Abstract
Background and Aims
Foliar fertilization to overcome nutritional deficiencies is becoming increasingly widespread. However, the processes of foliar nutrient absorption and translocation are poorly understood. The present study aimed to investigate how cuticular leaf properties affect the absorption of foliar-applied nutrients in leaf tissues.
Methods
Given that methyl jasmonate (MeJA) can cause alterations in leaf properties, we applied 1 mm MeJA to sunflower (Helianthus annuus), tomato (Solanum lycopersicum) and soybean (Glycine max) to assess changes in leaf properties. Using traditionally analytical approaches and synchrotron-based X-ray fluorescence microscopy, the effects of these changes on the absorption and translocation of foliar-applied Zn, Mn and Fe were examined.
Key Results
The changes in leaf properties caused by the application of MeJA increased foliar absorption of Zn, Mn and Fe up to 3- to 5-fold in sunflower but decreased it by 0·5- to 0·9-fold in tomato, with no effect in soybean. These changes in the foliar absorption of nutrients could not be explained by changes in overall trichome density, which increased in both sunflower (86%) and tomato (76%) (with no change in soybean). Similarly, the changes could be not attributed to changes in stomatal density or cuticle composition, given that these properties remained constant. Rather, the changes in the foliar absorption of Zn, Mn and Fe were related to the thickness of the cuticle and epidermal cell wall. Finally, the subsequent translocation of the absorbed nutrients within the leaf tissues was limited (<1·3mm) irrespective of treatment.
Conclusions
The present study highlights the potential importance of the combined thickness of the cuticle and epidermal cell wall in the absorption of foliar-applied nutrients. This information will assist in increasing the efficacy of foliar fertilization.
Keywords: Biofortification, cuticle, foliar absorption, methyl jasmonate, nutrients, trichomes, sunflower, soybean, tomato
INTRODUCTION
Foliar fertilization is becoming increasingly widely used, with this approach potentially being more environmentally friendly and more efficient compared with soil-based applications of fertilizers (Fernández and Eichert, 2009). For example, Ram et al. (2016) found that foliar application of Zn significantly increased both grain Zn concentrations and grain yield for wheat (Triticum aestivum L.) and rice (Oryza sativa L.) when examined at 31 sites across seven countries. However, the processes of foliar absorption and the subsequent translocation of absorbed nutrients are not well understood. As a consequence, foliar fertilizers are generally not designed on a physiological basis, and hence potentially suffer from various limitations. For instance, the use of soluble Zn salts for foliar fertilization can result in leaf scorching (Drissi et al., 2015), while chelated forms of Zn, such as Zn-EDTA, are under increased scrutiny due to associated environmental concerns (Oviedo and Rodríguez, 2003).
The penetration of nutrients through the leaf surface has been demonstrated in both living plants and isolated cuticle membranes (Koontz and Biddulph, 1957; Schlegel and Schönherr, 2001; Riederer and Friedmann, 2006; Fernández and Eichert, 2009; Du et al., 2015). The process by which nutrients are absorbed through aerial tissues differs from uptake through roots, with the cell walls of leaves covered by a cuticle. Given that the cuticle is hydrophobic, it has been proposed that the cuticle has two separate pathways responsible for the transport of lipophilic and hydrophilic substances (Buchholz, 2006; Schönherr, 2006). The process by which lipid-insoluble (hydrophilic) ions penetrate through the cuticle has been studied extensively (Koontz and Biddulph, 1957; Schönherr, 1976; Buchholz et al., 1998; Schreiber, 2001; Schlegel et al., 2005, 2006; Schreiber, 2005; Fernández et al., 2014). It is generally assumed that the cuticle has small aqueous pores that can allow hydrated ions to pass. Although this hypothesis has been proven theoretically (Schönherr, 2006), the chemical composition, structure and existence of such pores have never been directly confirmed. Through the use of fluorescent dyes and silver nitrate to localize the foliar absorption pathway, it has also been proposed that the aqueous pores preferentially occur in guard cells, trichome bases (especially glandular trichomes) and cuticular anticlinal walls (Schlegel et al., 2005; Schönherr, 2006). Therefore, it is possible that the absorption of nutrients across leaf surfaces may occur via (1) the cuticle, (2) cuticular cracks and imperfections or (3) stomata, trichomes and lenticels (Fernández et al., 2013).
It is known that methyl jasmonate (MeJA), as a phytohormone, is involved in plant defence systems and can cause alterations in leaf properties, such as trichome density (Tian et al., 2012). In the present study, using sunflower (Helianthus annuus), tomato (Solanum lycopersicum) and soybean (Glycine max), we utilized 1 mm MeJA to alter leaf properties and examined whether changes in leaf properties influence foliar absorption and the subsequent translocation of Zn, Fe and Mn. First, the effects of 1 mm MeJA on leaf properties were assessed by examining changes in thickness of the cuticle (measured as the combined thickness of the cuticle and epidermal cell wall; see later), cuticle composition, trichome density and stomatal density. Next, the absorption of foliar-applied nutrients was assessed by examining changes in bulk nutrient concentrations of the leaf tissues. Finally, synchrotron-based X-ray florescence microscopy (μ-XRF) was used to provide in situ analyses of the distribution of nutrients within hydrated and fresh leaf tissues. The results of the present study will assist in understanding the process by which nutrients move across the leaf surface, and thereby provide underlying information required for improving the efficacy of foliar fertilizers.
MATERIALS AND METHODS
Experimental design and plant growth
The plant growth study was conducted at The University of Queensland (St Lucia, Australia), in a growth room at 25°C with high-pressure sodium lamps providing light (photon flux density of 1500μmol m–2 s–1) for 14h d–1. Seeds of soybean (‘Bunya’), sunflower (‘Hyoleic 41’) and tomato (‘Red Luck’) were germinated in rolled paper towel moistened with tap water for either 3 (soybean and sunflower) or 4 d (tomato) before being transferred to 11L black buckets. Each bucket had four holes in the lid with a total of either eight (tomato and soybean) or four (sunflower) plants, with each bucket forming one experimental unit. The buckets were filled with a basal nutrient solution which contained (μm): 910 N (94% and 6% ), 475 K, 20 P, 1126 Ca, 227Mg, 1251Cl, 556S, 25 Fe(III)EDTA, 3 B, 0·5Mn, 0·5 Zn, 0·2 Cu and 0·01Mo (Blamey et al., 2015). Nutrient solutions were changed after the first week, and then after every 4 d. After the plants had been growing for 10 d, 5mL of 44 mm KH2PO4 was added to each 11L bucket every second day thereafter progressively to replace P which had been taken up by the plants.
