Abstract
BACKGROUND:
To our knowledge, the correlation between ultrasonographic enthesopathy and severity in psoriatic arthritis (PsA) has been done before. However, the correlation between ultrasonography of enthesopathy and the Psoriatic Arthritis Disease Activity Score (PASDAS) have not been done.
AIM:
To compare the results of ultrasonographic enthesopathy of foot and PASDAS in PsA.
MATERIALS AND METHODS:
A total of 65 PsA patients were involved and divided into two groups. The first group of 35 active PsA and the second group of 30 ages and sex matched inactive PsA as a control group were recruited in this study. Both groups were evaluated by examination, radiological findings and ultrasonography.
RESULTS:
Of 70 entheses in 35 active PsA patients, the most entheseal abnormalities were tender plantar fascia (18.5%), tender Achilles tendon (37.8%). PASDAS was a direct highly significant correlated with plantar fascia and Achilles tendon thickness in in active PsA (r = 0.823 and r = 0.796, p < 0.001 respectively).
CONCLUSION:
Musculoskeletal US is an accurate and low-cost method for assessment of enthesopathy with significant correlation to disease activities in psoriatic arthritis.
Keywords: Psoriatic arthritis, Ultrasonographic enthesopathy, Psoriatic Activity
Introduction
To our knowledge, the correlation between ultrasonographic enthesopathy and severity in psoriatic arthritis (PsA) has been done before. However, the correlation between ultrasonography of enthesopathy in the foot and the Psoriatic Arthritis Disease Activity Score (PASDAS) has not been done. Thus, our aim is to compare the results of ultrasonographic enthesopathy of foot and PASDAS in PsA.
Psoriatic arthritis is rheumatic disease associated with psoriasis and included among seronegative forms of spondyloarthropathy. It is characterized clinically by the involvement of joints, tendons, and entheses, that is, sites of insertion of tendons, ligaments, and articular capsules into bone [1]. The functioning entheses dissipate stress over a wide area, including the insertion, immediately adjacent tendon and adjacent bone [2].
Heel pain is a common sign of psoriatic arthritis, and ten percent of psoriatic arthritis sufferers heel pain as a significant symptom [3]. In particular, Achilles tendinitis is fairly common in psoriatic arthritis and may associate with severe foot pain and difficulty walking with swelling and tenderness along the course of the tendon and at the calcaneal insertion [4]. The Leeds Enthesitis Index (LEI) is one of clinical assessment of disease activity were commonly used as enthesitis Index in psoriatic arthritis [5].
Musculoskeletal ultrasonography is thought to be the gold standard imaging technique for assessing tendons, inexpensive, and readily demonstrates superficial tissue such as entheseal and tendon abnormalities, fluid collections, soft tissue lesions as well as bone surface lesions [6]. Recently, PASDAS is new composite disease activity measurement for the psoriatic arthritis and it is a composite index based on patient (PtGA) and physician (PhGA) global VAS scores, tender (SJC66) and swollen (SJC68) joint counts, dactylitis and enthesitis, health-related QOL (SF36 − PCS), and CRP level [7].
Also, our aim of the present study was to study ultrasonographic evaluation of entheseal abnormalities in the foot including Achilles tendon, and plantar fascia insertions in the calcaneus in active and inactive PsA patients and moreover, to compare the results of ultrasonographic enthesopathy of the foot in the foot with PASDAS in active PsA patients.
Patients and Methods
A total of 65 PsA patients were involved and divided into two groups. The first group of thirty-five patients with active PsA were recruited from outpatient clinics, dermatology department together with the second group of thirty age and sex matched inactive PsA patient as a control group. Some patients underwent treatment with DMARD and biologic agents. CASPAR (classification of psoriatic arthritis study group) criteria for the classification of psoriatic arthritis were used [8]. A total of 70 entheses in the study group and 60 entheses in the control group were evaluated by clinical, radiological examination and ultrasonography of bilateral Achilles tendon and the plantar fascia insertions in the calcaneus.
Inclusion criteria for cases were age more 18 years; diagnosis of chronic plaque psoriasis lasting more one year; the presence of any clinical signs and symptoms of articular involvement and enthesopathy. Exclusion criteria for cases were rheumatoid arthritis, crystal induced arthritis, osteoarthritis, Reiter’s syndrome, obvious inflammatory bowel disease, other active inflammatory skin conditions, metabolic, and endocrine disorders, severe comorbidities, recent serious infection and drug- induced tendinopathy e.g. fluoroquinolones and retinoid. All patients gave their informed verbal voluntary consent to use the recorded data in their follow up sheets and to perform US assessment according to the protocol approved by the local ethics committee and in accordance with the ethical standards of the Helsinki declaration.
