Abstract
BACKGROUND:
Elderly population (≥ 65) are more prone to develop acute kidney injury (AKI) compared to younger, also elderly with AKI have an increased requirement for dialysis treatment and an elevated risk of short-term and long-term mortality.
AIM:
The objectives of this study were to examine the effect of treatment of short-term outcomes and mortality in elderly patients with AKI.
MATERIAL AND METHODS:
Seventy elderly AKI patients, that filled one of the criteria of AKI definition and had hospitalization over 24 hours, were enrolled in the study.
RESULTS:
The median age of patients was 74.28 ± 6.64, with mean CCI (Charlson Comorbidity Index) score of 6.94 ± 1.94. The majority of patients (70%) were classified at stage 3 of AKIN, 20% of patients were classified at stage 2 and 10% at stage 1. In the groups of patients with death outcome, the chronic cardiomyopathy was more frequently present (p = 0.034). Regarding treatment, 58.6% of the AKI patients underwent hemodialysis while 41.4% received conservative treatment. Mortality rate was 52.8%, out of which 28.6% was in-hospital mortality, while in 24.3% of patients death occurred in the follow-up period of 90 days.
CONCLUSION:
In our study, short- term survival is not related to different treatment options. Applied treatment in elderly patients with AKI should be assessed by measuring the long term outcome.
Keywords: Acute kidney injury, elderly, treatment, outcome, hemodialysis
Introduction
Acute kidney injury (AKI), previously termed as acute renal failure (ARF), is a common acute syndrome characterized by an abrupt or rapid decline in renal filtration function [1]. The elderly (≥ 65) are particularly vulnerable to renal insults which are explained with age related structural and functional changes that lead to decreased glomerular filtration rate, vascular dysautonomia, and alter diminished renal reserve [2]. Increased longterm life expectancy is commonly is accompanied with chronic diseases such as hypertension, diabetes mellitus, and cardiovascular diseases. Also, elderly patients are exposed to multiple medications with related toxicity and invasive procedures. These risk factors together make elderly susceptible to AKI. The incidence of AKI in the elderly population is higher than in younger, and age as a predictive factor of outcomes and mortality is reported by multiple studies [3-5].
An increased requirement has also been reported for dialysis treatment in the elderly with AKI, and the incidence is up to 10 times higher compared to patients under 65 years of age [6-8]. Older patients with AKI have an elevated risk of both short-term and long-term mortality [9, 10] and survivors are often left with multiple chronic conditions which make prognosis significantly poor at the time after hospital discharge. Elderly patients with AKI have significantly higher chance of non-recovery of renal function [5] and 3-20 fold higher risk of subsequent chronic kidney disease (CKD) [11] that eventually progresses to end stage renal disease (ESRD), particularly in the case of AKI and prior CKD [12]. The objectives of this study were to examine the effect of treatment of short-term outcomes and mortality in elderly patients with AKI.
Material and Methods
The prospective observational study has been conducted at the University clinic of nephrology Skopje. In the study, seventy patients that were treated at the Clinic of nephrology Skopje over the period of 8 months were enrolled in the study. The study protocol was approved by the ethic commission of Medical Faculty in Skopje, and written consents were obtained from the patients enrolled in the study or by a family member.
Inclusive criteria were: Age ≥65, filling out one of the criteria of the definition of AKI according to Kidney Disease Improving Global Outcome (KDIGO) and hospitalization above 24 hours. Patients with chronic kidney disease (CKD) on maintenance dialysis or peritoneal dialysis and renal transplantation were excluded.
Laboratory parameters, urea, serum creatinine, sodium, potassium, calcium, phosphate, serum albumin, C-reactive protein (CRP) levels were measured at admission for all subjects. Clinical data include patient’s age, gender, admission and discharge data, causes of AKI and applied treatment. Data about co-existing comorbidities was taken from patient’s medical records or were diagnosed during the hospitalization. The burden of comorbidities was estimated by Charlson comorbidity index (CCI). This index incorporates age and 19 different medical conditions, each weight from 1-6 according to its impact on mortality [13]. The age is adjusted by calculating each decade after 40 years of age at one point. The total sum of all the weights results in a single comorbidity score for a patient. The standard algorithm proposed by Quan et al. was used to define the Charlson comorbidity index (CCI) score [14].
