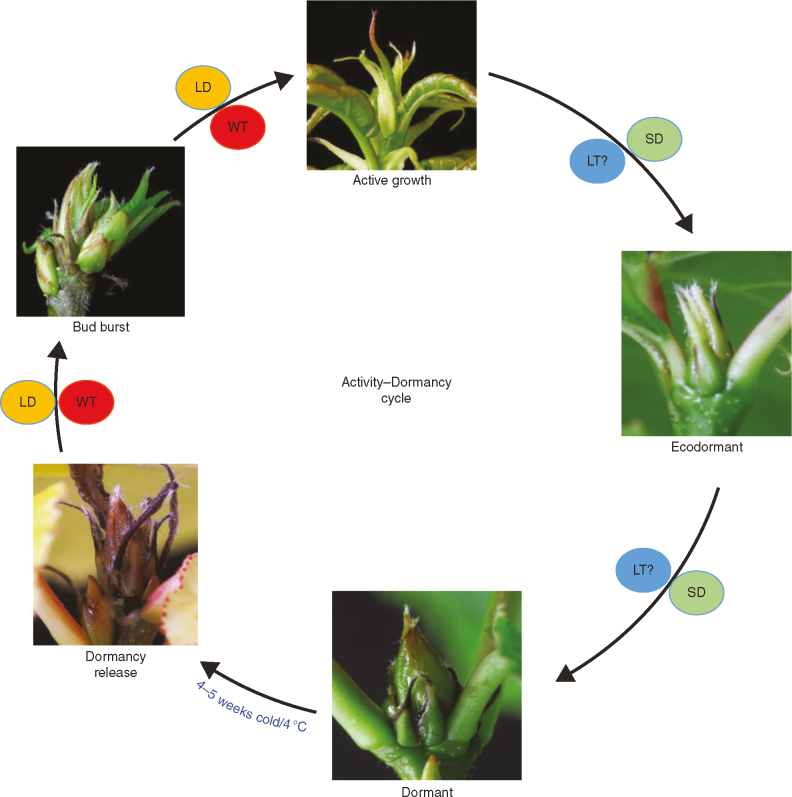
Fig. 1.
Seasonal changes that occur in the apex of hybrid aspen during the activity–dormancy cycle. Under long-day (LD) and warm temperature (WT) conditions, such as those experienced during the summer, trees grow actively. They stop their growth upon sensing short days (SDs) during early autumn. Initially, growth cessation is reversible by exposure to the growth-promoting LDs, as the buds are in an ecodormant state. SDs induce dormancy in the buds during late autumn. Once dormancy is established, growth becomes insensitive to any growth-promotive signals and the buds are endodormant. Chilling temperatures during the winter periods promote the release of dormancy and buds become ecodormant again. Relatively warmer temperatures in the spring promote bud burst, which is followed by active growth in the summer.
SIGNAL PERCEPTION AND GROWTH CESSATION
Trees such as poplar sense the advent of winter through a reduction in daylength, and certain trees may also use temperature to gauge the changing of seasons (Junttila et al., 2003; Heide and Prestrud, 2005; Mølmann et al., 2005; Kalcsits et al., 2009). Here we focus mainly on perception of SD signal and how this induces growth cessation, bud set and dormancy establishment. The perception of SDs involves a complex regulatory network that includes photoreceptors, the circadian clock and photoperiodic components. The photoreceptor phytochromes act early in the SD perception that leads to growth cessation (Olsen et al., 1997). The poplar genome has three phytochrome genes: PHYA, PHYB1 and PHYB2 (Howe et al., 1998; Olsen, 2010). Alterations in the expression of PHYA in poplar affect circadian rhythm, timing of bud set and cold acclimatization responses (Olsen et al., 1997; Kozarewa et al., 2010). Various circadian clock components have also been shown to play a role in growth cessation. Downregulation of LHY1 and TOC1, two key components of the circadian clock, reduces the critical daylength (daylength below which growth cessation is induced) required for growth cessation in hybrid aspen (Ibáñez et al., 2010). Böhlenius et al. (2006) offered insight into how a reduction in daylength results in the induction of growth cessation by showing that the downregulation of a poplar orthologue of the arabidopsis flowering time regulator FLOWERING LOCUS T (FT) results in a faster SD response relative to the wild type. In contrast to arabidopsis, the poplar genome has two FT orthologues, FT1 and FT2. Overexpression of either of the FT genes delays growth cessation in response to SDs (Hsu et al., 2006, 2011; Azeez et al., 2014), whereas the downregulation of FT expression leads to earlier growth cessation under SD conditions (Böhlenius et al, 2006; Hsu et al, 2006, 2011). These results strongly suggest that the external coincidence model, which mechanistically explains the photoperiodic control of flowering in arabidopsis, may also apply to the photoperiodic control of growth in trees such as poplar. CONSTANS (CO) is one of the key components mediating photoperiodic control of flowering, and CO expression displays a diurnal pattern. The CO expression peaks during the day, leading to an accumulation of CO protein that then activates the transcription of its target, FT. In contrast, when CO expression peaks during dark periods (i.e. SDs), CO protein is degraded because it is unstable in the dark and FT expression decreases. As a result, no flowering occurs (Suarez-Lopez et al., 2001). Likewise, CO expression also follows a diurnal pattern in poplar; and when the hybrid aspen clone T89 was shifted from long days to the SDs that induce growth cessation, CO expression peaked in the dark (Böhlenius et al., 2006). Assuming that poplar has a similar mechanism to arabidopsis for the regulation of FT by CO, FT2 expression would decrease rapidly after SDs, resulting in growth cessation. Indeed, FT2 expression is rapidly reduced after exposure to SDs (Böhlenius et al., 2006). Thus, it is the absence of a growth-promotive signal, e.g. FT2, and not SD-driven generation of signals that results in the induction of growth cessation in poplar. Interestingly, an FT/TFL-based mechanism for growth cessation has also been observed in conifers such as spruce (Karlgren et al., 2013). Studies on FLOWERING LOCUS T/TERMINAL FLOWER1-like genes (PaFTL) suggest that they are involved in growth cessation and bud setting (Karlgren et al., 2013). However, in spruce, the induction of PaFTL2 induces growth cessation and bud set. Thus, in spruce, SDs may actually generate the signals that induce growth cessation. However, these studies in spruce relied on the overexpression of PaFTL2, and it is vital to know what phenotype results from PaFTL2 loss of function before concluding that SDs generate the signals for growth cessation in spruce.
