SUMMARY
We report analytic and consensus processes that produced recommendations for pathologic stage groups (pTNM) of esophageal and esophagogastric junction cancer for the AJCC/UICC cancer staging manuals, 8th edition. The Worldwide Esophageal Cancer Collaboration provided data for 22,654 patients with epithelial esophageal cancers; 13,300 without preoperative therapy had pathologic assessment after esophagectomy or endoscopic treatment. Risk-adjusted survival for each patient was developed using random survival forest analysis to identify data-driven pathologic stage groups wherein survival decreased monotonically with increasing group, was distinctive between groups, and homogeneous within groups. The AJCC Upper GI Task Force, by smoothing, simplifying, expanding, and assessing clinical applicability, produced consensus pathologic stage groups. For pT1-3N0M0 squamous cell carcinoma (SCC) and pT1-2N0M0 adenocarcinoma, pT was inadequate for grouping; subcategorizing pT1 and adding histologic grade enhanced staging; cancer location improved SCC staging. Consensus eliminated location for pT2N0M0 and pT3N0M0G1 SCC groups, and despite similar survival, restricted stage 0 to pTis, excluding pT1aN0M0G1. Metastases markedly reduced survival; pT, pN, and pM sufficiently grouped advanced cancers. Stage IIA and IIB had different compositions for SCC and adenocarcinoma, but similar survival. Consensus stage IV subgrouping acknowledged pT4N+ and pN3 cancers had poor survival, similar to pM1. Anatomic pathologic stage grouping, based on pTNM only, produced identical consensus stage groups for SCC and adenocarcinoma at the cost of homogeneity in early groups. Pathologic staging can neither direct pre-treatment decisions nor aid in prognostication for treatment other than esophagectomy or endoscopic therapy. However, it provides a clean, single therapy reference point for esophageal cancer.
Keywords: epidemiology, esophagectomy, esophagogastric junction, oncology
INTRODUCTION
TNM is a means of recording anatomic facts about a cancer; stage grouping assembles these facts into broad classes with similar prognosis.1 Data necessary for staging esophageal and esophagogastric junction cancer in the AJCC and UICC cancer staging manuals, 8th edition, is based on the 6-continent Worldwide Esophageal Cancer Collaboration (WECC).2,3 Cancer facts, patient characteristics, and survival data served as substrate for a machine-learning analysis producing data-driven pathologic stage groups. The AJCC Upper GI Task Force reviewed these facts and prognosis groupings and, by smoothing, simplifying, expanding, and assessing clinical applicability, produced consensus pathologic stage groups. An additional machine-learning analysis and consensus produced anatomic pathologic stage groups based only on traditional pT, pN, and pM categories.
This manuscript reports these recommendations for pathologic staging of esophageal and esophagogastric junction cancer.
PATIENTS AND METHODS
Patients
At 33 WECC institutions (Supporting Information Appendix S1), 22,654 patients were treated for epithelial esophageal cancers.2 Of these, 22,123 had treatment, survival, and pT data available. Among the latter, 13,300 patients had pathologic staging after esophagectomy, endoscopic mucosal resection, or ablation without preoperative therapy. Patient, cancer, and survival data have been published.3
Endpoint
The endpoint was all-cause mortality from first management decision. Median potential follow-up was 8.9 years4; however, median actual follow-up for surviving patients was 2.5 years, with 25% followed more than 5.1 years and 10% more than 8.4 years.2
Data
Data were collected using a common format with standardized definitions (Supporting Information Table S1). The Case Cancer Institutional Review Board of Case Western Reserve University and Cleveland Clinic Institutional Review Board approved the entire project.
Data analysis
Analytic strategy
Risk-adjusted survival for each patient was developed. Data-driven groups were formulated such that (1) survival decreased monotonically with increasing group number, and (2) survival between groups was distinctive, with no more than a 5% difference. pTNM categories comprising each group were then exposed. Homogeneity of survival within groups was determined. Subgroup analyses were performed as necessary.
Risk-adjusted survival
Random survival forest (RSF) methodology was used to estimate nonparametric risk-adjusted and cross-validated survival for each patient.5,6 Thirty-nine dichotomous, polytomous, ordinal, and continuous variables were used (Supporting Information Table S2) in separate analyses for squamous cell carcinoma (SCC) and adenocarcinoma.
