Abstract
AIM:
The role of behavioural factors and sleep duration and quality is important in the pathogenesis of obesity. The aim of our study was to evaluate the effects of behavioural risk factors on melatonin secretion in women.
SUBJECTS AND METHODS:
In total, 120 female patients were enrolled in the study and divided into two groups according to the body mass index. Detailed history, anthropometric measurements, urine and blood samples were evaluated for each patient.
RESULTS:
Two groups significantly differed in weight, BMI, and waist circumference, and were 94.2 ± 14.9 kg, 33.4 ± 5.23 kg/m2 and 99.2 ± 12.6 cm for the study group and 56.0 ± 5.2 kg, 20.0 ± 1.8 kg/m2 and 60.1 ± 10.4 cm for the control group, respectively, sleep disruptions were detected in 48 patients from study group, with mean score 6.76 ± 3.6, and only 10 patients were detected in the control group, with mean score 4.42 ± 1.68. Eating disturbances were revealed in 66 patients from the study group and 21 patients from the control group. Melatonin levels were 17% higher in the study group, compared to control group.
CONCLUSION:
Higher melatonin levels in patients with obesity and concomitant behavioural impairments may be due to its protective effect to fight free radicals and to induce vasodilatation. Further studies are needed to confirm our finding.
Keywords: Obesity, Eating Disturbances, Sleep disorders, Melatonin, Behavioral disorders
Introduction
Obesity is a major medical, social and economical health problem of modern century, contributing to impairment of quality of life, lost productivity, development of serious comorbidities and invalidation. Statistically, obesity prevalence is three times higher in women, than in men and the prognosis is even pessimistic; by 2025, every second female with childbearing potential will be obese [1]. Increased incidence of obesity among females with childbearing potential is especially important because it influences not only reproductive capacity, including infertility but also causes early development of serious chronic diseases such as arterial hypertension, ischemic heart disease, atherosclerosis and diabetes mellitus [2]. Moreover, overweight and obesity have been demonstrated to be important risk factors for several types of cancers including those that involve the reproduction- related tissues [1].
Among multiple etiological factors, including, genetics, age, sex, profession, health conditions (pregnancy, lactation, menopause), endocrine disorders, environmental factors and many others [1, 2], published studies over the last years, have provided considerable evidence regarding the important role of behavioral factors in development of obesity. Behavioral factors, including inadequate dietary habits and night eating syndrome, sedentary lifestyle and sleep disturbance, are responsible for development of obesity “pandemic” [3].
Numerous studies have shown the correlation between sleep disturbance and development of cardio- metabolic disorders [3]. According to the data of longitudinal studies, the prevalence of insomnia among the obese population is 3.9-22.1% [4]. Therefore, many authors recommend adding sleep disturbance, and mostly obstructive sleep apnea, as one of the components of metabolic syndrome [3, 5].
Obesity and sleep disturbances are comorbid conditions, with underling neuroendocrine genesis [6]. However, the molecular mechanisms that are involved in the association between them remain unclear. Melatonin is an important regulator of circadian rhythm and its action on sleep-wake phase has already been proven [7]. Furthermore, melatonin has an important positive affect on immunomodulation and oncostatin, and it has also shown to have antiproliferative and antihypertensive effects [4, 8].
After entering the circulation, melatonin acts as an endocrine factor and a chemical messenger of light and darkness. It regulates a variety of important central and peripheral actions related to circadian rhythms [9]. It also affects the brain, immune, gastrointestinal, cardiovascular, renal, bone and endocrine functions and acts as an oncostatic and anti-aging molecule. Growing scientific evidence of melatonin, also suggests that it has some role in anorexia nervosa and bulimia [10, 11].
Therefore, the aim of our study is to evaluate the effects of behavioural risk factors on melatonin secretion in women with childbearing potential.
Subjects and Methods
The study population was derived from a database of National Institute of Endocrinology, Tbilisi, Georgia, during 2013 -2016 yy. The local ethics committee has approved the study.
The following inclusion criteria used to identify the study cohort was: (1) female patients of childbearing potential (age 16-35 years); (2) body mass index > 25 kg/m2; (3) Stable course of concomitant disease; (4) Signed informed consent before participation in the study.
Patients, with endocrine disorders, chronic or acute liver and kidney disease or cancer and with the history of corticosteroid use for three months before the informed consent was obtained, were excluded from the study.