FLOWERING LOCUS T (FT1 AND FT2) AND ITS DOWNSTREAM TARGET GENES
In poplar, FT1 and FT2 are proposed to have distinct and diverse functions. FT1 has been proposed to be involved in the control of reproductive onset (Hsu et al., 2011). In contrast, FT2 has been shown to control vegetative growth under long-day conditions (Böhlenius et al., 2006; Hsu et al., 2006, 2011). However, they are functionally interchangeable since overexpression of both FT1 and FT2 delays the growth cessation response under SD conditions and both can promote early flowering when overexpressed (Böhlenius et al., 2006; Hsu et al., 2006, 2011). The first major change that occurs after SD perception is the downregulation of FT2 (Resman et al., 2010). In arabidopsis, FT expression is controlled by a molecular network consisting of miR156, miR172, SPL proteins and certain AP2-like transcription factors such as AP2, SMZ (SCHLAFMUTZE), TOE1–TOE3 (TARGET OF EARLY ACTIVATION TAGGED 1–3) and SNZ (SCHNARCHZAPFEN) that responds to the perception of photoperiodic signals (Aukerman and Sakai, 2003; Schmid et al., 2003; Jung et al., 2007). The SPL proteins positively regulate miR172 levels, which in turn represses the levels of AP2-like transcription factors (Aukerman and Sakai, 2003; Jung et al., 2007; Wu et al., 2009). The upregulation of SPLs promotes flowering through an increase in FT expression. A similar mechanism may underlie the regulation of FT2 expression during growth cessation and dormancy in poplar. For example, a recent study has reported that eight SPL-like genes are downregulated during the transition from paradormancy to endodormancy in poplar (Howe et al., 2015). The downregulation of SPL genes is associated with the downregulation of miR172, upregulation of AP2, SMZ, TOE and SNZ genes, and reduction in FT2 expression during growth cessation, bud setting and the establishment of endodormancy (Howe et al., 2015). However, to the best of our knowledge, no functional studies of these genes have yet been performed, and there is thus a lack of conclusive evidence concerning how the aforementioned genes are involved in growth cessation and endodormancy.
Tissue-specific expression patterns from leafy spurge (Euphorbia esula L.) suggest that FT levels are inversely related to the expression of DORMANCY ASSOCIATED MADS-BOX (DAM) genes. Under dormancy-inducing conditions, leafy spurge clearly accumulates more DAM gene transcripts (DAM1 and DAM2) than FT transcripts. DAM-like proteins have also been shown to bind to the CArG boxes of the FT promoter in endodormant crown buds of leafy spurge during chromatin immunoprecipitation (ChIP) experiments (Hao et al., 2015). In the Japanese pear (Pyrus pyrifolia), the expression of another dormancy-associated MADS-box gene, PpMADS13-1, increases during dormancy induction, reaching a maximum level during December, after which expression decreases during periods of endodormancy release. However, ChIP assays showed that the translated protein does not bind to the promoter of either FT-like gene, PpFT1a or PpFT2a (Saito et al., 2015). These results are interesting, but again functional analysis is needed to confirm the role of DAM genes in dormancy regulation.
The downstream target genes of FT1 and FT2 are not yet well characterized. Nevertheless, microarray experiments in Populus deltoides have identified many common and distinct downstream target genes of FT1 and FT2 (Hsu et al., 2011). This microarray analysis identified 18 potential downstream targets of FT1 that are specifically involved in the reproductive process. One such target is MADS49, which is upregulated upon FT1 overexpression. MADS49 is a homologue of the arabidopsis SEPALLATA that is involved in floral organ formation. Unlike MADS49, MADS7 expression is inversely related to FT1 expression. MADS7 is functionally similar to arabidopsis SHORT VEGETATIVE PHASE (SVP) and is downregulated during FT1 overexpression (Hsu et al., 2011). Additionally, 15 auxin-related genes are downregulated during FT1 overexpression. Moreover, FT1 upregulation leads to the downregulation of many methyltransferase and histone genes, which indicates that FT1 may act via chromatin remodelling.
In contrast to FT1, which has been suggested to participate primarily in reproductive development, FT2 has been proposed to act in stress response in addition to its known role in the control of vegetative growth (Hsu et al., 2011). This suggestion is based on microarray data from FT2-overexpressing plants since many of the genes whose expression is altered in FT2 transgenics are related to the stress defence responses in the plants. These include JASMONATE-ZIM-DOMAIN PROTEIN 1, ZF14 (an antimicrobial extrusion efflux protein), MAPK3 and ETHYLENE RESPONSE FACTOR-APETALA 2.
THE FT–FDL COMPLEX PARTICIPATES IN PHOTOPERIODIC CONTROL OF GROWTH
Over the course of evolution, FT has developed functional diversity depending on interacting partners (Taoka et al., 2011; Niwa et al., 2013; Tylewicz et al., 2015; Tsuji et al., 2015). Since FT lacks DNA binding activity, interaction with other proteins enables FT to participate in modulation of distinct downstream signalling processes. The best characterized FT-interacting protein is FD, which interacts with FT in the shoot apex of plants (Abe et al., 2005; Wigge et al., 2005). FD belongs to the bZIP class of transcription factors and regulates the expression of genes involved in flowering and growth cessation (Abe et al., 2005; Hu et al., 2014; Tylewicz et al., 2015). The poplar genome has two closely related homologues of FD, FD-Like 1 (FDL1) and FD-Like 2 (FDL2) (Tylewicz et al., 2015). Both FDL1 and FDL2 interact with FT. The interaction between FDL1 and FT is important for photoperiodic control of the growth cessation process in hybrid aspen (Tylewicz et al., 2015). However, FDL1 also has additional FT-independent functions. For example, it has been shown that FDL1 is involved in the transcriptional regulation of adaptive response and bud maturation pathways (Tylewicz et al., 2015). Surprisingly, FDL2 does not appear to be involved in the growth cessation response in hybrid aspen even though it is able to interact with FT (Tylewicz et al., 2015). This suggests that FDL2 probably has some hitherto unknown function. Thus, the FT–FD complex promotes flowering in arabidopsis and mediates photoperiodic growth and the activation of adaptive response pathways in the apical buds of poplar.
IS FT MOVEMENT IMPORTANT FOR PHOTOPERIODIC CONTROL OF GROWTH?
An important facet of FT function during flowering is its movement from leaves to the apex. It is produced in the leaves, but then moves to the apex to regulate flowering (Corbesier et al., 2007; Jaeger and Wigge, 2007; Turck et al., 2008). The movement of FT from leaves to the apex has become better understood in recent years through the identification of FT-interacting proteins. A novel protein, NaKR1 (SODIUM POTASSIUM ROOT DEFECTIVE 1), which mediates the long-distance movement of FT in arabidopsis, was recently identified (Zhu et al., 2016). NaKR1 is a heavy metal-associated (HMA) domain-containing protein and is activated by CO under long-day conditions. It forms a complex with FT that is translocated through the sieve elements of phloem to the apex, where FT then dissociates from NaKR1. As in arabidopsis, FT2 expression in poplar is almost exclusively restricted to the leaves (P. Miskolczi et al., unpubl. res). However, FT clearly impacts growth in the apex and how FT expression in leaves controls responses at the apex must be clarified to truly understand FT function. There are, as yet, no confirmed reports of FT movement in poplar or spruce. Thus, an important aspect of FT function in the photoperiodic control of growth remains unclear and clearly warrants further attention.
