Abstract
AIM:
In this study, Epithelial Growth Factor Receptor and Platelet Endothelial Cell Adhesion Molecule-1 were localised to investigate the healing effects of a flavonoid-rich fraction of M. paradisiaca fruit in the gastric corpus of Wistar rats following aspirin-induced gastric lesion.
MATERIALS AND METHODS:
Mature, unripe fruits of M. paradisiaca were peeled; air dried, pulverised, extracted with 70% methanol, concentrated and partitioned. Ninety male Wistar rats were randomly assigned into 6 groups of 15 rats each. The gastric lesion was induced in groups B, C, D, E and F rats by administration of 400 mg/kg aspirin in distilled water. Group A received distilled water. After 24 hours, flavonoid fraction of M. paradisiaca was administered to groups C, D and E at 100, 200 and 400 mg/kg respectively for 21 days. Group F rats received omeprazole at 1.8 mg/kg for 21 days. Five rats from each group were anaesthetized with ketamine on days 14, 21 and 28. Gastric tissues were excised and fixed in Neutral buffered formalin. This was followed by paraffin wax embedding method and sections stained with haematoxylin and eosin and for immunolocalisation of EGFR and PECAM-1. Data were analysed using descriptive and inferential statistics.
RESULTS:
There was a significant difference in the ulcer index in the corpus of control and treated rats throughout the experimental period (p = 0.0001). H&E stained sections showed a gradual restoration of the epithelial lining in the treated groups. Immunohistochemical examination showed that M. paradisiaca significantly increased (p < 0.05) reactivity for both EGFR and CD31 across the treatment groups.
CONCLUSION:
The efficacy of Musa paradisiaca in attenuating the damaging effects of aspirin on the gastric mucosa was observed as there was a significantly increased reactivity for EGFR and PECAM-1 in the gastric corpus in a dose-dependent manner.
Keywords: Aspirin, EGFR, PECAM-1, Gastric lesions, Musa paradisiaca
Introduction
Gastric ulcer is a defect in the gastric wall running through the entire mucosal thickness and penetrating the muscularis mucosae. A variety of factors has been implicated in the aetiology of gastric ulcers. These include humoral, genetic, neural, therapy induced (e.g. use of NSAIDs) and Helicobacter pylori infection [1]. Gastric ulcer is precipitated by an imbalance between endogenous aggressive factors in the gastric lumen (such as hydrochloric acid, pepsin, refluxed bile, leukotrienes, reactive oxygen species) and cytoprotective factors; these include the function of the mucus-bicarbonate barrier, surface active phospholipids, prostaglandins, mucosal blood flow, cell renewal and migration, non-enzymatic and enzymatic antioxidants and some growth factors [2]. It has a worldwide prevalence of about 5% [3]. Nwokediuko et al. suggested that in Nigeria, there has been an increase in the prevalence of gastric ulcers over a 15-year period [4].
The remodelling of the mucosal architecture involves an active process of filling the mucosal defect with proliferating and migrating epithelial cells and connective tissue components [5]. This entails an intricate interaction of different tissues and cellular systems. Re-epithelization is an important process of ulcer healing in which epithelial cells migrate from the margin of the ulcer. Asides other factors responsible for ulcer healing, the Epidermal growth factor is cardinal for intensifying cell proliferation at the mucosa of the ulcer margin; it is also responsible for the restoration of the glandular structures by epithelial tubules originating from the mucosa adjacent to the ulcer crater base [1]. Epidermal growth factor is a cell-surface protein primarily responsible for increased cell proliferation at the mucosa of the ulcer margin and re-epithelialization; hence its receptors are expressed as gastric glands dilate and the lining epithelial cells de-differentiate [5].
Angiogenesis an important process in ulcer healing as it is essential for proliferation of endothelial cells as well as connective tissues involved in the formation of granulation tissue [5]. Through the formation of a capillary network, angiogenesis in granulation tissue facilitates nutrient and oxygen delivery to the ulcer.
PECAM-1 is a protein involved in cell adhesion and angiogenesis which demonstrates the presence of endothelial cells and hence, evaluates the degree of angiogenesis. Connective tissue cells are supplied by granulation tissue to develop lamina propria and micro vessels which form micro vascular network in an ulcer scar tissue [6].
The healing process depends on a progressive interaction between the epithelial part of the ’healing’ zone at the ulcer margin and the connective tissue component (including microvessels) originating from the granulation tissue [6].
Musa paradisiaca (M. paradisiaca) is a tropical plant which is called plantain or vegetable banana and has to be cooked to be edible. It is herbaceous (up to 9 m high) with oblong and fleshy fruits; 5-7cm long in wild form and longer in the cultivated varieties. In traditional folk medicine, different parts of this plant are used for numerous purposes. It is known to have varying medicinal properties [7, 8]. Studies have shown that the extract of unripe M. paradisiaca have high antioxidant activity which is attributed to the presence of phenols and phytochemicals which are potent antioxidants with free radical scavenging activities [9]
Asides other phytochemicals, several flavonoids and related compounds have been isolated from the unripe pulp of plantain [10]. A natural flavonoid from the unripe MP pulp, Leucocyanidin and the synthetic analogues were found to protect the gastric mucosa in aspirin-induced erosions in the rat by increasing gastric mucus thickness [11]. Also, MP pulp powder showed significant antiulcerogenic activity in aspirin-, indomethacin-, phenylbutazone-, prednisolone-induced gastric ulcers in rats. The authors attributed the effect to increased mucosal thickness and increased [3H] thymidine incorporation into mucosal DNA that results in mucosal cellular proliferation and healing.
