Abstract
Dynemicin is a novel anthraquinone-fused member of the 10-membered enediyne antitumor antibiotic family. The development of a genetic system for the dynemicin producer Micromonospora chersina confirmed, for the first time, the requirement of the putative enediyne core biosynthetic genes (dynE8, U14 and U15) and a tailoring oxidase gene (orf23) for dynemicin production. Cloning and sequence analysis of a 76 kb of genomic sequence region containing dynE8 revealed a variety of genes conserved among known enediyne loci. Surprisingly, this fragment and flanking chromosomal DNA lacked any obvious genes encoding for the biosynthesis of the anthraquinone, suggesting that the location of genes encoding for the biosynthesis of the dynemicin enediyne core and the dynemicin anthraquinone are chromosomally distinct. The demonstrated trace production of a shunt product from mutant strain QGD23 (Δorf23) also sets the stage for subsequent studies to delineate the key steps in enediyne core biosynthesis and tailoring.
Keywords: dynemicin, enediyne, biosynthesis, polyketide synthase, gene disruption, cancer
Introduction
The enediyne antibiotics are appreciated for their unique molecular architectures, fascinating mechanisms of action and remarkably potent biological activities (Thorson et al., 2000; Shen, 2003; Galm et al., 2005). Thirteen naturally occurring enediynes have been structurally characterized, all of which encompass the signature di-acetylenic core conjugated by (or to) a double bond. Despite their structural distinctions, all enediyne antibiotics damage DNA via a rapid enediyne cycloaromatization to form highly reactive diradical species capable of inducing oxidative DNA strand scission. Conjugation of enediynes to tumor-specific monoclonal antibodies and the application of polymer-assisted delivery devices have also led to the clinical success of enediynes (Abe & Otsuki, 2002; Damle, 2004; Jedema et al., 2004; Wu & Senter, 2005).
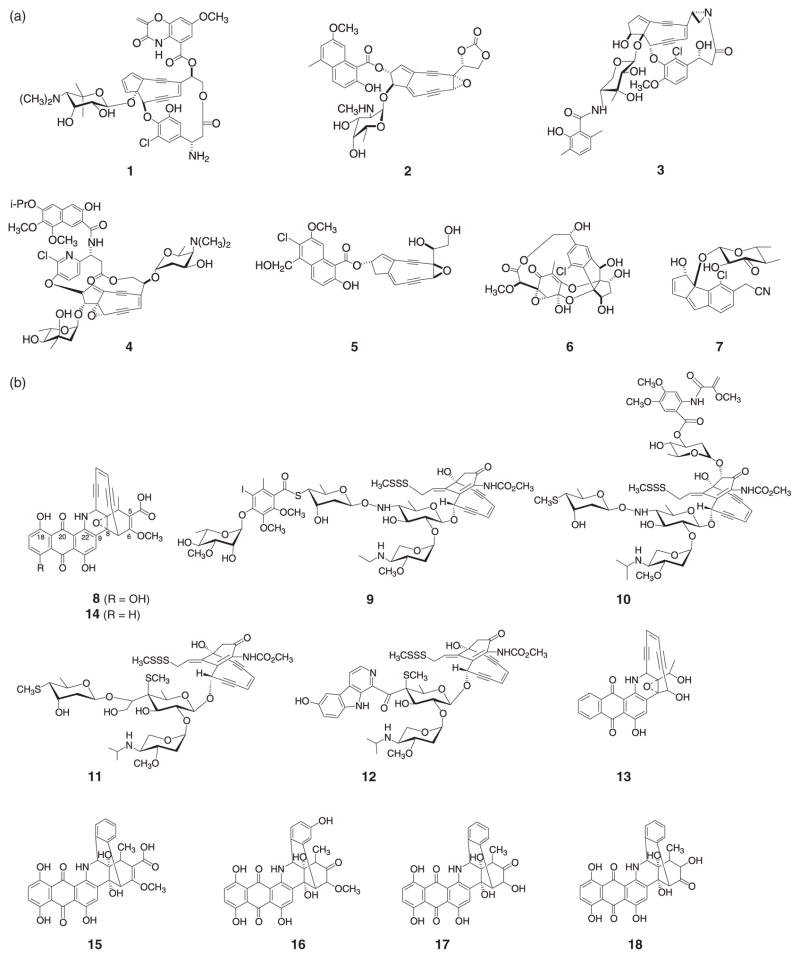
There exist two enediyne structural subfamilies –chromoprotein (or nine-membered) enediynes and 10-membered enediynes. The chromoprotein enediynes C-1027 (1), neocarzinostatin (NCS 2), maduropeptin (MAD 3), kedarcidin (4) and N1999A2 (5) contain a bicyclo[7.3.0]enediyne nine-membered core and, with one exception (5), require a specific protein for enediyne stabilization and transport (for a recent review, see Galm et al., 2005). The recently isolated sporolides (6) and cyanosporasides (7) (Fig. 1a) have also been proposed to derive from nine-membered enediyne precursors (Buchanan et al., 2005; Oh et al., 2006). The 10-membered enediynes share a common bicyclo[7.3.1]enediyne core and include calicheamicin (9), esperamicin (10), namenamicin (11) shishijimicin (12) dynemicin (8) and uncialamycin (13) (Fig. 1b) (for a recent review, see Galm et al., 2005). While the 10-membered enediynes lack protein stabilizers, some producing organisms of this family rely upon a novel ‘self-sacrifice’ resistance protein (as exemplified by the calicheamicin protein, CalC) as a self-resistance mechanism (Biggins et al., 2003; Singh et al., 2006). This latter subgroup can be further divided into the glycosylated analogs (9–12) and anthraquinone-fused members (8 and 13).
Fig. 1.
Representative naturally occurring enediynes. (a) The nine-membered enediynes: C-1027 (1), neocarzinostatin (2), maduropeptin (3), kedarcidin (4), N1999A2 (5) and putative members sporolide A (6) and cyanosporaside A (7). (B) The 10-membered enediynes: dynemicin A (8), calicheamicin (9), esperamicin A1 (10), namenamicin (11), shishijimicin A (12), uncialamycin (13), deoxydynemicin A (14), dynemicin N (15), dynemicin O (16), dynemicin P (17), dynemicin Q (18).