The experiment consisted of three plant species and two concentrations of MeJA [0 (control) and 1 mm] with four replicates, yielding a total of 24 experimental units. Application of the MeJA occurred after the plants had been growing for 7 (sunflower), 8 (soybean) or 10 d (tomato). The MeJA (0 or 1 mm in 0·8% ethanol) was sprayed onto the foliage until the leaves were fully saturated (approx. 300mL per container) (Boughton et al., 2005; Tian et al., 2012), with the growth lamps switched off for 4h following the application of the MeJA. Thereafter, plants were grown until the youngest leaves were fully expanded (approx. 10 d after application of MeJA). The youngest fully expanded leaves (YFELs) were then used for examining leaf morphology (stomatal and trichome density), the thickness of the cuticle and epidermal cell wall, and cuticle composition.
Assessment of leaf characteristics
Using the YFELs, leaf (adaxial) stomatal density and trichome density were examined using scanning electron microscopy (SEM). Fresh materials (approx. 3 × 3mm) were collected from the YFELs of each treatment and immediately fixed in 3% glutaraldehyde in 0·1 m sodium cacodylate, followed by a microwave processing which was performed using a Pelco BioWave (Ted Pella Inc., CA, USA) with a ColdSpot water recirculating device. Then the samples were post-fixed with 1% osmium tetroxide, subjected to a dehydration series using ethanol (30, 50, 70, 90, 100 and 100%) and processed using the BioWave. The tissues were then processed using a critical point dryer (Autosamdri-815 CPD, USA) before being coated with Au. Finally, samples were examined using a scanning electron microscope (JEOL NEOSCOPE, Japan) at 10kV accelerating voltage, with the density of trichomes and stomata determined.
Cuticle composition was examined using Fourier transform infrared spectroscopy (FTIR). Briefly, the abaxial side of each YFEL was scraped as clean as possible using a scalpel, and the remaining adaxial cuticles were placed into an enzyme solution containing 2% pectinase and 0·1% cellulase in 50 mm sodium acetate buffer to remove the residual cell debris from the cuticle. This enzymatic digestion was performed overnight at room temperature (25°C) before the cuticles were washed twice using deionized water. The samples were examined using light microscopy to ensure that the underlying tissues were completely removed before being air-dried and analysed by FTIR (the obtained spectra were then normalized) (Guzmán et al., 2014).
Although the cuticle has traditionally been thought to be independent of the underlying epidermal cell wall, it has recently been proposed that the cuticle is an extension of the epidermal cell wall (Guzmán et al., 2014; Fernández et al., 2016). Therefore, in the present study, we measured the combined thickness of the cuticle and epidermal cell wall using light microscopy by examining sections cut from resin-embedded leaf samples. Briefly, the fresh YFELs of each treatment were cut into pieces approx. 1 × 4mm. The segments were fixed in 3% glutaraldehyde in 0·1 m sodium cacodylate at 4°C overnight, rinsed twice with 0·1 m sodium cacodylate, post-fixed with 1% OsO4 in 0·1 m sodium cacodylate for 4h and dehydrated in a graded ethanol series of 20, 30, 40, 50 (10min each), 70, 90, 100 (30min each) and 100% (overnight). A series of graded Epon mixtures (Epon in ethanol) were used to infiltrate the tissues: 10, 20, 30, 40 (90min), 50 (overnight), 65, 80 (4h) and 100% (overnight). The tissues were then embedded in 100% Epon blocks and polymerized at 60°C overnight. Sections (1μm thick) were cut using an ultramicrotome (LEICA EM UC6), stained by Toluidine blue (0·5% in 1% borax) and observed by light microscopy (Obrien, 1995).
Foliar application of Zn, Mn and Fe
Bulk leaves (six replicates) were analysed by inductively coupled plasma mass spectrometry (ICP-MS) in order to obtain total elemental concentrations. A half-leaf loading method was used (Vu et al., 2013), with half of each leaf receiving nutrients and the other half receiving deionized water. To increase contact with the leaf surface, 0·05% Tween-20 was used as a mild non-ionic surfactant (Reuveni et al., 1994). Specifically, to one half of each leaf, 20 droplets (5μL per droplet) of 1000mg L–1 Zn (15·4 mm, pH 5·2, supplied as ZnSO4·7H2O), Mn (18·2 mm, pH 4·1, supplied as MnSO4·4H2O) and Fe (17·9 mm, pH 3·8, supplied as FeSO4·7H2O) were applied with 0·05% Tween-20, while the other half of each leaf received 60 droplets (5μL per droplet) of deionized water with 0·05% Tween-20 (pH 5·3). The leaves were incubated in Petri dishes for 6h before being cut from the plants and then blotted dry with filter paper. Thereafter, the leaves were cut in half along the mid-vein, rinsed separately using 2% HNO3, followed by 3% ethanol, and deionized water (Du et al., 2015). The samples were oven dried, digested using a 5:1 mixture of nitric acid and perchloric acid, and analysed using ICP-MS. Blanks and reference materials were included to ensure accuracy.
Finally, to examine the in situ distribution of foliar absorbed Zn, Mn and Fe in leaf tissues, the same three plant species were grown at La Trobe University (Bundoora, Australia) and exposed to either 0 or 1 mm MeJA as described earlier. Two droplets (5μL each) of each nutrient (1000mg L–1 Zn, Mn or Fe with 0·05% Tween-20) were applied to the adaxial YFELs of each of the three plant species. For sunflower and soybean, six droplets were applied near the tip of the leaf, with the three nutrients applied on one side of the mid-rib and three replicate droplets on the other side of the mid-rib. For tomato, the six droplets were applied on the base, middle or tip of a leaflet of a compound leaf with three nutrients on each side (see later). Following application of the droplets, the leaves were sealed inside Petri dishes containing moist filter paper for 6h, with lights on. For this entire incubation period, the leaves remained attached to the plants, with the petiole passing through a small hole in the side of the Petri dish (Supplementary Data Fig. S1). After incubation for 6h, leaves were cut and rinsed thoroughly (as described earlier) before analysis using μ-XRF at the XFM beamline at the Australian Synchrotron (Melbourne, Australia).
Details of the XFM beamline as used to analyse plant tissues have been provided previously (Kopittke et al., 2011; Paterson et al., 2011). Briefly, X-rays were selected by a Si (111) monochromator and focused (approx. 2 × 2μm) on the specimen by a pair of Kirkpatrick–Baez mirrors. The X-ray fluorescence emitted by the specimen was collected using the 384-element Maia detector system in a backscatter position at an excitation energy of 12900eV. Washed leaves were carefully blotted dry and mounted between two pieces of 4μm thick Ultralene film, forming a tight seal around the leaf to limit dehydration. There was generally <5min between excision of the leaf and commencement of the μ-XRF analysis. For each sample, two scans were performed. The first scan (‘survey scan’) was comparatively rapid and aimed to examine the entire sample and identify the portion of the leaf surface to which the Zn, Mn and Fe had been applied. The second scan (‘detailed scan’) was performed on a smaller area of the tissues surrounding the portion of the leaf to which the nutrients had been applied. This detailed scan was conducted with a smaller step size to increase spatial resolution (see details below).