Patients were divided into groups of survivors and nonsurvivors based on their survival status at 90 days. Depending on the treatment patients were divided into groups of patients treated with hemodialysis and groups of patients treated conservatively. The indications for the initiation of hemodialysis were volume overload inadequately controlled with diuretics, refractory hyperkalaemia to conservative measures, the presence of insufficient urinary output, uraemic signs or symptoms and clinical severity of illness of patients. The less severe patients with preserved diuresis were treated conservatively. Pre-existing chronic kidney disease (CKD) was estimated by the value of serum creatinine (sCr) over the last three months before the current episode of acute injury as it is recommended by KDIGO. When the value sCr was not available, the consensus committee recommended the use of the back-calculation [15] with the Modification of Diet in Renal Disease (MDRD) to estimate patients GFR, assuming all patients had a normal GFR of 75 ml/min, or the lowest value of sCr during hospitalization or respectively at discharge. We defined short-term patient’s mortality as a death during the hospital stay or death within follow up 90 days of diagnosis of AKI.
Definition and classification of AKI
AKI was defined as an increase in SCr by 26.5ulmol/l within 48 hours or a 50% increase in SCr from the baseline within seven days according to the KDIGO criteria (16). By KDIGO criteria, AKI is classified at three stages by severity determined using the peak sCr level after AKI onset.
Statistical analysis
The results are presented with descriptive statistics expressed as the mean ± standard deviation. For comparison of the two groups, Student’s test was used. χ2 test or Fisher’s test was used to compare the two groups with categorical (nominal) variables. Cumulative proportion surviving Kaplan Meier (significance test, log-rank) was used for statistical processing of the data where mortality was the outcome. P values less than 0.05 were considered statistically significant.
Results
Seventy patients (53% female) who met inclusion criteria were included in the study, with average age 74.28 ± 6.64 years and with mean CCI score of 6.94 ± 1.94. The most common comorbid condition was chronic heart failure with 47% of patients and diabetes mellitus with 42.8%. Pre-existing chronic kidney diseases was present in 44.3%. The majority of the patients (70%) were classified as stage 3 of AKIN, 20% of patients were classified as stage 2 and 10% as stage 1. Regarding treatment, 58.6% of the AKI patients underwent hemodialysis while 41.4% received conservative treatment. Mortality rate was 52.8%, out of which 28.6% was in-hospital mortality, while in 24.3% of patients death occurred in follow up 90 days. Baseline and demographic characteristics of the enrolled patients are presented in Table 1.
Table 1.
Characteristics of AKI population
| Characteristics | |
|---|---|
| Age (years) | 74.28 ± 6.64 (65-91) |
| Gender male/female) | 33 (47%)/37 (53%) |
| Length of stay (LOS) days | 9.13 ± 5.69 (1-30) |
| CCI score | 6.94 ± 1.94 |
| sCr (admission) | 667.81 ± 286.72 (202-1623) |
| sCr (discharge) | 238.57 ± 175.06 (58-844) |
| GFR (ml/min) | 38.33 ± 24.96 (7-108) |
| Pre- existing CKD | 31 (44.3 %) |
| CHF | 33 (47%) |
| Diabetes mellitus | 30 (42.8 %) |
| Urine output (ml) | 989.38 ± 746.25 |
| Anuria | 6 (8.6%) |
| Oliguria | 14 (20%) |
| Diuresis ≥ 500ml | 50 (71.4%) |
| AKIN | |
| Stage 1 | 7 (10%) |
| Stage 2 | 14 (20%) |
| Stage 3 | 49 (70%) |
| Treatment (HD/Conservative) | 41 (58.6%)/ 29 (41.4%) |
| Mortality | |
| Intra hospital | 20 (28.6%) |
| During 90 days follow-up | 17 (24.3%) |
| Total | 37 (52.8%) |
| Survived ≥ 90 days | 33 (47.1%) |
Abbreviations: CCI, Charlson comorbidity index; SCr, serum creatinine; GFR, glomerular filtration rate; AKIN, CHF, chronic heart failure; Acute Kidney Injury Network; LOS Length of hospital stay. Data are presented as means and standard deviation.