Early metabolic labeling studies suggested the nine- and 10-membered enediynes to derive from distinct biosynthetic pathways (Hensens et al., 1989; Tokiwa et al., 1992; Lam et al., 1993). In contrast, the recent cloning and characterization of gene clusters encoding both nine-membered and 10-membered enediynes revealed a common enediyne polyketide synthase (PKSE) gene and thereby established the first unified, divergent polyketide paradigm for enediyne core biosynthesis (Ahlert et al., 2002; Liu et al., 2002). Subsequent genomic scanning with nine-membered PKSE-specific or 10-membered PKSE-specific primers were consistent with a predicted biosynthetic divergence (Liu et al., 2003), and PKSE genes were found to be remarkably prevalent in bacteria (Zazopoulos et al., 2003). Yet, while gene loci encoding for chromoprotein (1 and 2) and saccharide-appended 10-membered (9) enediynes have been characterized (Ahlert et al., 2002; Liu et al., 2002, 2005), loci for the anthraquinone-fused enediynes 8 or 13 remain surprisingly elusive. Moreover, although a putative 8 PKSE-encoding gene (dynE8) has been identified by degenerate PCR (Liu et al., 2003), this gene has not been confirmed to play a role in 8 biosynthesis.
Herein we report cloning, sequencing and characterization of a 76-kb contiguous genomic DNA region containing dynE8 from Micromonospora chersina ATCC53710. The subsequent successful implementation of gene replacements for the 8-producing M. chersina confirmed, for the first time, dynE8 and other localized genes (dynU14, U15 and orf23) as essential for the biosynthesis of the 8. While analysis of the isolated genomic region surprisingly lacked additional PKS-encoding genes anticipated for the biosynthesis of the 8 anthraquinone moiety, the demonstrated trace production of a shunt product from mutant strain QGD23 (Δorf23) clearly sets the stage for subsequent studies to delineate the key steps in enediyne core biosynthesis and tailoring.
Materials and methods
For details regarding bacterial strains, culture conditions, plasmids, reagents, cloning, genomic library construction and screening, DNA sequencing and analysis, gene inactivation, and product isolation and characterization, see supplemental online material. The DNA sequence reported in this paper has been deposited in GenBank under the accession number EF552206.
Results and discussion
Cloning of the dynE8 -containing genomic region from M. chersina ATCC 53710
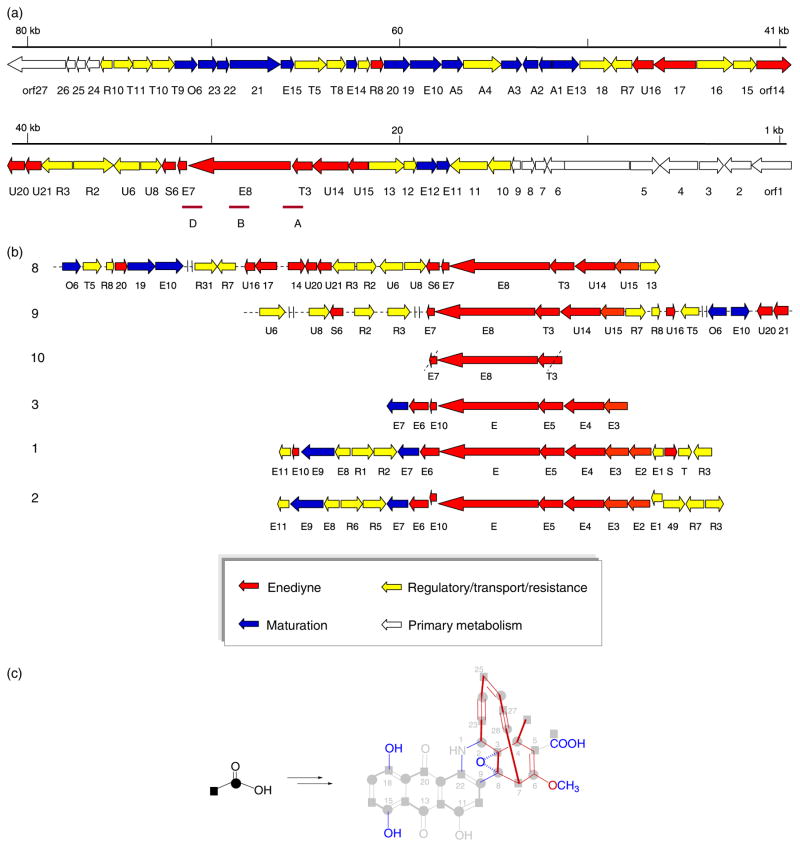
For the current study, three distinct probes based upon the putative dynE8 sequence (Liu et al., 2003, Zazopoulos et al., 2003), designated A, B and D (Fig. 3a), were used to screen c. 4800 colonies from a M. chersina genomic cosmid library constructed in pOJ446 (Kieser et al., 2000). All positive clones were confirmed by secondary hybridization and as templates for the amplification of the dyn A, B and D amplicons. From this analysis, seven dynE positive clones spanning c. 90 kb (based upon restriction mapping) were identified.
Fig. 3.
Enediyne biosynthetic genes. (a) The organization of dynE8-containing sequenced region. (b) Comparison of nine and 10-membered enediyne biosynthetic genes. Numbers on the left correspond to enediyne numbering in Fig. 1. Arrows represent the relative ORF size and direction of transcription. Colors designate putative functionality based upon homology to gene products of known or putative functions (see Table 1). (c) Summary of metabolic labeling in the context of genes found clustered with dynE8. The anthraquinone and enediyne core derive from distinct intermediates, the latter of which is constructed by the minimal enediyne machinery encoded within the dynE8-containing sequence region (highlighted in red). While the core genes responsible for the anthraquinone biosynthesis are not clustered with dynE8, a variety of putative maturation genes (highlighted in blue) located adjacent to dynE8 are likely involved in oxidation and possibly the key enediyne–anthraquinone fusion event.