Elemental mapping of each specimen involved continuous ‘on the fly’ analysis in the horizontal direction and discrete steps in the vertical direction. For the survey scan, the step size (i.e. pixel size) was 200μm with a horizontal stage velocity of 18mm s–1 (resulting in a pixel transit time of 11ms). For the detailed scan, the step size was 20μm with a horizontal stage velocity of 3mm s–1 (resulting in a pixel transit time of 6·7ms). The full X-ray fluorescence spectra were analysed using the CSIRO Dynamic Analysis method in GeoPIXE (http://www.nmp.csiro.au/dynamic.html) which enables quantitative, true-elemental images (Ryan and Jamieson, 1993; Ryan, 2000). All images were corrected for variations in leaf thickness through the use of Compton scattering as an internal standard.
Statistical analyses
Data were analysed using IBM SPSS Statistics version 24 (IBM Corporation, NY, USA). Comparisons between means were made using the t-test (95%).
RESULTS
Effects of methyl jasmonate on leaf properties
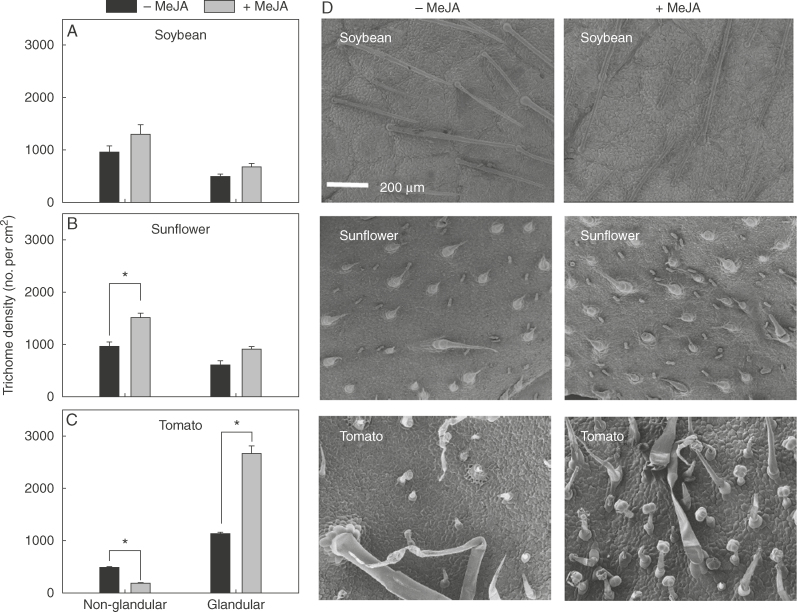
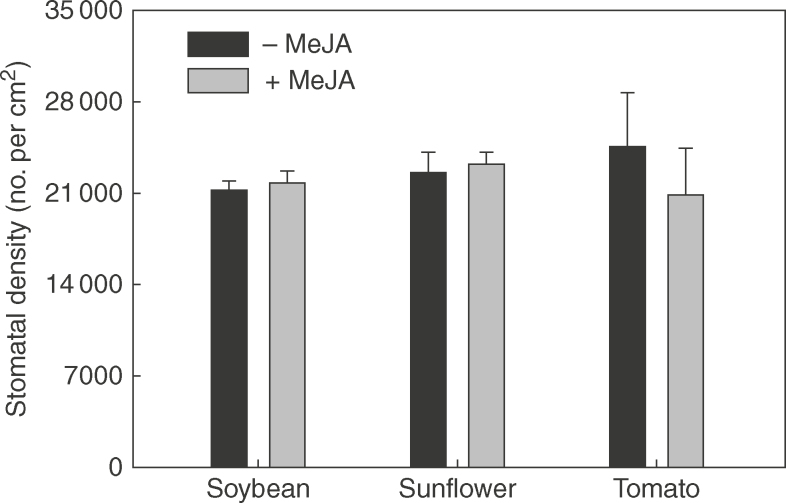
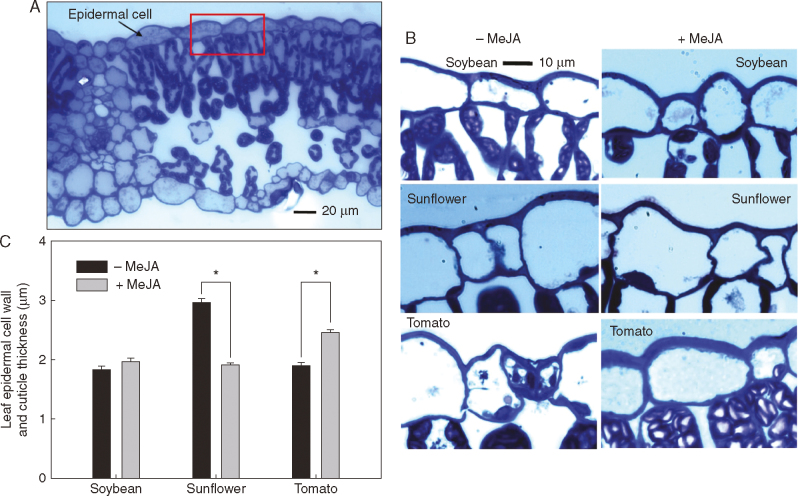
Application of 1 mm MeJA resulted in changes in leaf properties, with these changes varying among the three plant species. First, in regard to trichome density, it was observed that 1 mm MeJA significantly increased non-glandular trichome density in sunflower by 56% and glandular trichome density in tomato by 134%, but had no significant effect on trichome density in soybean (Fig. 1). Secondly, in contrast to trichome density, 1 mm MeJA had no significant effect on stomatal density in any of the three plant species (Fig. 2). Next, consideration was given to the thickness of the cuticle and epidermal cell wall. For sunflower, application of 1 mm MeJA significantly decreased thickness by 35%, but in direct contrast, the thickness of the cuticle and epidermal cell walls in tomato actually increased by 30%, while no significant differences were observed for soybean (Fig. 3). Finally, FTIR was used to examine changes in cuticle composition, but no marked differences were observed following the application of 1 mm MeJA for any of the three plant species (Fig. 4).
Fig. 1.
Effects of methyl jasmonate (MeJA) on leaf trichome densities. (A–C) Density of non-glandular and glandular trichomes following the application of 1 mm MeJA in soybean (A), sunflower (B) and tomato (C). Values are means with the SD (n = 4). Within each plant species, P < 0·05 between 0 and 1 mm MeJA is indicated with an asterisk (*). (D) Scanning electron micrographs showing leaf trichome densities of 0 and 1 mm MeJA of soybean, sunflower and tomato. The scale bar applies to all images.
Fig. 2.
Comparison of leaf stomatal density of 0 and 1 mm methyl jasmonate (MeJA)-treated leaves of soybean, sunflower, and tomato.
Fig. 3.
Effects of methyl jasmonate (MeJA) on the thickness of the cuticle and epidermal cell wall. (A) A light micrograph showing a cross-section of soybean leaf, with the red rectangle showing the area in (B), (B) Light micrographs showing the cuticle and epidermal cell wall thickness (the blue layer above the epidermal cells) of 0 and 1 mm MeJA-treated leaves of soybean, sunflower and tomato (the scale bar applies to all images), (C) Comparison of leaf cuticle and epidermal cell wall thickness of 0 and 1 mm MeJA-treated leaves of soybean, sunflower and tomato. Values are the mean with the SD (n = 4). Within each plant species, P-values < 0·05 between 0 and 1 mm MeJA are indicated with an asterisk (*).