All computations used open-source randomForestSRC R-software under default settings.7 Missing data were pre-imputed without using outcome information by random forest (RF) imputation methodology, missForest.8,9 Thereafter, 1,000 survival trees were grown using log-rank splitting. Each tree was constructed using an independent bootstrap sample of size 22,123; on average, each bootstrap sample contained 63% of the patients (in-sample bootstrapped data). The remaining unused patients (37%) were referred to as out-of-bag (OOB) observations. Each tree and its corresponding OOB observations were used to calculate a patient-specific risk-adjusted OOB (cross-validated) survival function for stage grouping and patient-specific mortality value (integrated cumulative hazard for each patient) for analysis of homogeneity.5
Data-driven staging
From the individual survival estimates, 27 subsets of SCC patients and 19 of adenocarcinoma were produced (Supporting Information Table S3 and Figs. S1,S2). Stage groups were then formed by iteratively merging these subsets by closeness, defined as the root-mean-squared-error difference between OOB survival functions from 3–10 years, until no more than a 5% difference between adjacent groups, above and below, was observed.
Consensus pathologic staging
The AJCC Upper GI Task Force reviewed cancer categories and data-driven stage groupings. By consensus request, because pT1a/b subcategories were not available on all pT1 patients, separate RSF analysis of pT1a-1bN0M0 subcategories was performed. Consensus streamlined data-driven groups and subcategories by merging and splitting while maintaining clinical relevance and minimizing drift from data-driven groups. Finally, the AJCC Upper GI Task Force requested data-driven placement of GX and LX cancers in the consensus pathologic stage groups.
Anatomic pathologic staging
To meet UICC requirements for pure TNM groups, a regrouping of survival curves was performed to produce data-driven anatomic pathologic stage groups. Grade and location were used only in risk adjustment. The consensus process produced consensus anatomic pathologic stage groups.
Homogeneity
Within each data-driven, consensus, and anatomic stage group, homogeneity of survival was assessed as follows. OOB survival was analyzed with respect to the 39 independent variables using RF regression. Regression forests of 1,000 random regression trees were grown using mean-squared-error splitting. From each RF regression, variable importance (VIMP) of each variable was calculated by measuring the increase in OOB mean-squared error when the variable was removed from the analysis,6 and standardized by dividing VIMP by the variance of OOB patient mortality multiplied by 100. Standardized VIMP measured the relative importance of each independent variable in predicting OOB mortality within a stage group. If a group is homogeneous, standardized VIMP for all cancer facts should be near zero. Values under 5% were deemed non-significant.10
RESULTS
Data-driven pathologic stage groups
Eight data-driven stage groups, presented simply as cardinal numbers, were identified for SCC (Table 1) and 9 for adenocarcinoma (Table 2). Risk-adjusted survival for SCC (Fig. 1A) and adenocarcinoma (Fig. 1B) monotonically decreased, was distinctive, and was generally homogeneous. Homogeneity was excellent except for groups 7 and 8 for SCC and 9 for adenocarcinoma (Supporting Information Figs. S3A,S4A). However, refinement in these groups was not possible nor clinically meaningful because survival was so poor.
Table 1.
Data-driven and consensus pathologic stage grouping for squamous cell carcinoma
| Data-driven group | pT | pN | pM | pGrade | pLocation | Consensus stage group |
|---|---|---|---|---|---|---|
| 1 | Tis | N0 | M0 | N/A | Any | 0 |
| T1a | N0 | M0 | G1 | Any | IA† | |
| T1b | N0 | M0 | G1 | IB† | ||
| 2 | T1 | N0 | M0 | G2-3 | Any | |
| T2 | N0 | M0 | G1 | Any | ||
| T3 | N0 | M0 | G1 | Lower | IIA | |
| 3 | T2 | N0 | M0 | G2-3 | Lower | |
| 4 | T2 | N0 | M0 | G2-3 | Upper/middle | |
| T3 | N0 | M0 | G2-3 | Lower | ||
| T3 | N0 | M0 | G1 | Upper/middle | ||
| 5 | T3 | N0 | M0 | G2-3 | Upper/middle | IIB |
| T1 | N1 | M0 | Any | Any | ||
| 6 | T1 | N2 | M0 | Any | Any | IIIA |
| T2 | N1 | M0 | Any | Any | ||
| 7 | T2 | N2 | M0 | Any | Any | IIIB |
| T3 | N1-2 | M0 | Any | Any | ||
| T4a | N0-1 | M0 | Any | Any | ||
| 8 | T4a | N2 | M0 | Any | Any | IVA |
| X‡ | T4b | N0-2 | M0 | Any | Any | |
| Any | N3 | M0 | Any | Any | ||
| Any | Any | M1 | Any | Any | IVB |
From subset analysis.