SIGNALLING COMPONENTS DOWNSTREAM OF THE FT–FDL COMPLEX
The downstream components of the FT–FD module in flowering are the floral meristem identity gene such as APETALA 1 (AP1) in arabidopsis and OsMADS1 in rice (Irish et al., 1990; Mandel et al., 1992; Chung et al., 1994; Riechmann et al., 1997). The orthologue of arabidopsis AP1 in poplar is LAP1 (Like-AP1) (Azeez et al., 2014). Transgenic plant lines over- and underexpressing LAP1 show delayed and faster growth cessation responses under SD conditions, respectively (Azeez et al., 2014). Moreover, plants that overexpress FDL1 show attenuation in SD-mediated downregulation of LAP1 expression (Tylewicz et al., 2015). Thus, LAP1 acts downstream of FT–FDL1 and the downregulation of LAP1 is required for SD-induced growth cessation to occur in hybrid aspen. In poplar, the downstream target of LAP1 during the SD-mediated growth cessation response is the transcription factor gene AINTEGUMENTA-like 1 (AIL1) (Azeez et al., 2014). Inability to respond to SDs, e.g. due to overexpression of PHYA and FT1 cDNA, resulted in a corresponding failure in downregulating AIL1 expression in hybrid aspen, and plants with AIL1 overexpression and downregulation show delayed and earlier growth cessation responses, respectively, in comparison with the wild type (Karlberg et al., 2011). These findings suggest that AIL1 downregulation is necessary for growth cessation under SD conditions. Moreover, LAP1 was shown to bind to the AIL1 promoter (Azeez et al., 2014), suggesting that AIL1 is a direct target of LAP1. It has been suggested that AIL1 mediates cell proliferation genes, e.g. D-type cyclin genes, during growth cessation (Karlberg et al., 2011). This suggestion is supported by the finding that, under SD conditions, AIL1 overexpression reduces the downregulation of CYCD3:2 and CYCD6:1. Moreover, AIL1 can directly interact with the promoters of these genes. These results suggest that AIL1 presumably participates in the regulation of cell division in meristematic tissues at the shoot apex (Karlberg et al., 2010; Randall et al., 2015).
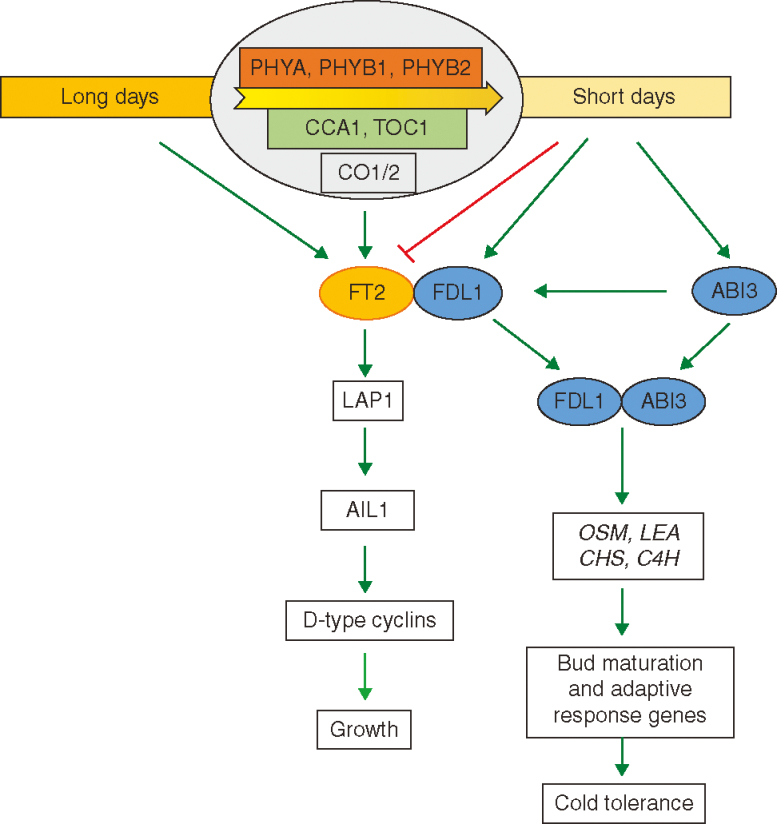
We summarize these findings in a schematic model of the photoperiodic control of growth in trees (Fig. 2). According to this model, during long days, FT2 is expressed and interacts with FDL1, which ensures that LAP1 and its target AIL1 are expressed. AIL1 positively controls the expression of cell proliferation genes to promote the activity of the SAM. Upon a shift to SDs, FT2 expression is rapidly downregulated, which leads to a repression of the entire pathway and, thus, growth cessation. In the absence of FT2 as in SDs, FDL1 interacts with ABI3 and activates cold hardiness-related genes to ensure the survival of the meristem and leaf primordia, thereby temporally co-ordinating the regulation of growth cessation and activation of adaptive responses such as cold hardiness.
Fig. 2.
Molecular networks involved in the photoperiodic control of growth cessation and bud set in poplar. Long days (LDs) promote the expression of FT via CO. The downstream target of FT is LAP1, which in turn controls AIL1. AIL1 regulates the expression of core cell regulatory genes, e.g. D-type cyclins, that are involved in the control of cell division. These D-cyclins promote the division of meristematic cells in the apex, which results in active apical growth. The perception of SDs inhibits FT expression and, thus, the rest of the signalling pathway, which leads to growth cessation and dormancy establishment. SDs also induce the expression of FDL1 and ABI3. Both of these proteins are able to interact physically with each other and regulate the expression of genes involved in bud maturation and adaptive responses for cold tolerance (see text for details).
TEMPERATURE CONTROL OF SEASONAL GROWTH
Many reports suggest that in addition to photoperiod, temperature also acts as an environmental cue for seasonal growth cessation and bud set in some tree species (Junttila et al., 2003; Heide and Prestrud, 2005; Mølmann et al., 2005; Kalcsits et al., 2009; Heide, 2011). It has been suggested that temperature modifies sensitivity to daylength signals during the growth cessation process and affects the timing of bud set in poplar (Rohde et al., 2011). Warm temperatures increase the rate of SD-induced growth cessation and dormancy induction in poplar (Kalcsits et al., 2009). In contrast, low temperature, regardless of photoperiodic signal, is sufficient for the induction of growth cessation and dormancy induction in apple, pear and sorbus (Heide and Prestrud, 2005; Heide, 2011). When two genotypes of Sorbus aucuparia L. were grown at 15 and 21°C with either a 20 or 10h photoperiod, they continued to grow for 8 and 9weeks. However, the growth stopped immediately when the temperature was decreased to 9°C but the photoperiodic conditions remained unchanged (Heide, 2011). It is currently unknown how temperature can mediate the control of seasonal growth. Nevertheless, with the knowledge gained from photoperiodic control of growth in poplar and spruce, it should be interesting to investigate whether similar or unique pathways mediate the temperature control of seasonal growth in trees such as apple.
HORMONAL CONTROL OF GROWTH CESSATION
Phytohormones are key developmental regulators that play pivotal roles at various stages of plant growth and life cycles (Davies, 1995). Among the phytohormones, gibberellins (GAs) are the best characterized in terms of the seasonal control of growth in trees. Earlier studies in the bay willow (Salix pentandra) have suggested that reduced GA levels are a prerequisite for the initiation of growth cessation under SD conditions (Olsen et al., 1995a, b). In contrast, the exogenous application of GA1 under SD conditions induces shoot elongation (Junttila and Jensen, 1988) and, similarly, hybrid aspen trees overexpressing GA20 oxidase with high GA levels do not undergo growth cessation in response to SDs (Eriksson and Moritz, 2002). It has recently been suggested that GA acts in parallel with the FT2 pathway to control shoot elongation in hybrid aspen (Eriksson et al., 2015). Unexpectedly, transgenic poplar plants with reduced response to GA levels do not respond significantly differently to SDs compared with the wild-type controls (Zawaski and Busov, 2014). As a result, the role of GAs in the photoperiodic control of growth cessation still remains poorly understood.