Fig. 4.
Fourier transform infrared spectroscopy (FTIR) spectra obtained from isolated cuticles from the adaxial leaf surfaces of tomato, soybean and sunflower, either from 0 or 1 mm methyl jasmonate (MeJA)-treated leaves.
Comparison of foliar absorption of Zn, Mn and Fe
Upon detailed quantitative examination of bulk tissue concentrations using ICP-MS (Table 1), it was found that foliar absorption occurred in all treatments, although differences were observed depending upon both the plant species and the MeJA treatment (control or 1 mm MeJA). Specifically, for soybean, the application of 1 mm MeJA did not have a significant influence on foliar absorption for any of the three nutrients (Table 1). In contrast, for sunflower, treatment with 1 mm MeJA resulted in marked increases (approx. 3- to 5- fold) compared with the controls (Table 1) while, for tomato, treatment with 1 mm MeJA actually decreased absorption of the three nutrients by 50–90% (Table 1). Across the control treatments of the three plant species, soybean (<1μg per leaf) had the lowest levels of absorption for all the three nutrients, followed by sunflower (approx. 4–6μg per leaf) and tomato (approx. 5–12μg per leaf) (Table 1).
Table 1.
Compa rison of foliar absorption of Mn, Zn and Fe in soybean, tomato and sunflower after 6h of foliar application
| Treatment | Nutrient absorption (μg per leaf) | ||
|---|---|---|---|
| Mn | Zn | Fe | |
| Soybean | |||
| – MeJA | 0·11 ± 0·05a | 0·11 ± 0·07a | 0·69 ± 0·27a |
| + MeJA | 0·18 ± 0·06a | 0·26 ± 0·15a | 0·82 ± 0·03a |
| Sunflower | |||
| – MeJA | 6·45 ± 0·40a | 4·15 ± 0·35a | 5·77 ± 1·16a |
| + MeJA | 25·6 ± 3·66b | 18·4 ± 3·22b | 36·5 ± 6·85b |
| Tomato | |||
| – MeJA | 7·44 ± 1·41a | 5·32 ± 0·93a | 11·5 ± 4·52a |
| + MeJA | 3·69 ± 0·66b | 0·88 ± 0·12b | 1·47 ± 0·63b |
Plants were treated with either 0 mm (control) or 1 mm methyl jasmonate (MeJA).
Elemental absorption (μg per leaf)=(elemental concentration in the treated half – elemental concentration in the control half) × dry weight of the treated half of the leaf.
Values are the mean ± SD (n = 6).
Where P < 0·05 occurred between control and 1 mM MeJA for each species is indicated with different lower case letters.
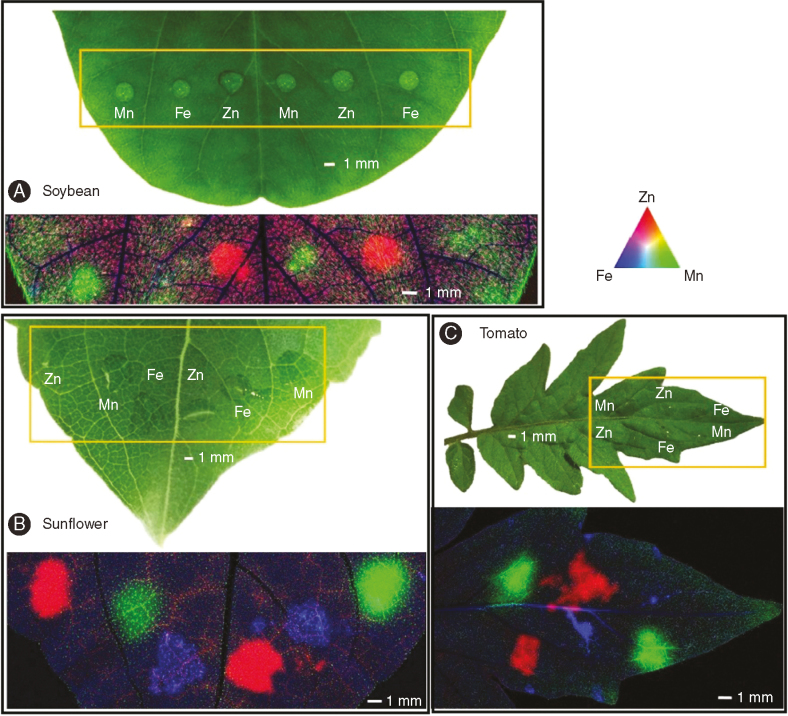
Using μ-XRF analysis, it was found that foliar application of Zn, Mn and Fe resulted in substantial increases in their concentrations in the underlying leaf tissues, indicating that after 6h, the three nutrients had crossed the leaf cuticle and penetrated into leaf tissues of soybean (Fig. 5A), sunflower (Fig. 5B) and tomato (Fig. 5C). In these tri-colour images (Fig. 5), red (Zn), green (Mn) and blue (Fe) indicate the concentrations of these nutrients. It was noted that, below the site of application, the concentration of the nutrients in the veins was higher than in the surrounding interveinal tissues. Indeed, for all three nutrients in both sunflower and tomato, projected concentrations in the veins were 2- to 4-fold higher than in the corresponding interveinal tissues (Supplementary Data Figs S2 and S3).
Fig. 5.
Zn, Mn, and Fe distribution (after 6h of foliar application) in control leaf of soybean (A) and tomato (C), and 1 mm methyl jasmonate (MeJA)-treated leaf of sunflower (B). In each image, the upper images are light microscopy before μ-XRF analysis, with the orange rectangle indicating the area examined by μ-XRF. The images below are tricolour μ-XRF maps of Zn (red), Mn (green) and Fe (blue) distribution.