Sequencing and organization of the M. chersina dynemicin core biosynthetic genes
Shotgun sequencing of cosmids pJST1009 and pJST1059 (Supplementary Fig. S1) at approximately fivefold coverage provided 76 kb of genomic sequence (deposited in Genbank under accession number EF552206). Analysis of the sequence (72.5% GC content) by FramePlot (Ishikawa & Hotta, 1999) revealed 60 ORFs (Fig. 3a), the preliminary annotation of which derived from BLAST and NCBI Conserved Domain Database (Altschul et al., 1997; Marchler-Bauer et al., 2007) analysis. The putative functional assignments for each ORF, and their closest homologs, are listed in Table 1. Of the putative ORFs identified, 24 genes were notably homologous to counterparts within 9 biosynthetic gene cluster (Fig. 3b). For readers’ convenience, gene denominations correlate to activity groups, E for enediyne biosynthesis, A for maturation or fusion, T for uptake and transport, R for regulation and U for unknown.
Table 1.
Deduced functions of the dynemicin ORFs illustrated in Fig. 3
| Gene | Amino acid | Putative function | Closest homology (Identity, similarity %) | Accession number |
|---|---|---|---|---|
| orf1 | 698 (partial) | Unknown protein | Strop_0190 (50/65) | ABP52675 |
| orf2 | 409 | Unknown protein | Strop_0188 (54/63) | ABP52673 |
| orf3 | 306 | Putative NUDIX hydrolase | Strop_0187 (66/57) | ABP52672 |
| orf4 | 605 | Putative resistance protein | Conserved hypothetical protein (71/83) | CAG38681 |
| orf5 | 215 | NADP oxidoreductase, F420-dependent | ORF MecP21.23c (75/88) | CAG38682 |
| rRNA | 7244-12 447 | rRNA operon with 5S, 16S and 23S rRNA genes | M. echinospora strain DSM 43036 | AJ628149 |
| orf6 | 159 | Hypothetical protein | GntT (52/58) | AAR98538 |
| orf7 | 116 | Putative alkylated DNA repair protein | TobX protein (76/88) | YP_035491 |
| orf8 | 155 | Unknown protein | – | – |
| orf9 | 151 | Unknown protein | SAV740 (60/72) | BAC68450 |
| orf10 | 352 | Tryptophanyl tRNA synthetase | GntU (83/90) | AAR98539 |
| orf11 | 572 | Arginyl-tRNA synthetase | SareDRAFT_0146 (73/83) | EAX25545 |
| dynE11 | 144 | Putative glyoxalase/bleomycin resistance protein/dioxygenase | YraH (34/46) | CAB46394 |
| dynE12 | 386 | Putative dehydrogenases maturation factor; XdhC/CoxF family | MSMEG_0743 (61/72) | ABK69650 |
| orf12 | 195 | Hypothetical protein | SareDRAFT_0187 (60/66) | EAX25586 |
| orf13 | 623 | Transcriptional regulatory | CalR2 (45/59) | AAM94777 |
| dynU15 | 328 | Unknown conserved protein | CalU15 (39/49) | AAM70330 |
| dynU14 | 632 | Unknown conserved protein | CalU14 (47/57) | AAM70329 |
| dynT3 | 329 | Unknown conserved protein | CalT3 (53/66) | AAM94795 |
| dynE8 | 1900 | Enediyne core forming iterative polyketide synthase | CalE8 (44/54) | AAM94794 |
| 4–453 | KS domain | CalE8-KS (59/70) | ||
| 476–895 | AT domain | CalE8-AT (49/55) | ||
| 933–1003 | ACP domain | CalE8-ACP (45/59) | ||
| 1148–1402 | KR domain | CalE8-KR (49/59) | ||
| 1409–1549 | DH domain | CalE8-DH (50/59) | ||
| 1685–1891 | Putative PPTase domain | CalE8-Sfp | ||
| dynE7 | 144 | Putative thioesterase | CalE7 (48/64) | AAM94793 |
| dynS6 | 219 | NADP oxidoreductase, F420-dependent | CalS6 (44/54) | AAM94776 |
| dynU8 | 224 | Putative transcriptional regulator | CalU8 (66/79) | AAM94775 |
| dynU6 | 307 | Putative transporter | CalU6 (61/75) | AAM94767 |
| dynR2 | 627 | Putative transcriptional regulator | CalR2 (43/58) | AAM94777 |
| dynR3 | 454 | Putative regulator | CalR3 (49/61) | AAM94780 |
| dynU21 | 188 | Hypothetical protein | CalU21 (65/78) | AAM70363 |
| dynU20 | 191 | Unknown protein | CalU20 (31/46) | AAM70362 |
| orf14 | 405 | Unknown protein | CalU16 (29/41) | AAM70339 |
| orf15 | 210 | Putative aminopeptidase | CAB720 (22/41) | CAH64167 |
| orf16 | 441 | Hypothetical protein | Strop_0601 (53/71) | ABP53081 |
| orf17 | 516 | Ketone reductase | CalE8-KR (42/51) | AAM94794 |
| dynU16 | 283 | Unknown protein | CalU16 (29/43) | AAM70339 |
| dynR7 | 283 | Putative transcriptional regulator | CalR7 (37/47) | AAM70331 |
| orf18 | 418 | Putative regulator | CalR3 (46/60) | AAM94780 |
| dynE13 | 378 | FAD-dependent oxidoreductases | MXAN_3398 (32/47) | ABF93079 |
| dynA1 | 142 | Putative hydroxylase | AclR protein (41/64) | CAJ87106 |
| dynA2 | 167 | Putative hydroxylase | SnoL (32/45) | AAF01808 |
| dynA3 | 298 | NmrA family protein | Aave_3935 (38/50) | ABM34478 |
| dynA4 | 509 | Putative peptidases and hydroxylases | ORF9 (36/48) | AAP85358 |
| dynA5 | 370 | Putative hydroxylase | RdmB | AAA83421 |
| orf19 | 403 | Cytochrome P450 hydroxylase | PdmJ (45/61) | ABM21756 |
| dynE10 | 400 | Cytochrome P450 hydroxylase | LnmA (49/65) | AAN85514 |
| orf20 | 146 | Unknown protein | CalU16 (29/39) | AAM70339 |
| dynR8 | 114 | Putative transcriptional regulator | CalR8 (39/55) | AAM70335 |
| dynE14 | 123 | Putative glyoxalase/dioxygenase | NB231_07547 (42/57) | EAR21006 |