The most frequent causes of AKI in our population were prerenal causes (36 patients, 51.4%, volume depletion, hypotension), postrenal (14 patients, 2%, obstruction, malignancy), cardiovascular event (11 patients, 15.7%, cardiorenal syndrome- CRS), sepsis with 11.4% and renal cause was less frequent, with 1.4%.
Figure 1.
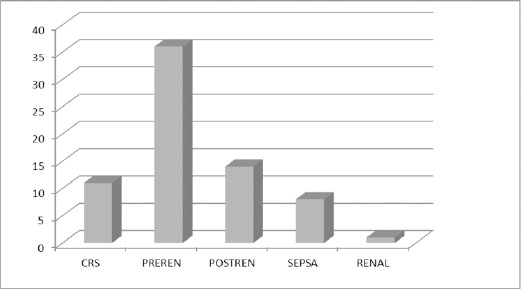
Causes of AKI
Comparison of clinical and laboratory parameters between survivor and no- survivor AKI patients is shown in Table 2.
Table 2.
Comparison of clinical and laboratory parameters between survivor and no- survivor AKI patients
| Characteristics | Survivor N = 33 | Non survivor N = 37 | p |
|---|---|---|---|
| Age (years) | 73.76 ± 6.96 | 74.76 ± 6.39 | n.s |
| CCI scor | 6.55 ± 2.02 | 7.9 ± 1.83 | n.s |
| DM vs non DM | 12 (17.14%)/21 (30.0%) | 18 (25.7%)/19 (27.14%) | n.s |
| Preexisting CKD vs without CKD | 17 (24.3%)/16 (22.9%) | 14 (20%)/23 (32.8%) | n.s |
| HD vs Conservative treatment | 19 (27.1%)/14 (20%) | 22 (31.3%)/15 (21.4%) | n.s |
| CHF vs non CHF | 11 (15.7%)/22 (31.4%) | 22 (31.4%)/15 (21.4%) | 0.025 |
| sCr (µmol/l) | 684.91 ± 297.04 | 652.57 ± 280.41 | n.s |
| BUN (mmol/l) | 37.38 ± 18.19 | 40.19 ± 14.03 | n.s |
| GFR (ml/min) | 37.95 ± 22.08 | 38.69 ± 27.71 | n.s |
| Hb (g/l) | 109.79 ± 20.69 | 108.83 ± 20.49 | n.s |
| Plt (×109/l) | 247.42 ± 101.34 | 223.89 ± 113.36 | n.s |
| CRP (mg/L) | 96.19 ± 101.01 | 132.93 ± 97.46 | n.s |
| Na (mmol/l)) | 136.67 ± 6.83 | 135.54 ± 7.54 | n.s |
| K (mmol/l) | 5.23 ± 1.50 | 5.06 ± 1.23 | n.s |
| Ca (mmol/l) | 2.11 ± 0.20 | 2.01 ± 0.20 | n.s |
| Alb (g/l) | 33.29 ± 5.84 | 31.15 ± 6.19 | n.s |
| Du (ml) | 1222.76 ± 779.75 | 801.39 ± 671.14 | 0.022 |
| LOS (days) | 11.78 ± 5.87 | 6.84 ± 4.45 | 0.000 |
Abbreviations: CCI, Charlson comorbidity index; SCr, serum creatinine; BUN, blood urea nitrogen; GFR, glomerular filtration rate; Hgb, haemoglobin; Plt, platelets; CRP, C-reactive protein; Na, sodium; K, Potassium; Alb, albumin; Du, urine output LOS Length of hospital stay.
Deceased AKI patients had a higher percentage of chronic heart failure (CMP) (p = 0.025) as well as lower diuresis and shorter length of stay which is statistically significantly different compared to the group of survivors. (p = 0.022). A significant association was not registered among the other compared clinical and laboratory variables in the two groups of patients, survivors and non-survivors.