Not in data-driven analysis.
Table 2.
Data-driven and consensus pathologic stage grouping for adenocarcinoma
| Data-driven group | pT | pN | pM | pG | Consensus stage group |
|---|---|---|---|---|---|
| 1 | Tis | N0 | M0 | N/A | 0 |
| T1a | N0 | M0 | G1 | IA† | |
| T1a | N0 | M0 | G2 | IB† | |
| T1b | N0 | M0 | G1-2 | ||
| 2 | T1 | N0 | M0 | G3 | IC |
| 3 | T2 | N0 | M0 | G1-2 | |
| 4 | T2 | N0 | M0 | G3 | IIA |
| 5 | T1 | N1 | M0 | Any | IIB |
| T3 | N0 | M0 | Any | ||
| 6 | T1 | N2 | M0 | Any | IIIA |
| T2 | N1 | M0 | Any | ||
| T4a | N0 | M0 | Any | IIIB | |
| 7 | T2 | N2 | M0 | Any | |
| 8 | T3 | N1-2 | M0 | Any | |
| T4a | N1 | M0 | Any | ||
| T4a | N2 | M0 | Any | IVA | |
| 9 | T4b | N0-2 | M0 | Any | |
| X‡ | Any | N3 | M0 | Any | |
| Any | Any | M1 | Any | IVB |
From subset analysis.
Not in data-driven analysis.
Fig. 1.
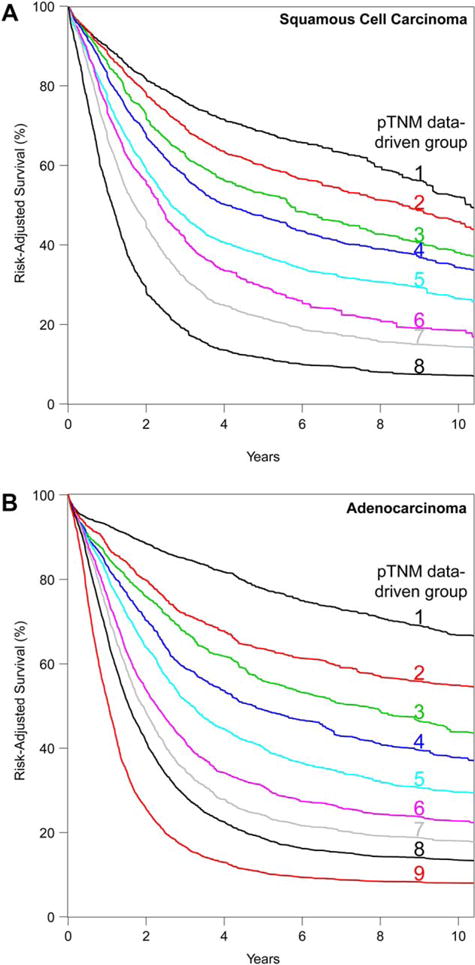
Risk-adjusted survival of data-driven pathologic stage groups. (A) Squamous cell carcinoma. (B) Adenocarcinoma.
Survival for pT1 adenocarcinoma was much better than for pT1 SCC. pT alone was insufficient for grouping pT1-2N0M0 adenocarcinoma and pT1-3N0M0 SCC; addition of histologic grade improved these groupings (see Tables 1 and 2). However, addition of location was necessary for grouping pT2-3N0M0 SCC. The uncommon pT1N1M0 cancer was grouped with pT3N0M0G2-3 upper/middle thoracic esophagus for SCC and pT3N0M0 for adenocarcinoma.
The adverse effect of regional lymph node and distant metastases on survival was remarkable (see Fig. 1); thus, advanced SCC and adenocarcinoma (pT4N0M0, pTanyN + M0, and pM1) shared stage groupings (see Tables 1 and 2).
Data for pT4b and pM1 were insufficient and required consensus grouping.
Consensus pathologic stage groups
By consensus, G4 was combined with G3, with further pathologic analysis required to determine histopathologic cell type. The anatomic boundary between esophagus and stomach was redefined such that cancers with epicenter no more than 2 cm into the proximal stomach are staged as esophageal cancers.
