Abstract
Background and Aims
Selected beneficial Pseudomonas spp. strains have the ability to influence root architecture in Arabidopsis thaliana by inhibiting primary root elongation and promoting lateral root and root hair formation. A crucial role for auxin in this long-term (1week), long-distance plant–microbe interaction has been demonstrated.
Methods
Arabidopsis seedlings were cultivated in vitro on vertical plates and inoculated with pathogenic strains Pseudomonas syringae pv. maculicola (Psm) and P. syringae pv. tomato DC3000 (Pst), as well as Agrobacterium tumefaciens (Atu) and Escherichia coli (Eco). Root hair lengths were measured after 24 and 48h of direct exposure to each bacterial strain. Several Arabidopsis mutants with impaired responses to pathogens, impaired ethylene perception and defects in the exocyst vesicle tethering complex that is involved in secretion were also analysed.
Key Results
Arabidopsis seedling roots infected with Psm or Pst responded similarly to when infected with plant growth-promoting rhizobacteria; root hair growth was stimulated and primary root growth was inhibited. Other plant- and soil-adapted bacteria induced similar root hair responses. The most compromised root hair growth stimulation response was found for the knockout mutants exo70A1 and ein2. The single immune pathways dependent on salicylic acid, jasmonic acid and PAD4 are not directly involved in root hair growth stimulation; however, in the mutual cross-talk with ethylene, they indirectly modify the extent of the stimulation of root hair growth. The Flg22 peptide does not initiate root hair stimulation as intact bacteria do, but pretreatment with Flg22 prior to Psm inoculation abolished root hair growth stimulation in an FLS2 receptor kinase-dependent manner. These early response phenomena are not associated with changes in auxin levels, as monitored with the pDR5::GUS auxin reporter.
Conclusions
Early stimulation of root hair growth is an effect of an unidentified component of living plant pathogenic bacteria. The root hair growth response is triggered in the range of hours after bacterial contact with roots and can be modulated by FLS2 signalling. Bacterial stimulation of root hair growth requires functional ethylene signalling and an efficient exocyst-dependent secretory machinery.
Keywords: Root hair, Arabidopsis, Pseudomonas, exocyst, Flg22, vesicle trafficking, dde2/ein2/pad4/sid2
INTRODUCTION
Plants are in contact during their life cycle with both beneficial and harmful bacteria. It is thus of key importance for a plant to distinguish between such bacteria and react accordingly. Some beneficial bacteria, so-called plant growth-promoting rhizobacteria (PGPR), may stimulate plant growth (Kremer et al., 1990; Lugtenberg and Kamilova, 2009). Recently, the ability of selected beneficial Pseudomonas spp. strains to influence root architecture in Arabidopsis thaliana by inhibiting primary root elongation and promoting lateral root and root hair formation was reported, with the role of auxin in this long-term (1week) and long-distance interaction clearly demonstrated (Rudrappa and Bais, 2008; Zamioudis et al., 2013). In addition, auxin-independent root architecture modifications caused by bacteria have been found, with bacterial quorum-sensing signalling N-acyl-homoserine lactones (AHLs) found to affect primary root growth, lateral root formation and root hair development (Ortíz-Castro et al., 2008).
Evidence that plants initially respond similarly to both PGPR and pathogenic bacteria has emerged in recent years (van Loon et al., 2008; reviewed by Zamioudis and Pieterse, 2012). In agreement with this, one of the most studied pathogen/microbe associated molecular patterns (P/MAMPs), the conserved peptide Flg22 derived originally from Pseudomonas aeruginosa flagellar protein, has also been shown to induce inhibition of primary root growth just as observed with PGPRs (Gómez-Gómez et al., 1999; Millet et al., 2010). Flg22 is recognized by the plant cell via the plant pathogen recognition receptor kinase FLS2 (FLAGELLIN SENSITIVE 2) and the co-receptor BAK1 (BRI1-ASSOCIATED KINASE 1). The activated FLS2 switches on a signalling cascade leading to activation of the transcription of defence genes and production of reactive-oxygen species (ROS) and salicylic acid (SA) which also function as signalling molecules (Chinchilla et al., 2007; Beck et al., 2012). SA is involved in the plant immune response alongside jasmonic acid (JA), ethylene (ET) and the protein PHYTOALEXIN DEFICIENT 4 (PAD4) (Tsuda et al., 2009, Kim et al. 2014). The SA-dependent transcriptional activation relies on NPR1 (NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1), a transcriptional co-activator that is relocated from the cytoplasm to the nucleus (Kinkema et al., 2000). One of the major outcomes of this gene activation is a modification of cell walls and the extracellular space. This is supported by efficient targeted secretion, which depends upon correct tethering and fusion of vesicles to the target plasma membrane. These processes are mediated, amongst others, by the exocyst and SNARE (soluble N-ethylmaleimide-sensitive fusion attachment protein receptors) protein complexes (Rothman and Warren, 1994; TerBush et al., 1996; Dörmann et al., 2014).
The exocyst, a secretory vesicle-tethering protein complex, was first discovered in yeast and animals, but in recent years it has also been found to function as a complex in plants (Guo et al., 1999; Hsu et al., 2004; Hála et al., 2008; Zárský et al., 2009, 2013; Kulich et al., 2013). The Arabidopsis exo70A1 mutant, as well as the maize sec3 mutant (roothairless1 – rth1) fails to properly elongate root hairs (Wen et al., 2005; Synek et al., 2006). The exocyst has also been found to be important for efficient plant defence against several pathogens (Pečenková et al., 2011; Stegmann et al., 2012; Du et al., 2015).
Whilst biotic interactions and plant defence reactions have been mostly studied in above-ground plant tissues, specific PAMP perception has also been identified in root tissues in Arabidopsis (Millet et al., 2010). When the transcriptional activation of reporter genes and callose deposition were monitored in roots after treatment with different PAMPs, including Flg22, strong tissue-specific upregulation of defence-related responses was triggered (Millet et al., 2010). These responses were ET- but not SA- or JA-signalling dependent. The sensitivity of roots to Flg22 may be explained by the fact that Flg22, as well as other elicitors, are derived from highly conserved bacterial components that are present in both phylosphere and soil.
The function and morphology of root hairs in relation to the acquisition of water and nutrients have been well studied. Tanaka et al. (2014) demonstrated that root hairs play significant roles in: the absorption of water, phosphorus, iron, calcium, zinc, copper and potassium; in the secretion of acid phosphatase, malate and citrate; and in penetration of the primary roots into gels. Root hairs have been found to be induced and their lengths more elongated under different nutrient deficiencies (phosphorus, potassium, magnesium, iron and manganese) (Bates and Lynch, 1996; Schikora and Schmidt, 2001; Yang et al., 2008; Jung et al., 2009; Niu et al., 2014). Although the role of root hairs in interactions with nitrogen-fixing bacteria has been investigated, little attention has been given to their role during plant–pathogen interactions.