When comparing the three groups of patients according to the outcome: survivors (≥ 90 days), the group of patients in which death occurred during hospitalization and during follow up period up to 90 days, we found that the age was similar in these three groups. The group of survivors had significantly higher diuresis compared to the other two groups (p = 0.001). Also, they had longer hospital stay (p = 0.000). In the groups of patients with death outcome, the chronic cardiomyopathy was more frequently present (p = 0.034). The value of serum Cr at admission was significantly higher in the group of patients in which death occurred during hospitalization (p = 0.029). These statistical differences among the AKI patients that were divided according to the outcomes are presented in Table 3.
Table 3.
Statistical significant differences among the AKI patients according to the outcome
| Characteristics | In-hospital mortality N=20 | Deceased during the follow-up N=17 | Survivor No=33 | p |
|---|---|---|---|---|
| Age (years) | 73.80 ± 6.53 | 75.88 ± 6.22 | 73.75 ± 6.95 | n.s |
| Diuresis (ml) | 497.36 ± 466.98 | 1141.17 ± 712.44 | 1222.75 ± 779.75 | 0.001 |
| LOS (days) | 4.65 ± 3.86 | 9.41 ± 3.73 | 11.78 ± 5.87 | 0.000 |
| CHF | 28.6% | 24.3% | 47.1% | 0.034 |
| sCr-admission | 349.30 ± 226.52 | 192.02 ± 104.71 | 217.65 ± 167.09 | 0.029 |
Abbreviations: LOS Length of hospital stay; CHF, chronic heart failure; SCr, serum creatinine.
We plotted Kaplan–Meier survival curves stratified by urine output at the time of initiation of renal replacement therapy. Fig. 2 shows that a decreased urine output was associated with lower survival (p = 0.001 by the log-rank test).
Figure 2.
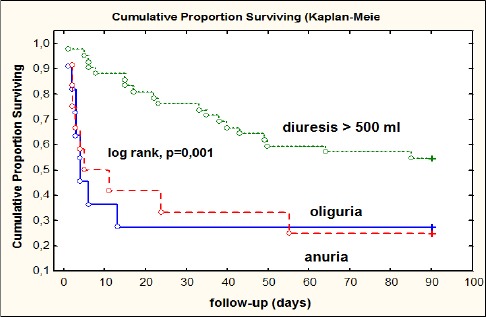
Survival curves according to the urine output at the time of initiation of renal replacement therapy
According to treatment patients were divided into 2 groups: group of patients treated with hemodialysis and group of patients treated conservatively. As shown in Table 4, significant difference between the hemodialysis vs conservatively treated group of patients was evident in sCr at admission (765.24 μmol/L vs. 530.06 μmol/L; p = 0.000), BUN at admission (43.20 mmol/L vs. 32.73 mmol/L; p = 0.006), diuresis (p = 0.032) and Potassium (5.44 mmol/L vs. 4.70 mmol/L; p = 0.022).
Table 4.
Comparison of laboratory parameters of elderly patients with AKI according to the treatment (hemodialysis / conservative)
| Characteristics | Hemodialysis N = 41 | Conservative treatment N = 29 | p |
|---|---|---|---|
| Age (year) | 74.00 ± 6.92 | 74.69 ± 6.29 | n.s |
| sCr- admission | 765.24 ± 288.00 | 530.06 ± 224.81 | 0.000 |
| sCr- discharge | 265.09 ± 203.05 | 207.31 ± 131.75 | n.s |
| BUN- admission | 43.20 ± 17.57 | 32.73 ± 11.35 | 0.006 |
| BUN- discharge | 25.54 ± 35.70 | 18.06 ± 11.28 | n.s |
| Hb (g/l) | 112.89 ± 21.07 | 104.44 ± 18.85 | n.s |
| CRP | 116.94 ± 114.59 | 115.57 ± 75.08 | n.s |
| Na (mmol/l)) | 134.92 ± 7.40 | 137.69 ± 6.65 | n.s |
| K (mmol/l) | 5.44 ± 1.40 | 4.70 ± 1.16 | 0.022 |
| Alb (g/l) | 31.42 ± 5.95 | 33.22 ± 6.20 | n.s |
| Du (ml) | 817.83 ± 819.85 | 1216.07 ± 574.65 | 0.032 |
Abbreviations: sCr, serum creatinine; BUN, blood urea nitrogen; Hgb, haemoglobin; Plt, platelets; Na, sodium; K, Potassium; Alb, albumin; Du, diuresis.