In total, 120 female patients were enrolled in the study. Eighty female patients of childbearing potential with body mass index (BMI) > 25 kg/m2 were selected for the study group. Forty females, with BMI 19.0-24.9 kg/m2, matched by sex and age, were selected for the control group.
Following components were evaluated for each patient: detailed history (including demography, genetic predisposition, behavioural disorders (sleep, eating, physical activity, alcohol and tobacco use) and concomitant disorders), anthropometric measurements, ultrasonography of abdominal cavity, urine and blood samples for analysis.
Several established measures of disordered eating and sleeping were included in the survey.
Pittsburg Sleep Quality Index was used to measure quality and patterns of sleep [12]. Seven areas are evaluated with this questionnaire, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the last month. A total score of “5” or greater is indicative of poor sleep quality.
Eating habit disorders were evaluated by DEBQ (Dutch Eating Behavior Questionnaire) that was developed in Netherlands in 1986. The questionnaire gives us the opportunity to distinguish between restrained, emotional, and external eating habits [13]. Emotional eating is defined as eating in response to emotional arousal states such as fear anger or anxiety, external eating arose in response to external food cues such as sight and the smell of food and under restraint eating is meant overeating after a period of slimming when the cognitive resolve to diet is abandoned. For assessment of eating habit, we also evaluated the patterns, numbers and characteristics of eating. Nighttime eating was assessed according the following criteria: ≥ 25% of total daily food intake at nighttime and/or two or more episodes of nighttime eating per weak and morning anorexia for at least three month period [14].
Anthropometric data, including height and weight, were collected for each patient using Seca Medical 763. Waist circumference was measured at the midpoint between the lower margin of the least palpable rib and the top of the iliac crest, using a stretch-resistant tape (Waist circumference and waist–hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008).
BMI was calculated using WHO classification, where BMI is defined as the weight in kilograms divided by the square of the height in meters (kg/m2).
Ultrasonography of abdominal cavity was performed by Philips Clearview 550.
Blood samples were drawn after 10 hour fasting period. Fasting glucose and lipid profile (including total cholesterol, triglycerides, HDL-cholesterol and LDL-cholesterol) were measured using BioSystem A-15 (automatic analyser). Melatonin metabolite six sulfatoxymelatonin levels were evaluated in morning urine sample and assessed by imunoenzyme method (Rayto RT-2100C), using IBL: melatonin sulphate 6–sulfatoxymelatonin, ELISA, DRG test system.
Significant level was set at p < 0.05. Odds ratio (OR) was defined for behavioural disorders, with 95%- confidence interval (CI) and positive and negative predictive value was calculated, using table (2X2). For comparative analysis of study and control groups (χ2) was identified with P critical value < 0.05. Pearson’s correlation coefficient (r) was calculated to determine the relationship between study parameters. Statistical analysis was performed using Microsoft Excel 2010 and SPSS/v15 software packages.
Results
Mean age of the total study population was 29.7 ± 5.01 years. Two groups significantly differed in weight, BMI, and waist circumference, and was 94.2 ± 14.9 kg, 33.4 ± 5.23 kg/m2 and 99.2 ± 12.6 cm for the study group and 56.0 ± 5.2 kg, 20.0 ± 1.8 kg/m2 and 60.1 ± 10.4 cm for the control group, respectively.
All patients from the study group had liver hepatosis of mild to a moderate degree; while no cases of liver hepatosis were seen in any subject from the control group.
The mean duration of obesity in the study group was 7.49 ± 7.04 years. Baseline characteristics of the study population are given in Fig. 1.
Figure 1.
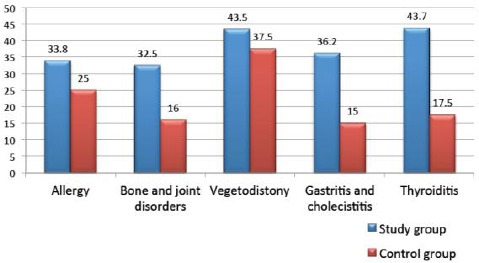
Concomitant somatic disease in study population (%)
The detailed characteristics of behavioural risk factors for both groups are presented in Fig. 2.
Figure 2.