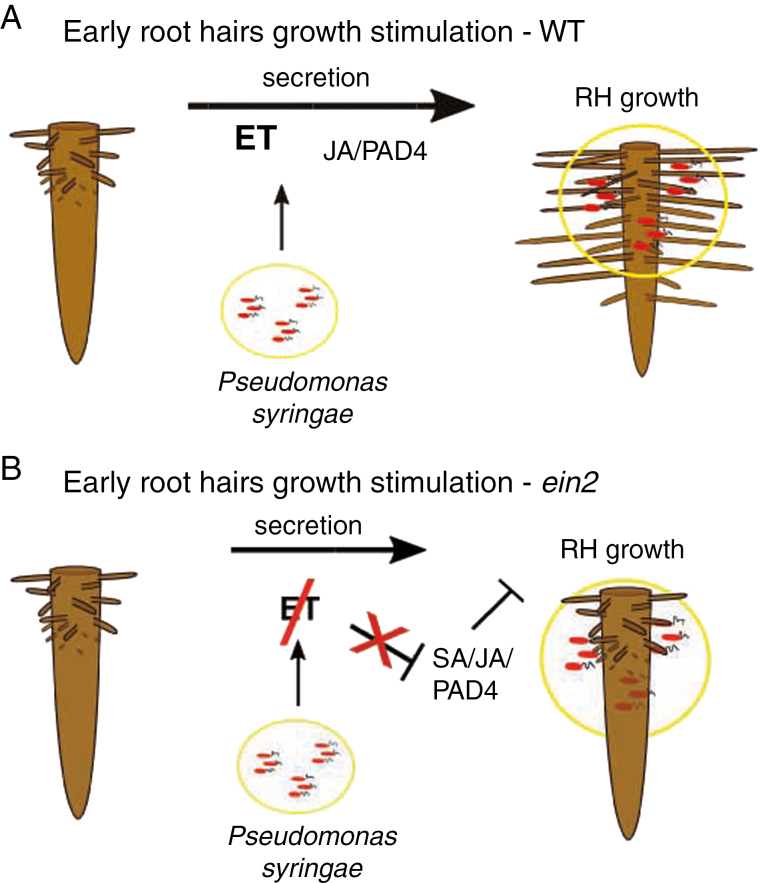
This study of the response of Arabidopsis seedlings to the attack by the pathogenic bacteria Pseudomonas syringae pv. maculicola (Psm) and Pseudomonas syringae pv. tomato DC3000 (Pst) revealed, in in vitro cultivated seedlings, primary root growth inhibition together with stimulation of rapid root hair growth. A detailed investigation of this observed behaviour was conducted by examining plant responses and the activity of an auxin-responsive promoter. Several Arabidopsis mutants were employed, comprising exocyst mutants, and a whole set of dde2/ein2/pad4/sid2 single to quadruple mutants. We show that the stimulation of root hair growth is triggered in a range of hours after contact of bacteria with roots, is dependent on unknown component(s) of living bacteria other than those described so far, and that the stimulation can be modified by FLS2 signalling. We also show that correct functioning of the EIN2 ET signal transducer and the exocyst complex play important roles in this stimulation of root hair growth. This appears to be in contrast to SA/JA/PAD4. Although in mutual cross-talk these pathways indirectly modify the outcome, independently they do not influence this plant response.
MATERIAL AND METHODS
Plant material
The A. thaliana T-DNA insertional mutant lines that were used in this study comprised: N8844 (At5g03280, ein2), SALK_122399 (At3g25070), npr1-1 [donated by Saskia van Wees (Utrecht University; Cao et al., 1994)] and SALK_014826 (At5g03540, exo70A1) and set of the mutants connected with the quadruple mutant (dde2-2/ein2-1/pad4-1/sid2-2), triple mutants (dde2-2/ein2-1/pad4-1, dde2-2/ein2-1/sid2-2, dde2-2/pad4-1/sid2-2 and ein2-1/pad4-1/sid2-2), double mutants (dde2-2/ein2-1, dde2-2/pad4-1, dde2-2/sid2-2, ein2-1/pad4-1, ein2-1/sid2-2 and pad4-1/sid2-2) and single mutants (dde2-2, ein2-1, pad4-1 and sid2-2) all donated by Dr Kenichi Tsuda (Max Planck Institute of Plant Breeding Research in Cologne; Tsuda et al., 2009).
For experiments with Psm, seeds were surface sterilized and plated on half strength Murashige and Skoog medium (1/2MS), 1·6 % plant agar. Germinated plants were propagated in vitro for 7 d on vertical plates. In addition, the GUS gene under the control of the DR5 promoter carrying line was used to check for changes in the expression of auxin-related genes (Ulmasov et al., 1997). For experiments with Pst, seeds were surface sterilized and plated on 1/2MS containing 1% sucrose (pH 5·7 adjusted with KOH) and 1·2 % plant agar. The plants were propagated in vitro for 4 d on Petri dishes (diameter 6cm) vertically. The cultivation conditions were 16/8h light/dark at 21–22°C.
Inoculation of plant material with bacteria
For inoculation with P. syringe pv. maculicola strain 795 (Psm; Dong et al., 1991), bacteria were prepared as described by Katagiri et al. (2002). Briefly, freshly inoculated plates (2–3 d old; Luria–Bertani medium with 25mg L–1 streptomycin) were used to prepare liquid overnight culture (40mL), without streptomycin, with incubation at 28°C on an orbital shaker at 130 r.p.m. Bacteria were centrifuged at 1500 g for 10min and the pellet was resuspended in dH2O and diluted to an OD600 of 0·3 (108 CFU mL–1). Approximately 10-μL droplets were applied to cover root tips within the elongation and differentiation zone. As a mock control, dH2O was applied in the same manner and, 48h later, the root growth and architecture was inspected. Experiments with other bacteria, namely, Atu – Agrobacterium tumefaciens GV3101, Eco – Escherichia coli DH5a, Psp – Pseudomonas savastanoi pv. phaseolicola and Pst – Pseudomonas syringae pv. tomato DC3000, were performed as described for experiments with Psm.
For experiments involving inoculation with Pst (Xin and He, 2013), bacteria were incubated overnight on plates (King’s B medium with 50mg L–1 rifampicin) and inoculum removed from the plate and diluted to an OD600= of 0·01 in MS/2 + 1 % sucrose (pH 5·7 adjusted with KOH). Seedlings were flooded with 3mL of the bacterial solution and the plates were placed in horizontal position for 24h.
Flg22 treatment
Flg22 is a 22-amino-acid peptide derived from P. aeruginosa flagellin, a structural protein that forms the major portion of flagellar filaments (QRLSTGSRINSAKDDAAGLQIA, synthesized by Vidia, Vestec, Czech Republic). The peptide was diluted in dH2O to a final concentration 10 μm and applied in 10-μL drops to cover the root tips of approx. 1-week-old seedlings and then left for 48h until evaluation. The peptide was applied at a concentration 10 times higher than usually employed for such treatments, given the potential diffusion of the peptide from the roots on the agar plate.
BFA treatment
For measurement of root hair lengths, the 4-d-old seedlings were flooded with a Pst solution containing 0·1% DMSO or 50 µm brefeldin A (BFA) for 24h.
Histochemical GUS staining procedure
After the treatment (including a mock to serve as a control), whole seedlings were submerged for 3h at 37°C in a solution consisting of 25 mm sodium phosphate buffer (pH 7·0), 10 mm EDTA, 2 mm X‐gluc, 0·5 mm ferricyanide and 0·5 mm ferrocyanide. The GUS staining solution was replaced with 70 % ethanol to bleach the tissues, and the seedlings roots were then examined.
Microscopic examination of inoculated roots
The plant responses to pathogens were monitored by microscopy and documented by photography. Microscopic analysis was performed using an Olympus BX51 microscope with attached DP50 camera (Olympus; Psm) or Zeiss AxioImager ApoTome2 microscope (Pst). In the Psm experiments, five plants were analysed for each genotype and photographed. Root hair lengths were determined for the first 20 root hairs starting from the root tip for each plant; for statistical analysis, the 40 longest root hairs for each line were used. In addition, root hairs were also observed under a confocal microscope (Zeiss LSM Duo510) 20min after staining with the membrane dye FM 4-64. For the Pst experiments, at least 10 seedlings were analysed for each variant. After Pst inoculation, five root hairs were measured, beginning approx. 1mm from the site of root hair initiation. The distance of the first root hairs from the root cap was also analysed.
Statistical evaluation
Image processing software was employed for image data quantification. Roots and root hair sizes were analysed using AnalySIS (Soft Imaging System GmbH, Germany) or ImageJ (Schneider et al., 2012) software. The numerical data obtained were processed using Microsoft Excel. To determine statistical significance, Student’s t-test and ANOVA tests were conducted either using Excel or on-line calculators (http://in-silico.net, and http://statpages.info/anova1sm.html). P-values of less than 0·05 indicated a significant difference among the various groups. For multiple-comparison experiments, the Tukey Honestly Significant Difference (HSD) post hoc test was used.