Squamous cell carcinoma
Consensus produced seven changes from the data-driven stage groups, which increased the number of stage subgroups from 8 to 9 and the number of entries from 19 to 20 (Table 1).
By consensus request, pT1 was subcategorized as pT1a and pT1b (Supporting Information Fig. S5A), producing three subsets: pTis, pT1aN0M0G1, and pT1aN0M0G2-3 plus pT1bN0M0G1-3.
Despite similar survival to pT1N0M0G1 cancer, pTis was placed alone into consensus stage 0 (Fig. 2A). The remaining data-driven group 1 was divided by pT1 subcategories and grade into consensus stage groups IA (pT1aN0M0G1) and IB (pT1bN0M0G1). Consensus stage group IB was completed by placing data-driven group 2 members pT1N0M0G2-3 and pT2N0M0G1 into consensus group IB. Data-driven stage groups 3 and 4 were consolidated into consensus stage group IIA along with pT3N0M0G1 lower thoracic esophageal cancer from data-driven stage group 2. These two changes removed location as a category for pT2N0M0 and pT3N0M0G1 cancers.
Fig. 2.
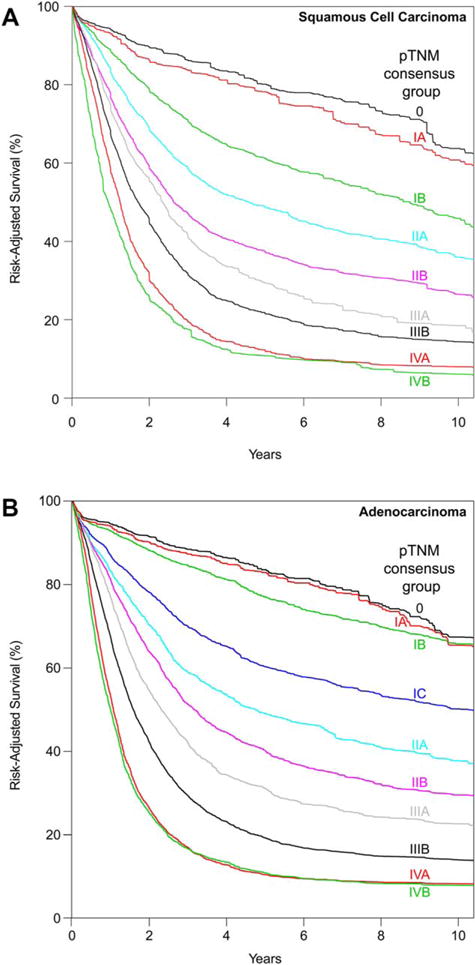
Risk-adjusted survival of consensus pathologic stage groups. (A) Squamous cell carcinoma. (B) Adenocarcinoma.
Data-driven stage groups 5-7 were identical to consensus stage groups IIB, IIIA, and IIIB. The consensus process divided data-driven group 8 into two subgroups: IVA and IVB. pT4bN1-2N0M0 cancer was placed into consensus stage group IVB.
For any location, if grade is unknown, pT1aN0M0GX is pStage IA, pT1bN0M0GX is pStage IB, pT2N0M0GX is pStage IIA, and pT3N0M0GX is pStage IIB. For any grade, if location is unknown, pT3N0M0LX is pStage IIB.
The consensus process caused deterioration in homogeneity in stages 0, IIA, IIIB, IVA, and IVB (Supporting Information Fig. S3B).
Adenocarcinoma
Consensus produced nine changes from the data-driven stage groups, which increased the number of stage subgroups for adenocarcinoma from 9to10 and entries from 15to 18 (Table 2).
By consensus request, pT1 was subcategorized as pT1a and pT1b (Supporting Information Fig. S5B), producing survival curves clearly clustered into three subsets: pTis and pT1aN0M0G1, pT1aN0M0G2 and pT1bN0M0G1-2, and pT1a/T1bN0M0G3. Survival of patients with pT1 adenocarcinoma was much better than for SCC.