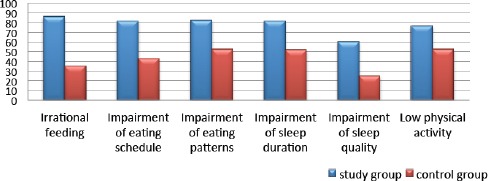
Differences in behavioural risk factor distribution among studied groups (%)
According to Pittsburg Sleep Quality Index, 48 patients from study group (60%) were detected with sleep disruptions, with mean score 6.76 ± 3.6, whereas, only ten patients were detected in the control group (25%), with mean score 4.42 ± 1.68. The sleep disruptions were in correlation with According to DEBQ (Dutch Eating Behavior Questionnaire), eating disturbances were revealed in 66 patients (89.2%) from the study group and 21 patients (67.7%) from the control group. Detailed characteristics of eating impairments are given in Fig. 3.
Figure 3.
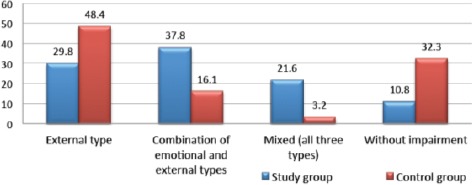
Distribution of types of eating disorders between studied groups (%)
Odds ratio with 95% CI was measured for behavioural factors, as potential risk factors for obesity. The data on quantitive indicators of behavioural risk factors are presented in Table 1.
Table 1.
Quantitative indicators of behavioural risk factors in total study population (n=120)
| Behavioral risk factors | Study group N = 80 | Control group N = 40 | OR | CI | Pv+ | Pv– | χ2 | P |
|---|---|---|---|---|---|---|---|---|
| Irrational eating | 69 (86.3%) | 14 (35.0%) | 11.65 | 4.31-32.38 | 0.83 | 0.70 | 30.48 | 0.0001 |
| Impairment of eating schedule | 65 (81.3%) | 17 (42.3%) | 5.86 | 2.34–14.92 | 0.79 | 0.61 | 16.76 | 0.0001 |
| Impairment of eating patterns | 66 (82.5%) | 21 (52.5%) | 4.27 | 1.69-10.90 | 0.76 | 0.58 | 10.58 | 0.002 |
| Sleep disorders | 65 (81.3%) | 20 (52.0%) | 4.33 | 1.74–10.93 | 0.76 | 0.57 | 11.14 | 0.001 |
| Impairment of sleep quality | 48 (60%) | 10 (25%) | 4.50 | 1.80–1.47 | 0.83 | 0.48 | 11.71 | 0.001 |
| Decreased physical activity | 61 (76.3%) | 21 (52.5%) | 2.91 | 1.20–7.06 | 0.74 | 0.5 | 5.90 | 0.015 |
Insulin resistance and disorders in lipid profile were seen in 49 and 48 patients from the study group (61.3 and 60%, respectively) and were not seen in any patient in the control group. Interestingly, statistically significant correlation was seen between dislipidemia status and obesity duration for more than ten years (P = 0.027), excessive intake of products rich in fat (P = 0.019), impairment of eating schedules (P = 0.045) and sleep disruptions (P = 0.010). Melatonin levels were 17% higher in the study group, compared to control group (130.6 ± 124.1 and 107.5 ± 103.9 ng/ml). Because of inconsistent melatonin levels in the present study, there was seen no correlation of melatonin with any study components.
Discussion
Statistically, a significant association was seen between overweight and obesity with impaired behavioural risk factors. The odds ratio was especially high for irrational eating (OR–11.65), eating schedule impairments (OR–5.86) and disruptions in sleep quality (OR–4.50). Furthermore, the prognostic value of positive response was also high in above-mentioned impairments and was 83%, 79% and 83%, respectively.
The two studied groups significantly differed according to the disturbances in eating patterns. The external type was the primary eating impairment in the control group, while the combination of two or three impairment types was seen in the study group, indicating the greater effect of overweight and obesity on behavioural factors. Our results are in line with the majority of publications, were more then half of female patients with obesity are prone to have obvious impaired eating patterns and the rest have hidden disruptions, that are covered by comorbid neuro-psychological disorders, including depression, hypochondria, anxiety, etc. Literature data indicate that obese people, among other eating disorder, also have impaired external eating behavior, indicating that they respond not only on internal (glucose and free fatty acid levels, gastric fulness), but also on external stimuli (advertisement), while lean individuals respond to external stimuli only in case of hunger [15]. The current study revealed that people without obesity also have the external eating disorders that might be explained by the fact, that lean person; do not have to control their appetite because of not having a weight problem, unlike obese individuals, who suppress their appetite, not always realizing it.