RESULTS
Early stimulation of root hair growth by bacteria in Arabidopsis WT and selected mutants
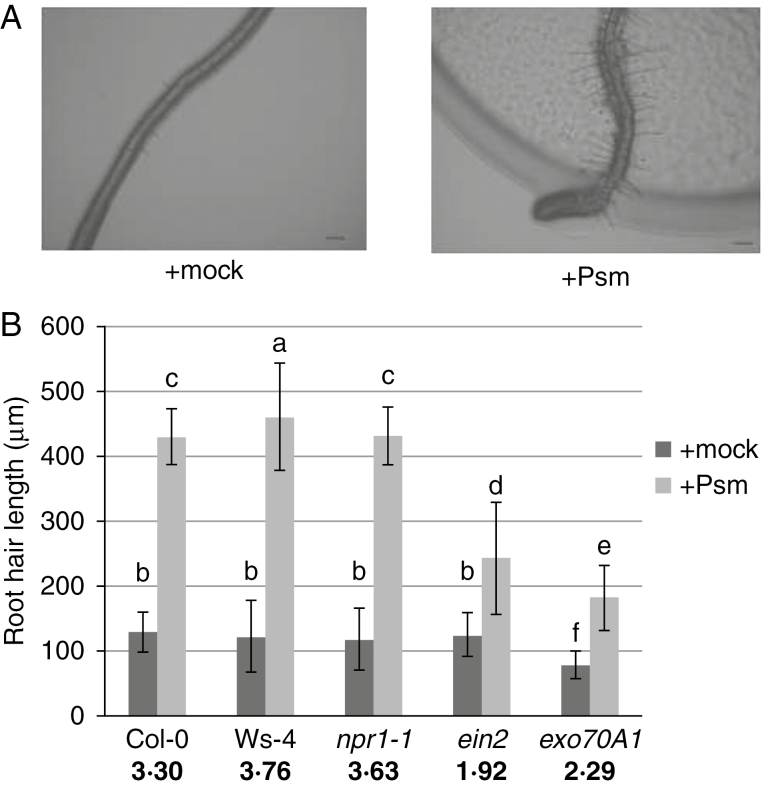
When roots of Arabidopsis seedlings were exposed to direct contact with the plant pathogenic bacterium Psm, a rapid modification of root architecture was observed. One unexpected but significant change involved root hair growth within 48h of exposure to Psm. We quantified root hair growth using 1-week-old seedlings grown on vertical plates and inoculated either with mock (sterile deionized water) or with Psm. Psm-treated root hairs were approx. 3·3 times longer than those of mock-treated plants (Fig. 1A). As expected, we also found that primary root growth was inhibited; the ratio of Psm-treated to mock-treated average root lengths of wild type (WT) Col-0 was approx. 0·82. A small but significant difference in root hair density between Psm- and mock-treated plants was also observed, with average values, respectively, of 37±8 and 29±6 root hairs per 1mm region closest to the root tip. The inhibition of root growth may be a consequence of the reduced length of the elongation/differentiation zone, while the size of the meristematic zone remained unchanged (Supplementary Data, Fig. S1).
Fig. 1.
Stimulation of root hair growth 48h post-inoculation with Psm. (A) Appearance of root hairs after mock and Psm treatment (scale bar=200μm). (B) Length of root hairs of mock- (dark grey) and Psm-treated (light grey) seedlings for different mutant lines. Ratios calculated for Psm vs. mock-treated seedlings are shown in bold for each mutant line under the respective columns in the graph. Different letters indicate significant differences in length (P<0·05).
Next we analysed these root growth responses to Psm using three types of mutants: a line carrying a mutation in the gene responsible for SA-dependent activation of pathogenesis-related genes (npr1-1); a well-studied mutant affected in ET perception, ein2 (Guzmán and Ecker, 1990; Rahman et al., 2002); and a line carrying a mutation in a gene encoding the exocyst vesicle-tethering complex, exo70A1. Both ein2 and exo70A1, when cultivated under the described conditions without bacterial treatment, produce fewer and shorter root hairs than the wild type plant. In addition, along with Col-0, we also studied the Wassilewskija (Ws-4) ecotype, which is a natural mutant in the bacterial flagellin receptor FLS2 (with a STOP codon in the kinase domain; Gómez-Gómez et al., 1999; Zipfel et al., 2004).
Average root hair lengths differed significantly after 48h of treatments (two to three times) between the mock- and bacteria-treated plants for all tested lines (Fig. 1B). As root hair lengths in the mock treatment varied across mutant lines, we calculated the ratio of the root hair lengths from bacteria-treated versus mock-treated plants, for each of the lines, and compared this to the ratio calculated for the wild type. The most prominent differences were found for the exo70A1 and ein2 mutants, which had shorter root hairs than Col-0. For each of the mutant lines, deviations from the Col-0 ratio were found to be statistically significant, with the exception of npr1-1.
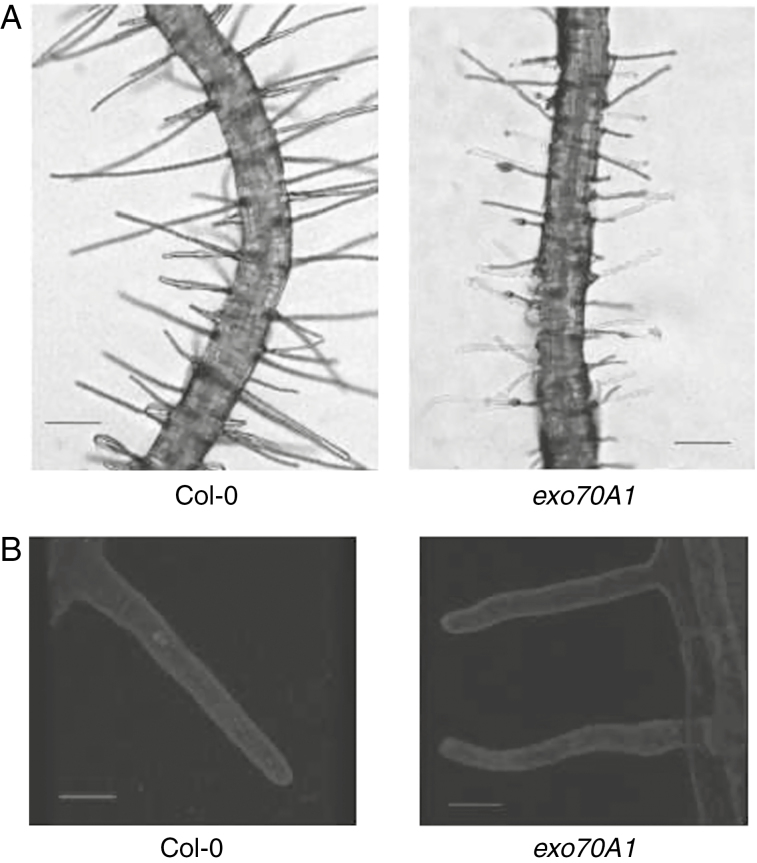
Exocyst functioning is important for stimulation of root hair growth, possibly due to an enhanced requirement for exocytosis in root hair elongation. Root hairs of mutant exo70A1 after exposure to bacteria were not only shorter, but also appeared with a severely distorted wavy shape (Fig. 2A). Although these root hairs were further examined using confocal microscopy after staining with FM-dye (Fig. 2B), no difference in endomembrane structures between the mutant and the WT was found after stimulation of root hair growth by Psm.
Fig. 2.
Appearance of the exo70A1 root hairs stimulated by Psm, in comparison with WT root hairs. (A) Optical microscopy (scale bar=200μm). (B) Confocal images taken after staining of the plasma membrane with FM4-64 dye (scale bar=20μm).
We also quantified changes in primary root growth after the application of bacteria. Average primary root lengths for all tested lines after 48h are shown in Supplementary Data Fig. S2. The strongest deviation from the WT response of growth inhibition of primary roots was found in the npr1-1 mutant, which showed weaker inhibition. The exo70A1 also showed lower inhibition of root growth after pathogen treatment compared to WT plants. Primary root lengths were found to be significantly different between mock- and bacteria-treated seedlings; however, differences in lengths between the WT and each mutant line (with the exception of Ws-4) within a given treatment were not statistically significant.