Clinical and laboratory assessment of disease activity included the following instruments: clinical examination of bilateral Achilles tendon, and plantar fascia insertions in the calcaneus; a measure of acute-phase response (CRP and ESR); the Leeds Enthesitis Index (LEI) and the Psoriatic Arthritis Disease Activity Score (PASDAS) [7].
Measures of enthesitis included the Leeds Enthesitis Index (LEI) score which consisted of bilaterally six sites: right and left Achilles tendon insertions, medial femoral condyles superior to the joint line, and lateral epicondyles of the humerus at the common extensor origin. The pressure was exerted at the enthesis sufficient to blanch the finger nail of the examiner (approximately 4 Kg). In addition, the examiner assessed the presence of soft-tissue swelling at the enthesis [5].
PASDAS was done and included a composite index based on patient and physician global VAS scores, tender and swollen joint counts, dactylitis and enthesitis, health-related QOL, and CRP level. The PASDAS was calculated from the following variables: patient global VAS (rescaled from 0–10 to 0–100), physician global VAS (rescaled from 0–10 to 0–100), 66 swollen joint count, 68 tender joint count, CRP level (rescaled from mg/dl to mg/liter), enthesitis (measured in GO- REVEAL as modified Maastricht Ankylosing Spondylitis Enthesitis Score and rescaled to a 0–6 range by multiplying by a factor of 6/15 for this analysis), tender dactylitis count (the GO-REVEAL study scored each digit from 0–3 and these were recoded to 0–1, where any score > 0 equaled 1), and, finally, the physical component summary (PCS) scale of the short form 36 health survey (SF-36) [7]. The PASDAS is computed by the following equation: PASDAS = (((0. 18 √ (PhGA) + 0.159 √ (PtGA) − 0.253 √ (SF36 − PCS) + 0.101 lognat (SJC66 + 1)) + 0.048 × lognat (SJC68 + 1)) + 0.23 × lognat (Leeds enthesitis index + 1)) + 0.37 × lognat (tender dactylitis count + 1) + 0.102 × lognat (CRP + 1) + 2 × 1.5. The physical component summary (PCS) scale of the Short Form 36 health survey (SF-36) was done [7, 9].
Laboratory assessments including a complete blood cell count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were done. Also, Rheumatoid Factor (RF) titers were determined. ESR was measured by the standard Westergren method (mm/h). CRP levels were measured by standard nephelometry (mg/l). RF was measured by enzyme-linked immunosorbent assay (ELISA) and results are expressed in titers of 1/40 and higher. Lateral radiographs of heels were obtained using standardized techniques as well as US scans. Measuring and detection of enthesitis by ultrasonography evaluations of bilateral Achilles tendon and plantar fascia insertions in the calcaneus in both groups were performed. Ultrasound (US) examinations were carried out with patients in the prone position with their feet hanging off the examination table in a neutral position (90 degrees of flexion) using a Hitachi Aloka Ultrasound Machine (the Hitachi Aloka medical, Ltd, Tokyo, Japan) using a 10–15 MHz linear transducer. In all patients, we predominantly used 90° flexion of the feet during examination of bilateral Achilles tendon and plantar fascia insertions. The longitudinal and transverse planes were used to assess the enthesis. The mean values of three measurements were used for statistical analysis. All of the sonographic examinations were performed by a radiologist who was expert in soft tissue sonography.
Statistical analysis
Study data were analysed using the SPSS (Statistical Package from Social Science Program) (version15.0) statistical package. The normality of the population was done during statistical analysis. The Student’s t test indicates the magnitudes of the differences of means ± SD and therefore the magnitude of the observation. Thus, the unpaired t-test was used to assess the difference between patients and control subjects. Before data analysis, the level of significance was established at P < 0.05. A p value of < 0.05 was used as significance.
We used SPSS (Statistical Package from Social Science Program) version 15.0 for data processing (SPSS Inc, Chicago, IL, USA). Quantitative data were presented as mean (± SD). Correlation between variables was done, and Pearson correlation coefficient was calculated. All tests were 2-tailed and considered statistically significant at p < 0.05.
Results
There was significant differences between ESR, CRP and US thickness of plantar fascia (mm) and Achilles tendon (mm) between active PsA patients and controls (p< 0.05). Moreover, there were a significant difference PASDAS between active PsA patients and controls (p< 0.05) (Table 1).
Table 1.