Effects of MeJA on subsequent movement of nutrients following their absorption
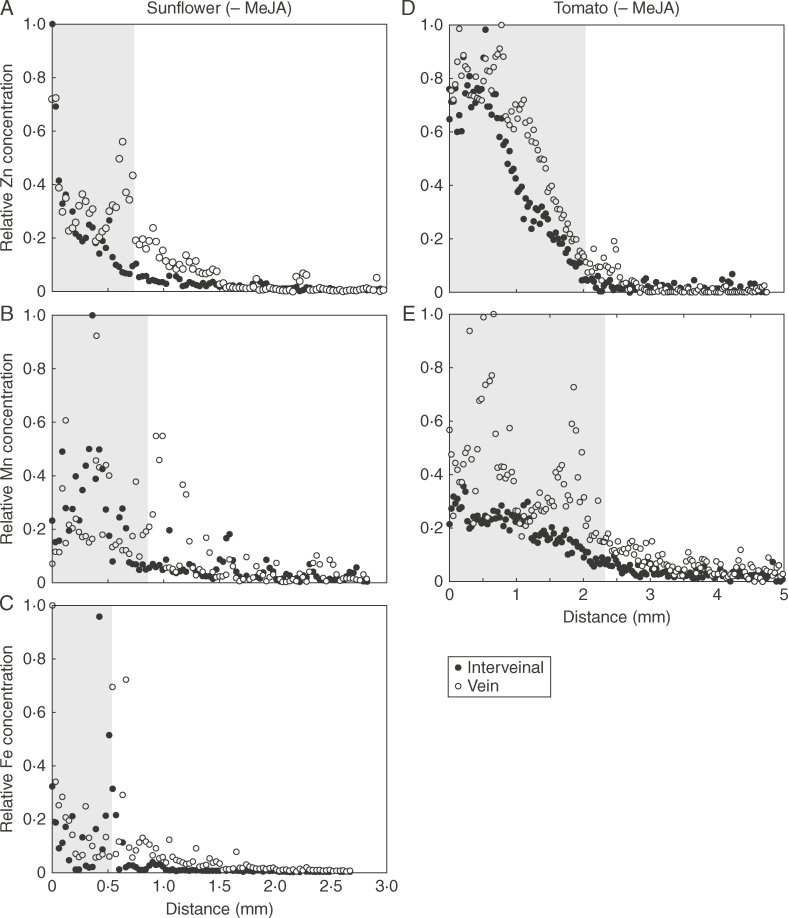
Using μ-XRF, it was possible to examine the movement of Zn, Fe and Mn by comparing their concentrations at increasing distance from the surface-applied droplets (Fig. 6). The distance that nutrients moved in the interveinal tissues and veins was calculated based upon measurements of droplet size from light microscopy and from maps of elemental distribution obtained from μ-XRF analyses (Fig. 6). As shown in Table 2, except for a significant reduction in the movement of Fe in the veins of sunflower, MeJA had no significant effect on the movement of Zn, Fe or Mn in either the interveinal tissues or the veins from the site of application. In all instances, the extent to which Zn, Fe and Mn moved from the site of their application was small, ranging from 0·22 to 1·32mm regardless of the plant species or the nutrient, with the movement distance in veins being slightly greater than in the interveinal tissues. For both sunflower and tomato, it was noted that Mn had a similar movement distance within the vein and the interveinal tissues, whereas Zn had a greater movement distance within the vein (approx. 2-fold longer) than the interveinal tissues (Table 2; Fig. 7). However, of the three nutrients, Mn had a slightly greater movement in interveinal tissues (mean 0·76mm) than Zn (mean 0·45mm) and Fe (mean 0·32mm) (Table 2).
Fig. 6.
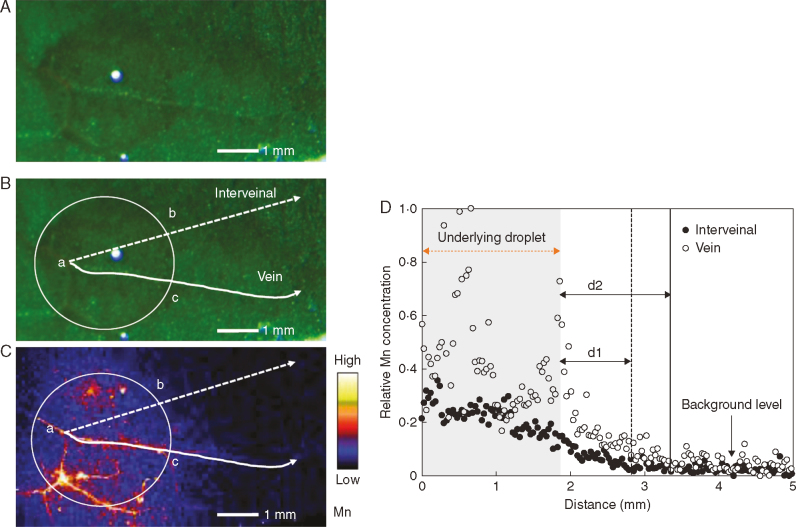
Comparison of Mn movement through the vein and interveinal tissues of a tomato control leaf after 6h of foliar application. (A) Light micrograph showing the Mn-containing droplet. (B) Same image as (A), showing the corresponding analysed area using μ-XRF in (C). White circles indicate the edge of the Mn droplet, while dashed lines represent a position of interveinal tissue and solid lines indicate the position of is of the same length in the droplet. (C) Compton-corrected Mn concentration of the area of (A) and (B). Brighter colour corresponds to higher Mn concentrations. The projected Mn concentrations underlying the solid (vein) and dashed (interveinal) line are shown in (D). (D) Compton-corrected Mn concentration in vein and interveinal tissue of tomato control leaf with the shaded part (shaded distance = ab = ac) representing the section under the Mn droplet; According to the Mn concentration of the background level, d1 is the Mn movement distance in the interveinal tissues, while d2 is the Mn movement distance in the vein.
Table 2.
Movement distance of Zn, Fe and Mn in leaves of sunflower, tomato and soybean after 6h of foliar application
| Treatments | Movement distance (mm) | |||||
|---|---|---|---|---|---|---|
| Mn | Zn | Fe | ||||
| Interveinal | Vein | Interveinal | Vein | Interveinal | Vein | |
| Sunflower | ||||||
| – MeJA | 0·72 ± 0·25a | 0·95 ± 0·19a | 0·53 ± 0·03a | 0·96 ± 0·26a | 0·41 ± 0·08a | 1·31 ± 0·19a |
| + MeJA | 0·62 ± 0·02a | 0·79 ± 0·10a | 0·40 ± 0·22a | 0·83 ± 0·03a | 0·22 ± 0·02a | 0·27 ± 0·05b |
| Tomato | ||||||
| – MeJA | 1·32 ± 0·11a | 1·27 ± 0·14a | 0·43 ± 0·05a | 0·88 ± 0·13a | NA | NA |
| + MeJA | 0·76 ± 0·23a | 1·01 ± 0·13a | 0·57 ± 0·10a | 0·95 ± 0·13a | NA | NA |
| Soybean | ||||||
| – MeJA | 0·44 ± 0·08a | NA | 0·30 ± 0·04a | NA | NA | NA |
| + MeJA | 0·69 ± 0·12a | NA | 0·46 ± 0·06a | NA | NA | NA |
Values are the mean ± SD (n = 4).
Where P < 0·05 occurred between control and 1 mM MeJA for each species is indicated with different lower case letters.
NA, data not available due to the comparatively low concentrations under the droplet.
Fig. 7.
Comparison of nutrient diffusion in vein and interveinal tissues of sunflower control (A–C) and tomato control (D–E) leaves after 6h of foliar application. The shaded area represents the portion of the leaf under the droplet. Soybean control and Fe for tomato control are not shown due to the comparatively low absorption or no detectable veins under the droplets.