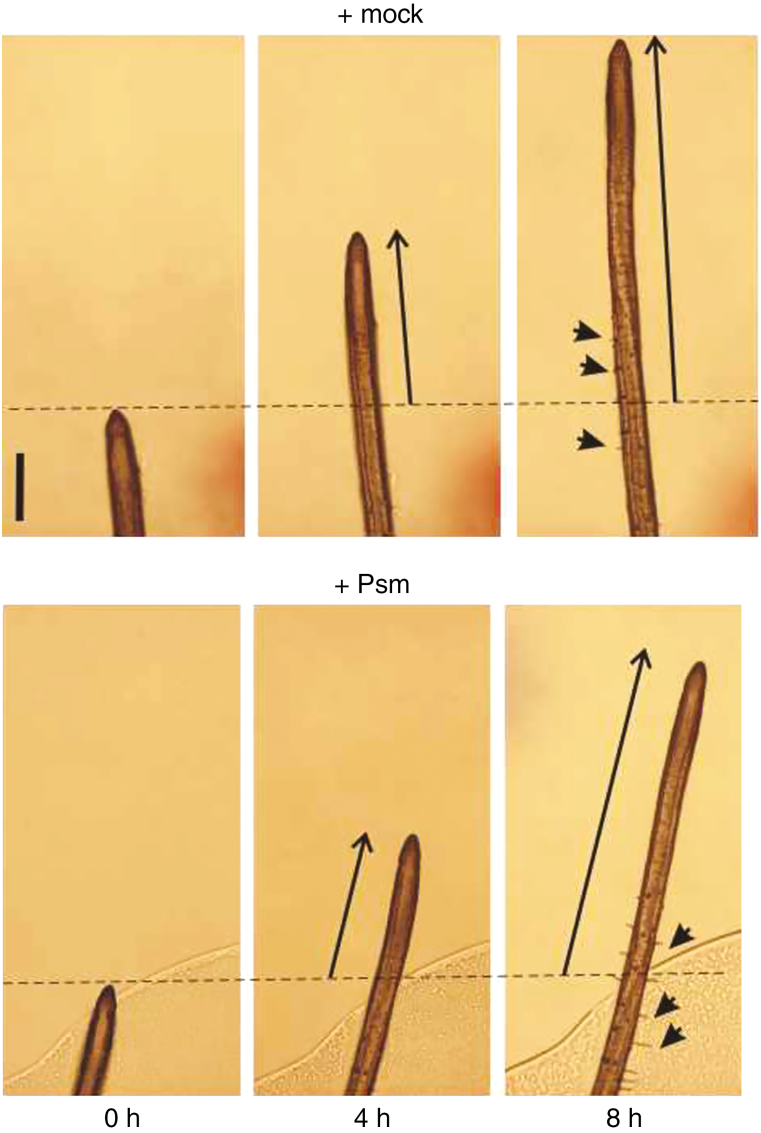
Continuous root tip imaging was used to observe the sequence of events after contact between the seedling roots and Psm. We found that stimulation of root hair growth and inhibition of root growth were noticeable 8 or 10h post-inoculation (hpi), respectively, while a more detailed time-course analysis revealed that root hair growth may be stimulated by 4 hpi (Fig. 3, Supplementary Data Figs S3 and S7).
Fig. 3.
Continuous root tip imaging after the Psm treatment, in comparison with mock-treated roots. The primary root growth from 0 to 4 and 8h are marked with arrows; some of the root hairs appearing and growing in this time range and region are indicated as well (marked with arrowheads; scale bar=0·2mm).
Flg22 pretreatment suppresses stimulation of root hair growth by Psm
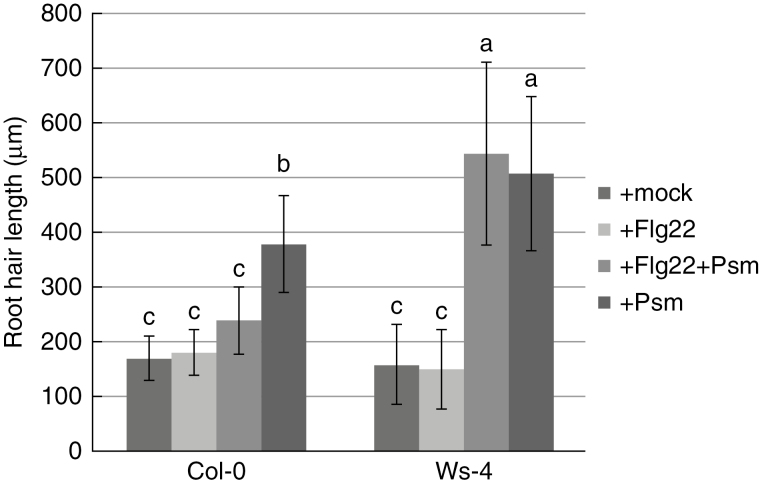
To test whether the observed pathogen-induced stimulation of root hair growth could be triggered or affected by the Flg22 peptide, we treated seedling roots with 10 µm Flg22 alone or with 10 µm Flg2212h prior to inoculation with Psm. Treatment with Flg22 alone failed to induce enhanced root hair growth, while primary root growth inhibition was observed as previously reported (Bauer et al., 2001). Pretreatment of Col-0 with Flg22 led to moderated stimulation of root hair growth and the difference compared to growth in Psm-treated Col-0 was significant. However, these results differed significantly from those found for Ws-4. In the case of Ws-4, an FLS2 null mutant, pretreatment had no impact on root hair and primary root growth (Fig. 4, Supplementary Data Fig. S4).
Fig. 4.
Stimulation of root hair growth by Psm after pretreatment with the Flg22 peptide. Seedling roots were treated with 10 µm Flg22 alone, or with 10 µm Flg22 followed by Psm inoculation 12h later. Mock and Psm inoculations were used as negative and positive controls, respectively. Pretreatment of Col-0 with Flg22 moderates stimulation of root hair growth, while in Ws-4, the pretreatment had no effect. Different letters indicate significant differences (P<0·05).
Activity of an auxin-responsive promoter in the root tip was not affected early after the contact with bacteria
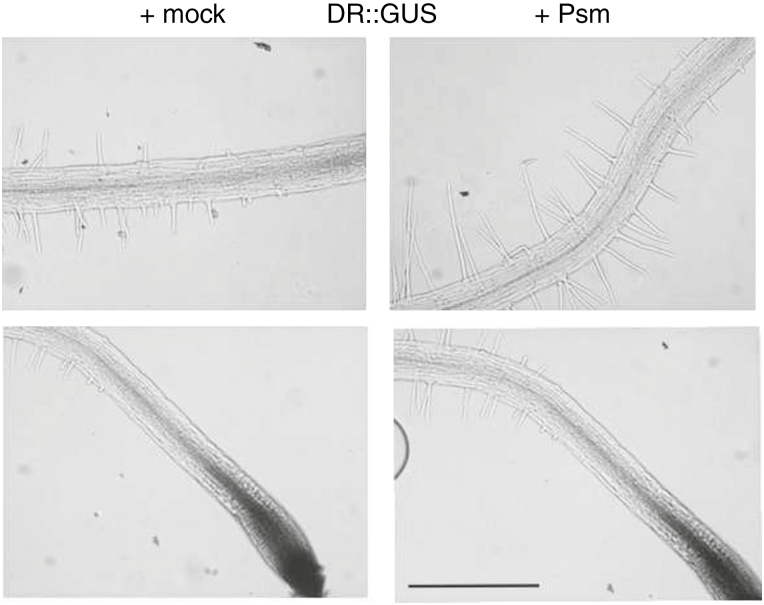
We examined whether auxin is involved in early stimulation of root hair growth, as it is involved in long-term stimulation (Zamioudis et al., 2013). Using the GUS assay in seedlings carrying the auxin-sensitive pDR5::GUS reporter (Ulmasov et al., 1997) and inoculation with Psm, no upregulation of the auxin-responsive reporter was observed within the time frame of our experiments (Fig. 5). This result indicates that these early root reactions are possibly regulated by different mechanisms to those described by Zamioudis et al. (2013), in which the bacteria were placed 5cm from younger seedlings and the effects were quantified after a much longer period of exposure (8 d).
Fig. 5.
GUS assay on seedlings carrying pDR5::GUS and inoculated with Psm. There was no detectable upregulation of the auxin responsive reporter expression after Psm inoculation (scale bar=0·5mm).