| dynT8 | 269 | Putative ABC transporter | ABC-2 (44/59) | ABD10850 |
| dynT5 | 333 | Transporter ATP-ATPase subunit | CalT5 (46/64) | AAM7034 |
| dynE15 | 123 | Putative glyoxalase/dioxygenase | RHA1_ro02544 (45/58) | ABG94349 |
| orf21 | 786 | Molybdenum-containing oxidoreductase-large subuit, molybdopterin binding | F.DRAFT_4713 (47/62) | EAN16489 |
| orf22 | 164 | Molybdenum-containing oxidoreductase-small subunit, (2Fe–2S)-binding subunit | Mjls_4986 (67/76) | ABO00752 |
| orf23 | 292 | Molybdenum-containing oxidoreductase-medium subunit, FAD-binding subunit | kdhM (42/56) | CAD47945 |
| dynO6 | 349 | O-methyltransferase | CalO6 (37/51) | AAM70356 |
| dynT9 | 339 | Transmembrane transport protein | ORF31 (55/72) | ABD65951 |
| dynT10 | 305 | ABC transporter ATP-binding protein | CcmA (70/83) | ABD65952 |
| dynT11 | 331 | Transmembrane transport protein | ORF31 (53/68) | ABD65951 |
| dynR10 | 189 | Two-component system sensor kinase | ORF43(48/65) | ABD65963 |
| orf24-orf26 | 218 141 141 | Unknown protein | – | – |
| orf27 | 1138 | Putative restriction enzyme | Mflv_0803 (60/75) | ABP43287 |
Disruption of dynE8, orf8, dynU15, dynU14 and orf23 in M. chersina
Before this work, a genetic system for the 8-producing M. chersina was unavailable. Using PCR-targeting-mediated gene replacement (Gust et al., 2003), the entire dynE8 in cosmid pQG9B01 (a subclone carrying a 32 kb insert of pJST1009, see Supplementary Table S1) was replaced by an apramycin resistance gene to give pQGD9001 (Supplementary Fig. S2). Conjugation using pQGD9001 led to 8 exconjugants – two of which were double crossovers. Although the conjugation efficiency was low (8 × 10−8 exconjugants per recipient spores), the desired M. chersina dynE8::AmR mutant strain QGD01 was confirmed by both PCR and Southern hybridization (Supplementary Fig. S2) thereby establishing, for the first time, a gene replacement for M. chersina. Similar apramycin replacement experiments for genes orf8, dynU15, dynU14 (in cosmid pQG9B01) and orf23 (in cosmid pQG59B01) provided constructs pQGD9008, pQGD9U15, pQGD9U14 and pQGD5923, respectively (Supplementary Fig. S2). Upon conjugation, this set of mutant cosmids ultimately presented M. cherisina mutant strains QGD08 (orf8::AmR), QGDU15 (dynU15::AmR), QGDU14 (dynU14:: AmR) and QGD23 (orf23:: AmR) (Supplementary Table S1).
Dynemicin production in wild-type and mutant strains of M. chersina
Using a slight modification of previous 8 fermentation protocols (Miyoshisaitoh et al., 1991), optimal 8 production (4.2 mg L−1) by M. chersina strain ATCC53710 was provided on the third day in production media containing 1% Diaion HP-20 (Lam et al., 1995). The identity of 8 was further confirmed by matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) analysis (m/z calculated for C30H19NO9 [M+H]+ 538.1133, observed 538.1155) and by coelution with a standard for 8. MALDI-MS analysis ultilized an IonSpec HiResMALDI FT-MS equipped with a 7 T superconducting magnet and a nitrogen laser. For this analysis, 0.4 μL of 8 (1 mg mL−1) in 50: 50 H2O: MeOH was spotted onto a plate containing 0.4 μL of a saturated solution of a 2,5-dihydroxybenzoic acid (DHB) matrix in 50: 50 H2O: MeOH and allowed to air dry. Seven laser shots of the sample spot were taken, and accumulated in the analyzer cell. The Fourier transformed spectrum was peak matched with a dimer of DHB to a mass accuracy of 4.24 p.p.m.
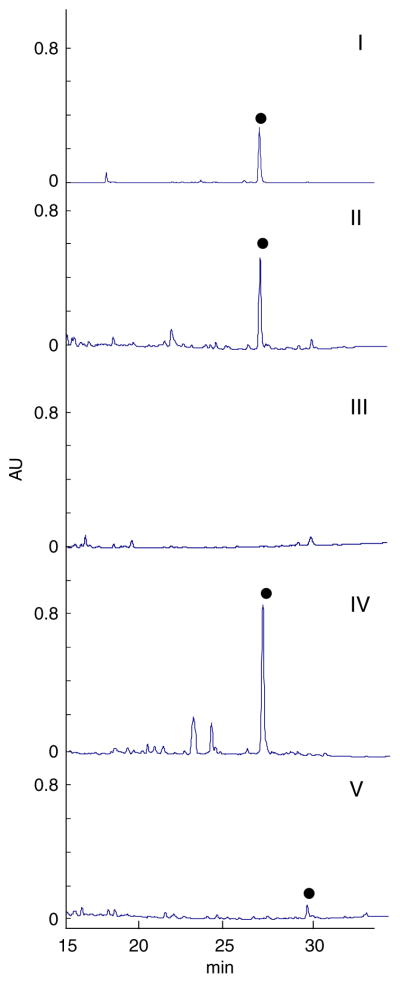
As highlighted in Fig. 2, disruption of dynE8 completely abolished 8 production (Fig. 2, panel iii), consistent with dynE8 as encoding the 8 PKSE. It is important to note that while the disruption of dynU14 and U15 presented identical phenotypes (Supplementary Fig. S3), due to the gene organization of dynU15, dynU14, dynT3, dynE8, dynE7 and dynS6, the ΔdynU15, ΔdynU14 and/or ΔdynE8 disruptions could potentially induce polar effects upon other downstream genes within this small operon. In contrast, mutant strain QGD08 (orf8) retained antibiotic production (Fig. 2, panel iv) at a level similar to the wild type strain (Fig. 2, panel ii) (4.3 mg L−1) while mutant strain QGD23 (Δorf23) accumulated a new compound with a similar color, solubility and UV spectrum to 8 (Fig. 2, panel v). LC-MS analysis of this new compound was consistent with the loss of an oxygen from 8. Notably, these results validate the genetic system and highlight the essential nature of dynE8 (and nonessential nature of orf8) for 8 biosynthesis. Given the lack of validated plasmids for complementation experiments in enediyne producing Micromonospora (Takada et al., 1994; Ahlert et al., 2002; Li et al., 2004; Hosted et al., 2005), the orf8 and orf23 replacement mutants are particularly noteworthy examples as they illustrate M. chersina genotype alterations without abolishing metabolite production.