The major therapeutic approach for a gastric ulcer is the control of gastric acid secretion and re-inforcement of gastric mucosal production using antacids, H2-receptor blockers, proton pump inhibitors, anticholinergics and cytoprotective agents [12]. The management of gastric ulcer is challenging because of the drawbacks with major therapies; these include high recurrence rate associated side effects from most drugs ranging from diarrhoea, itching and dizziness to arrhythmia, impotence, gynecomastia and hematopoietic changes [13, 14].
Recently, there has been an evolution to the use of alternative therapies and natural products especially those derived from plants as medicinal plants remain one of the promising sources of drugs and have produced propitious results in the treatment of various diseases including gastric ulcer.
Against the backdrop of previous studies and claims by traditional medical practitioners in South Western Nigeria on the efficacy of MP in the treatment of gastric ulcer; this work was initiated to verify if MP induces the expression of EGFR and PECAM-1 whose dynamic interaction is essential in gastric ulcer healing.
Materials and Methods
Plant Material
Mature, unripe fruits of Musa paradisiaca Linn (Musaceae) from a farmland were harvested in September 2014 between the hours of 16.00 and 17.00. The plant was identified by a taxonomist at the Department of Plant Science and Forestry, Ekiti State University, Ado-Ekiti, Nigeria (UHAE 2014/84).
The fruit pulp was cut into pieces, air-dried and pulverised using an electric grinder. 3kg of the powdered sample was extracted three times with 70% methanol (20% w/v) with continuous stirring using an Orbital shaker at room temperature for 48 hours each. This was filtered and the filtrate concentrated at 40 °C in a vacuum rotary evaporator under reduced pressure to obtain a dark brown extract of 98.51g. The crude extract was partitioned by solvent- solvent extraction with n-hexane, dichloromethane, ethyl acetate and n-butanol to obtain the flavonoid-rich fraction [15]. The resulting flavonoid fraction weighing 28.28g was concentrated, freeze-dried in a vacuum freeze drier and stored in a desiccator until needed. The flavonoid fractions were subjected to phytochemical analysis using Shibata’s reaction and Ferric chloride test [16].
Animal Care and Management
Ninety healthy male Wistar rats weighing between 120-150 g used for this study were procured from the Animal House of the College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria. They were on a diet of standard rat pellets procured from Ladokun feeds, Ibadan and given tap water ad libitum. The rats were acclimatised for a week in plastic cages at the Animal Holding of the Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria under standard laboratory conditions of natural light/dark cycle at room temperature and humidity. All animals were handled by the Guidelines for Animal research as detailed in the NIH Guidelines for the Care and Use of Laboratory Animals [17].
Ethical Approval for this work was obtained from the Health Research Ethics Committee (HREC), Institute of Public Health, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria (IPHOAU/12/187).
Experimental Design, Induction of Gastric Lesion and Treatment
The rats were randomly assigned into 6 groups (A, B, C, D, E and F) of 15 rats each. At the commencement of the experiment, stool samples of each animal were obtained and tested for the presence of H. pylori antigen using the H. pylori Stool Antigen Test Kit (Biotech, China) as described by Olufemi et al. [18].
Before the induction of gastric lesions, rats in the experimental group were starved overnight so as to ensure complete gastric emptying and a steady state gastric acid secretion. The animals were allowed free access to water during this period. Gastric lesions were induced in Groups B, C, D and E rats by a single oral administration of 400 mg/kg reference standard aspirin (Sigma Chemicals, USA) dissolved in 0.5 ml of distilled water. Group A rats received an equivalent volume of distilled water used in dissolving aspirin. Twenty-four hours later, flavonoid fraction of M. paradisiaca dissolved in distilled water was administered by gavage to the rats in groups C, D and E at graded doses of 100, 200 and 400 mg/kg respectively at a particular time (10.00-11.00 hours) for 21 days. Group F rats received Omeprazole (Divine Essential Formulations, Lagos, Nigeria) dissolved in distilled water at 1.8 mg/kg by gavage for 21 days. The rats in group A received equivalent volumes of distilled water used in dissolving the extract and drug for the same period.
On the 14th and 21st days of treatment, 5 rats from each group were sacrificed under ketamine anaesthesia at a dose of 75 mg/kg while the remaining were left untreated for another 7 days before sacrifice. Each of the animals was quickly dissected through a mid-line incision in the anterior abdominal wall.
Tissue Processing
Samples of the gastric corpus in each group of animals were fixed in 10% neutral buffered formalin and processed according to the general technique of tissue processing and microtomy as described by Bancroft and Gamble [19]. Sections of 4-5 µm thickness were produced on a Rotary microtome. Histopathology of gastric tissues was performed using Hematoxylin and Eosin (H&E); EGF receptor and PECAM-1 were localised using appropriate primary monoclonal antibodies respectively [20, 21].
Photomicrography and Image Analysis
The stained sections were examined under OMAX 40X-2000X Digital Light microscope. Digital photomicrographs of the tissue sections were taken at various magnifications.
The immunisation plugin in the Image J software was used to analyse and quantify areas of immunoreactivity in stained sections.
Determination of Ulcer Index
Microscopic ulcer index was obtained separately by two pathologists, and a mean index was calculated as described by Pandit et al. [22].
Normal tissue = 0; Local damage to gastric pits cells = 1; Local damage to gastric glands = 2; Deep damage to gastric glands = 3.
Microscopic ulcer index = (number of lesion 1) + (number of lesion 2) × 2 + (number of lesion 3) ×3.
Statistical Analysis
The results were expressed as mean ± SEM. Using the GraphPad Prism version 5.00 software, two-way ANOVA was used for comparative analysis of the data, followed by Bonferroni tests for multiple comparisons. Statistical significance was set at p < 0.05.
Results
Tests for Flavonoids in the Extract
Phytochemical analysis of the ethyl acetate fraction of Musa paradisiaca for the presence of flavonoid was positive as there was a coloration of red for the Shibata’s reaction and bluish green for the Ferric chloride test respectively.