DISCUSSION
Given that the leaf surface is covered with a cuticle, the underlying process whereby foliar-applied nutrients are absorbed through the leaf surface has been debated for many years. In the present study, we have utilized MeJA to alter leaf properties in order to examine the effects of leaf properties on foliar nutrient absorption. We found that foliar nutrient absorption was not related to overall trichome density – the application of 1 mm MeJA increased trichome density in both sunflower (86%) and tomato (76%) (Fig. 1), but the foliar absorption of Zn, Mn and Fe increased for sunflower but actually decreased in tomato (Table 1). Similarly, changes in foliar absorption could not be attributed to changes in either stomatal density or cuticle composition (Figs 2 and 4), given that these leaf properties remained constant. Rather, we suggest that the foliar absorption was related to the combined thickness of the cuticle and epidermal cell wall. Indeed, in sunflower, foliar absorption of the three nutrients increased by 300–530%, corresponding to a decrease in the cuticle and epidermal thickness from 3·0 to 1·9μm (Table 1; Fig. 3). Similarly, an increase in the thickness of the cuticle and epidermal cell wall from 1·9 to 2·5μm was accompanied by a decrease (50–90%) in the absorption of the three nutrients in tomato (Table 1; Fig. 3). Finally, in soybean, we observed no changes in either nutrient absorption or the thickness of the cuticle and epidermal cell wall. We also found that following their movement across the leaf surface, the absorbed Zn, Fe and Mn moved only a very limited distance (0·22–1·32mm) from the site of application before concentrations decreased to background levels, with MeJA (and the concomitant changes in leaf properties) having no effect on this translocation regardless of the plant species (Table 2).
The role of the cuticle in the foliar absorption of nutrients
Given that the cuticle is generally defined as a lipid-rich layer with its outer compartment dominated by waxes (Koch and Ensikat, 2008; Fernández et al., 2013), it is generally assumed that the cuticle is a layer distinct from the underlying epidermal cell wall. As a result, almost all studies examining the foliar absorption of nutrients have utilized isolated cuticles, with the underlying epidermal cell wall not taken into account (Baur et al., 1997; Riederer and Schreiber, 2001; Schreiber, 2005; Buchholz, 2006; Riederer and Friedmann, 2006; Schönherr, 2006; Jetter and Riederer, 2016). However, given that it has recently been reported that the cuticle and epidermal cell wall have similar chemical constitution and function, it has been proposed that the cuticle was actually an extension of the epidermal cell wall region (Guzmán et al., 2014; Fernández et al., 2016). To the best of our knowledge, the present study is the first to relate changes in foliar absorption of nutrients (Zn, Mn and Fe) to the combined thickness of the epidermal cell wall and thickness of cuticle.
We found that the absorption of these three nutrients, in all the three plant species, was related to changes in cuticle and epidermal cell wall thickness (Fig. 3; Table 1). Nevertheless, in contrast to these findings, some previous studies have suggested that cuticle thickness is not related to the cuticular permeability, although it must be noted that these previous studies have utilized isolated cuticles without also considering the underlying epidermal cell wall (Riederer and Schreiber, 2001; Jetter and Riederer, 2016).
In this present study, we also found differences in absorption of foliar nutrients among the three plant species. Overall, soybean (approx. 0·1–0·7μg per leaf) had a markedly lower absorption of the three nutrients than sunflower or tomato (approx. 4–12μg per leaf) (Table 1). We suggest that this is potentially because the soybean cuticle has a higher wax content than sunflower and tomato, as evidenced by the FTIR analyses (Supplementary Data Fig. S4), with a relatively high content of waxes (two bands at approx. 2900cm–1) and a low content of polysaccharides (the band at 3400cm–1) which have been reported previously in soybean compared with sunflower and tomato (Guzmán et al., 2014; Heredia-Guerrero et al., 2014). Also, we noted that it was substantially more difficult to apply the droplets to the leaves of soybean than it was to either sunflower or tomato because of higher surface tension (Fig. 5A), consistent with the analyses using SEM revealing a waxy layer covering leaves of soybean (Supplementary Data Fig. S5). This is in agreement with the previous studies that have reported the role of cuticular waxes in determining the permeability of plant cuticles (Riederer and Schreiber, 1995; Zhang et al., 2005; Bondada et al., 2006). In addition, by using μ-XRF, we found that the nutrient concentrations in the tissues underlying the droplets were higher in veins compared with the interveinal tissues (Figs 6 and 7). Given the low mobility of nutrients in the leaf tissues, this could be due to nutrient absorption being higher in veins than in the interveinal leaf tissues.
Leaf cuticle composition is expected to be important for the foliar absorption of nutrients. Indeed, it has been reported that MeJA increased polysaccharide content in the callus of Rhodiola sachalinensis (Li et al., 2014) and Dendrobium officinale (Yuan et al., 2016) through upregulating the metabolism enzymes and biosynthetic genes of sucrose. Similarly, MeJA can influence lipid metabolism, especially for fatty acyl metabolism (Cao et al., 2016). In the present study, however, FTIR analysis did not identify any marked changes in cuticle composition among the three plant species upon treatment with MeJA (Fig. 4). The effects of MeJA on leaf properties varied among different species. For example, MeJA increased the trichome density of sunflower and tomato, but did not change the leaf trichome for soybean (Fig. 1). Therefore, the effects of MeJA on leaf cuticle composition are probably species dependent.
The role of trichomes in the foliar absorption of nutrients
Trichomes are appendages that originate from epidermal cells and develop outwards on the surface of various plant organs, classified either as non-glandular or glandular trichomes, with one of the well-accepted functions being protecting the plant from herbivories (Werker, 2000). In the present study, the foliar absorption was not related to the overall trichome density – although application of 1 mm MeJA increased the overall trichome density in both sunflower (86%) and tomato (76%) (Fig. 1) – foliar absorption of Zn, Mn and Fe increased for sunflower but actually decreased in tomato (Table 1). However, the changes in nutrient absorption were consistent with changes in the densities of non-glandular trichomes. Specifically, 1 mm MeJA increased non-glandular trichome density of sunflower by 56% while it decreased non-glandular trichome density of tomato by 62%, and no changes occurred in non-glandular trichome density of soybean under the application of MeJA. We contend, however, that it is unlikely that these non-glandular trichomes play an important role in the foliar absorption of nutrients based on the fact that non-glandular trichomes are just appendages at the leaf surface which have no connections to the inside leaf tissues. However, it has been suggested that trichomes can change the surface roughness and in turn influence leaf wettability (Bickford, 2016). This could potentially impact the foliar absorption of nutrients, with leaf wettability decreasing with increasing trichome density (Brewer et al., 1991). In the present study, the non-glandular trichomes of both sunflower and tomato were larger than the glandular trichomes (Supplementary Data Fig. S6) and therefore non-glandular trichomes would be expected to have a larger impact on leaf wettability than glandular trichomes. Regardless, the change in leaf wettability induced by trichome density could not explain the differences in foliar absorption of nutrients in the present study, due to the fact that although leaf wettability decreased in sunflower (and increased in tomato), the foliar absorption of Zn, Mn and Fe increased for sunflower (and decreased for tomato).