Fig. 2.
HPLC analysis of fermentation extracts from wild-type, ΔdynE8 (QGD01), Δorf8 (QGD08) and Δorf23 (QGD23) Micromonospora chersina strains: (i) 8 authentic standard, (ii) wild-type, (iii) QGD01, (iv) QGD08, and (v) QGD23. The violet color, solubility and UV-vis signature of the QGD23 putative 8 shunt metabolite [CH3CN: 233.4, 279 (sh), 543 nm] are similar to that of 8 [CH3CN: 237, 280 (sh), 543 nm]. Parameters for analytical HPLC and product characterization are described in Materials and methods.
Biosynthesis of the dynemicin enediyne precursor
Sequence analysis of the M. chersina genes clustered with dynE8 revealed six gene products, DynU15-DynS6 with homologs in 9 biosynthesis (% identity/% similarity ranging from 39/49 to 53/66; Table 1), believed critical for enediyne core formation. The genes for five of these (U15, U14, T3, E8, E7, Fig. 3b) reside within what has now become known as the ‘minimal enediyne cassette’ (Ahlert et al., 2002; Liu et al., 2002, 2003, 2005; Zazopoulos et al., 2003). Consistent with other known enediyne loci, three conserved proteins of unknown function were also found to be encoded within the 8 minimal enediyne gene cassette (DynT3, DynU14 and DynU15). Unique in comparison with other enediyne loci, the characterized sequenced region contained a distinct gene (designated orf17) encoding a protein homologous to the PKSE KR domain (see Table 1). Beyond the genes related to minimal enediyne cassette, there exist only four additional genes (dyn/calS6, dyn/calU16, dyn/calU20 and dyn/calU21) uniquely conserved among the enediyne loci characterized to date. Based upon homology, DynS6/CalS6 has been annotated as a putative F420 – dependent NADP oxidoreductase (see Table 1) while the remaining gene products (U16 and U20 and U21) have no homologs outside putative enediyne-associated pathways. Interestingly, three U16-like proteins (U16, Orf14 and Orf20) are encoded by the 8 locus. While these three vary in size and share modest homology, they all contain two common domains – a COG3832 (uncharacterized) and ASHA1 (activator of Hsp90 ATPase homolog 1-like protein) domain.
Dynemicin fusion/tailoring/maturation
Consistent with the postulated separation of the biosynthesis of the 8 bicyclo[7.3.1.]diyne unit and the anthraquinone unit (Tokiwa et al., 1992), preliminary degenerate PCR and Southern hybridization failed to identify candidate anthraquinone biosynthetic genes near (within 20 kb) either end of 76-kb sequenced region (data not shown). It should also be noted that precedent does exist for the chromosal separation of biosynthetic loci in bacteria (Yu et al., 2002; Ostash et al., 2007). Based upon metabolic labeling, three putative oxygenation steps can be predicted (C18, C20 and possibly C22) as required for anthraquinone maturation/tailoring. In addition, the fact that deoxydynemicin (14) (Fig. 1b) and 8 were coproduced by M. globosa MG-331-FG (Shiomi et al., 1990), and the recent elucidation of uncialamycin (13) (Fig. 1b), implicate the potential for C5 oxidation. Consistent with this, a number of candidate genes encoding oxidative enzymes were found to be colocalized with dynE8. As the most likely candidates for C18/C22 oxidation, DynA1 and DynA2 (the genes for which are transcriptionally coupled) are homologs of the small ‘cofactorless’ hydroxylases AclR (see Table 1) and SnoL (see Table 1), respectively. AclR and SnoL (also designated as SnoaL2) catalyze the C1 aromatic hydroxylation of cinerubin (Streptomyces galilaeus) and nogalamycin (Streptomyces nogalater) (Torkkell et al., 2001), respectively, and also share distant sequence and architectural similarity to members of polyketide cyclases (Beinker et al., 2006). Consistent with a lack of anthraquinone core PKS genes, a gene encoding a signature quinone-forming cofactor-less anthrone monoxygenase (as exemplified by TcmH) (Shen & Hutchinson, 1993; Fetzner, 2002) was not found within the sequenced region highlighted in Fig. 3.