Despite similar survival to pT1N0M0G1 cancer, pTis was placed alone into consensus stage group 0 (Fig. 2B). The remaining data-driven group 1 was divided by T1 subcategories into consensus stage groups IA (pT1aN0M0G1) and IB (pT1aN0M0G2 and pT1bN0M0G1-2). Data-driven groups 2 and 3 were amalgamated into consensus group IC, and groups 4 and 5 became consensus groups IIA and IIB, respectively. Data-driven group 6, minus pT4aN0M0, became consensus group IIIA. Data-driven groups 7 and 8 plus pT4aN0M0 from data-driven group 6 were amalgamated into consensus group IIIB after removing pT4aN2M0.
Data-driven group 9 was divided into two subgroups: IVA and IVB. pT4aN2M0 and pT4bN1-2M0 were placed into consensus stage group IVA.
If grade is unknown, pT1aN0M0GX is pStage IA, pT1bN0M0GX is pStage IB, and pT2N0M0GX is pStage IIA.
The consensus process had little effect on the homogeneity of stage groupings compared with the data-driven groupings (Supporting Information Fig. S4B).
Anatomic pathologic stage groups
Despite slightly different data-driven stage groupings for SCC and adenocarcinoma, anatomic pathologic stage groupings were identical for both histopathologic cell types (Table 3).
Table 3.
Data-driven and consensus anatomic pathologic stage grouping for squamous cell carcinoma
| Data-driven group | pT | pN | pM | Consensus stage group |
|---|---|---|---|---|
| 1 | Tis | N0 | M0 | 0 |
| 2 | T1a | N0 | M0 | IA† |
| T1b | N0 | N0 | IB† | |
| 3 | T2 | N0 | M0 | IIA |
| 4 | T3 | N0 | M0 | IIB |
| T1 | N1 | M0 | ||
| 5 | T1 | N2 | M0 | IIIA |
| T2 | N1 | M0 | ||
| 6 | T2 | N2 | M0 | IIIB |
| T3 | N1-2 | M0 | ||
| T4a | N0-1 | M0 | ||
| 7 | T4a | N2 | M0 | IVA |
| X‡ | T4b | N0-2 | M0 | |
| Any | N3 | M0 | ||
| Any | Any | M1 | IVB |
From subset analysis.
Not in data-driven analysis.
Squamous cell carcinoma
Data-driven anatomic pathologic staging produced seven groups (Table 3). Consensus increased the number of stage subgroups to 9, with the number of entries increasing from 13 to 15.
Survival of pTis was much better than all pT1N0M0 cancers (Fig. 3A). Thus, data-driven group 1 became anatomic pathologic stage group 0. Data-driven group 2 was divided by T1 subcategories into consensus anatomic stage groups IA (pT1aN0M0) and IB (pT1bN0M0). Stage IIA solely comprised pT2N0M0 patients. Stage IIB was pT3N0M0 and pT1N1M0 cancers. Stage grouping of advanced cancers (III and IV) was identical to consensus pathologic stage grouping recommendations.
Fig. 3.
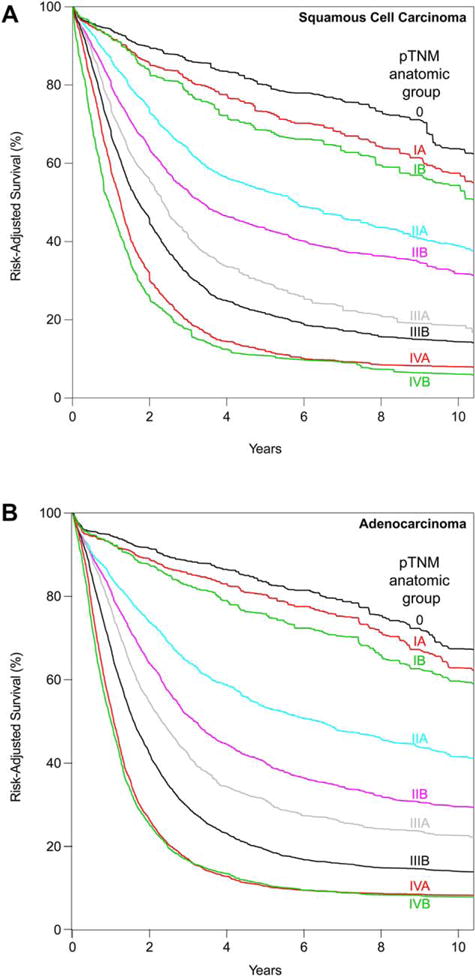
Risk-adjusted survival of anatomic pathologic stage groups. (A) Squamous cell carcinoma. (B) Adenocarcinoma.