Testing with other bacteria and treatments for stimulation of root hair growth
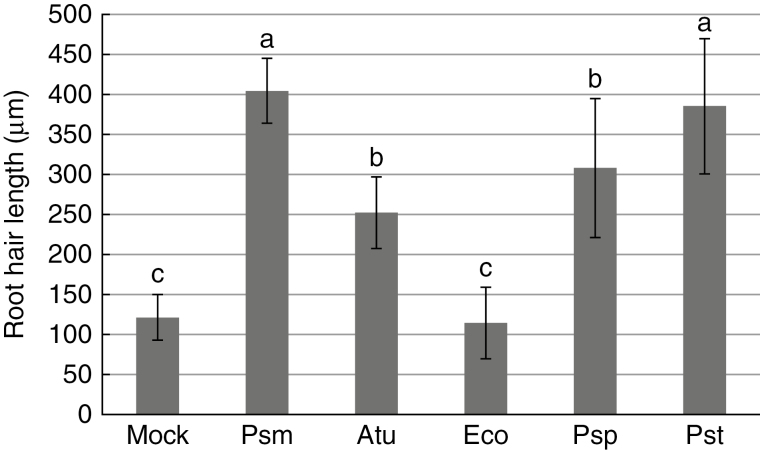
To examine whether the root responses described above are specific to particular bacterial species, we performed the same test with Col-0 Arabidopsis plants and four other bacteria species: Agrobacterium tumefaciens GV3101 (Atu), Escherichia coli DH5α (Eco), P. syringae pv. tomato DC3000 (Pst) and Pseudomonas savastanoi pv. phaseolicula CCM 2861 (Psp). Exposure to Atu resulted in stimulation; the root hairs were twice as long as those exposed to the mock treatment. The reaction to Psp and Pst was comparable to the reaction to Psm: root hairs were approx. 2·5–3 times longer than those in the mock treatment. Exposure to Eco resulted in slight inhibition of root hair growth (Fig. 6). These results suggest that the stimulation of root hair growth is similar for different soil- and plant-adapted bacteria, while the response to animal-adapted E. coli is different.
Fig. 6.
Stimulation of root hairs growth by Atu, Eco, Psm, Pst and Psp. Root hair growth in Col-0 seedlings treated with bacteria: Agrobacterium tumefaciens GV3101 (Atu), Escherichia coli DH5α (Eco), Pseudomonas syringae pv. tomato DC3000 (Pst) and Pseudomonas savastanoi pv. phaseolicula strain CCM 2861 (Psp), in comparison with Psm. All treatments except Eco stimulated root hair growth. Different letters indicate significant differences (P<0·05).
Interestingly, treatment with bacteria inactivated by heat or sonication, with filtrate from the bacterial culture, with supernatant from culture of Psm-infected plants, with elf18, chitin, peptidoglycan, coronatine and root tissue powdered in liquid N2 [from infected and non-infected seedlings, as a source of DAMPs (danger-associated molecular patterns); data not shown] had no effect on root hairs. Additionally, we tested two bacterial quorum-sensing AHLs that were previously shown to enhance root hair lengths, N-decanoyl-HLs, namely C10-HL and oxo-C10-HL (Sigma; Ortíz-Castro et al., 2008). These chemicals failed to stimulate root hair growth, even over a wide range of tested concentrations (data not shown). Thus, only living bacteria at high inocula were capable of provoking stimulation of root hair growth. Interestingly, type 3 secretion system mutants of Pseudomonas provoked the same range of root hair growth stimulation as did its WT counterpart (in agreement with Maketon et al., 2012; data not shown).
SA, JA, ET and PAD4 interplay and root hair growth
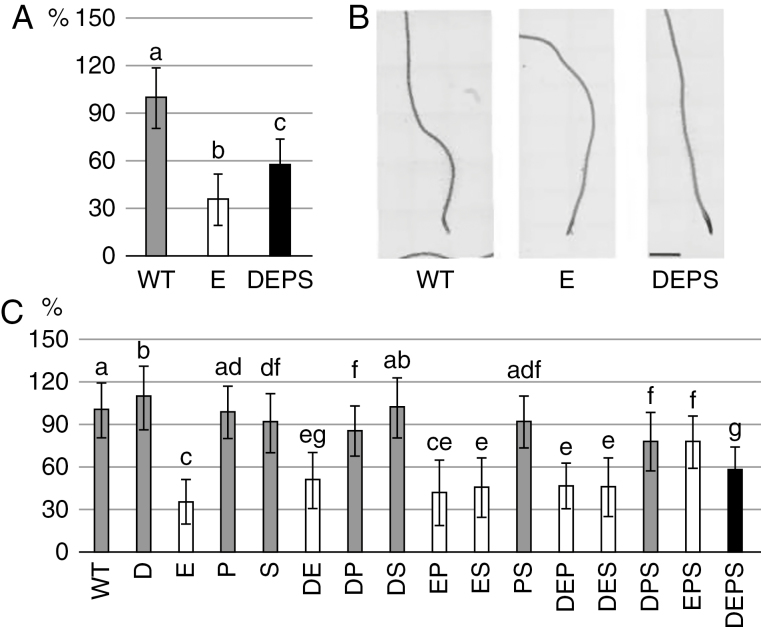
A mutant impaired in JA, ET and SA signalling pathways and PAD4-dependent immunity, dde2/ein2/pad4/sid2 (deps) was described by Tsuda et al. (2009). Dde2 is a knock-out mutant of the AOS (ALLENE OXIDE SYNTHASE) gene, which is responsible for the biosynthesis of JA (von Malek et al., 2002). The sid2 mutant is a knock-out of the ICS1 (ISOCHORISMATE SYNTHASE 1), gene which is a crucial component in the pathway responsible for the biosynthesis of SA after pathogen attack (Wildermuth et al., 2001). Interestingly, the quadruple mutant deps has never been used previously to study root responses to pathogenic bacteria. Here, we employed the entire set of mutant combinations related to deps and treated them with Pst by the flooding method for 24h. We examined the stimulation of root hair growth in Arabidopsis Col-0 (WT) seedlings after flooding with Pst (OD600 of 0·01; Supplementary Data Figs S5, S6 and S8, and Tables S1 and S2). This method was appropriate for analysis of very large numbers of seedlings of all deps mutants. We showed that impaired ET signalling is responsible for the shorter root hairs in deps (Fig. 7C). Interestingly, SA-, JA- and PAD4-related pathways enhance the effect of impaired ET signalling such that the ein2 single mutant had the shortest root hairs of all studied mutants (Fig. 7C). The deps mutant displayed root hairs almost twice as long as those in ein2 after Pst stimulation (Fig. 7).
Fig. 7.
The influence of SA, JA, ET and PAD4 on root hair growth. Four-day-old seedlings were treated with P. syringae pv. tomato DC3000 and root hair lengths were measured 24 hpi. (A) Lengths of root hairs of selected genotypes – WT, ein2 (E) and dde2/ein2/pad4/sid2 (DEPS). (B) Representative images of WT and selected mutants after Pst treatment. Scale bar=1000 µm. (C) Lengths of root hairs of all ‘DEPS’ collection mutants. D, dde2; E, ein2; P, pad4; S, sid2. For A and C, n = 10–200 for each genotype. Values represent the mean, and error bars represent s.d. Small letters indicate statistically significant (P < 0·01) differences between treatments. Root hair lengths are given as %, with value for WT set as 100%.