No statistically significant difference was evident between the hemodialysis and conservatively treated group in term of comorbidities, Diabetes mellitus, Chronic Heart Failure and pre-existing Chronic Kidney Disease, as shown in Table 5. Figure 3 presents Kaplan-Meier survival curve between the group of patients treated with hemodialysis and group of a patient treated conservatively, with no significant difference in survival during the follow-up period up to 90 days (p = 0.633 by the log rank test).
Figure 3.
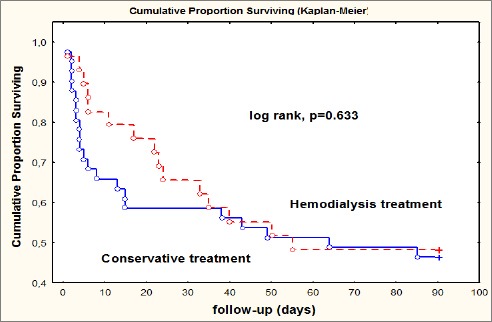
Kaplan-Meier survival curve according to the treatment
Discussion
AKI is a growing disease for the elderly patients associated with morbidity and mortality, increased requirement for dialysis treatment and significantly worse clinical outcome. Our study was performed to evaluate the effect of applied treatment on short-term outcomes in elderly patients with AKI. Seventy patients who met AKI criteria were enrolled in the study, with a median age of 74 years and meant CCI score of 6.55 ± 2.02 in the group pf survivors and even higher (7.9 ± 1.83) in the group of non-survivors which indicates high burden of comorbidities. Pre-existing CKD was present with 44.3%. The most common comorbid condition was chronic/acute heart failure in 47% of patients. Even in 11% of patients “cardiorenal syndrome” (CRS) was the cause of AKI which speaks about the bidirectional interaction between these two organs. Resent study confirmed heart failure as an independent predictor of deterioration of renal function [17]. Our results showed statistically significant differences regarding heart failure between surviving and not a surviving group. The second most common comorbidity condition was diabetes mellitus which may significantly affect kidneys and may potentially increase AKI risk and long-term mortality/morbidity of AKI [18] but was not confirmed by our study. Significant lower diuresis was noted in the group of non-survivors, which could be explained by the severity of renal impairment, preexisting kidney disease or presence of cardiovascular disease that could be associated with lower “output”. Also, significant shorter hospitalization was registered in no surviving group due to an early in- hospital death as a result of an overall critical condition of these patients by the worse CCI score, or multiple organ failure conditions.
Comparing the tree groups of patients divided according to the outcomes: group with in-hospital mortality group with death occurred during follow up period up to 90 days and group of survivors (≥ 90 days), we found significant differences among diuresis, the length of stay, the value of sCr at admission and the presence of CHF. Diuresis was lowest in the group of patients with in-hospital death, which also has registered the highest value sCr admission. The sCr was with the lowest value in the group of patients in which death occurred during the follow-up period; hence the cause of death in this group could be explained by the severity of pre-existing comorbid conditions. The median age between surviving and not a surviving group of patients also showed no significant statistical difference that is in contrary with referenced data from EACH study conducted on a large group of the adult population [19].
The majority of patients were classified at AKIN stage 3 (70%) and 20% of patients at AKIN stage 2, which points out the severity of AKI in our group of patients. Regarding treatment, patients were divided into a group with dialysis treatment and group with conservative treatment. A high percentage of patients (58.6%) underwent hemodialysis, which was by severity of AKI. It is expected, significantly higher values of BUN and hyperkalemia were noted in the hemodialysis treated group.