Earlier data suggested that ABA (abscisic acid) participates in the photoperiodic control of growth (Eagles and Wareing, 1964). However, transgenic plants with a reduced response to ABA display a normal response to SDs, which indicates that, at least in hybrid aspen, ABA does not mediate SD-induced growth cessation (S. Tylewicz et al., unpubl. res.). Nevertheless, in addition to growth cessation, SDs also control maturation of buds, as does ABA. For example, transgenic hybrid aspen with a reduced ABA response display a severe perturbation of bud maturation (Petterle, 2011). Additionally, overexpression of the poplar ABSCISICACID-INSENSITIVE3 (PtABI3) homologue affects the size and ratio of embryonic leaves and bud scales/stipules that differentiate from the primordia under SD conditions (Rohde et al. 2002; Ruttink et al. 2007). However, it should be emphasized that ABI3 is not ABA inducible in poplar, and there is currently no evidence to suggest that ABI3 mediates ABA response in poplar. Ethylene may also mediate bud maturation since overexpression of the dominant-negative allele of arabidopsis ETHYLENE RESPONSE1 (ETR1) in birch plants (Ruonala et al., 2006) results in open bud structure resembling ABI3 overexpressors. Previous research has not comprehensively studied whether cytokinins are involved in the SD-induced apical growth cessation and dormancy establishment. Similarly, the role of indole acetic acid (IAA), which mediates cambial growth cessation and dormancy (Bhalerao et al., 2003; Baba et al., 2011), has not been studied in apical growth and bud dormancy.
INDUCTION OF BUD DORMANCY
Continued exposure to SDs after growth cessation leads to the establishment of dormancy in buds. The mechanistic basis of SD-induced bud dormancy still remains poorly understood. One hypothesis for the establishment of bud dormancy is based on symplastic isolation of the SAM via closure of plasmodesmata (Jian et al., 1997; Rinne and van der Schoot, 1998). Plasmodesmata are intercellular conduits that symplastically connect neighbouring cells (Maule, 2008; Burch-Smith and Zambryski, 2012). Several transcription factors and other signalling compounds, such as microRNAs and hormones, are known to traffick via plasmodesmata to control plant development (Lucas and Lee, 2004; Urbanus et al., 2010; Yoo et al., 2013; Han et al., 2014). It has been reported that the plasmodesmata close via the deposition of callose-rich dormancy sphincters following SD treatment (Rinne et al., 2005; Levy et al., 2007; Simpson et al., 2009). This would limit the access of growth-promotive signals to the meristem and could thereby explain the establishment of dormancy. So far, genetic evidence to support this hypothesis has been lacking, as closure of plasmodesmata is only temporally associated with bud dormancy, but causality has remained unproven. An important step in elucidating the control of bud dormancy comes from analysis of transgenic hybrid aspen with reduced response to the plant hormone ABA or altered polycomb function which fail to establish bud dormancy, as they also lack these dormancy sphincters (S. Tylewicz et al., unpubl. res.). Although these results do not conclusively prove the role of plasmodesmata in dormancy, they nevertheless identify genetic components that mediate dormancy formation and the establishment of dormancy sphincters in response to SDs.
THE ROLE OF CHROMATIN REMODELLING IN THE CONTROL OF SD-INDUCED DORMANCY
The epigenome of plants is very sensitive to changes in the external environment, and variations in the epigenetic silencing mechanisms are one of the key factors that help plants adapt to changing environmental conditions (Baulcombe and Dean, 2014). The release of bud dormancy by prolonged exposure to low temperature shares certain features with the vernalization-mediated promotion of flowering in arabidopsis. During vernalization, epigenetic silencing of the floral repressor FLC (Flowering Locus C) by low temperature promotes flowering (Amasino, 2004; Bastow et al., 2004). This observation has led researchers to hypothesize that epigenetic modifications also influence bud dormancy in trees. DNA methylation and histone modifications associated with chromatin remodelling are the main epigenetic changes that occur during dormancy transitions (Howe et al., 2015). Two chromatin-associated genes which are similar to SPT (SUPPRESSOR OF TY) showed strong upregulation during dormancy. Interestingly, polycomb function, which is known to be critical for vernalization, is also involved in dormancy. In arabidopsis, vernalization induces chromatin changes at the MADS-box of FLC, a floral repressor, to relieve the repression of flowering (Amasino, 2004; Bastow et al., 2004). Interestingly, the expression of several DAM genes that encode MADS box transcription factors such as FLC is upregulated during dormancy induction and downregulated at the time of dormancy release, and the promoters of DAM genes undergo chromatin modifications that are similar to those of FLC in arabidopsis (Horvath et al., 2010; Jimenez et al., 2010; Sasaki et al., 2011; Yamane et al., 2011; Leida et al., 2012; Yamane, 2014). Moreover, many plant species, for example poplar, blackcurrant, raspberry and leafy spurge, have been shown to have high expression levels of DAM-related genes in dormant buds (Ríos et al., 2014). ChIP sequencing has verified that there are significantly enriched levels of the histone H3 lysine-27 trimethylation (H3K27me3) mark at the chromatin of DAM1, DAM4, DAM5 and DAM6 in dormancy-released buds of peach (de la Fuente et al., 2015). However, it is not yet clear whether these DAM genes are involved in dormancy regulation since there are no mutants available that can functionally connect these DAM genes with dormancy regulation. Nevertheless, some studies do hint that DAM5 and DAM6 could be involved in terminal bud dormancy in peach (Prunus persica). For example, heterologous expression of Japanese apricot DAM6 under the control of the 35S promoter (35S:PmDAM6) in a T89 hybrid aspen plant resulted in earlier apical growth cessation and bud formation under long-day conditions (16h light/8h dark) than in the control plant (Sasaki et al., 2011; Yamane, 2014).
Many genes, for example CDC48-LIKE, HISTONE1-3, FERTILIZATION INDEPENDENT ENDOSPERM (FIE), a component of the polycomb repression complex, and PICKLE (PKL) that are involved in chromatin remodelling show differential expression patterns after exposure to SD conditions. FIE and PKL were strongly upregulated after SD perception (Druart et al., 2007; Ruttink et al., 2007). Interestingly, the apical bud of a FIE-RNA interference (RNAi) hybrid aspen plant did not develop dormancy (A. Petterle et al., unpubl. res.). However, the SD-induced growth cessation and bud set processes were not affected in these plants (Petterle, 2011). Genes encoding putative histone deacetylases (HDA08 and HDA14) and histone lysine methyltransferases (SUVR3), as well as a gene for histone ubiquitination, are upregulated during exposure to SD conditions; on the other hand, many trithorax-like genes, e.g. Demeter, ATX1 and ATXRG, were down-regulated after exposure to SD conditions for 5–11weeks (Karlberg et al., 2010; Cooke et al., 2012). Several other genes, such as HDA9-, DML2- and SIN3-like, are upregulated in response to exposure to low temperatures, and this makes them interesting targets for analyses of their roles in dormancy release. RNA-directed DNA methylation (RdDM) is another epigenetic mechanism that results in transcriptional gene silencing (TGS) (Howe et al., 2015). This process involves the methylation and demethylation of DNA at target loci during both TGS and post-transcriptional gene silencing (PTGS). Certain ARGONAUTE (AGO) and DICER-like (DCL) genes, such as AGO4 and DCL4, have been identified in leafy spurge, and were found to be downregulated during endodormancy (Horvath et al., 2008). Besides these studies, there are many genes which are associated with epigenetic regulation during dormancy in poplar and have been listed by Shim et al. (2014). Moreover, our unpublished data indicate that several thousand genes undergo changes in chromatin status during the dormancy release that follows prolonged exposure to low temperature (P. Miskolczi et al., unpubl. res.), yet it is unlikely that all of these changes are involved in dormancy control, highlighting the problem of drawing conclusions regarding the role of specific genes in dormancy regulation based primarily on changes in chromatin status. Thus, further research is needed to understand clearly the role of chromatin modifications and the mediators of these modifications in the SD-induced dormancy establishment process.