Correlation of root hair stimulation with overall growth of bacteria
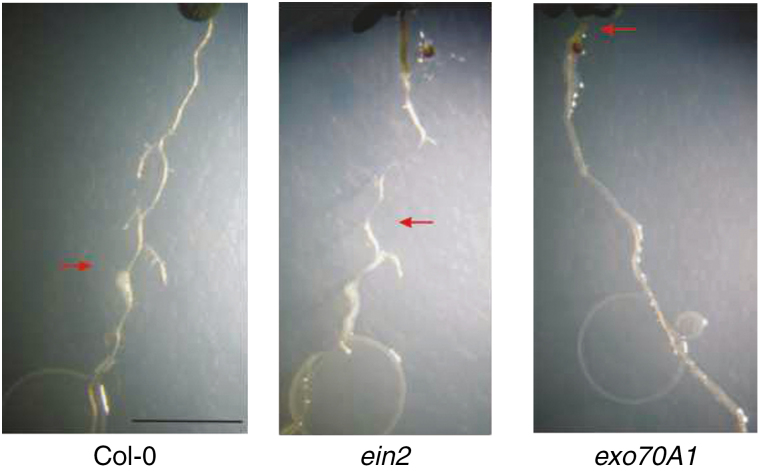
To understand how the stimulation of root hair growth reflects the ability of a plant to limit the spread of a pathogen, we analysed the spread of the bacteria on the inoculated plants after a further 3 d of exposure. The most remarkable result was found for the exo70A1 mutant, which, unlike ein2 and Col-0WT, displayed roots that were often completely colonized by Psm (Fig. 8). As root hair stimulation is impaired in exo70A1 and ein2, the root hair growth response is not simply correlated with the ability of the plant to limit the growth of bacteria in the rhizosphere.
Fig. 8.
Psm colonization of roots and growth during prolonged co-cultivation. After 5 d of growth of bacteria on roots, Psm completely colonized only exo70A1. The red arrows indicate the uppermost positions on primary roots that were colonized by Psm spreading from the root tip (scale bar=0·5mm).
DISCUSSION
We describe the early growth response of root hairs in Arabidopsis to bacterial contact. We found that the general responses of the root and root hairs to pathogenic bacteria resemble previously described reactions to the presence of PGPR microbes – root hair growth is stimulated and primary root growth is inhibited (e.g. López-Bucio et al., 2007; reviewed by Vacheron et al., 2013).
We show here that stimulation of root hair growth and inhibition of primary root growth begin after the first few hours of direct contact with bacteria. Both these phenomena appear to be initiated simultaneously; however, as Flg22 inhibits primary root growth without stimulating growth of root hairs, we hypothesized that these processes are independent. In contrast to the auxin-dependent long-term (i.e. 1week) and long-distance responses described by Zamioudis et al. (2013), our results suggest no major involvement of auxin signalling during the short time-frame responses studied here. Stimulation of root hair growth with A. tumefaciens and P. savastanoi provoke similar effects to those produced by Psm and Pst, suggesting that the enhanced root hair growth may be a general response of roots to contact with living plant- and soil-adapted bacteria.
Our results show that functional ET perception is necessary for efficient pathogen-induced stimulation of root hair growth. In addition, vesicle trafficking plays a role in this phenomenon, as seen not only in the example with the exo70A1 mutant but also in the exocyst subunit mutant sec8 (Supplementary Data Fig. S8) and in the co-treatment with Pst and BFA, which blocks the stimulation of root hair growth (Supplementary Data Fig. S9). In the ein2 and exo70A1 knockout mutants, stimulation of root hair growth is largely compromised compared to the WT, but bacterial growth along the roots of the two mutants differs – bacteria overgrow on exo70A1 but not on ein2 seedlings. The exo70A1 mutant plants display a strong defect in secretion that results in dwarfed plants with smaller cotyledons and delayed lateral roots. Therefore, it is possible that the exo70A1 mutant plants during co-cultivation with bacteria produce lower levels of defence compounds that limit bacterial growth when compared to WT plants and the mutant lines that do not have a defective growth phenotype. Interestingly, our previous tests with Psm leaf inoculation showed the same level of bacterial growth in leaves of exo70A1 and WT plants (data not shown). The contrasting results obtained for exo70A1 clearly indicate that substantially different plant–microbe interaction mechanisms operate in leaves and roots (Balmer and Mauch-Mani, 2013).
The prominent role of EXO70A1 in the bacterial root hair stimulation phenomenon confirms that a secretion/exocytosis mechanism that is supported by the exocyst complex is important for root hair tip growth. The wavy appearance of root hairs of the exo70A1 mutant after treatment with Psm could reflect inefficient secretion and a deficiency in the mutant’s ability to support fast tip growth; unfortunately, the specific mechanism remains unexplained. The wavy root hair phenotype resembles that found in the Arabidopsis rhd3 (root hair-defective 3) mutant, which carries a mutation in a large GTP binding protein; however, we are currently unable to speculate whether there might be a functional connection between RHD3 and EXO70A1 other than involvement of the both in the endomembrane dynamics (Brands and Ho, 2002; Stefano et al., 2012).
In parallel to vesicle trafficking, we focused on the involvement of plant immune signalling pathways in the stimulation of root hair growth. For this we used a quadruple mutant (deps) that is impaired in the key SA, JA and ET phytohormonal pathways, as well as in the immune signalling dependent on the PAD4 protein (Tsuda et al., 2009; Kim et al., 2014). Tsuda et al. (2009) have defined the SA, JA, ET and PAD4 pathways as signalling sectors (portions of plant immunity signalling; Tsuda and Katagiri, 2010) and described their roles in PAMP- and effector-triggered immunity. SA signalling is commonly involved in defence responses against biotrophic and hemibiotrophic pathogens (e.g. P. syringae), while JA and ET are involved in immune responses to necrotrophic pathogens (Glazebrook, 2005; Pieterse et al., 2009). PAD4 forms heterodimers with EDS1 (ENHANCED DISEASE SUSCEPTIBILITY 1) after pathogen attack and triggers the plant immune responses that are mostly connected with induction of the SA signalling pathway, but EDS1/PAD4 may also be involved in SA-independent immune responses (Rietz et al., 2011; Janda and Ruelland, 2015; Cui et al., 2017). According to the general model of phytohormonal crosstalk in defence responses, JA and ET inhibit SA signalling and vice versa. However, new evidence shows that the relationships between these pathways are much more complicated than previously supposed. Kim et al. (2014) proposed a model that shows a synergistic relationship between SA and JA with a positive feedback loop that was further elaborated by the epistatic role of PAD4 over the SA sector (Mine et al., 2017).
Using 16 combinatorial genotypes related to the deps quadruple mutant we showed that ET is directly responsible for the stimulation of root hair growth after treatment with Psm/Pst, as expected based on the known positive role of ET in root hair cell expansion (Fig. 7; Dolan, 2001). Based on the fact that the deps mutant has longer root hairs than the single ein2 mutant, we concluded that the full inhibitory effect of the ein2 mutation on root hair growth requires functional JA, PAD4 and SA sectors. Our data support a negative regulatory relationship with ET on the one side, and SA and JA on the other side in the rhizosphere (Figs 7 and 9 B). When ET, which is the most important factor for root hair growth enhancement, is non-functional (ein2), the unbalanced phytohormonal condition allows the three SA/JA/PAD4 sectors to inhibit root hair growth, which can be explained only by the negative control of ET over these three sectors. However, the JA and PAD4 sectors also have minor and indirect influence in the pathogen induction of root hair growth (Fig. 9A). This is observable on the behaviour of the double dp mutant and triple dps mutant (both with functional ET signalling) root hairs, which were shorter than WT root hairs, probably due to an overall slower recognition of Pst (Fig. 7). The dp and dps, but not ds and ps, mutants have shorter root hairs, which indicates that there might be a cooperation between JA and PAD4pathways/sectors in root hair growth stimulation. This is also in agreement with the suggested dominant role of the PAD4 sector over the SA sector in immunity (Mine et al., 2017; Fig. 7). Interestingly, in the deps mutant, the greater distance between the initiation zone of root hairs and the root tip (Supplementary Data Fig. S6) indicates that traditional immune pathways are involved in the primary root growth inhibition caused by Pseudomonas.
Fig. 9.
Model of stimulation of root hair growth by Psm/Pst and role of four phytohormonal/immunity sectors in this model. Ethylene (ET) is of key importance in root hair growth in the developmental context. The other sectors, especially the jasmonic acid (JA) and PAD4 pathways, also have minor and indirect influences on the pathogen-related induction of root hair growth (A). When the most important sector, ethylene signalling, is missing (ein2), the phytohormonal misbalance allows salicylic acid (SA)/JA/PAD4 to inhibit root hair growth, which can be explained only by the possible negative control of ethylene over these three sectors (B).