Mortality rate registered in our study was 52.8% which is by the results of another study which referred mortality rate from 50.7% up to 66% [20, 21]. In-hospital mortality was 28.6%, and in 24.3% of cases, death occurred during the follow-up period of 90 days. In our study, the comparative outcome in patients with conservative and RRT treatment did not show significant differences in survival (log rank p = 0.633) during the follow-up period which is by reports of Van Berendoncks et al. [22-24] that also showed no differences in long-term survival according to different treatment options. But our study results also deviate from the data of the other study which referred poor prognosis and increased mortality (after correction for disease severity) among elderly patients who developed dialysis requiring AKI [21, 25, 26].
Our findings revealed that decreased volume of urine was associated with lower survival as its shown with Kaplan–Meier survival curves stratified by urine volumes at the time of initiation of renal replacement therapy (log rank p = 0.001), which is in accordance with previous reports where authors identified oliguria/anuria as predictor of worse outcome [27, 28].
In conclusion, high comorbidity burden is common in elderly patients who are at higher risk for the development of AKI. In our study survival is not related to different treatment options, but decreased urine volume is associated with lower survival. Applied treatment in elderly patients with AKI should be assessed by measuring the long term outcome.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Eknoyan G, Lameire N, Eckardt K, Kasiske B. Kidney disease:improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 2.Carlos G Musso, Dimitrios G Oreopoulos. Aging and Physiological Changes of the Kidneys including Changes in Glomerular Filtration Rate. Nephron Physiol. 2011;119(Suppl 1):1–5. doi: 10.1159/000328010. https://doi.org/10.1159/000328010 PMid:21832859. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Kader K, Palevsky P. Acute Kidney Injury in the Elderly. Clin Geriatr Med. 2009;25(3):331–358. doi: 10.1016/j.cger.2009.04.001. https://doi.org/10.1016/j.cger.2009.04.001 PMid:19765485 PMCid:PMC2748997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CY, McCulloch CE, Fan D, Ordo-ez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. https://doi.org/10.1038/sj.ki.5002297 PMid:17507907 PMCid:PMC2673495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly:a systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–71. doi: 10.1053/j.ajkd.2008.03.005. https://doi.org/10.1053/j.ajkd.2008.03.005 PMid:18511164. [DOI] [PubMed] [Google Scholar]
- 6.Pascual J, Orofino L, Liano F, et al. Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc. 1990;38(1):25–30. doi: 10.1111/j.1532-5415.1990.tb01592.x. https://doi.org/10.1111/j.1532-5415.1990.tb01592.x PMid:2295766. [DOI] [PubMed] [Google Scholar]
- 7.Lia-o F, Pascual J. Epidemiology of acute renal failure:a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group Kidney Int. 1996;50(3):811–8. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 8.Baraldi A, Ballestri M, Rapana R, et al. Acute renal failure of medical type in an elderly population. Nephrol Dial Transplant. 1998;13(Suppl 7):25–29. doi: 10.1093/ndt/13.suppl_7.25. https://doi.org/10.1093/ndt/13.suppl_7.25 PMid:9870433. [DOI] [PubMed] [Google Scholar]
- 9.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. https://doi.org/10.1681/ASN.2005060668 PMid:16495381. [DOI] [PubMed] [Google Scholar]
- 10.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure:a population-based study. Crit Care. 2005;9:700–709. doi: 10.1186/cc3879. https://doi.org/10.1186/cc3879 PMid:16280066 PMCid:PMC1414056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. https://doi.org/10.1681/ASN.2007080837 PMid:19020007 PMCid:PMC2615732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80:1222–30. doi: 10.1038/ki.2011.259. https://doi.org/10.1038/ki.2011.259 PMid:21832983. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies:development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. https://doi.org/10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. https://doi.org/10.1097/01.mlr.0000182534.19832.83 PMid:16224307. [DOI] [PubMed] [Google Scholar]