From metabolic labeling, minimally a single oxygenation step (C5) is required during maturation of the enediyne core. The basis for this postulation derives from the identification of minor fermentation products dynemicins M, O, P and Q (15, 16, 17, and 18, respectively) (Fig. 1b), all of which bear a C5 carbonyl oxygen (Konishi et al., 1991; Miyoshisaitoh et al., 1991). Isotopic labeling also revealed the C5 carboxylate to derive from a separate acetate condensation with the cyclodiynene polyketide followed by decarboxylation/oxidation and a likely enzymatic route to this convergent condensation parallels the many recently elucidated HMG-CoA-like β-alkylation pathways (Simunovic et al., 2003; Chang et al., 2004; Edwards et al., 2004; Pulsawat et al., 2007; Wu et al., 2007). While a signature HMG-CoA gene cassette was not found within the existing sequenced region, various candidates were identified that may play a role in subsequent decarboxylation/oxidation. For example, the encoded DynA5 shares similarities to RdmB, a S-adenosyl-L-methionine (AdoMet)-dependent aclacinomycin 10-hydroxylase responsible for decarboxylation and subsequent oxidation en route to aclacinomycin biosynthesis (Jansson et al., 2005). Genes orf21-orf23, encode for a set of protein homologs to those known to comprise various prokaryotic molybdenum containing hydroxylases (Lehmann et al., 1994; Okamoto et al., 2004), the putative maturation factor for which is encoded by dynE12 (see Table 1) (Ivanov et al., 2003). Interestingly the trace production of a new product with similar UV signature to 8 was detected in mutant strain QGD23 (Δorf23), the structural elucidation of which was restricted by limited abundance (see Fig. 2, panel v). The encoded DynE11, DynE14 and DynE15 also all contain conserved domains found in glyoxalase/bleomycin resistance protein/dioxygenase superfamily (Moran, 2005) while DynA3, contains a short chain dehydrogenase domain common to members of the dehydrogenase/isomerase family but also displays weak homology with proteins involved in controlling nitrogen metabolism (NmrA) and azoreductases. Finally, DynE13, a homolog of FAD dependent monooxygenases such as salicylate hydroxylase (39% identity, 49% similarity) and zeaxanthin epoxidase (see Table 1), serves as a likely candidate for the C3/C8 epoxidase (Fig. 3c), while DynO6, a homolog of CalO6 (a phenolic O-methyltransferase involved in calicheamicin biosynthesis; see Table 1) (Ahlert et al., 2002), is the plausible dynemicin C6 O-methyltransferase.
While prior metabolic labeling (Tokiwa et al., 1992) or the current gene sequence annotation fails to shed any additional light upon the amino donor for the key anthraquinone–enediyne bridging nitrogen, we postulate two putative cytochrome P450s may be involved in forming the C8/C9 C–C bond, and possibly, the C2–N1–C22 bridge. Specifically, DynE10 and Orf19, show strong similarity to PdmJ (Table 1), NocL (DynE10- 43% identity, 57% similarity; Orf19- 49% identity, 62% similarity; AAT09797) and CalE10 (DynE10- 41% identity, 53% similarity; Orf19-37% identity, 50% similarity). While the role of PdmJ (also refered to as PrmJ) in pradimicin biosynthesis remains undetermined (Kim et al., 2007), NocL catalyzes a rare conversion of a primary amine to an oxime in nocardicin A biosynthesis (Kelly & Townsend, 2002). while CalE10 catalyzes a novel sugar amine oxidation in calicheamicin biosynthesis (H.D. Johnson and J.S. Thorson, unpublished data). Similar P450s are critical to C–C (e.g. RebP) (Onaka et al., 2002; Sanchez et al., 2002; Hyun et al., 2003; Howard-Jones & Walsh, 2006) and C–N (e.g. StaN) (Onaka et al., 2005) bond formation within indolocarbazole biosyntheses.
Conclusions
Cumulatively, this study highlights the first genetic system for M. chersina, direct confirmation of the participation of dynU14, dynU15, dynE8 and orf23 in 8 biosynthesis. Disruption of the putative tailoring oxidase gene orf23 or the nonstructural gene orf8 importantly illustrated the ability for M. chersina genetic manipulation while retaining production of 8 or corresponding shunt metabolites and thereby sets the stage for the application of genetics in delineating key biosynthetic steps. In addition, consistent with the postulation that 8 bicyclo[7.3.1.]diyne unit and the anthraquinone unit are biosynthesized separately and later assembled (Tokiwa et al., 1992), this preliminary study suggests that the genes encoding for these two distinct dynemicin precursors may also be physically separated in M. chersina genome.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants GM70637 and CA84374 and a National Cooperative Drug Discovery Group grant from the National Cancer Institute (U19 CA113297). J.S.T. is a H.I. Romnes fellow. The authors want to thank Drs Byron R. Griffith and Randy Goff for technical assistance. The authors also wish to acknowledge the Analytical Instrumentation Center of the School of Pharmacy, UW-Madison, for MS and NMR support and the John Innes Center, Norwich, United Kingdom, for providing the REDIRECT Technology Kit and Bristol-Myers-Squibb for graciously providing the 8 standard.
Footnotes
The following supplementary material is available for this article:
Table S1. Bacterial strains, plasmids and primers used in this study.
Fig. S1. Overview of the key cosmids and probes highlighted in this study.
Fig. S2. Inactivation of dynE8, dynU14, dynU15, orf8, and orf23.
Fig. S3. HPLC analysis of fermentation extracts from wild-type, ΔdynU14 (QGDU14), and ΔdynU15 (QGDU15) M. chersina prodigy.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1574-6968.2008.01112.x (This link will take you to the article abstract.)