The present study revealed that prevalence of the sleep disruptions was 1.5 times higher in the study group, compared to the control group and was seen in 66 (82.5%) vs. 19 (47.5%) patients, respectively (χ2–10.580, P = 0.002). Most common complaints were a reduction of sleep duration, frequent awakening during nighttime and somnolence during the daytime. Mean duration of sleep was 7.4 ± 1.3 hour.
Several studies have reported an association between chronic sleep deprivation and long-term weight gain. As of today, over 40 studies describe either an inverse or U-shaped relationship between self-reported sleep disorders and weight gain. Severe sleep deprivation has been shown to reduce insulin sensitivity via increased cortisol, cytokines, and other endocrine and immune mechanisms and increase appetite via decreased leptin and increased ghrelin levels [16].
Our findings are in contrast with some publications, were melatonin secretion deficiency is considered as the primary reason for sleep disruptions [11]. It is important to note, that melatonin hypersecretion is often seen in the pathogenesis of such disorders, as menopause syndrome, nighttime hypertension and hyperplastic processes of uterus and ovaries [15]. According to some authors, hypermethioninemia, similarly to low serum melatonin levels, is associated with neuro-vegetative and metabolic disorders and worsens the course of primary disease [17].
Our results according to melatonin levels were inhomogenous in the study groups. Nineteen patients in the study group had decreased melatonin levels (mean level 32.2 ± 14.1 ng/ml) and sleep deprivations expressed by late sleep or night eating syndrome. It is well known that night eating syndrome is not a just psychological disorder, but has underlying hormonal disbalance of decreased melatonin and increased cortisol levels [14, 4].
Controversial results, concerning the elevated melatonin levels in the study group, were seen in the majority of patients. Increased melatonin levels were associated with eating behaviour disorders (136.6 ± 132.2 ng/ml), low physical activity level (139.4 ± 117.5 ng/ml) and sleep deprivation (141.9 ± 133.5 ng/ml). According to various authors, melatonin hypersecretion during metabolic syndrome is induced due to increased sympathetic activity on suprachiasmatic nuclei. Furthermore, chronic insulin resistance studies, melatonin has been shown proven antioxidant capacity, expressed by strong free radical scavenging properties and vasodilation activity, indicating that hypersecretion of this hormone may be compensatory, due to protective effect [17]. There are some data, where people are divided into normal, hypo and hypermelatoninemics. Beside similar sleep patterns, people may have significantly different melatonin levels in the urine, indicating that other mechanisms, then sleep, are involved in the metabolism of melatonin. Our results are also in line with abovementioned data, confirming the multifactorial pathogenesis of melatonin secretion and the importance of its quality of action, rather than quantity.
Prevalence of melatonin hypersecretion was high in patients with obesity and concomitant behavioural impairments, such as eating disorders, sleep disruption and reduced physical activity. As other hormones, including leptin, ghrelin and cortisol, are also involved in the pathogenesis of obesity, it will be interesting to evaluate the effect of all these hormones on behavioural impairments. More detailed evaluation will have practical value and might help to develop new ways of diagnosis and treatment of obesity. More studies are needed to better understanding of this mechanism.