RELEASE OF DORMANCY AND BUD FLUSH
Prolonged exposure to low temperature gradually breaks dormancy, and sets the stage for the subsequent activation of growth in the spring. However, the understanding of the molecular signalling mechanism underlying dormancy release is still quite basic. The opening of plasmodesmata and removal of dormancy sphincters is associated with dormancy release, which is initiated by prolonged exposure to low temperature in birch and hybrid aspen (Rinne and Van der Schoot, 1998; Rinne et al., 2011). The opening of plasmodesmata could potentially restore the supply of growth-promotive signals to the meristem. However, these signals are currently unknown, although upregulation of GA biosynthesis and FT1 expression occur concomitantly with the opening of plasmodesmata (Karlberg et al., 2010; Rinne et al., 2011), and may contribute to dormancy release and/or bud flush (Fig. 3). However, dormancy release in grape (Vitis vinifera) has been suggested to involve molecular mechanisms that resemble those that occur during oxidative stress conditions, i.e. increased calcium signalling, hypoxia and altered ABA and ethylene metabolism (Keilin et al., 2007; Pang et al., 2007; Halaly et al., 2008; Ophir et al., 2009; Meitha et al., 2015; Zheng et al., 2015). Both exposing endodormant grape buds to stimuli such as heat shock and treating them with hydrogen cyanamide result in dormancy release. Both treatments were characterized by increased levels of catalase, alcohol dehydrogenase, pyruvate decarboxylase, glutathione reductase, ascorbate peroxidase and glutathione S-transferase transcripts (Or et al., 2002; Halaly et al., 2008; Ophir et al., 2009). These changes also resulted in impaired mitochondrial function, hypoxic conditions and the swelling of cells in dormant grape buds, consequently leading to increased glycolysis and fermentation, both of which may be involved in the activation of downstream genes that regulate dormancy release. Hydrogen cyanamide and sodium azide (NAN3) treatment also activates the expression of 1,3-β-d-glucanase, which may promote dormancy release (Perez et al., 2009), possibly through the opening of plasmodesmata as described above.
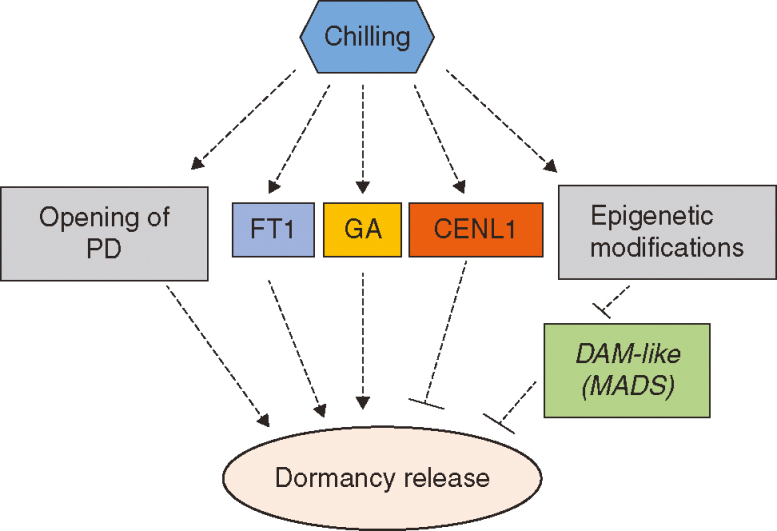
Fig. 3.
Schematic representation of the release of dormancy in trees. Exposing dormant buds to low temperatures is a prerequisite for dormancy release. This induces the opening of plasmodesmata (PD) and simultaneously promotes the expression of FT1 and other genes involved in gibberellin (GA) metabolism. On the other hand, CENL1 counteracts dormancy release. Additionally, exposure to cold causes epigenetic modifications in DAM-like genes, which may result in their downregulation and the release of dormancy. However, this model remains largely speculative in the absence of functional evidence. Note: this model is based on results mostly from poplar and some other deciduous species.
It is worth mentioning that bud flush is delayed in transgenic poplars that overexpress CENTRORADIALIS (CENL1), and these plants need prolonged chilling treatment relative to wild-type plants. This suggest that downregulation of CENL1 may be necessary for either the release of dormancy or the promotion of bud burst, or both of these processes (Mohamed et al., 2010). EARLY BUD BREAK 1 (EBB1) is another candidate that has been identified as a potential mediator of bud break in poplar (Yordanov et al., 2014). It encodes an APETALA2/Ethylene-responsive transcription factor (AP2/ERF). Over- and underexpression of EBB1 results in early and delayed bud break, respectively. Furthermore, EBB1 is highly expressed in growing apices, but is not detectable during the dormancy period. However, its expression increases during bud break, suggesting that it is involved in post-dormancy release activation of bud flush rather than dormancy release. Transcriptomic analyses identified 971 differentially expressed genes that correspond to the EBB1 expression changes, and all of these could potentially be the downstream targets and/or influence bud flush (Yordanov et al., 2014). EBB1 orthologues in apple and grape have similar expression patterns to that in poplar, and this suggests that EBB1 has a conserved role in the bud burst of trees (Busov et al., 2016). Interestingly, downregulation of the circadian clock component LATE ELONGATED HYPOCOTYL1 (PttLHY1 and PttLHY2) in hybrid aspen results in delayed bud burst (Ibáñez et al., 2010). Since bud burst, at least in poplar, is not affected by photoperiod, the mechanism of how the downregulation of a key component of the circadian clock impacts bud burst needs to be elucidated.
COMPARISON BETWEEN BUD AND SEED DORMANCY
The regulation of SD-induced dormancy shares many features with the control of seed dormancy. Thus, an important question is whether similar signalling pathways are involved in the regulation of seed and bud dormancy. Dormancy establishment in seeds is mediated by ABA, and our unpublished data indicate that this is also the case for bud dormancy in poplar (Bewley, 1997; Finkelstein et al., 2008; Tylewicz et al., 2015). Moreover, the release of SD-induced dormancy requires prolonged exposure of buds to low temperatures, which is similar to the low temperature treatment of seeds to initiate germination. The vernalization of cereal seeds alters the expression of many genes such as VERNALIZATION1 (VRN1), VRN2 and VRN3 (Trevaskis et al., 2007; Distelfeld et al., 2009; Sasani et al., 2009), which suggests that epigenetic control may be involved in seed dormancy. The data presented above also indicate that chromatin modification could be involved in bud dormancy. In arabidopsis, PHYTOCHROME INTERACTING FACTOR 1 (PIF1) and SPATULA (SPT) repress seed germination, and are required for dormancy maintenance. Both inhibit seed germination and maintain dormancy by regulating the levels of active GA. They inhibit the expression of GA3ox1 and GA3ox2, which are responsible for converting GA precursors to their active form. Cold temperature inhibits the ability of SPT to induce germination (Jiao et al., 2007). PIFs are also induced during the growth cessation and dormancy establishment processes (Ruttink et al., 2007), and it would be interesting to investigate whether PIFs play a similar role in bud dormancy regulation. Another study in leafy spurge (Euphorbia esula L.) reported that the transitions of buds and seeds may involve similar mechanisms. Both types of dormancy include ABA and auxin signalling and transport, AP2/ERF transcription factors and many cell cycle components (Chao et al., 2014). A comparison of endodormant buds and dormant seeds identified that transcripts associated with the cell cycle (HisH4), ABA and auxin response (ABA1, IAA7, ARF1 and TFL1), some stress-responsive factors (ERFB4/ABR1 and ICE2) and multiple proteins involved in carbohydrate and protein metabolism had similar differential expression patterns in both buds and seeds (Chao et al., 2014). Thus, a comparative analysis of seed and bud dormancy could be an effective way to identify regulators of bud dormancy in the future.