Ulcer Index of the Gastric Corpus of Control and Treated Rats
There was a significant difference in the ulcer index in the corpus of control and treated rats throughout the experimental period (p=0.0001). As seen in Table 1, in the gastric corpus, administration of aspirin resulted in a significant increase in ulcer index (p < 0.05) in both group B and groups C, D and E when compared with the control. This indicates that aspirin-induced ulceration and hemorrhagic lesions in the gastric corpus of the rats that received it. On the 14th and 21st days of the experiment, there was a significant increase (p < 0.05) in ulcer index in all the treated groups when compared with the control but on the 28th day of the experiment, apart from the group C rats, there was no significant difference (p > 0.05) in the ulcer index between the control and groups D, E and F rats. This indicates the efficacy of both MP (200 mg/kg and 400 mg/kg) and OMZ in the reduction of aspirin-induced gastric lesions over a period. Also, throughout the experimental period, a significant increase (p < 0.05) was observed in the ulcer index of the group B rats when compared with all the treated groups (C, D, E and F). On the 14th day of the experiment, there was no significant difference (p < 0.05) in ulcer index among the treated groups of rats (C, D, E and F). At the end of the treatment period, a significant increase (p < 0.05) was however observed when group C was compared with groups D, E and F. This shows the efficacy of MP at doses of 200 mg/kg, 400 mg/kg and OMZ over MP at doses of 100 mg/kg in reducing the ulcer index.
Histological Observation of the Gastric Corpus
On day 14 of the experimental period, as seen in Fig. 1, the gastric corpus of group A presented with a normal histoarchitecture characterised by a well-organized outline of the gastric layers. A continuous epithelial lining was also observed. In group B, there was discontinuation of the epithelial lining as evidenced by the ulcer craters. In the treated groups, a gradual restoration of the epithelial lining was observed. Groups D and E presented with a proper alignment of the connective tissue fibres similar to what is observable in control. However, some pockets of ulcerations were still observed in groups C and F. As seen in Fig. 2, on Day 21, the control group displayed a well-structured histoarchitecture with the gastric layers well arranged. In group B, there were severe ulcerations as characterised by epithelial distortions. Evidence of distortion of the connective tissue fibres within the mucosa was also observed. The treated groups displayed a normal histoarchitecture as compared with the control. No ulcerations were observed, and the gastric layers were in proper alignment.
Figure 1.
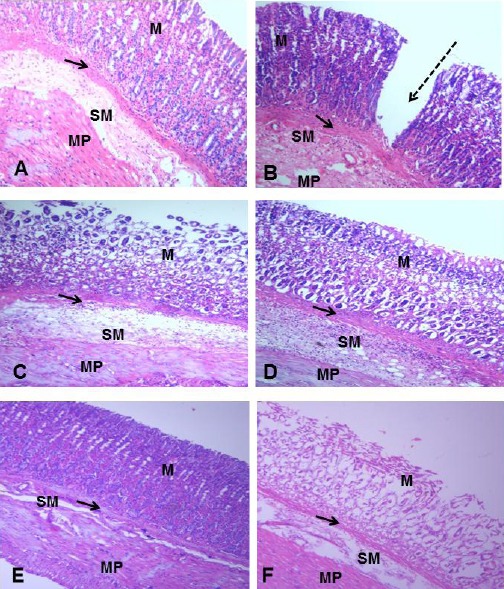
Photomicrograph of the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 14. H&E, X100. Mucosa (M), Muscularis mucosa (arrows), Submucosa (SM), Muscular propia (MP), Ulcer crater (dashed arrow)
Figure 2.
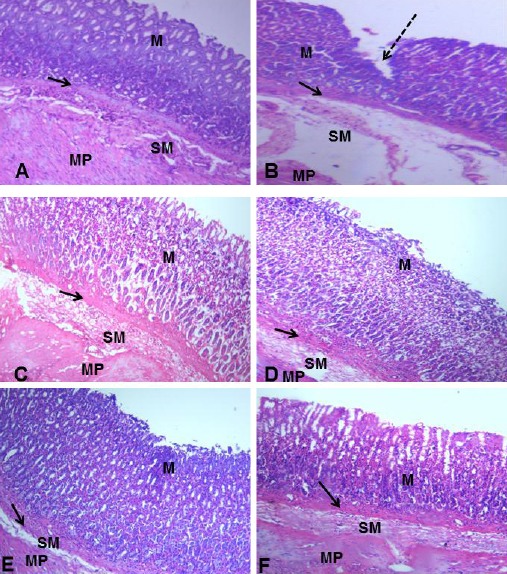
Photomicrograph of the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 21. H&E, X100. Mucosa (M), Muscularis mucosa (arrows), Submucosa (SM), Muscularis propia (MP), Ulcer crater (dashed arrow)
On Day 28, a well-organized histoarchitecture was observed in the gastric layers of the control and treated groups. Group B, however, showed severe ulceration with irregularly arranged connective tissue fibres in the submucosa (Fig. 3).
Figure 3.