As trichomes vary markedly in structure, morphology and function, it is difficult to generalize regarding the functions of all trichomes. Interestingly, several studies have examined foliar absorption by trichomes in epiphytic Bromeliaceae (Benzing et al., 1976, 1985; Winkler and Zotz, 2010). These studies have, for example, reported that foliar absorption of water, Ca and Zn was associated with leaf trichome densities of Bromeliaceae (Benzing and Burt, 1970; Ohrui et al., 2007; Winkler and Zotz, 2010). However, given that these epiphytic Bromeliaceae are generally highly drought resistant and do not have absorbent roots, the leaf trichomes of Bromeliaceae could potentially be highly specialized, with the peltate trichomes different from trichomes of other species (Ohrui et al., 2007).
Translocation of Zn, Fe and Mn away from the site of application
The efficacy of foliar nutrient application not only depends upon the rate at which the nutrients move through the leaf surface, but also the mobility of nutrients from the application site of the treated leaves to other parts of the plant. The mobility of these nutrients depends upon three factors: (1) the ability of the nutrients to enter the phloem; (2) the ability of the nutrients to move within the phloem; and (3) the ability of the nutrients to move out of the phloem into the sink tissues (Fernández et al., 2013). In the present study, we examined the distribution of Zn, Fe and Mn 6h after their application. It was observed that these three nutrients moved only a limited distance from their site of application, regardless of the plant species. This result is in agreement with previous studies which have reported that Zn, Mn or Fe have only a low mobility in plants (Eddings and Brown, 1967; Kannan and Charnel, 1986; Ferrandon and Chamel, 1988; Zhang and Brown, 1999; Marešová et al., 2012; Du et al., 2015). The reason for the low mobility of these nutrients remains unclear, although Fernández and Brown (2013) suggested that the low mobility of Zn may be due to the binding of Zn to the negative charge of the apoplastic space. Further studies are required to examine the location and movement of foliar-applied nutrients at a cellular and sub-cellular level.
In conclusion, using three plant species (soybean, sunflower and tomato), we utilized 1 mm MeJA to induce changes in leaf properties in order to examine the effects of these changes on absorption of foliar-applied nutrients. For all three plant species, we found that the foliar absorption of Zn, Mn and Fe was related to the thickness of the cuticle and epidermal cell wall. In contrast, foliar absorption of the nutrients was not related to changes in the total trichome density. In addition, using μ-XRF to provide in situ data from hydrated and fresh leaves, we found that the translocation of Zn, Mn and Fe away from the sites of application was limited (0·22–1·32mm) either in the vein or in the interveinal tissues, although the concentration of three nutrients within the underlying leaf tissues were higher in the veins than in the surrounding interveinal tissues.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: Foliar application of Zn, Mn and Fe droplets on a sunflower leaf for X-ray fluorescence microscopy (μ-XRF) analysis. Fig. S2: Compton-corrected μ-XRF images showing the distribution of Mn, Zn and Fe in a control leaf of tomato after 6h of foliar application. Fig. S3: Compton-corrected μ-XRF images showing the distribution of Mn, Zn and Fe in a control leaf of sunflower after 6h of foliar application. Fig. S4: Fourier transform infrared spectroscopy (FTIR) spectra obtained from isolated cuticles from the adaxial leaf surfaces of tomato, soybean and sunflower (all controls). Fig. S5: Scanning electron micrographs showing the surfaces of the leaves of soybean, tomato and sunflower. Fig. S6: Scanning electron micrographs showing non-glandular and glandular trichomes on the leaf surface of tomato and sunflower.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by Sonic Essentials (Melbourne, Australia) and through the Australian Research Council's Linkage Projects funding scheme (LP130100741). Support was also provided to P.M.K. as a recipient of an ARC Future Fellowship (FT120100277) and to C.L. who received support through the China Scholarship Council and through The University of Queensland (UQ International Scholarship). We thank Kathryn Green (Centre for Microscopy and Microanalysis, The University of Queensland) for assistance with the operation of the scanning electron microscope and the ultramicrotome. Parts of this research were undertaken on the XFM beamline at the Australian Synchrotron (AS152/XFM/9487), Victoria, Australia, for which we thank the assistance of Daryl Howard.
LITERATURE CITED
- Baur P, Buchholz A, Schönherr J.. 1997. Diffusion in plant cuticles as affected by temperature and size of organic solutes: similarity and diversity among species. Plant, Cell & Environment 20: 982–994. [Google Scholar]
- Benzing DH, Burt KM.. 1970. Foliar permeability among twenty species of the Bromeliaceae. Bulletin of the Torrey Botanical Club 97: 269–279. [Google Scholar]
- Benzing DH, Henderson K, Kessel B, Sulak J.. 1976. The absorptive capacities of bromeliad trichomes. American Journal of Botany 63: 1009–1014. [Google Scholar]
- Bickford CP. 2016. Ecophysiology of leaf trichomes. Functional Plant Biology 43: 807–814. [DOI] [PubMed] [Google Scholar]
- Blamey FPC, Hernandez-Soriano MC, Cheng M. et al. 2015. Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiology 169: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondada RB, Petracek DP, Syvertsen PJ, Albrigo L.. 2006. Cuticular penetration characteristics of urea in citrus leaves. Journal of Horticultural Science and Biotechnology 81: 219–224. [Google Scholar]
- Boughton AJ, Hoover K, Felton GW.. 2005. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. Journal of Chemical Ecology 31: 2211–2216. [DOI] [PubMed] [Google Scholar]
- Brewer C, Smith W, Vogelmann T.. 1991. Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant, Cell & Environment 14: 955–962. [Google Scholar]
- Buchholz A. 2006. Characterization of the diffusion of non-electrolytes across plant cuticles: properties of the lipophilic pathway. Journal of Experimental Botany 57: 2501–2513. [DOI] [PubMed] [Google Scholar]
- Buchholz A, Baur P, Schönherr J.. 1998. Differences among plant species in cuticular permeabilities and solute mobilities are not caused by differential size selectivities. Planta 206: 322–328. [Google Scholar]
- Cao J, Li M, Chen J, Liu P, Li Z.. 2016. Effects of MeJA on Arabidopsis metabolome under endogenous JA deficiency. Scientific Reports, 6: 37674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drissi S, Houssa AA, Bamouh A, Benbella M.. 2015. Corn silage (Zea mays L.) response to zinc foliar spray concentration when grown on sandy soil. Journal of Agricultural Science 7: 68–79. [Google Scholar]
- Du Y, Kopittke PM, Noller BN. et al. 2015. In situ analysis of foliar zinc absorption and short-distance movement in fresh and hydrated leaves of tomato and citrus using synchrotron-based X-ray fluorescence microscopy. Annals of Botany 115: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddings JL, Brown A.. 1967. Absorption and translocation of foliar-applied iron. Plant Physiology 42: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V, Brown PH.. 2013. From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Frontiers in Plant Science 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V, Eichert T.. 2009. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Critical Reviews in Plant Sciences 28: 36–68. [Google Scholar]