- 15.John W Pickering, Zoltán H Endre. Back-Calculating Baseline Creatinine with MDRD Misclassifies Acute Kidney Injury in the Intensive Care Unit. Clin J Am Soc Nephrol. 2010;5(7):1165–1173. doi: 10.2215/CJN.08531109. https://doi.org/10.2215/CJN.08531109 PMid:20498242 PMCid:PMC2893073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KDIGO Clinical Practice Guideline for Acute Kidney Injury, Kidney International Supplements. 2012;2,3 https://doi.org/10.1038/kisup.2012.3 PMid:25028632 PMCid:PMC4089615. [Google Scholar]
- 17.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–39. doi: 10.1016/j.jacc.2008.07.051. https://doi.org/10.1016/j.jacc.2008.07.051 PMid:19007588. [DOI] [PubMed] [Google Scholar]
- 18.Girman CJ, Kou TD, Brodovicz K, et al. Risk of acute renal failure in patients with Type 2 diabetes mellitus. Diabetic Medicine. 2012;29(5):614–621. doi: 10.1111/j.1464-5491.2011.03498.x. https://doi.org/10.1111/j.1464-5491.2011.03498.x PMid:22017349. [DOI] [PubMed] [Google Scholar]
- 19.Ge S, Nie S, Liu Z, Chen C, Zha Y, Qian J, Liu B, Teng S, Xu A, Bin W, Xu X. Epidemiology and outcomes of acute kidney injury in elderly chinese patients:a subgroup analysis from the EACH study. BMC Nephrology. 2016;17(1) doi: 10.1186/s12882-016-0351-2. https://doi.org/10.1186/s12882-016-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elseviers MM, Lins RL, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J. Renal replacement therapy is an independent risk factor for mortality in critically ill patients with acute kidney injury. Critical Care. 2010;14(6):R221. doi: 10.1186/cc9355. https://doi.org/10.1186/cc9355 PMid:21122146 PMCid:PMC3219996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucuvic EM, Ponce D, Balbi AL. Risk factors for mortality in acute kidney injury. Rev Assoc Med Bras (1992) 2011;57(2):158–63. doi: 10.1590/s0104-42302011000200012. https://doi.org/10.1016/S0104-4230(11)70037-9. [DOI] [PubMed] [Google Scholar]
- 22.Van Berendoncks AM, Elseviers MM, Lins RL SHARF Study Group. Outcome of acute kidney injury with different treatment options:long-term follow-up. Clin J Am Soc Nephrol. 2010;5(10):1755–62. doi: 10.2215/CJN.00770110. https://doi.org/10.2215/CJN.00770110 PMid:20634328 PMCid:PMC2974373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lins RL, Elseviers MM, Daelemans R. Severity scoring and mortality 1 year after acute renal failure. Nephrol Dial Transplant. 2006;21(4):1066–8. doi: 10.1093/ndt/gfk094. https://doi.org/10.1093/ndt/gfk094 PMid:16476721. [DOI] [PubMed] [Google Scholar]
- 24.Rhee H, Jang KS, Park JM, Kang JS, Hwang NK, Kim IY, Song SH, Seong EY, Lee DW, Lee SB, Kwak IS. Short- and Long-Term Mortality Rates of Elderly Acute Kidney Injury Patients Who Underwent Continuous Renal Replacement Therapy. PLoS One. 2016;11(11):e0167067. doi: 10.1371/journal.pone.0167067. https://doi.org/10.1371/journal.pone.0167067 PMid:27875571 PMCid:PMC5119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayatas K, Sahin G, Tepe M, Kaya ZE, Apaydin S, Demirtunç R. Acute kidney injury in the elderly hospitalized patients. Ren Fail. 2014;36(8):1273–7. doi: 10.3109/0886022X.2014.934693. https://doi.org/10.3109/0886022X.2014.934693 PMid:24986184. [DOI] [PubMed] [Google Scholar]
- 26.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–8. doi: 10.1097/00003246-200209000-00016. https://doi.org/10.1097/00003246-200209000-00016 PMid:12352040. [DOI] [PubMed] [Google Scholar]
- 27.Choi HM, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. Etiology and outcomes of anuria in acute kidney injury:a single center study. Kidney Res Clin Pract. 2015;34(1):13–9. doi: 10.1016/j.krcp.2014.11.002. https://doi.org/10.1016/j.krcp.2014.11.002 PMid:26484014 PMCid:PMC4570603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal Failure Study Group. Journal of the American Society of Nephrology. 1998;9(4):692–8. doi: 10.1681/ASN.V94692. PMid:9555672. [DOI] [PubMed] [Google Scholar]