Homogeneity was disrupted within anatomic pathologic groups 0, IIA, and IIB when G and location were removed from staging (Supporting Information Fig. S3C).
Adenocarcinoma
Data-driven anatomic pathologic staging produced eight groups (Table 4). Consensus increased the number of stage subgroups to 9, with the number of entries increasing from 14 to 16.
Table 4.
Data-driven and consensus anatomic pathologic stage grouping for adenocarcinoma
| Data-driven group | pT | pN | pM | Consensus stage group |
|---|---|---|---|---|
| 1 | Tis | N0 | M0 | 0 |
| 2 | T1a | N0 | M0 | IA† |
| T1b | N0 | M0 | IB† | |
| 3 | T2 | N0 | M0 | IIA |
| 4 | T3 | N0 | M0 | IIB |
| T1 | NI | M0 | ||
| 5 | T1 | N2 | N0 | IIIA |
| T2 | N1 | M0 | ||
| T4a | N0 | M0 | IIIB | |
| 6 | T2 | N2 | M0 | |
| 7 | T3 | N1-2 | M0 | |
| T4a | N1 | M0 | ||
| T4a | N2 | M0 | IVA | |
| 8 | T4b | N0-2 | M0 | |
| X‡ | Any | N3 | M0 | |
| Any | Any | M1 | IVB |
From subset analysis.
Not in data-driven analysis.
Changes were similar for SCC except that pT4aN0M0 in data-driven group 5 was amalgamated with data-driven groups 6 and 7, producing anatomic stage group IIIB after removal of T4aN2M0 (Table 2 and Fig. 3B). Stage grouping of advanced cancers (III and IV) was identical to consensus pathologic stage grouping.
For adenocarcinoma, homogeneity was disrupted within anatomic pathologic group I when G was removed from staging (Supporting Information Fig. S4C).
DISCUSSION
Principal findings
TNM categories are unchanged except for addition of peritoneal invasion to T4a. However, non-anatomic category G4 was eliminated and anatomic boundary of cancers in the esophagogastric junction was narrowed. Survival of stage I adenocarcinoma was better than SCC. For pT1-3N0M0 SCC and pT1-2N0M0 adenocarcinoma, T alone was inadequate. Stage grouping for N0M0 cancers was enhanced by subdividing pT1 into pT1a and pT1b subcategories and by adding histologic grade. For pT2-3N0M0 SCC, location was also important. Consensus removed location as a category for pT2N0M0 and pT3N0M0G1 SCCs. For both SCC and adenocarcinoma, stage IIA and IIB cancers had somewhat different category compositions, but they were associated with similar survival. Metastases markedly reduced survival, so that T, N, and M were sufficient for grouping advanced cancers. Advanced cancers had similar survival regardless of histopathologic cell type, histologic grade, or cancer location.
Consensus maintained the definition of stage 0, ignoring that pTis survival was similar to early invasive carcinoma. In contrast, broadening the definition of stage IV acknowledged that pT4N+ and pN3 cancers had dismal survival equivalent to distant metastases. However, the subgroups of stage IVA and IVB were consensus driven.
Anatomic pathologic staging based on pTNM alone rendered stage groups of SCC and adenocarcinoma identical, but with loss of homogeneity for earlier stage cancers.
7th versus 8th edition
Generally, the 7th edition11,12 was an improvement over the 6th, as single institution reviews attest13–17; however, shortcomings were also noted.18–21 Aware of deficiencies in the 7th edition, four goals for pathologic staging were realized22: improving homogeneity of stage I and IV by broadening definitions, improving homogeneity of stage IIA and IIB SCC and stage IIB adenocarcinoma by amassing more data, assessing other non-anatomic cancer characteristics, and adding treatment other than esophagectomy. Changes from edition 7 are summarized in Box 1.