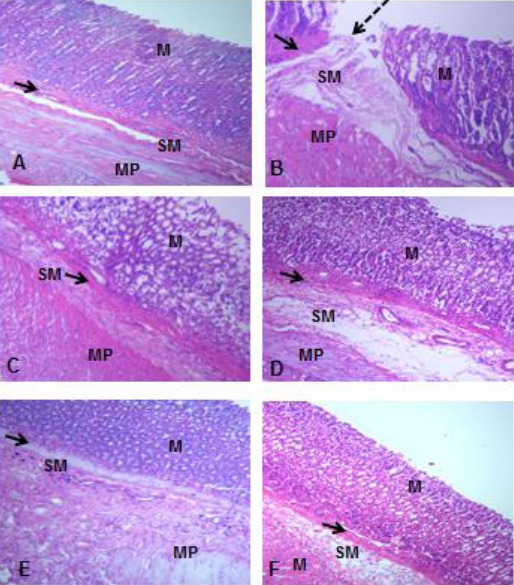
Photomicrograph of the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 28. H&E, X100. Mucosa (M), Muscularis mucosa (arrows), Submucosa (SM), Muscularis propia (MP), Ulcer crater (dashed arrow)
Epidermal Growth Factor Receptor (EGFR)
On days 14, 21 and 28 of the study, immunohistochemical staining of the granulation tissue and ulcer margin of the gastric mucosal of rats in group B did not display significant immunoreactivity for EGFR (Fig. 4B, 5B, 6B).
Figure 4.
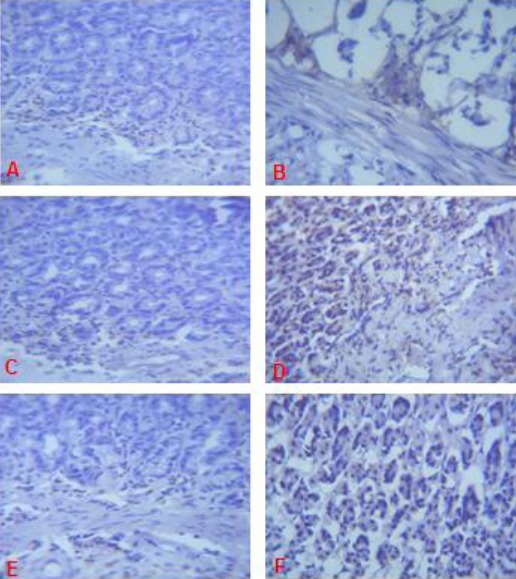
Expression of EGFR (brown colour) in the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 14 Note the EGFR immunoreactivity in the treatment groups. X400
Figure 5.
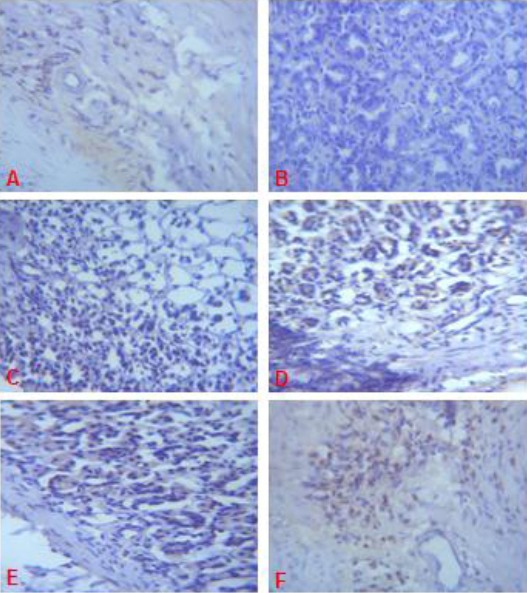
Expression of EGFR (brown colour) in the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 21. Note the EGFR immunoreactivity in the control and treatment groups. X400
Figure 6.
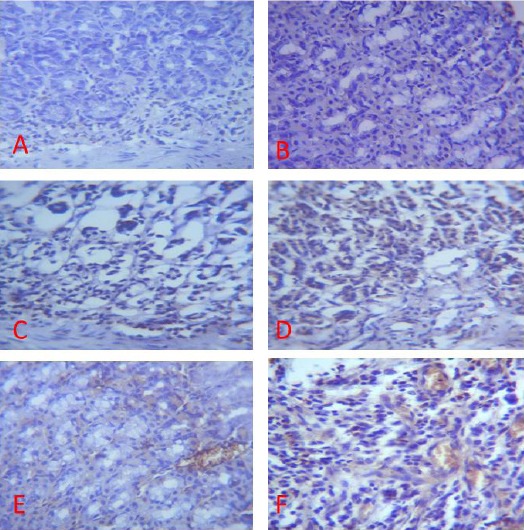
Expression of EGFR (brown colour) in the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 28. Note the EGFR immunoreactivity in the control and treatment groups. X400
When compared with the control, the treated groups (C, D, E and F) displayed a significant EGFR immunoreactivity (Fig. 4, 5, 6).
Quantitative Analysis of EGFR Positive Cells in the Gastric Corpus of Control and Treated Rats
Using the Image J immunisation analysis tool, immunohistochemical studies of the gastric body showed that there was a significant difference in the number of EGFR positive cells among groups A to F on days 14, 21 and 28 (p = 0.0001). There was no significant difference (p > 0.05) in the number of EGFR positive cells in group B compared with the control (Fig. 7). In contrast, all the treatment groups had a significantly increased (p < 0.01) number of EGFR positive cells when compared with control throughout the experimental period. Similar findings were made when group B was compared with all the treatment groups as there was a significant increase (p < 0.05) in the number of EGFR immunoreactive cells in the treatment groups.
Figure 7.