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abe S, Otsuki M. Styrene maleic acid neocarzinostatin treatment for hepatocellular carcinoma. Curr Med Chem Anticancer Agents. 2002;2:715–726. doi: 10.2174/1568011023353679. [DOI] [PubMed] [Google Scholar]
- Ahlert J, Shepard E, Lomovskaya N, et al. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science. 2002;297:1173–1176. doi: 10.1126/science.1072105. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinker P, Lohkamp B, Peltonen T, Niemi J, Mantsala P, Schneider G. Crystal structures of SnoaL2 and AclR: two putative hydroxylases in the biosynthesis of aromatic polyketide antibiotics. J Mol Biol. 2006;359:728–740. doi: 10.1016/j.jmb.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Biggins JB, Onwueme KC, Thorson JS. Resistance to enediyne antitumor antibiotics by CalC self-sacrifice. Science. 2003;301:1537–1541. doi: 10.1126/science.1086695. [DOI] [PubMed] [Google Scholar]
- Buchanan GO, Williams PG, Feling RH, Kauffman CA, Jensen PR, Fenical W. Sporolides A and B: structurally unprecedented halogenated macrolides from the marine actinomycete Salinispora tropica. Org Lett. 2005;7:2731–2734. doi: 10.1021/ol050901i. [DOI] [PubMed] [Google Scholar]
- Chang Z, Sitachitta N, Rossi JV, Roberts MA, Flatt PM, Jia J, Sherman DH, Gerwick WH. Biosynthetic pathway and gene cluster analysis of curacin A, an antitubulin natural product from the tropical marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2004;67:1356–1367. doi: 10.1021/np0499261. [DOI] [PubMed] [Google Scholar]
- Damle NK. Tumour-targeted chemotherapy with immunoconjugates of calicheamicin. Expert Opin Biol Thera. 2004;4:1445–1452. doi: 10.1517/14712598.4.9.1445. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, Gerwick WH. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem Biol. 2004;11:817–833. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Fetzner S. Oxygenases without requirement for cofactors or metal ions. Appl Microbiol Biotechnol. 2002;60:243–257. doi: 10.1007/s00253-002-1123-4. [DOI] [PubMed] [Google Scholar]
- Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105:739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensens OD, Giner JL, Goldberg IH. Biosynthesis of NCS Chrom-A, the chromophore of the antitumor antibiotic neocarzinostatin. J Am Chem Soc. 1989;111:3295–3299. [Google Scholar]
- Hosted TJ, Jr, Wang T, Horan AC. Characterization of the Micromonospora rosaria pMR2 plasmid and development of a high G+C codon optimized integrase for site-specific integration. Plasmid. 2005;54:249–258. doi: 10.1016/j.plasmid.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Howard-Jones AR, Walsh CT. Staurosporine and rebeccamycin aglycones are assembled by the oxidative action of StaP, StaC, and RebC on chromopyrrolic acid. J Am Chem Soc. 2006;128:12289–12298. doi: 10.1021/ja063898m. [DOI] [PubMed] [Google Scholar]
- Hyun CG, Bililign T, Liao JC, Thorson JS. The biosynthesis of indolocarbazoles in a heterologous E. coli host. Chembiochem. 2003;4:114–117. doi: 10.1002/cbic.200390004. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Hotta K. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G+C content. FEMS Microbiol Lett. 1999;174:251–253. doi: 10.1111/j.1574-6968.1999.tb13576.x. [DOI] [PubMed] [Google Scholar]
- Ivanov NV, Hubalek F, Trani M, Edmondson DE. Factors involved in the assembly of a functional molybdopyranopterin center in recombinant Comamonas acidovorans xanthine dehydrogenase. Eur J Biochem. 2003;270:4744–4754. doi: 10.1046/j.1432-1033.2003.03875.x. [DOI] [PubMed] [Google Scholar]
- Jansson A, Koskiniemi H, Erola A, Wang J, Mantsala P, Schneider G, Niemi J. Aclacinomycin 10-hydroxylase is a novel substrate-assisted hydroxylase requiring S-adenosyl-L-methionine as cofactor. J Biol Chem. 2005;280:3636–3644. doi: 10.1074/jbc.M412095200. [DOI] [PubMed] [Google Scholar]
- Jedema I, Barge RMY, van der Velden VHJ, Nijmeijer BA, van Dongen JJM, Willemze R, Falkenburg JHF. Internalization and cell cycle-dependent killing of leukemic cells by gemtuzumab ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004;18:316–325. doi: 10.1038/sj.leu.2403205. [DOI] [PubMed] [Google Scholar]
- Kelly WL, Townsend CA. Role of the cytochrome P450 NocL in nocardicin A biosynthesis. J Am Chem Soc. 2002;124:8186–8187. doi: 10.1021/ja025926g. [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner M, Chater K, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation; Norwich: 2000. [Google Scholar]
- Kim BC, Lee JM, Ahn JS, Kim BS. Cloning, sequencing, and characterization of the pradimicin biosynthetic gene cluster of Actinomadura hibisca p157-2. J Microbiol Biotechnol. 2007;17:830–839. [PubMed] [Google Scholar]
- Konishi M, Ohkuma H, Matsumoto K, Saitoh K, Miyaki T, Oki T, Kawaguchi H. Dynemicins, new antibiotics with the 1,5-diyn-3-ene and anthraquinone subunit. 1. Production, isolation and physicochemical properties. J Antibiot. 1991;44:1300–1305. doi: 10.7164/antibiotics.44.1300. [DOI] [PubMed] [Google Scholar]
- Lam KS, Veitch JA, Golik J, Krishnan B, Klohr SE, Volk KJ, Forenza S, Doyle TW. Biosynthesis of esperamicin-A1 an enediyne antitumor antibiotic. J Am Chem Soc. 1993;115:12340–12345. [Google Scholar]
- Lam KS, Veitch JA, Lowe SE, Forenza S. Effect of neutral resins on the production of dynemicins by Micromonospora chersina. J Ind Microbiol. 1995;15:453–456. [Google Scholar]
- Lehmann M, Tshisuaka B, Fetzner S, Roger P, Lingens F. Purification and Characterization of isoquinoline 1-oxidoreductase from Pseudomonas diminuta, a novel molybdenum-containing hydroxylase. J Biol Chem. 1994;269:11254–11260. [PubMed] [Google Scholar]
- Li X, Zhou X, Deng Z. Isolation and characterization of Micromonospora phage PhiHAU8 and development into a phasmid. Appl Environ Microbiol. 2004;70:3893–3897. doi: 10.1128/AEM.70.7.3893-3897.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Christenson SD, Standage S, Shen B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science. 2002;297:1170–1173. doi: 10.1126/science.1072110. [DOI] [PubMed] [Google Scholar]