CONCLUSIONS
Although the photoperiod- and temperature-mediated control of the seasonal growth of trees has become an intensively studied topic in recent years, we are far from addressing several key issues in this area. One interesting insight from studies so far is how similar the photoperiodic signalling pathway in the control of growth cessation in boreal tree species is to the pathway that regulates flowering in annual plants such as arabidopsis. In this way, over the course of evolution, similar molecular players have maintained pivotal roles in the regulation of diverse biological processes. The molecular mechanisms underlying dormancy release and bud burst are still poorly understood and should be investigated using the model tree poplar, in which functional genomics approaches are feasible. Substantial progress has been made in elucidating certain aspects of the seasonal control of growth in model trees such as poplar and spruce, and in the coming years it should be interesting to investigate the extent to which these pathways are conserved in other tree species.
ACKNOWLEDGEMENTS
This work was supported by grants from Vetenskapsrådet (VR), FORMAS and Knut, and the Alice Wallenberg foundation to R.P.B.
LITERATURE CITED
- Abe M, Kobayashi Y, Yamamoto S. et al. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056. [DOI] [PubMed] [Google Scholar]
- Amasino R. 2004. Vernalization, competence, and the epigenetic memory of winter. The Plant Cell 16: 2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H. 2003. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. The Plant Cell 15: 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeez A, Miskolczi P, Tylewicz S, Bhalerao RP. 2014. A tree ortholog of APETALA1 mediates photoperiodic control of seasonal growth. Current Biology 24: 717–724. [DOI] [PubMed] [Google Scholar]
- Baba K, Karlberg A, Schmidt J. et al. 2011. Activity–dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proceedings of the National Academy of Sciences, USA108: 3418–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. 2004. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427: 164–167. [DOI] [PubMed] [Google Scholar]
- Baulcombe DC, Dean C. 2014. Epigenetic regulation in plant responses to the environment. Cold Spring Harbor Perspectives in Biology 6: a019471. doi: 10.1101/cshperspect.a019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao R, Nilsson O, Sandberg G. 2003. Out of the woods: forest biotechnology enters the genomic era. Current Opinion in Biotechnology 14: 206–213. [DOI] [PubMed] [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L. et al. 2006. CO/FT regulatory module controls timing of flowering andseasonal growth cessation in trees. Science 312: 1040–1043. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Evans LM, Hsu CY, Sheng X. 2014. Vernalization and the chilling requirement to exit bud dormancy: shared or separate regulation? Frontiers in Plant Science 5: 732. doi: 10.3389/fpls.2014.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Zambryski PC. 2012. Plasmodesmata paradigm shift: regulation from without versus within. Annual Review of Plant Biology 63: 239–260. [DOI] [PubMed] [Google Scholar]
- Busov V, Carneros E, Yakovlev I. 2016. EARLY BUD-BREAK1 (EBB1) defines a conserved mechanism for control of bud-break in woody perennials. Plant Signaling and Behavior 11: e1073873. doi: 10.1080/15592324.2015.1073873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao WS, Doğramaci M, Anderson JV, Foley ME, Horvath DP. 2014. The resemblance and disparity of gene expression in dormant and non-dormant seeds and crown buds of leafy spurge (Euphorbia esula). BMC Plant Biology 14: 216. doi: 10.1186/s12870-014-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YY, Kim SR, Finkel D, Yanofsky MF, An G. 1994. Early flowering and reduced apical dominance result from ectopic expression of a rice MADS box gene. Plant Molecular Biology 26: 657–665. [DOI] [PubMed] [Google Scholar]
- Cooke JE, Eriksson ME, Junttila O. 2012. The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant, Cell and Environment 35: 1707–1728. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S. et al. 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 18: 1030–1033. [DOI] [PubMed] [Google Scholar]
- Davies PJ. (ed.). 1995. Plant hormones: physiology, biochemistry and molecular biology. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J. 2009. Regulation of flowering in temperate cereals. Current Opinion in Plant Biology 12: 178–184. [DOI] [PubMed] [Google Scholar]
- Druart N, Johansson A, Baba K. et al. 2007. Environmental and hormonal regulation of the activity–dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. The Plant Journal 50: 557–573. [DOI] [PubMed] [Google Scholar]
- Eagles CF, Wareing PF. 1964. The role of growth substances in the regulation of bud dormancy. Physiologia Plantarum 17: 697–709. [Google Scholar]
- El-Kayal El, Allen CCG, Ju CJT. et al. 2011. Molecular events of apical bud formation in white spruce, Picea glauca. Plant, Cell and Environment 34: 480–500. [DOI] [PubMed] [Google Scholar]
- Eriksson ME, Moritz T. 2002. Daylength and spatial expression of a gibberellin 20-oxidase isolated from hybrid aspen (Populus tremula L. × P. tremuloides Michx.). Planta 214: 920–930. [DOI] [PubMed] [Google Scholar]
- Eriksson ME, Hoffman D, Kaduk M, Mauriat M, Moritz T. 2015. Transgenic hybrid aspen trees with increased gibberellin (GA) concentrations suggest that GA acts in parallel with FLOWERING LOCUS T2 to control shoot elongation. New Phytologist 205: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. 2008. Molecular aspects of seed dormancy. Annual Review of Plant Biology 59: 387–415. [DOI] [PubMed] [Google Scholar]
- Fu YH, Piao S, Vitasse Y. et al. 2015. Increased heat requirement for leaf flushing in temperate woody species over 1980–2012: effects of chilling, precipitation and insolation. Global Change Biology 21: 2687–2697. [DOI] [PubMed] [Google Scholar]
- de la Fuente L, Conesa A, Lloret A, Badenes ML, Ríos G. 2015. Genome-wide changes in histone H3 lysine 27 trimethylation associated with bud dormancy release in peach. Tree Genetics and Genomes 11: 45. doi: 10.1007/s11295-015-0869-7. [Google Scholar]
- Gillespie LM, Volaire FA. 2017. Are winter and summer dormancy symmetrical seasonal adaptive strategies? The case of temperate herbaceous perennials. Annals of Botany 119: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet MC, Larson PR. 1981. Structural changes in Populus deltoides terminal buds and in the vascular transition zone of the stems during dormancy induction. American Journal of Botany 68: 118–129. [Google Scholar]
- Halaly T, Pang X, Batikoff T. et al. 2008. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 228: 79–88. [DOI] [PubMed] [Google Scholar]
- Han X, Kumar D, Chen H, Wu S, Kim JY. 2014. Transcription factor-mediated cell-to-cell signalling in plants. Journal of Experimental Botany 65: 1737–1749. [DOI] [PubMed] [Google Scholar]
- Hannerz M, Ekberg I, Norell L. 2003. Variation in chilling requirements for completing bud rest between provenances of Norway spruce. Silvae Genetica 52: 161–168. [Google Scholar]
- Hao X, Chao W, Yang Y, Horvath D. 2015. Coordinated expression of FLOWERING LOCUS T and DORMANCY ASSOCIATED MADS-BOX-like genes in leafy spurge. PLoS One 11: e0126030. doi: 10.1371/journal.pone.0126030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide OM, Prestrud AK. 2005. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiology 25: 109–114. [DOI] [PubMed] [Google Scholar]
- Heide OM. 2011. Temperature rather than photoperiod controls growth cessation and dormancy in Sorbus species. Journal of Experimental Botany 62: 5397–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP, Chao WS, Suttle JC, Thimmapuram J, Anderson JV. 2008. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genomics 9: 536. doi:10.1186/1471-2164-9-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP, Sung S., Kim D, Chao W, Anderson J. 2010. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Molecular Biology 73: 169–179. [DOI] [PubMed] [Google Scholar]