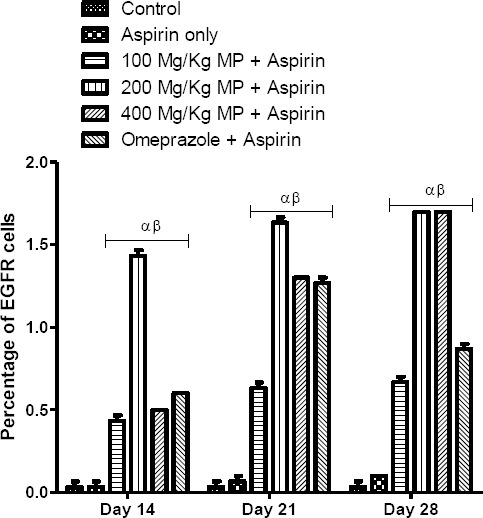
Immunization Plugging Analysis of EGFR Positive Cells in the Gastric Corpus of Control and Treated rats. *p < 0.05. α and β - significant difference compared to control and group B respectively
On the 21st and 28th days of the experiment, there was a significant increase (p < 0.05) in the number of EGFR positive cells in groups D and E when compared with group C. This indicates a better healing effect of MP in groups D and E when compared with group C. At the end of the experimental period, there was no significant difference (p > 0.05) in the number of EGFR immunoreactive cells between groups C and F. Also; there was no significant difference (p > 0.05) between the number of EGFR immunoreactive cells in groups D and E throughout the experimental period. This indicates similar healing effects of MP at the dose of 200 mg/kg and 400 mg/kg. As seen in figure 1, group E had a significantly increased (p < 0.05) number of EGFR immunoreactive cells when compared with group F at the end of the experimental period.
PECAM-1 Immunohistochemistry
Immunohistochemical staining of the gastric mucosal tissue of rats in group B did not display significant immunoreactivity for PECAM-1 (Fig 8B, 9B, 10B).
Figure 8.
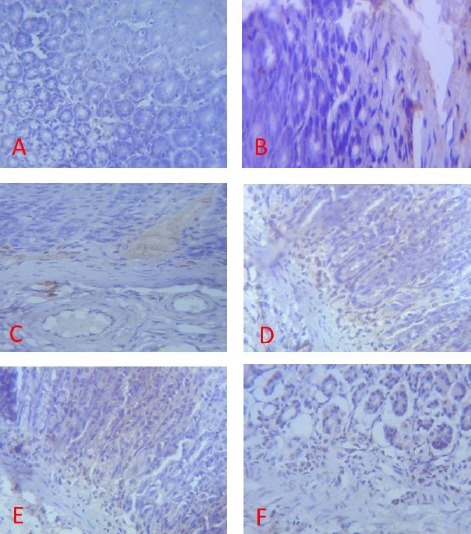
Expression of PECAM-1 (brown colour) in the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 14. X400
Figure 9.
Expression of PECAM-1 (brown colour) in the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 21. X400
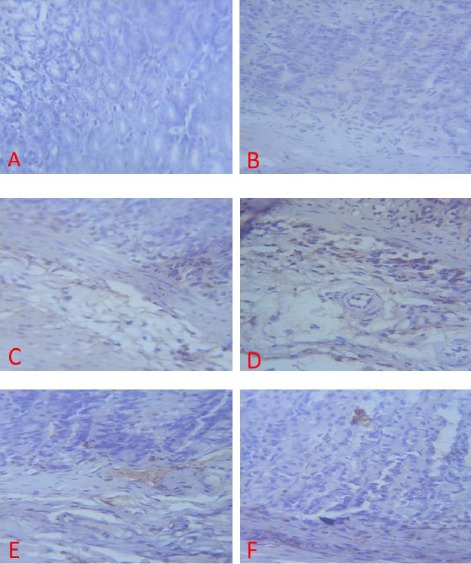
Figure 10.
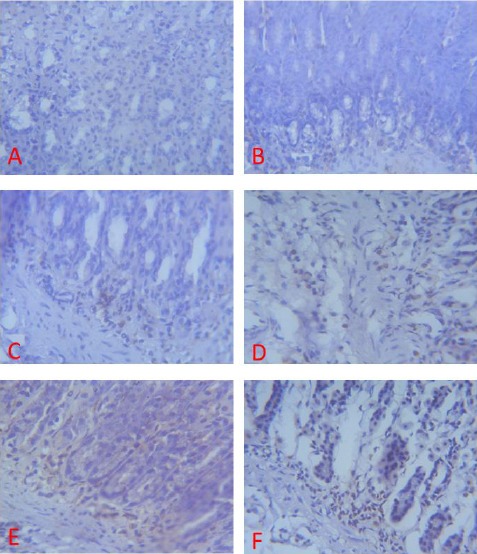
Expression of PECAM-1 (brown colour) in the Gastric Corpus of Control (A), ASA Only (B), MP Treated (C, D, E) and OMZ Treated (F) Groups on Day 28. X400
When compared with the control, the treated groups (C, D, E and F) displayed a significant PECAM-1 immunoreactivity (Fig 8, 9, 10). There was little or no staining for PECAM-1 in the gastric tissues of the control group.
Quantitative Analysis of PECAM-1 Positive Cells in Gastric Corpus of Control and Treated Rats
In the gastric corpus, Image J immunisation analysis tool showed that there was a significant difference in the number of PECAM-1 immunoreactive cells throughout the experimental period (p = 0.0001). There was no significant difference (p > 0.05) in the number of PECAM-1 positive cells between the control and group B (Fig. 11).
Figure 11.
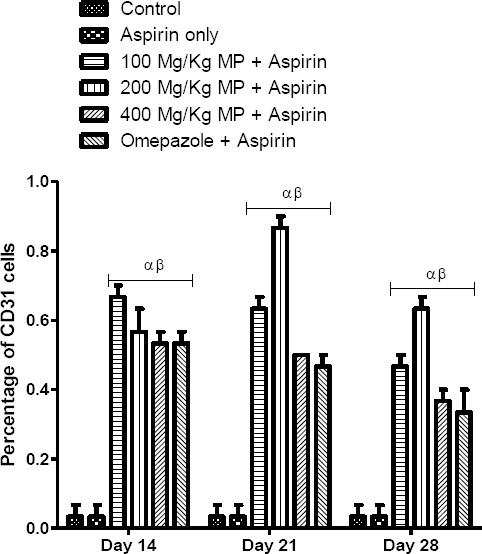
Immunization Plugging Analysis of PECAM-1 Positive Cells in Corpus of Control and Treated Rats. *p < 0.05, α and β - significant difference compared to control and group B respectively
This indicates a delay in the process of angiogenesis. In contrast, throughout the experimental period, there was a significant increase (p < 0.05) in the number of PECAM-1 positive cells in the treatment groups (C, D, E and F) when compared with the control. This shows the restoration of microvessels in the treatment groups.