In conclusion, people with same sleep patterns may have decreased normal or elevated levels of melatonin that might be due to several mechanisms. In present studies, data regarding melatonin levels were also inconsistent. Higher melatonin levels in patients with obesity and concomitant behavioural impairments may be due to its protective effect to fight free radicals and to induce vasodilatation. Further studies are needed to confirm our finding.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Crujeiras AB, Casanueva FF. Obesity and the reproductive system disorders:epigenetics as a potential bridge. Hum Reprod Update. 2014:dmu060. doi: 10.1093/humupd/dmu060. PMid:25413685. [DOI] [PubMed] [Google Scholar]
- 2.Pinkhasov BB. Pathogenetic features of primary obesity and its types in women of reproductive age. International Journal of Endocrinology. 2011;8(40) [Google Scholar]
- 3.Strueva NV, Poluektov MG, Savelieva LV. Obeisty and sleep. Obesity and metabolism. 2013;3 [Google Scholar]
- 4.Lemoine P, Nir T, Laudon M, et al. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372–80. doi: 10.1111/j.1365-2869.2007.00613.x. https://doi.org/10.1111/j.1365-2869.2007.00613.x PMid:18036082. [DOI] [PubMed] [Google Scholar]
- 5.Jonge L, Zhao X, Mattingly MS, Zuber SM, Piaggi P, et al. Poor Sleep Quality and Sleep Apnea Are Associated with Higher Resting Energy Expenditure in Obese Individuals with Short Sleep Duration. J Clin Endocrinol Metab. 2012;97(8):2881–2889. doi: 10.1210/jc.2011-2858. https://doi.org/10.1210/jc.2011-2858 PMid:22689694 PMCid:PMC3410277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunz D, Mahlberg R, Muller C, Tilmann A, Bes F. Melatonin in patients with reduced REM sleep duration:two randomized controlled trials. The Journal of Clinical Endocrinology and Metabolism. 2004;89(1):128–134. doi: 10.1210/jc.2002-021057. https://doi.org/10.1210/jc.2002-021057 PMid:14715839. [DOI] [PubMed] [Google Scholar]
- 7.Pirozzi FF, Bonini-Domingos CR, Ruiz MA. Metabolic Actions of Melatonin on Obesity and Diabetes:A Light in the Darkness. Cell Biol:Res Ther. 2015;4:2. https://doi.org/10.4172/2324-9293.1000119. [Google Scholar]
- 8.Zhang HM, Zhang Y. Melatonin:a well-documented antioxidant with conditional pro- oxidant actions. J Pineal Res. 2014;57(2):131–46. doi: 10.1111/jpi.12162. https://doi.org/10.1111/jpi.12162 PMid:25060102. [DOI] [PubMed] [Google Scholar]
- 9.Tan DX, Manchester LC, Fuentes-Broto L, et al. Significance and application of melatonin in the regulation of brown adipose tissue metabolism:relation to human obesity. Obes Rev. 2011;12:167–188. doi: 10.1111/j.1467-789X.2010.00756.x. https://doi.org/10.1111/j.1467-789X.2010.00756.x PMid:20557470. [DOI] [PubMed] [Google Scholar]
- 10.Burchakov D.I. Melatonin–adaptogen of female reproductive system. “Effective pharmacotherapy Obstetrics and gynecology”. 2015;1(5) [Google Scholar]
- 11.Mahlberg R, Kunz D. Melatonin excretion levels and polysomnographic sleep parameters in healthy subjects and patients with sleep-related disturbances. Sleep Med. 2007;8(5):512–6. doi: 10.1016/j.sleep.2006.11.001. https://doi.org/10.1016/j.sleep.2006.11.001 PMid:17581781. [DOI] [PubMed] [Google Scholar]
- 12.Buysse D.J, Reynolds C.F, Monk T.H, Berman S.R, Kupfer D.J. The Pittsburgh Sleep Quality Index:A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. https://doi.org/10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Van Strein T. The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional and external eating behavior. Int J Eating Disord. 1986;2:188–204. https://doi.org/10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T. [Google Scholar]
- 14.Allison KC, Lundgren JD, O’reardon JP, Geliebter A, Gluck ME, Vinai P, Mitchell JE, Schenck CH, Howell MJ, Crow SJ, Engel S. Proposed diagnostic criteria for night eating syndrome. International Journal of Eating Disorders. 2010;43(3):241–7. doi: 10.1002/eat.20693. PMid:19378289 PMCid:PMC4531092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvetnaia TV, Antropova OE, Golubitskaia EG, Burimenko EI, Mursalov CU, Bolshakov AA. Levels of melatonin in the elderly people with metabolic syndrome. Gerontology. 2013;2:50. [Google Scholar]
- 16.Cizza G, Requena M, Galli G, De Jonge L. Chronic sleep deprivation and seasonality:implications for the obesity epidemic. Journal of endocrinological investigation. 2011;34(10):793. doi: 10.3275/7808. PMid:21720205 PMCid:PMC3297412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voznesenskaia TG. Eating disorders in obesity and their correction. Obesity and metabolism. 2004;2 [Google Scholar]