- Howe GT, Bucciaglia PA, Hackett WP, Furnier GR, Cordonnier-Pratt MM, Gardner G. 1998. Evidence that the phytochrome gene family in black cottonwood has one PHYA locus and two PHYB loci but lacks members of the PHYC/F and PHYE subfamilies. Molecular Biology and Evolution 15: 160–175. [DOI] [PubMed] [Google Scholar]
- Howe GT, Horvath DP, Dharmawardhana P, Priest HD, Mockler TC, Strauss. 2015. Extensive transcriptome changes during natural onset and release of vegetative bud dormancy in Populus. Frontiers in Plant Science 6: 989. doi: 10.3389/fpls.2015.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Liu Y, Luthe DS, Yuceer C. 2006. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. The Plant Cell 18: 1846–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Adams JP, Kim H. et al. 2011. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proceedings of the National Academy of Sciences, USA 108: 10756–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Jin Y, Shi H, Yang W. 2014. GmFLD, a soybean homolog of the autonomous pathway gene FLOWERING LOCUS D, promotes flowering in Arabidopsis thaliana. BMC Plant Biology 14: 263. doi: 10.1186/s12870-014-0263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez C, Kozarewa I, Johansson M, Ogren E, Rohde A, Eriksson ME. 2010. Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiology 153: 1823–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. 1990. Function of the apetala-1 gene during Arabidopsis floral development. The Plant Cell 2: 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. 2007. FT protein acts as a long-range signal in Arabidopsis. Current Biology 17: 1050–1054. [DOI] [PubMed] [Google Scholar]
- Jian LC, Li PH, Sun LH, Chen THH. 1997. Alterations in ultrastructure and subcellular localization of Ca2+ in poplar apical bud cells during the induction of dormancy. Journal of Experimental Botany 48: 1195–1207. [Google Scholar]
- Jiao Y, Lau OS, Deng XW. 2007. Light-regulated transcriptional networks in higher plants. Nature Reviews. Genetics 8: 217–230. [DOI] [PubMed] [Google Scholar]
- Jiménez S, Reighard GL, Bielenberg DG. 2010. Gene expression of DAM5 and DAM6 is suppressed by chilling temperatures and inversely correlated with bud break rate. Plant Molecular Biology 73: 157–167. [DOI] [PubMed] [Google Scholar]
- Jung JH, Seo YH, Seo PJ. et al. 2007. The GIGANTEA-regulated MicroRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. The Plant Cell 19: 2736–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila O, Jensen E. 1988. Gibberellins and photoperiodic control of shoot elongation in Salix. Physiologia Plantarum 74: 371–376. [Google Scholar]
- Junttila O, Nilsen J, Igeland B. 2003. Effect of temperature on the induction of bud dormancy in ecotypes of Betula pubescens and Betula pendula. Scandinavian Journal of Forest Research 18: 208–217. [Google Scholar]
- Kalcsits LA, Silim S., Tanino K. 2009. Warm temperature accelerates short photoperiod-induced growth cessation and dormancy induction in hybrid poplar (Populus × spp.). Trees 23: 971–979. [Google Scholar]
- Karlberg A, Bako L, Bhalerao RP. 2011. Short day-mediated cessation of growth requires the downregulation of AINTEGUMENTALIKE1 transcription factor in hybrid aspen. PLoS Genetics 7: e1002361. doi:10.1371/journal.pgen.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg A, Englund M, Petterle A. et al. 2010. Analysis of global changes in gene expression during activity–dormancy cycle in hybrid aspen apex. Plant Biotechnology 27: 1–16. [Google Scholar]
- Karlgren A, Gyllenstrand N, Clapham D, Lagercrantz U. 2013. FLOWERING LOCUS T/TERMINAL FLOWER1-like genes affect growth rhythm and bud set in Norway spruce. Plant Physiology 163: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin T, Panga X, Venkateswari J. et al. 2007. Digital expression profiling of a grape-bud EST collection leads to new insight into molecular events during grape-bud dormancy release. Plant Science 173: 446–457. [Google Scholar]
- Kozarewa I, Ibanez C, Johansson M. et al. 2010. Alteration of PHYA expression change circadian rhythms and timing of bud set in Populus. Plant Molecular Biology 73: 143–156. [DOI] [PubMed] [Google Scholar]
- Leida C, Conesa A, Llácer G, Badenes ML, Ríos G. 2012. Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytologist 193: 67–80. [DOI] [PubMed] [Google Scholar]
- Levy A, Guenoune-Gelbart D, Epel BL. 2007. β-1,3-glucanases: plasmodesmal gate keepers for intercellular communication. Plant Signaling and Behavior 2: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Lee JY. 2004. Plasmodesmata as a supracellular control network in plants. Nature Reviews. Molecular Cell Biology 5: 712–726. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. 1992. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277. [DOI] [PubMed] [Google Scholar]
- Maule AJ. 2008. Plasmodesmata: structure, function and biogenesis. Current Opinion in Plant Biology 11: 680–686. [DOI] [PubMed] [Google Scholar]
- Meitha K, Konnerup D, Colmer TD, Considine JA, Foyer CH, Considine MJ. 2015. Spatio-temporal relief from hypoxia and production of reactive oxygen species during bud burst in grapevine (Vitis vinifera). Annals of Botany 116: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R, Wang CT, Ma C. et al. 2010. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. The Plant Journal 62: 674–688. [DOI] [PubMed] [Google Scholar]
- Mølmann JA, Asante DK, Jensen JB. et al. 2005. Low night temperature and inhibition of gibberellin biosynthesis override phytochrome action and induce bud set and cold acclimation, but not dormancy in PHYA overexpressors and wild-type of hybrid aspen. Plant, Cell & Environment 28: 1579–1588. [Google Scholar]
- Nitsch JP. 1957. Photoperiodism in woody plants. Proceedings of the American Society for Horticultural Science 70: 526–544. [Google Scholar]
- Niwa M, Daimon Y, Kurotani K. et al. 2013. BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis. The Plant Cell 25: 1228–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JE. 2010. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol Biol 73: 37–47. [DOI] [PubMed] [Google Scholar]
- Olsen JE, Jensen E, Junttila O, Moritz T. 1995a. Photoperiodic control of endogenous gibberellins in seedlings of Salix pentandra. Physiologia Plantarum 93: 639–644. [Google Scholar]
- Olsen JE, Junttila O, Moritz T. 1995b. A localized decrease of GA1 in shoot tips of Salix pentandra seedlings precedes cessation of shoot elongation under short photoperiod. Physiologia Plantarum 95: 627–632. [Google Scholar]
- Olsen JE, Junttila O, Nilsen J. et al. 1997. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. The Plant Journal 12: 1339–1350. [Google Scholar]
- Ophir R, Pang X, Halaly T. et al. 2009. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene–ABA interplay and cell enlargement. Plant Molecular Biology 71: 403–423. [DOI] [PubMed] [Google Scholar]
- Or E, Vilozny I, Fennell A, Eyal Y, Ogrodovitch A. 2002. Dormancy in grape buds: isolation and characterization of catalase cDNA and analysis of its expression following chemical induction of bud dormancy release. Plant Science 162: 121–130. [Google Scholar]
- Pang X, Halaly T, Crane O. et al. 2007. Involvement of calcium signalling in dormancy release of grape buds. Journal of Experimental Botany 58: 3249–3262. [DOI] [PubMed] [Google Scholar]
- Pérez FJ, Vergara R, Or E. 2009. On the mechanism of dormancy release in grapevine buds: a comparative study between hydrogen cyanamide and sodium azide. Plant Growth Regulation 59: 145–152. [Google Scholar]
- Petterle A, Karlberg A, Bhalerao RP. 2013. Daylength mediated control of seasonal growth patterns in perennial trees. Current Opinion in Plant Biology 16: 301–306. [DOI] [PubMed] [Google Scholar]
- Petterle A. 2011. ABA and chromatin remodeling regulate the activity–dormancy cycle in hybrid aspen. Doctoral Thesis, No. 2011:17, Swedish University of Agricultural Sciences, Umeå.