In groups C, D, E and F, there was a significant increase (p < 0.05) in the number of PECAM-1 positive cells when compared with group B. The reduced rate of angiogenesis and hence the effectiveness of healing was also observed as there was a significant difference in the number of PECAM-1 positive cells between group C and groups D, E and F on days 21 and 28 of the experimental period. However, there was no significant difference in the number of PECAM-1 positive cells among the other treatment groups.
Discussion
Many growth factors including Epidermal Growth Factor (EGF) contribute to the mechanism of ulcer healing as a result of the stimulatory effect on the process of mucosal repair, cell migration and proliferation as well as angiogenesis. Konturek et al. [23] observed that EGF which is normally present in saliva and gastric juice exerts potent mitogenic and gastroprotective activities. EGFR enhances proliferation which is important for ulcer healing as it supplies essential epithelial cells for re-epithelization of mucosa surface and reconstruction of gastric gland. Results from this study show that in the gastric corpus, the number of EGFR positive cells in the treatment groups significantly increased when compared with control and the ASA only groups throughout the experimental period. Recent research indicated that EGFR was closely related to the healing of impaired gastric mucosa and was of great importance in gastric mucosal protection [24, 25]. Other studies indicated that there was an elevated EGFR expression during the healing course of damaged gastric mucosa [25, 27]. The observed effect on the expression of EGFR in MP-treated groups was however in a dose-dependent manner. There was a significantly increased expression of EGFR in the gastric tissues of rats treated with MP200 and MP400 when compared with MP100. This shows the efficacy of MP200 and MP400 over MP100 in the healing of gastric ulcers. A comparable effect was also observed in the expression of EGFR between MP100 and OMZ groups as there was no significant difference in EGFR expression in both groups at the end of the experimental period. Several studies have shown that in gastric mucosa of the ulcer margin, epithelial cells form a characteristic “recovery zone” and dilated gastric glands undergo differentiation and express EGFR [24, 28]. In another study, following aspirin-induced gastric damage, fucoidan-treated rats demonstrated significant EGFR immunoreactivity around the submucosal connective tissue [20]. The present study complements those above.
Results from this study showed a significantly reduced expression of EGFR in the ASA only group when compared with the treatment groups. Suppression of endogenous NO synthesis leads to delayed ulcer healing and high doses of aspirin impair normal NO activity, thereby suppressing the expression of growth factors (such as EGFR) in ulcer progression [29]. In another study, aspirin-treated rats showed a weak positive EGFR immunoreactive signal, confirming that EGF produced locally activate re-epithelialization and migration of epithelial cells from the ulcer margin to restore mucosal epithelial continuity.
PECAM- 1 is an endothelial marker involved in cell adhesion and angiogenesis [30]. The nsaids-induced gastric injury is associated with vascular changes that cause hypoxia in the mucosa and activation of leukocytes [31]. Konturek et al. [32] noted that impairment in gastric blood flow (GBF) at an ulcer area and excessive cytokine expression and release, as well as a reduction in the mucosal antioxidizing enzyme activity induced by NSAIDs, may contribute to the delay in ulcer healing. When there is damage to the vascular endothelium, the decreased blood flow which reduces oxygen supply and consequently the nutrient transport results in the appearance of erosion and ulceration in the mucous membrane [33].
In this study, throughout the experimental period, there was a significantly increased PECAM-1 immunoreactivity in the corpus when the treated groups were compared with control. Remodelling of the destroyed vascular network within the ulcerated site is integral to healing. A reduced efficacy of MP100 in angiogenesis was observed as there was a significant reduction in PECAM-1 immunoreactivity between MP100 and the other treatment groups. In the ASA only group, there was a significant reduction in the PECAM-1 immunoreactivity when compared with the treated groups. However, there was a significant increase in PECAM-1 immunoreactivity in MP200 and MP400 groups when compared with MP100 groups. This indicates the ability of MP to initiate angiogenesis in impaired gastric tissues at 200 mg/kg and 400 mg/kg. The delay in gastric ulcer healing with ASA administration has been associated with diminished angiogenesis and impaired blood flow at the ulcer margin [34, 35]. Results from this study showed that a marked reduction in immunoreactivity to PECAM-1 was observed in the ASA only group. Our results are in agreement with the work of Hudson et al. [36] who investigated the relationship between NSAID therapy and gastroduodenal ulcer healing.
In conclusion, this study have demonstrated that oral administration of flavonoid fraction of mature and unripe fruit extract of MP at a dose of 200 mg/kg and 400 mg/kg significantly (compared with placebo and omeprazole) attenuated the damaging effects of aspirin on the gastric mucosa as it enhances expression of EGFR and PECAM-1.
At these doses, results obtained for MP was better than OMZ. Omeprazole is used for the treatment of gastric ulcers due to its ability to irreversibly inhibit the H+/K+/ATPase pump. In this study, MP was efficacious in restoring the integrity of the damaged mucosa by increasing the expression of EGFR and PECAM-1. Activation of these markers appears to promote gastric ulcer healing, thereby facilitating tissue repair.