Squamous cell carcinoma
AJCC consensus would not relax the definition of stage 0. Subcategorizing T1 allowed Stage IA to be more distinctive in the 8th edition and restricted to pT1aN0M0G1. This change relegates all other T1N0M0 to stage IB. pT2N0M0 and pT3N0M0 cancers, grouped identically in the 7th edition, are more homogeneous in the 8th edition. In the 7th edition, pT2-3N0M0 cancers occupied three subgroups: IB, IIA, and IIB. In the 8th edition, location is removed as a category for pT2N0M0 cancers, and they are either stage IB (pT2N0M0G1) or stage IIA (pT2N0M0G2-3). pT3N0M0 cancers are confined to stage IIA and IIB in the 8th edition. All but pT3N0M0G2-3 upper/middle (stage IIB) thoracic esophageal cancer are now placed into stage IIA. 8th edition stage IIB is restricted to pT3N0M0G2-3 upper/middle thoracic esophageal cancers and pT1N1M0 cancers. pT2N1M0 cancers are moved from stage IIB in the 7th edition to join pT1N2M0 and form stage IIIA in the 8th edition, again improving homogeneity. 7th edition stage IIIA members pT3N1M0 and pT4aN0M0, stage IIIB pT3N2M0 cancers, and stage IIIC pT4aN1M0 cancers form stage IIIB in the 8th edition. All other stage IIIC cancers of the 7th edition become stage IVA in the 8th edition.
Adenocarcinoma
AJCC consensus would not relax the definition of stage 0. Subcategorizing T1 required an increase from 2 stage I subgroups in the 7th edition to 3 in the 8th edition, improving distinctiveness. 7th edition stage IA pT1N0M0G1-2 cancers are now stage IA (pT1aN0M0G1) and stage IIB (pT1aN0M0G2 and pT1bN0M0G1-2) cancers in the 8th edition. 7th edition stage IB becomes 8th edition IC. Stage IIA is unchanged. All 8th edition changes from IIB on are identical to those reported for SCC.
Strengths and limitations
Many believe that cancer-specific survival represents the ultimate disease-specific survival. However, this soft endpoint is plagued by our ability to detect persistence of cancer after treatment. Once patient demographics, comorbidities, and treatments are accounted for (risk-adjusted survival), and in this analysis, continent and institution as well, the residual information contained in all-cause mortality becomes a better end-point23–25 (Supporting Information Fig. S6).
Although the strength of WECC is worldwide representation, this also imposes limitations similar to those of large national and international registries. In WECC, these related to institutional, country, and continent heterogeneity of etiology, diagnosis, treatment, and pathologic characterization of esophageal cancer. All data were provided by individual institutions with no independent audit against medical record documentation. Similarly, there was no core pathology laboratory to review pathologic specimens for histopathology of the cancer, histologic grade, lymphovascular invasion, lymph node count, cancer genetics, or other special studies. There was, however, intense cross-tabulation of the data at the data center for interval and cross-institutional consistency. Data elements with clear inconsistencies were reported to the institution and, when possible, the data were corrected. Symptomatic differences were also identified; treatment of adenocarcinoma, for example, appeared to be more consistent across continents and sites than SCC (Supporting Information Fig. S6).
Imputation was necessary for missing data in some risk-adjustment variables, and some patients who did not have complete pTNM data or survival information were excluded. Although we attempted to incorporate less invasive treatments in the analysis, the number of patients receiving such treatment was small.
Clinical implications
Consensus and anatomic pathologic staging erased some important cancer variables. Despite the rigid definition of stage 0, pTis behaves like a pT1N0M0G1 cancer. Although location was used only to stage pT3N0M0G2-3 SCCs, it is an essential prognosticator for all pT2-3N0M0 SCCs. Stage IIB is an interesting group wherein ‘T meets N’; survival of the uncommon superficial pT1 cancers with one or two regional nodal metastases is similar to the more common, deeply invasive pT3 cancers free of regional nodal metastases. Stage III further defines the T and N axis. Confirmation that survival of patients with most locally advanced cancers was similar to that of patients with distant metastases allowed relaxation of the formerly rigid definition of Stage IV.
CONCLUSIONS
Pathologic staging can neither direct pre-treatment decisions after the fact nor aid in prognostication for treatment other than esophagectomy or endoscopic treatment, and it must be remembered that what is important for stage grouping of populations is not necessarily helpful for the individual patient. This separation of decision-making and prognostication is supported by WECC data2 and will be addressed in future papers. However, 8th edition pathologic staging provides an important, clean, single therapy reference point for esophageal cancer.
Supplementary Material
Acknowledgments
Funding: Funded in part by (1) the International Society for Diseases of the Esophagus (ISDE), (2) the Daniel and Karen Lee Chair in Thoracic Surgery at Cleveland Clinic (TWR), (3) the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research at Cleveland Clinic (EHB), and (4) the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Services component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the ISDE, Cleveland Clinic, or NIH.