- Randall RS, Miyashima S, Blomster T. et al. 2015. AINTEGUMENTA and the D type cyclin CYCD3;1 regulate root secondary growth and respond to cytokinins. Biology Open 4: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resman L, Howe G, Jonsen D. et al. 2010. Components acting downstream of short day perception regulate differential cessation of cambial activity and associated responses in early and late clones of hybrid poplar. Plant Physiology 154: 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM. 1997. Determination of floral organ identity by Arabidopsis MADS domain homeotic proteins AP1, AP3, PI, and AG is independent of their DNA-binding specificity. Molecular Biology of the Cell 8: 1243–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne PLH, van der Schoot C. 1998. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125: 1477–1485. [DOI] [PubMed] [Google Scholar]
- Rinne PLH, van den Boogaard R, Mensink MGJ. et al. 2005. Tobacco plants respond to the constitutive expression of the tospovirus movement protein NSM with a heat reversible sealing of plasmodesmata that impairs development. The Plant Journal 43: 688–707. [DOI] [PubMed] [Google Scholar]
- Rinne PL, Welling A, Vahala J. et al. 2011. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. The Plant Cell 23: 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos G, Leida C, Conejero A, Badenes ML. 2014. Epigenetic regulation of bud dormancy events in perennial plants. Frontiers in Plant Science 5: 247. doi: 10.3389/fpls.2014.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Bastien C, Boerjan W. 2011. Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiology 31: 472–482. [DOI] [PubMed] [Google Scholar]
- Rohde A, Bhalerao RP. 2007. Plant dormancy in the perennial context. Trends in Plant Science 12: 217–223. [DOI] [PubMed] [Google Scholar]
- Rohde A, Prinsen E, De Rycke R, Engler G, Van Montagu M, Boerjan W. 2002. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. The Plant Cell 14: 1885–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruonala R, Rinne PLH, Baghour M, Moritz T, Tuominen H, Kangasjärvi J. 2006. Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involve ethylene. The Plant Journal 46: 628–640. [DOI] [PubMed] [Google Scholar]
- Ruttink T, Arend M, Morreel K. et al. 2007. A molecular timetable for apical bud formation and dormancy induction in poplar. The Plant Cell 19: 2370–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Bai S, Imai T, Ito A, Nakajima I, Moriguchi T. 2015. Histone modification and signalling cascade of the dormancy-associated MADS-box gene, PpMADS13-1, in Japanese pear (Pyrus pyrifolia) during endodormancy. Plant, Cell & Environment 38: 1157–1166. [DOI] [PubMed] [Google Scholar]
- Sasaki R, Yamane H, Ooka T. et al. 2011. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiology 157: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasani S, Hemming MN, Oliver SN. et al. 2009. The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). Journal of Experimental Botany 60: 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saure M. 1985. Dormancy release in deciduous fruit trees. Horticultural Reviews 7: 239–300. [Google Scholar]
- Schmid M, Uhlenhaut NH, Godard F. et al. 2003. Dissection of floral induction pathways using global expression analysis. Development 130: 6001–6012. [DOI] [PubMed] [Google Scholar]
- Shim D, Ko JH, Kim WC, Wang Q, Keathley DE, Han KH. 2014. A molecular framework for seasonal growth–dormancy regulation in perennial plants. Horticulture Research 1: 14059. doi: 10.1038/hortres.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. 2009. An Arabidopsis GPI anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. The Plant Cell 21: 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Svystun T, AlDahmash B, Jönsson AM, Bhalerao RP. 2017. Photoperiod- and temperature-mediated control of phenology in trees – a molecular perspective. New Phytologist 213: 511–524. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. 2001. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120. [DOI] [PubMed] [Google Scholar]
- Taoka K, Ohki I, Tsuji H. et al. 2011. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476: 332–335. [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. 2007. The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science 12: 353–357 [DOI] [PubMed] [Google Scholar]
- Tsuji H, Tachibana C, Tamaki S, Taoka K, Kyozuka J, Shimamoto K. 2015. Hd3a promotes lateral branching in rice. The Plant Journal 82: 256–266. [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. 2008. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annual Review of Plant Biology 59: 573–594. [DOI] [PubMed] [Google Scholar]
- Tylewicz S, Tsuji H, Miskolczi P. et al. 2015. Dual role of tree florigen activation complex component FD in photoperiodic growth control and adaptive response pathways. Proceedings of the National Academy of Sciences, USA 112: 3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus SL, Martinelli AP, Dinh QD. et al. 2010. Intercellular transport of epidermis expressed MADS domain transcription factors and their effect on plant morphology and floral transition. The Plant Journal 63: 60–72. [DOI] [PubMed] [Google Scholar]
- Weiser CJ. 1970. Cold resistance and injury in woody plants. Science 169: 1269–1278. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE. et al. 2005. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059. [DOI] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. 2009. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. 2014. Regulation of bud dormancy and bud break in Japanese apricot (Prunus mume Siebold & Zucc.) and peach [Prunus persica (L.) Batsch]: a summary of recent studies. Journal of the Japanese Society for Horticultural Science 83: 187–202. [Google Scholar]
- Yamane H, Ooka T, Jotatsu H, Hosaka Y, Sasaki R, Tao R. 2011. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. Journal of Experimental Botany 62: 3481–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SC, Chen C, Rojas M. et al. 2013. Phloem long-distance delivery of FLOWERINGLOCUS T (FT) to the apex. The Plant Journal 75: 456–468. [DOI] [PubMed] [Google Scholar]
- Yordanov YS, Ma C, Strauss SH, Busov VB. 2014. EARLY BUD-BREAK 1 (EBB1) is a regulator of release from seasonal dormancy in poplar trees. Proceedings of the National Academy of Sciences, USA 111: 10001–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawaski C, Busov VB. 2014. Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS One 9: e86217. doi:10.1371/journal. pone.0086217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Halaly T, Acheampong AK. et al. 2015. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. Journal of Experimental Botany 66: 1527–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu L, Shen L, Yu H. 2016. NaKR1 regulates long-distance movement of FLOWERING LOCUS T in Arabidopsis. Nature Plants 2: 16075. doi: 10.1038/nplants.2016.75. [DOI] [PubMed] [Google Scholar]