Demographic, clinical and laboratory findings in 35 patients with active PsA and 30 inactive PsA as control group
| Characteristics (Mean ± SD) n (%) | Active PsA) (N = 35) | Inactive PsA (N = 30) | p –value |
|---|---|---|---|
| Age (years) | 44.77± 10.11 | 46.30 ± 12.6 2 | - |
| Gender (male/female) | 20/15 | 17/13 | - |
| Disease duration (years) | 8.18 ± 3.27 | 8.00 ± 2.49 | - |
| BMI (kg/m^2) | 26.42 ± 4.80 | 24.90 ± 4.67 | - |
| Clinical presentations of arthritis, n (%) | - | - | - |
| Low back pain | 13/35 (37.1%) | 8/30 (26.7%) | - |
| Oligoarthritis | 21/35 (60.0%) | 16/30 (53.3%) | - |
| Polyarthritis | 7/35 (20.0%) | 6/30 (20.0%) | - |
| Visual Analogue Scale (VAS) | - | - | - |
| Patient global VAS scores(range 0–100) | 40.57 ± 16.67 | 26.20 ± 7.74 | p<0.05 S |
| Physician global VAS scores(range 0–100) | 29.68 ± 7.53 | 17.19 ± 4.63 | p<0.05 |
| Peripheral joint counts | - | - | - |
| 68 tender joint count (range 0-68) | 42.80 ± 12.80 | 17.93 ± 5.94* | p<0.05 S |
| 66 swollen joint count (range 0-66) | 29.16 ± 3.62 | 17.16 ± 5.06* | p<0.05 S |
| Enthesis/dactylitis counts | - | - | - |
| Leeds Enthesitis Index range 0–6) | 3.28 ± 1.52 | 2.30 ± 0.83* | p<0.05 S |
| Dactylitis count (range 0–20) | 8.94 ± 480 | 6.43 ± 2.40* | p<0.05 S |
| Health-related quality of life and functions: (the mean ± SD), SF36 PCS (range 0–100) | - | - | - |
| 35.11 ± 16.07 | 7.92 ± 6.74* | p<0.05 S | |
| Acute phase reactants | - | - | - |
| ESR (mm/hour) | 42.14 ±16.02 | 8.20 ± 5.67* | p<0.05 S |
| CRP (mg/dl) | 19.08 ± 8.07 | 7.33 ± 6.46* | p<0.05 S |
| PASDAS (the mean ± SD) | 3.50 ± 0.81 | 1.92 ± 0.48* | p<0.05 S |
| US thickness of Plantar fascia (mm) | 4.63 ± 1.16 | 3.62 ± 0.51* | p<0.05 S |
| US thickness of Achilles tendon (mm) | 5.42 ± 1.13 | 4.03 ± 0.36* | p<0.05 S |
| Medications, n (%) | - | - | |
| NSAID | 32/35 (91.4%) | 21/30 (70.0%) | - |
| Methotrexate (7.5–17.5 mg/week) | 30/35 (85.7%) | 20/30 (66.7%) | - |
| Anti-tumor necrosis factor | 4/35 (11.4%) | 2/30 (6.7%) | - |
| Combined therapy | 3/35 (8.6%) | 3/30 (10.0%) | - |
p < 0.0001 = highly significant,
p<0.05=significant. p>0.05=non-significant (NS). ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; PASDAS: Psoriatic Arthritis Disease Activity Score, SF36 PCS: The Short Form 36 Scale (SF-36) health survey -Physical component scale of SF36.
Of 70 entheses in 35 active PsA patients, the most entheseal abnormalities were tender plantar fascia insertion (18.5%), tender Achilles tendon insertion (37.8%), incidence of US tendon thickening of plantar fascia insertion (55.5%), incidence of US Tendon thickening of Achilles tendon insertion (34.5%), osteophyte (spur) of plantar fascia insertion (12.6%) and osteophyte (spur) of Achilles tendon insertion (16.1%) (Table 2, Figure 1).
Table 2.
Clinical, radiological findings and ultrasonography of plantar fascia insertion and Achilles tendon insertion in calcaneus in 35 patients with active PsA patients
| Data, n (%) | Plantar fascia insertion in calcaneus (n = 70 entheses) | Achilles tendon insertion in calcaneus (n = 70 entheses) |
|---|---|---|
| Clinical presentations, n (%) | - | - |
| Tender | 17/70 (19.5%) | 33/70 (37.9%) |
| Swollen | 0/70 (0 %) | 3/70 (3.4%) |
| Pain x-ray of heel, n (%) | - | - |
| Osteophyte (spur) | 11/70 (12.6%) | 14/70 (16.1%) |
| Erosions | 3/70 (3.4%) | 2/70 (2.3%) |
| Ultrasonography, n (%) | - | - |
| US tendon thickened* | 16/70 (55.2%) | 10/70 (34.5%) |
| US Enthesophyte | 9/70 (10.3%) | 13/70 (14.9%) |
| US Bone erosion | 2/70 (6.9%) | 2/70 (2.3%) |
| US Bursitis | 0/70 (0%) | 2/70 (2.3%) |
Achilles tendon >5.29 mm, plantar aponeuroses >4.4 mm;.