Footnotes
Author contributions: Conception or design of the experiment(s), or collection and analysis or interpretation of data: all authors. Drafting the manuscript or revising its intellectual content: Rice, Ishwaran, and Blackstone. Approval of the final version of the submitted manuscript: all authors.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- 1.International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. Geneva: 1968. [Google Scholar]
- 2.Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide Esophageal Cancer Collaboration: clinical staging data. Dis Esophagus. 2016;7:707–14. doi: 10.1111/dote.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice TW, Chen L-Q, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration: pathologic staging data. Dis Esophagus. 2016;7:724–33. doi: 10.1111/dote.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman AI. Eventcharts: visualizing survival and other timed-event data. Am Stat. 1992;46:13–8. [Google Scholar]
- 5.Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat. 2008;2:841–60. [Google Scholar]
- 6.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 7.Ishwaran H, Kogalur UB. RandomForestSRC: random forests for survival, regression and classification (RF-SRC) R package version 2.1.0. 2016 http://cran.r-project.org.
- 8.Stekhoven DJ, Buhlmann P. MissForest-non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–8. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 9.Tang F, Ishwaran H. Random forest missing data algorithms. Stat Methods Med Res. 2016 doi: 10.1002/sam.11348. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the 7th edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer Staging Manual. 7th. New York: Springer-Verlag; 2010. [Google Scholar]
- 12.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours International Union Against Cancer. 7th. Oxford, England: Wiley-Blackwell; 2009. [Google Scholar]
- 13.Hsu P, Wu Y, Chou T, Huang C, Hsu W. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg. 2010;89:1024–31. doi: 10.1016/j.athoracsur.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th editions of the UICC-AJCC TNM classification for esophageal cancer. Ann Surg Oncol. 2012;19:2142–8. doi: 10.1245/s10434-012-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasaki M, Miyata H, Miyazaki Y, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol. 2014;21:2850–6. doi: 10.1245/s10434-014-3696-4. [DOI] [PubMed] [Google Scholar]
- 16.Zahoor H, Luketich JD, Weksler B, et al. The revised American Joint Committee on Cancer staging system (7th edition) improves prognostic stratification after minimally invasive esophagectomy for esophagogastric adenocarcinoma. Am J Surg. 2015;210:610–7. doi: 10.1016/j.amjsurg.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Situ D, Wei W, Lin P, et al. Do tumor grade and location affect survival in esophageal squamous cell carcinoma? Survival analysis of 302 cases of pT3N0M0 esophageal squamous cell carcinoma. Ann Surg Oncol. 2013;20:580–5. doi: 10.1245/s10434-012-2656-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Cheong J, Song K, et al. Staging of adenocarcinoma of the esophagogastric junction: comparison of AJCC 6th and 7th gastric and 7th esophageal staging systems. Ann Surg Oncol. 2013;20:2713–20. doi: 10.1245/s10434-013-2898-5. [DOI] [PubMed] [Google Scholar]
- 19.Ning Z, Wang Z, Chen J, et al. Proposed modification of nodal staging as an alternative to the seventh edition of the American Joint Committee on Cancer tumor-node-metastasis staging system improves the prognostic prediction in the resected esophageal squamous-cell carcinoma. J Thorac Oncol. 2015;10:1091–8. doi: 10.1097/JTO.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 20.Nafteux PR, Lerut AM, Moons J, et al. International multicenter study on the impact of extracapsular lymph node involvement in primary surgery adenocarcinoma of the esophagus on overall survival and staging systems. Ann Surg. 2015;262:809–16. doi: 10.1097/SLA.0000000000001463. [DOI] [PubMed] [Google Scholar]
- 21.Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin. 2013;23:461–9. doi: 10.1016/j.thorsurg.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen PJ, Kranse R, Hakulinen T, et al. Disease-specific mortality may underestimate the total effect of prostate cancer screening. J Med Screen. 2010;17:204–10. doi: 10.1258/jms.2010.010074. [DOI] [PubMed] [Google Scholar]
- 23.Black WC, Haggstrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94:167–73. doi: 10.1093/jnci/94.3.167. [DOI] [PubMed] [Google Scholar]
- 24.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–20. doi: 10.1016/s0735-1097(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 25.Rice TW, Lerut TEM, Orringer MB, et al. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;7:715–23. doi: 10.1111/dote.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