Despite the fact that MP has been found to have a stimulatory effect on mucosa growth, clinical studies in human are needed to provide evidence of its efficacy and explore the potential of development of leads that may contribute to drug development.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Tarnawski A, Tanoue K, Santos AM, Sarfeh IJ. Cellular and molecular mechanisms of gastric ulcer healing. Is the quality of mucosal scar affected by treatment? Scand J Gastroenterol. 1995;30(210):9–14. doi: 10.3109/00365529509090261. https://doi.org/10.3109/00365529509090261. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee M, Bhattacharjee S, Gupta A, Banerjee RK. Critical role of an endogenous gastric peroxidase. Biochemical Pharmacology. 2002;37:271–280. doi: 10.1016/s0891-5849(02)00757-8. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay D, Biswas K, Bhattacharyya M, Reiter RJ, Banerjee RK. Gastric toxicity and mucosal ulceration induced by oxygen-derived reactive species, protection by melatonin. Current Molecular Medicine. 2001;1(4):501–513. doi: 10.2174/1566524013363483. https://doi.org/10.2174/1566524013363483 PMid:11899094. [DOI] [PubMed] [Google Scholar]
- 4.Nwokediuko SC, Ijoma U, Obienu O, Picardo N. Time trends of upper gastrointestinal diseases in Nigeria. Ann Gastroenterol. 2012;25(1):52–56. [PMC free article] [PubMed] [Google Scholar]
- 5.Tarnawski A. Molecular mechanism of ulcer healing. Drug News & Perspective. 2000;13:158–68. doi: 10.1358/dnp.2000.13.3.858438. https://doi.org/10.1358/dnp.2000.13.3.858438. [DOI] [PubMed] [Google Scholar]
- 6.Syam AF, Sadikin M, Wanandi SI, Rani AA. Molecular Mechanism on Healing Process of Peptic Ulcer. Acta Med Indones-Indones J Intern Med. 2009;41(2):95–98. [PubMed] [Google Scholar]
- 7.Sharma PC, Yelne MB, Dennis JJ, Kadali IN. Data base on medical plants used in Ayurveda and Siddha. Vol. 5. New Delhi: Public printing; 2002. pp. 78–93. [Google Scholar]
- 8.Imam MZ, Akter S, Musa paradisiacal L, Musa sapientum L. A Phytochemical and Pharmacological Review. Journal of Applied and Pharmaceutical Science. 2011;1(5):14–20. [Google Scholar]
- 9.Eleazu CO, Okafor PN, Amajor JA, Awa E, Ikpeama AI, Eleazu KC. Chemical Composition, antioxidant activity, functional properties and inhibitory action of unripe plantain (M. paradisiacae) flour. African Journal of Biotechnology. 2011;10(74):16948–16952. https://doi.org/10.5897/AJB10.1180. [Google Scholar]
- 10.Ragasa CY, Martinez A, Chua JEY, Rideout JA. A Triterpene from Musa errans. Philippine Journal of Sciences. 2007;136(2):167–171. [Google Scholar]
- 11.Lewis DA, Shaw GP. A natural flavonoid and synthetic analogues protect the gastric mucosa from aspirin-induced erosions. Journal of Nutrition and Biochemistry. 2001;12:95–100. doi: 10.1016/s0955-2863(00)00133-9. https://doi.org/10.1016/S0955-2863(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 12.Rao CH, Ojha SK, Radhakrishnan K, Govndarajan R, Rastogi S, Mehrotra S. Antilcer activity of Utleriasalicifolia rhizome extract. Antiulcer activity of Utleriasalicifolia rhizome extract. Journal of Ethnopharmacology. 2004;91(2-3):243–249. doi: 10.1016/j.jep.2003.12.020. https://doi.org/10.1016/j.jep.2003.12.020 PMid:15120446. [DOI] [PubMed] [Google Scholar]
- 13.Bandyopadhyay D, Biswas K, Bhattacharyya M, Reiter RJ, Banerjee RK. Involvement of reactive oxygen species in gastric ulceration, protection by melatonin. Indian Journal of Experimental Biology. 2002;40:693–705. PMid:12587717. [PubMed] [Google Scholar]
- 14.Chan FK, Leung WK. Peptic ulcer disease. Lancet. 2002;360:933–41. doi: 10.1016/s0140-6736(02)11030-0. https://doi.org/10.1016/S0140-6736(02)11030-0. [DOI] [PubMed] [Google Scholar]
- 15.Owoyele BV, Oguntoye SO, Dare K, Ogunbiyi BA, Aruboula EA, Soladoye AO. Analgesic, anti-inflammatory and antipyretic activities from flavonoid fractions of Chromolaena odorata. Journal of Medicinal Plants Research. 2008;2(9):219–225. [Google Scholar]
- 16.Trease G, Evans SM. Pharmacognosy. 15th ed. Bailer Tindal: London; 2002. pp. 23–67. [Google Scholar]
- 17.National Institute of Health. Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press: Washington, D. C., USA; 2011. [Google Scholar]
- 18.Olufemi FO, Quadri R, Akinduti PA, Bamiro SA. Potential Risk Fcators and Prevalence of Infection of Helicobacter pylori in Nigeria. Journal of Scientific Research & Reports. 2015;7(1):42–48. https://doi.org/10.9734/JSRR/2015/16014. [Google Scholar]
- 19.Bancroft JD, Gamble G. Theory and Practice of Histological Techniques. 6th ed. Churchill Livingstone: Elsevier; 2008. [Google Scholar]
- 20.Raghavendran HRB, Srinivasan P, Rekha S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosa damage in rats. International Immunopharmacology. 2002;11:157–163. doi: 10.1016/j.intimp.2010.11.002. https://doi.org/10.1016/j.intimp.2010.11.002 PMid:21084063. [DOI] [PubMed] [Google Scholar]
- 21.Dudar GR, Luca DD, Di Stasi R, Pedone C, Wallace J. A vascular endothelial growth factor mimetic accelerates gastric ulcer healing in an iNOS-dependent manner. American Journal of Gastrointestinal Liver Physiology. 2008;295:374–381. doi: 10.1152/ajpgi.90325.2008. [DOI] [PubMed] [Google Scholar]