The downstream component of the SA signalling pathway, NPR1, is abundantly expressed in roots that are not under any biotic stress (Cao et al., 1994; Lamesch et al., 2010). However, the lack of NPR1 function did not affect root hair growth in our assays. Additionally, the missing immune receptor FLS2 had no major effect on stimulation of root hair growth in the Ws ecotype. FLS2 was, however, important for the desensitization of plants pretreated with Flg22, as it might have either decreased the sensitivity of plants following bacterial contact through the FLS2 degradation, as reported by Smith et al. (2014), or triggered PAMP-triggered immunity, slowing bacterial growth.
While we have identified at least some of the players in this phenomenon in plants, unfortunately we are currently unable to explain the cause of early stimulation of root hair growth by bacteria from the pathogen side, as shown by the list of chemicals and treatments that we tested without a positive result. The most promising candidates tested were AHLs (Ortíz-Castro et al., 2008); however, it is possible that because they quickly lose their activity, the effect was insufficient to enhance root hair growth under our experimental conditions. It has been recently shown that pyocyanin, a virulence factor of the plant and animal pathogen P. aeruginosa, affects the growth and development of Arabidopsis seedlings by inhibiting primary root growth and promoting lateral root and root hair growth without affecting meristem viability or causing cell death (Ortíz-Castro et al., 2014). This modulation works independently of auxin, cytokinin and abscisic acid but requires ET signalling (Ortíz-Castro et al., 2014). Based on this evidence, we speculate that the plant pathogenic Pseudomonas might contain a similar metabolite that could stimulate Arabidopsis root hair growth at certain concentrations. Alternatively, the presence of Pseudomonas bacteria around the root tips might temporarily change nutrient availability. It has recently been shown that pyoverdine, a siderophore produced by beneficial Pseudomonas, not only modulates the availability of soil iron to plants, but also positively regulates the expression of genes related to development and iron acquisition in plants grown under iron-deficient conditions and represses the expression of defence-related genes (Vansuyt et al., 2007; Trapet et al., 2016). This system demonstrates that there are many levels at which plant hosts and bacteria can influence each other and that plants need to trade-off between defence and growth to preserve their fitness.
To conclude, while the most common plant cell response to pathogens is growth arrest, root hair cells can perform the opposite reaction. Functional ET signalling and efficient exocyst-dependent vesicular trafficking are important for this stimulation of root hair growth. As the presence of high levels of bacteria in a soil patch might signal the proximity of a higher nutrient content, it is also possible that root systems, including root hairs, interpret contact with soil-adapted bacteria as a signal of a putative local nutrient maximum. This phenomenon also reflects the differences in defence strategies between shoots and roots, which warrant further examination of the Arabidopsis–Pseudomonas interaction.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: the root tip zones of mock- and Psm-treated plants. Fig. S2: inhibition of primary root growth 48h post-inoculation. Fig. S3: time-course experiment of the early effect of Psm treatment on root architecture. Fig. S4: inhibition of primary root growth after pretreatment with the Flg22 peptide. Fig. S5: stimulation of root hair growth by Pseudomonas syringae pv. tomato DC3000 (Pst). Fig. S6: the influence of the dde2/ein2/pad4/sid2 (DEPS) pathways on root growth. Fig. S7: characterization of the root hair growth induced by Pst over time. Fig. S8: additional set of exocyst sec8 and syp122 SNARE mutants. Fig. S9: the effect of brefeldin A on the execution of the root hairs growth stimulation. Table S1: characterization of the stimulation of root hair growth in WT seedlings after Pst flooding. Table S2: lengths of root hairs and primary roots after Pst flooding
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the GACR/CSF projects 15-14886S (T.P., J.O., V.Z.), 14-09685S (M.J., V.H., Z.S.) and GA UK project 1102214 (J.O.). T.P. would like to thank Dr Andrea Genre (University of Turin) for help with root hair microscopy, to collegues Dr Roman Pleskot for help with figure and statistical analysis, Dr Edita Drdová Janková for helpful discussion on exo70A1 mutant and Michal Kuchar for technical support. M.J., Z.S. and V.H. would like thank to leaders of their laboratories Prof. Olga Valentová, Assoc. Prof. Lenka Burketová and Dr Jan Martinec and to Lucie Lamparová and Petra Konopáčová for their great technical support. We also would like to thank Dr Kenichi Tsuda and Dieter Becker (from Max Planck Institute for Plant Breeding Research, Cologne) who kindly provided seeds of the whole ‘deps’ collection and also P. syringae pv. tomato DC3000. We thank Dr Emily Larson for critical reading of the manuscript and language editing.
LITERATURE CITED
- Balmer D, Mauch-Mani B. 2013. More beneath the surface? Root versus shoot antifungal plant defenses. Frontiers in Plant Science 4: 256. doi:10.3389/fpls.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. 1996. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell & Environment 19: 529–538. [Google Scholar]
- Bauer Z, Gómez-Gómez L, Boller T, Felix G. 2001. Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. The Journal of Biological Chemistry 276: 45669–45676. doi:10.1074/jbc.M102390200 [DOI] [PubMed] [Google Scholar]
- Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. 2012. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. The Plant Cell 24: 4205–4219. doi:10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands A, Ho TD. 2002. Function of a plant stress-induced gene, HVA22. Synthetic enhancement screen with its yeast homolog reveals its role in vesicular traffic. Plant Physiology 130: 1121–1131. doi:10.1104/pp.007716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. 1994. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. The Plant Cell 6: 1583–1592. doi:10.1105/tpc.6.11.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S. et al. 2007. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500. doi:10.1038/nature05999 [DOI] [PubMed] [Google Scholar]
- Cui H, Gobbato E, Kracher B, Qiu J, Bautor J, Parker JE. 2017. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytologist 213: 1802–1817. doi:10.1111/nph.14302. [DOI] [PubMed] [Google Scholar]
- Dolan L. 2001. The role of ethylene in root hair growth in Arabidopsis. Journal of Plant Nutrition and Soil Science 164: 141–145. [Google Scholar]
- Dong X, Mindrinos M, Davis KR, Ausubel FM. 1991. Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. The Plant Cell 3: 61–72. doi:10.1105/tpc.3.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörmann P, Kim H, Ott T. et al. 2014. Cell-autonomous defense, re-organization and trafficking of membranes in plant-microbe interactions. New Phytologist 204: 815–822. doi:10.1111/nph.12978. [DOI] [PubMed] [Google Scholar]
- Du Y, Mpina MH, Birch PRJ, Bouwmeester K, Govers F. 2015. Phytophthora infestans RXLR effector AVR1 interacts with exocyst component Sec5 to manipulate plant immunity. Plant Physiology 169: 1975–1990. doi:10.1104/pp.15.01169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43: 205–227. doi:10.1146/annurev.phyto.43.040204.135923 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. 1999. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. The Plant Journal 18: 277–284. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. 1999. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. The EMBO Journal 18: 1071–1080. doi:10.1093/emboj/18.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2: 513–523. doi:10.1105/tpc.2.6.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hála M, Cole R, Synek L. et al. 2008. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. The Plant Cell 20: 1330–1345. doi:10.1105/tpc.108.059105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S-C, TerBush D, Abraham M, Guo W. 2004. The exocyst complex in polarized exocytosis. International Review of Cytology 233: 243–265. doi:10.1016/S0074-7696(04)33006-8 [DOI] [PubMed] [Google Scholar]
- Janda M, Ruelland E. 2015. Magical mystery tour: salicylic acid signaling. Environmental and Experimental Botany, 114:117–128. doi:10.1016/j.envexpbot.2014.07.003 [Google Scholar]