- Liu W, Ahlert J, Gao Q, Wendt-Pienkowski E, Shen B, Thorson JS. Rapid PCR amplification of minimal enediyne polyketide synthase cassettes leads to a predictive familial classification model. Proc Natl Acad Sci USA. 2003;100:11959–11963. doi: 10.1073/pnas.2034291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Nonaka K, Nie L, et al. The neocarzinostatin biosynthetic gene cluster from Streptomyces carzinostaticus ATCC 15944 involving two iterative type I polyketide synthases. Chem Biol. 2005;12:293–302. doi: 10.1016/j.chembiol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Derbyshire MK, et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–D240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshisaitoh M, Morisaki N, Tokiwa Y, Iwasaki S, Konishi M, Saitoh K, Oki T. Dynemicin-O, dynemicin-P and dynemicin-Q – novel antibiotics related to dynemicin-A – isolation, characterization and biological-activity. J Antibiot. 1991;44:1037–1044. doi: 10.7164/antibiotics.44.1037. [DOI] [PubMed] [Google Scholar]
- Moran GR. 4-Hydroxyphenylpyruvate dioxygenase. Arch Biochem Biophys. 2005;433:117–128. doi: 10.1016/j.abb.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Oh DC, Williams PG, Kauffman CA, Jensen PR, Fenical W. Cyanosporasides A and B, chloro- and cyanocyclopenta[a]indene glycosides from the marine actinomycete “Salinispora pacifica”. Org Lett. 2006;8:1021–1024. doi: 10.1021/ol052686b. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Matsumoto K, Hille R, Eger BT, Pai EF, Nishino T. The crystal structure of xanthine oxidoreductase during catalysis: implications for reaction mechanism and enzyme inhibition. Proc Natl Acad Sci USA. 2004;101:7931–7936. doi: 10.1073/pnas.0400973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka H, Taniguchi S, Igarashi Y, Furumai T. Cloning of the staurosporine biosynthetic gene cluster from Streptomyces sp. TP-A0274 and its heterologous expression in Streptomyces lividans. J Antibiot. 2002;55:1063–1071. doi: 10.7164/antibiotics.55.1063. [DOI] [PubMed] [Google Scholar]
- Onaka H, Asamizu S, Igarashi Y, Yoshida R, Furumai T. Cytochrome P450 homolog is responsible for C–N bond formation between aglycone and deoxysugar in the staurosporine biosynthesis of Streptomyces sp. TP-A0274. Biosci Biotechnol Biochem. 2005;69:1753–1759. doi: 10.1271/bbb.69.1753. [DOI] [PubMed] [Google Scholar]
- Ostash B, Saghatelian A, Walker S. A streamlined metabolic pathway for the biosynthesis of moenomycin A. Chem Biol. 2007;14:257–267. doi: 10.1016/j.chembiol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsawat N, Kitani S, Nihira T. Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae. Gene. 2007;393:31–42. doi: 10.1016/j.gene.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Butovich IA, Brana AF, Rohr J, Mendez C, Salas JA. The biosynthetic gene cluster for the antitumor rebeccamycin: characterization and generation of indolocarbazole derivatives. Chem Biol. 2002;9:519–531. doi: 10.1016/s1074-5521(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Shen B. Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr Opin Chem Biol. 2003;7:285–295. doi: 10.1016/s1367-5931(03)00020-6. [DOI] [PubMed] [Google Scholar]
- Shen B, Hutchinson CR. Enzymatic synthesis of a bacterial polyketide from acetyl and malonyl coenzyme A. Science. 1993;262:1535–1540. doi: 10.1126/science.8248801. [DOI] [PubMed] [Google Scholar]
- Shiomi K, Iinuma H, Naganawa H, Hamada M, Hattori S, Nakamura H, Takeuchi T, Iitaka Y. New Antibiotic Produced by Micromonospora globosa. J Antibiot. 1990;43:1000–1005. doi: 10.7164/antibiotics.43.1000. [DOI] [PubMed] [Google Scholar]
- Simunovic V, Gherardini FC, Shimkets LJ. Membrane localization of motility, signaling, and polyketide synthetase proteins in Myxococcus xanthus. J Bacteriol. 2003;185:5066–5075. doi: 10.1128/JB.185.17.5066-5075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Hager MH, Zhang CS, Griffith BR, Lee MS, Hallenga K, Markley JL, Thorson JS. Structural insight into the self-sacrifice mechanism of enediyne resistance. ACS Chem Biol. 2006;1:451–460. doi: 10.1021/cb6002898. [DOI] [PubMed] [Google Scholar]
- Takada Y, Inouye M, Morohoshi T, Muto N, Kato F, Kizuka M, Tanaka M, Koyama Y. Establishment of the host-vector system for Micromonospora griseorubida. J Antibiot (Tokyo) 1994;47:1167–1170. doi: 10.7164/antibiotics.47.1167. [DOI] [PubMed] [Google Scholar]
- Thorson JS, Sievers EL, Ahlert J, Shepard E, Whitwam RE, Onwueme KC, Ruppen M. Understanding and exploiting nature’s chemical arsenal: the past, present and future of calicheamicin research. Curr Pharm Des. 2000;6:1841–1879. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- Tokiwa Y, Miyoshisaitoh M, Kobayashi H, Sunaga R, Konishi M, Oki T, Iwasaki S. Biosynthesis of dynemicin-a, a 3-ene-1,5-diyne antitumor antibiotic. J Am Chem Soc. 1992;114:4107–4110. [Google Scholar]
- Torkkell S, Kunnari T, Palmu K, Mantsala P, Hakala J, Ylihonko K. The entire nogalamycin biosynthetic gene cluster of Streptomyces nogalater: characterization of a 20-kb DNA region and generation of hybrid structures. Mol Genet and Genomics. 2001;266:276–288. doi: 10.1007/s004380100554. [DOI] [PubMed] [Google Scholar]
- Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- Wu J, Cooper SM, Cox RJ, Crosby J, Crump MP, Hothersall J, Simpson TJ, Thomas CM, Willis CL. Mupirocin H, a novel metabolite resulting from mutation of the HMG-CoA synthase analogue, mupH in Pseudomonas fluorescens. Chem Commun. 2007:2040–2042. doi: 10.1039/b700613f. [DOI] [PubMed] [Google Scholar]
- Yu TW, Bai L, Clade D, Hoffmann D, Toelzer S, Trinh KQ, Xu J, Moss SJ, Leistner E, Floss HG. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum. Proc Natl Acad Sci USA. 2002;99:7968–7973. doi: 10.1073/pnas.092697199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazopoulos E, Huang K, Staffa A, Liu W, Bachmann BO, Nonaka K, Ahlert J, Thorson JS, Shen B, Farnet CM. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat Biotechnol. 2003;21:187–190. doi: 10.1038/nbt784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.