- 22.Pandit S, Sur TK, Jana U, Bhattacharyya D, Debnath PK. Anti-ulcer effect of Shankha bhasma in rats:A preliminary study. Indian Journal of Pharmacology. 2000;32:378–380. [Google Scholar]
- 23.Konturek SJ, Brzozowski T, Stachura J, Dembinski A, Majka J. Role of gastric blood flow, neutrophil infiltration, and mucosal cell proliferation in gastric adaptation to aspirin in the rat. Gut. 1994;35:1189–1196. doi: 10.1136/gut.35.9.1189. https://doi.org/10.1136/gut.35.9.1189 PMid:7525421 PMCid:PMC1375692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarnawski AS, Jones MK. The role of epidermal growth factor (EGF) and its receptor in mucosal protection, adaptation to injury, and ulcer healing:involvement of EGF-R signal transduction pathways. Journal of Clinical Gastroenterology. 1998;27(1):SI2–20. doi: 10.1097/00004836-199800001-00004. https://doi.org/10.1097/00004836-199800001-00004. [DOI] [PubMed] [Google Scholar]
- 25.Jones NL, Day AS, Sherman PM. Determinants of disease outcome following Helicobacter pylori infection in children. Canadian Journal of Gastroenterology. 1999;13:613–7. doi: 10.1155/1999/545427. https://doi.org/10.1155/1999/545427 PMid:10519961. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WN, Zhang Y, Li JB. Effect of Jianwei Yuyang Granules on expression of epidermal growth factor receptor in gastric mucosa of gastric ulcer patients. Zhongxiyi Jiehe Xuebao. 2004;2:24–26. doi: 10.3736/jcim20040109. https://doi.org/10.3736/jcim20040109. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara Y, Higuchi K, Hamaguchi M, Takashima T, Watanabe T, Tominaga K, Oshitani N, Matsumoto T, Arakawa T. Increased expression of transforming growth factor-alpha and epidermal growth factor receptors in rat chronic reflux esophagitis. Journal of Gastroenterology and Hepatology. 2004;19:521–527. doi: 10.1111/j.1440-1746.2003.03332.x. https://doi.org/10.1111/j.1440-1746.2003.03332.x PMid:15086595. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Wang WP, Chow JY, Yuen ST, Cho CH. Reduction of EGF is associated with the delay of ulcer healing by cigarette smoking. American Journal of Gastrointestinal and Liver Physiology. 2000;278(1):10–7. doi: 10.1152/ajpgi.2000.278.1.G10. [DOI] [PubMed] [Google Scholar]
- 29.Lanas A, Bajador E, Serrano P, Fuentes J, Carreno S, Guardia J. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. New England Journal of Medicine. 2000;343(12):834–9. doi: 10.1056/NEJM200009213431202. https://doi.org/10.1056/NEJM200009213431202 PMid:10995862. [DOI] [PubMed] [Google Scholar]
- 30.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-celIs. Journal of Cell Science. 2013;126(11):2343–52. doi: 10.1242/jcs.124099. https://doi.org/10.1242/jcs.124099 PMid:23761922. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez C, Santamatilde E, McCreath KJ, Cervera AM, Diez I, Ortiz-Masia D, Matinez N, Calatayud S, Esplugues JV, Barrachina MD. Induction of trefoil factor (TFF) 1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1:implications for gastric mucosal healing. British Journal of Pharmacology. 2009;156:262–272. doi: 10.1111/j.1476-5381.2008.00044.x. https://doi.org/10.1111/j.1476-5381.2008.00044.x PMid:19076725 PMCid:PMC2697836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konturek SJ, Brzozowski T, Majka J, Dembinski A, Slomiany A, Slomiany B. L. Transforming growth factor and epidermal growth factor in protection and healing of gastric mucosal injury. Scandinavian Journal of Gastroenterology. 1992;27:649–655. doi: 10.3109/00365529209000134. https://doi.org/10.3109/00365529209000134 PMid:1439546. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho CA, Fernandes KM, Matta SLP, da Silva MB, de Oliveira LL, Fonseca CC. Evaluation of antiulcerogenic Activity of aqueous extract of Brassica oleracea var. Capitata (cabbage) on Wistar rat gastric ulceration. Experimental Gastoenterology. 2011;48(4):276–283. doi: 10.1590/s0004-28032011000400011. https://doi.org/10.1590/s0004-28032011000400011. [DOI] [PubMed] [Google Scholar]
- 34.Brzozowski T, Konturek PC, Sliwowski Z, Drozdowicz D, Hahn EG, Konturek SJ. Importance of nitric oxide and capsaicin-sensitive afferent nerves in healing of stress lesions induced by epidermal growth factor. Journal of Clinical Gastroenterology. 1997;25:S28–S38. doi: 10.1097/00004836-199700001-00007. https://doi.org/10.1097/00004836-199700001-00007 PMid:9479624. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi K, Kato S, Takehara K, Asada Y, Yasuiro T. Role of nitric oxide in mucosal blood flow response and the healing of HCl-induced lesions in the rat stomach. Digestion. 1997;5:19–27. doi: 10.1159/000201419. [DOI] [PubMed] [Google Scholar]
- 36.Hudson N, Balsitis M, Everitt S, Hawkey CJ. Angiogenesis in gastric ulcers:impaired in patients taking non-steroidal anti-inflammatory drugs. Gut. 1995;37(2):191–4. doi: 10.1136/gut.37.2.191. https://doi.org/10.1136/gut.37.2.191 PMid:7557566 PMCid:PMC1382716. [DOI] [PMC free article] [PubMed] [Google Scholar]