- Jung J-Y, Shin R, Schachtman DP. 2009. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. The Plant Cell 21: 607–621. doi:10.1105/tpc.108.063099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY. 2002. The Arabidopsis thaliana-Pseudomonas syringae interaction. Arabidopsis Book 1: e0039. doi:10.1199/tab.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Tsuda K, Igarashi D. et al. 2014. Mechanisms underlying robustness and tunability in a plant immune signaling network. Cell Host & Microbe 15: 84–94. doi:10.1016/j.chom.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkema M, Fan W, Dong X. 2000. Nuclear localization of NPR1 is required for activation of PR gene expression. The Plant Cell 12: 2339–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer RJ, Begonia MF, Stanley L, Lanham ET. 1990. Characterization of rhizobacteria associated with weed seedlings. Applied and Environmental Microbiology 56: 1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I, Pečenková T, Sekereš J. et al. 2013. Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14: 1155–1165. doi:10.1111/tra.12101 [DOI] [PubMed] [Google Scholar]
- Lamesch P, Dreher K, Swarbreck D, Sasidharan R, Reiser L, Huala E. 2010. Using the Arabidopsis information resource (TAIR) to find information about Arabidopsis genes. Current Protocols in Bioinformatics Suppl. 30: Chapter 1, Unit1.11. doi:10.1002/0471250953.bi0111s30 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E. et al. 2007. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Molecular Plant-Microbe Interactions 20: 207–217. doi:10.1094/MPMI-20-2-0207 [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annual Review of Microbiology 63: 541–556. doi:10.1146/annurev.micro.62.081307.162918 [DOI] [PubMed] [Google Scholar]
- Maketon C, Fortuna A, Okubara PA. 2012. Cultivar-dependent transcript accumulation in wheat roots colonized by Pseudomonas fluorescens Q8r1-96 wild type and mutant strains. Biological Control 60: 216-224. [Google Scholar]
- Millet YA, Danna CH, Clay NK. et al. 2010. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. The Plant Cell 22: 973–990. doi:10.1105/tpc.109.069658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine A, Nobori T, Salazar‐Rondon MC. et al. 2017. An incoherent feed‐forward loop mediates robustness and tunability in a plant immune network. EMBO Reports 18: 464–476. doi:10.15252/embr.201643051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Chai R, Liu L. et al. 2014. Magnesium availability regulates the development of root hairs in Arabidopsis thaliana (L.) Heynh. Plant, Cell & Environment 37: 2795–2813. doi:10.1111/pce.12362 [DOI] [PubMed] [Google Scholar]
- Ortíz-Castro R, Martínez-Trujillo M, López-Bucio J. 2008. N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant, Cell & Environment 31: 1497–1509. doi:10.1111/j.1365-3040.2008.01863.x [DOI] [PubMed] [Google Scholar]
- Ortíz-Castro R, Pelagio-Flores R, Méndez-Bravo A, Ruiz-Herrera LF, Campos-García J, López-Bucio J. 2014. Pyocyanin, a virulence factor produced by Pseudomonas aeruginosa, alters root development through reactive oxygen species and ethylene signaling in Arabidopsis. Molecular Plant-Microbe Interactions 27: 364–378. doi:10.1094/MPMI-08-13-0219-R [DOI] [PubMed] [Google Scholar]
- Pečenková T, Hála M, Kulich I. et al. 2011. The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. Journal of Experimental Botany 62: 2107–2116. doi:10.1093/jxb/erq402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. 2009. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology 5: 308–16. doi:10.1038/nchembio.164 [DOI] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. 2002. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiology 130: 1908–1917. doi:10.1104/pp.010546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietz S, Stamm A, Malonek S. et al. 2011. Different roles of Enhanced Disease Susceptibility1 (EDS1) bound to and dissociated from Phytoalexin Deficient4 (PAD4) in Arabidopsis immunity. New Phytologist 191: 107–119. doi:10.1111/j.1469-8137.2011.03675.x. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. 1994. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Current Biology 4: 220–233. [DOI] [PubMed] [Google Scholar]
- Rudrappa T, Bais HP. 2008. Rhizospheric pseudomonads: Friends or foes? Plant Signaling and Behavior 3: 1132–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Schmidt W, 2001. Iron stress-induced changes in root epidermal cell fate are regulated independently from physiological responses to low iron availability. Plant Physiology 125: 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Salamango DJ, Leslie ME, Collins CA, Heese A. 2014. Sensitivity to Flg22 is modulated by ligand-induced degradation and de novo synthesis of the endogenous flagellin-receptor FLAGELLIN-SENSING2. Plant Physiology 164: 440–454. doi:10.1104/pp.113.229179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G, Renna L, Moss T, McNew JA, Brandizzi F. 2012. In Arabidopsis, the spatial and dynamic organization of the endoplasmic reticulum and Golgi apparatus is influenced by the integrity of the C-terminal domain of RHD3, a non-essential GTPase. Plant Journal 69: 957–966. doi:10.1111/j.1365-313X.2011.04846.x [DOI] [PubMed] [Google Scholar]
- Stegmann M, Anderson RG, Ichimura K. et al. 2012. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. The Plant Cell 24: 4703–4716. doi:10.1105/tpc.112.104463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Schlager N, Eliás M, Quentin M, Hauser M-T, Zárský V. 2006. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant Journal 48: 54–72. doi:10.1111/j.1365-313X.2006.02854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Kato M, Tomioka R. et al. 2014. Characteristics of a root hair-less line of Arabidopsis thaliana under physiological stresses. Journal of Experimental Botany 65: 1497–1512. doi:10.1093/jxb/eru014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. 1996. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO Journal 15: 6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Trapet P, Avoscan L, Klinguer A. et al. 2016. The Pseudomonas fluorescens siderophore pyoverdine weakens Arabidopsis thaliana defense in favour of growth in iron-deficient conditions. Plant Physiology 171: 675–693. doi:10.1104/pp.15.01537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current Opinion in Plant Biology 13: 459–465. doi:10.1016/j.pbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. 2009. Network properties of robust immunity in plants. PLoS Genetics 5(12), doi:10.1371/journal.pgen.1000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9: 1963–1971. doi:10.1105/tpc.9.11.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron J, Desbrosses G, Bouffaud ML. et al. 2013. Plant growth-promoting rhizobacteria and root system functioning. Frontiers in Plant Science 4: 356. doi:10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Bakker PAHM, van der Heijdt WHW, Wendehenne D, Pugin A. 2008. Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Molecular Plant-Microbe Interactions 21: 1609–1621. doi:10.1094/MPMI-21-12-1609 [DOI] [PubMed] [Google Scholar]
- Vansuyt G, Robin A, Briat J-F, Curie C, Lemanceau P. 2007. Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Molecular Plant-Microbe Interactions 20: 441–447. doi:10.1094/MPMI-20-4-0441 [DOI] [PubMed] [Google Scholar]
- von Malek B, van der Graaff E, Schneitz K, Keller B, 2002. The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216: 187–192. doi:10.1007/s00425-002-0906-2 [DOI] [PubMed] [Google Scholar]
- Wen T-J, Hochholdinger F, Sauer M, Bruce W, Schnable PS. 2005. The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiology 138: 1637–1643. doi:10.1104/pp.105.062174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM, 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. doi:10.1038/35107108 [DOI] [PubMed] [Google Scholar]
- Xin XF, He SY. 2013. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annual Review of Phytopathology 51: 473-498. doi:10.1146/annurev-phyto-082712-102321 [DOI] [PubMed] [Google Scholar]
- Yang TJW, Perry PJ, Ciani S, Pandian S, Schmidt W. 2008. Manganese deficiency alters the patterning and development of root hairs in Arabidopsis. Journal of Experimental Botany 59: 3453–3464. doi:10.1093/jxb/ern195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamioudis C, Pieterse CMJ. 2012. Modulation of host immunity by beneficial microbes. Molecular Plant-Microbe Interactions 25: 139–150. doi:10.1094/MPMI-06-11-0179 [DOI] [PubMed] [Google Scholar]
- Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ. 2013. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiology 162: 304–318. doi:10.1104/pp.112.212597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárský V, Cvrčková F, Potocký M, Hála M. 2009. Exocytosis and cell polarity in plants – exocyst and recycling domains. New Phytologist 183: 255–272. doi:10.1111/j.1469-8137.2009.02880.x [DOI] [PubMed] [Google Scholar]
- Zárský V, Kulich I, Fendrych M, Pečenková T. 2013. Exocyst complexes multiple functions in plant cells secretory pathways. Current Opinion in Plant Biology 16: 726–733. doi:10.1016/j.pbi.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L. et al. 2004. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767. doi:10.1038/nature02485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.