- Fernández V, Sotiropoulos T, Brown P.. 2013. Foliar fertilization: scientific principles and field practices. Paris, France: International Fertilizer Industry Association (IFA; ). [Google Scholar]
- Fernández V, Sancho-Knapik D, Guzmán P. et al. 2014. Wettability, polarity, and water absorption of holm oak leaves: effect of leaf side and age. Plant Physiology 166: 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V, Guzmán-Delgado P, Graça J, Santos S, Gil L.. 2016. Cuticle structure in relation to chemical composition: re-assessing the prevailing model. Frontiers in Plant Science 7: 427. doi:10.3389/fpls.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon M, Chamel AR.. 1988. Cuticular retention, foliar absorption and translocation of Fe, Mn and Zn supplied in organic and inorganic form. Journal of Plant Nutrition 11: 247–263. [Google Scholar]
- Guzmán P, Fernández V, Graça J. et al. 2014. Chemical and structural analysis of Eucalyptus globulus and E. camaldulensis leaf cuticles: a lipidized cell wall region. Frontiers in Plant Science 5: 481. doi:10.3389/fpls.2014.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia-Guerrero JA, Benitez JJ, Dominguez E. et al. 2014. Infrared and Raman spectroscopic features of plant cuticles: a review. Frontiers in Plant Science 5: 305. doi:10.3389/fpls.2014.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter R, Riederer M.. 2016. Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiology 170: 921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Charnel A.. 1986. Foliar absorption and transport of inorganic nutrients. Critical Reviews in Plant Sciences 4: 341–375. [Google Scholar]
- Koch K, Ensikat H-J.. 2008. The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39: 759–772. [DOI] [PubMed] [Google Scholar]
- Koontz H, Biddulph O.. 1957. Factors affecting absorption and translocation of foliar applied phosphorus. Plant Physiology 32: 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Menzies NW, de Jonge MD. et al. 2011. In situ distribution and speciation of toxic copper, nickel, and zinc in hydrated roots of cowpea. Plant Physiology 156: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lian M, Shao C, Jin C, Piao X.. 2014. Effect of methyl jasmonate on salidroside and polysaccharide accumulation in Rhodiola sachalinensis callus. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 39: 4252–4257. [PubMed] [Google Scholar]
- Marešová J, Remenárová L, Horník M, Pipíška M, Augustín J, Lesný J.. 2012. Foliar uptake of zinc by vascular plants: radiometric study. Journal of Radioanalytical and Nuclear Chemistry 292: 1329–1337. [Google Scholar]
- Obrien S. 1995. Extrafloral nectaries in Chamelaucium uncinatum: a first record in the Myrtaceae. Australian Journal of Botany 43: 407–413. [Google Scholar]
- Ohrui T, Nobira H, Sakata Y. et al. 2007. Foliar trichome- and aquaporin-aided water uptake in a drought-resistant epiphyte Tillandsia ionantha Planchon. Planta 227: 47–56. [DOI] [PubMed] [Google Scholar]
- Oviedo C, Rodríguez J.. 2003. EDTA: the chelating agent under environmental scrutiny. Quimica Nova 26: 901–905. [Google Scholar]
- Paterson D, De Jonge M, Howard D. et al. 2011. . The X-ray Fluorescence Microscopy Beamline at the Australian Synchrotron. The 10th International Conference on X-ray microscopy. AIP Publishing. [Google Scholar]
- Ram H, Rashid A, Zhang W. et al. 2016. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant and Soil 403: 389–401. [Google Scholar]
- Reuveni R, Agapov V, Reuveni M, Raviv M.. 1994. Effects of foliar sprays of phosphates on powdery mildew (Sphaerotheca pannosa) of roses. Journal of Phytopathology 142: 331–337. [Google Scholar]
- Riederer M, Friedmann A.. 2006. Transport of lipophilic non-electrolytes across the cuticle. Annual Plant Reviews 23: 250–279. [Google Scholar]
- Riederer M, Schreiber L.. 1995. Waxes: the transport barriers of plant cuticles. Dundee, UK: The Oily Press. [Google Scholar]
- Riederer M, Schreiber L.. 2001. Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of Experimental Botany 52: 2023–2032. [DOI] [PubMed] [Google Scholar]
- Ryan C. 2000. Quantitative trace element imaging using PIXE and the nuclear microprobe. International Journal of Imaging Systems and Technology 11: 219–230. [Google Scholar]
- Ryan C, Jamieson D.. 1993. Dynamic analysis: on-line quantitative PIXE microanalysis and its use in overlap-resolved elemental mapping. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 77: 203–214. [Google Scholar]
- Schlegel T, Schönherr J.. 2001. Selective permeability of cuticles over stomata and trichomes to calcium chloride. Acta Horticulturae 594: 91–96. [Google Scholar]
- Schlegel TK, Schönherr J, Schreiber L.. 2005. Size selectivity of aqueous pores in stomatous cuticles of Vicia faba leaves. Planta 221: 648–655. [DOI] [PubMed] [Google Scholar]
- Schlegel TK, Schönherr J, Schreiber L.. 2006. Rates of foliar penetration of chelated Fe (III): role of light, stomata, species, and leaf age. Journal of Agricultural and Food Chemistry 54: 6809–6813. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 1976. Water permeability of isolated cuticular membranes: the effect of pH and cations on diffusion, hydrodynamic permeability and size of polar pores in the cutin matrix. Planta 128: 113–126. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 2006. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany 57: 2471–2491. [DOI] [PubMed] [Google Scholar]
- Schreiber L. 2001. Effect of temperature on cuticular transpiration of isolated cuticular membranes and leaf discs. Journal of Experimental Botany 52: 1893–1900. [DOI] [PubMed] [Google Scholar]
- Schreiber L. 2005. Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis. Annals of Botany 95: 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Tooker J, Peiffer M, Chung SH, Felton GW.. 2012. Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236: 1053–1066. [DOI] [PubMed] [Google Scholar]
- Vu DT, Huang L, V Nguyen A. et al. 2013. Quantitative methods for estimating foliar uptake of zinc from suspension-based Zn chemicals. Journal of Plant Nutrition and Soil Science 176: 764–775. [Google Scholar]
- Werker E. 2000. Trichome diversity and development. Advances in Botanical Research, 31: 1–35. [Google Scholar]
- Winkler U, Zotz G.. 2010. ‘And then there were three’: highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Annals of Botany 106: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Zhang J, Liu T.. 2016. Enhancement of polysaccharides accumulation in Dendrobium officinale by exogenously applied methyl jasmonate. Biologia Plantarum 1–7. [Google Scholar]
- Zhang JY, Broeckling CD, Blancaflor EB, Sledge MK, Sumner LW, Wang ZY.. 2005. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). The Plant Journal 42: 689–707. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Brown PH.. 1999. Distribution and transport of foliar applied zinc in pistachio. Journal of the American Society for Horticultural Science 124: 433–436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.