**p< 0.0001= highly significant,*p<0.05=significant.
Figure 1.
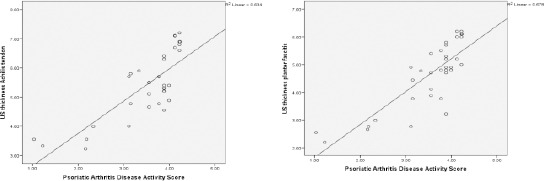
Linear regression correlation (r-) of PASDAS and plantar fascia thickness (mm) in active psoriatic arthritis (r =0.823**, p< 0.001) (left). Figure 2: Linear regression correlations (r-) of PASDAS and Achilles tendon thickness in active psoriatic arthritis (r =0.796**, p< 0.001) (right)
In our study, we did not find power Doppler signal entheseal in PsA patients. PASDAS was a direct significant correlation with Leeds Enteritis Index score in active PsA patients (r = 0. 0.452, p< 0.05).
Moreover, PASDAS was a direct highly significant correlation with the thickness of Plantar fascia and the thickness of Achilles tendon in active PsA patients (r = 0. 823, p< 0.001 and r = 0. 796, p < 0. 001 respectively) (Table 3, Figure 2).
Table 3.
Correlation (r) between PASDAS and Leeds Enthesitis Index as well as the US thickness of both plantar fascia and Achilles tendons (mm) in active PsA patients
| Leeds Enthesitis Index | r =0.452*s, p< 0.05 S |
| The plantar fascia thickness (mm) | r =0.823**, p< 0.001 HS |
| The Achilles tendon thickness (mm) | r =0.796**, p< 0.001 HS |
p< 0.001= Correlation is highly significant,
p<0.05= Correlation is significant. Psoriatic Arthritis Disease Activity Score (PASDAS).
Figure 2.
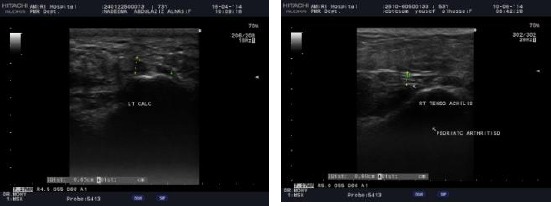
The ultrasound of left heel showed the plantar fascia thickness (4.5 mm) (left). The ultrasound of right tendoachilis showed the Achilles tendon thickness (4 mm) (right)
Discussion
Limitations in clinical assessment of the entheses have prompted the use of ultrasound (US) to detect the presence of enthesitis. Imaging techniques such as the US have been increasingly used in psoriatic arthritis (PsA) providing additional clues to severity and the pathogenesis of this peripheral, axial disease. In the last few years, composite measures of disease activity in PsA have been developed. PASDAS was better able to discriminate between high and low disease activity [7]. Acquacalda E et al. [10] found a high frequency of US enthesitis in psoriasis patients, with or without musculoskeletal symptoms. Also, enthesitis is common in PsA and considered important by affected patients [11].
In the present study, of 70 entheses in 35 active PsA patients, the most entheseal abnormalities were tender plantar fascia insertion (18.5%), tender Achilles tendon insertion (37.8%), US tendon thickening of plantar fascia insertion (55.5%), US Tendon thickening of Achilles tendon insertion (34.5%), osteophyte (spur) of plantar fascia insertion (12.6%) and osteophyte (spur) of Achilles tendon insertion (16.1%). Moreover, we found a significant difference of ESR, CRP, US thickness of plantar fascia, US thickness of Achilles tendon, PASDAS between active PsA patients and controls (p < 0.05). In our study, we did not find power Doppler signal entheseal in PsA patients.
Similar findings were reported by Galluzzo E et al. [4] who found that 71% of the 31 PsA patients showed sonographic features of tendon involvement in 27 cases of plantar fasciitis in 16 patients and 11 cases of Achilles tendon enthesopathy in 6 patients. The Achilles tendon is among the most frequent sites of enthesopathic involvement in psoriatic arthritis, producing heel pain, and difficulty walking. Also, heel pain is a common sign of psoriatic arthritis with ten percent of psoriatic arthritis sufferers reporting heel pain as a significant symptom [3].
Furthermore, Naredo E et al. [12] demonstrated that US enthesopathy was significantly more frequent in psoriatic patients than in controls in 11.6% of entheses in the psoriasis group and 5.3% of entheses in the control group. Similarly, Yang Q et al. [13] found that enthesitis was present in 26.8% of the patients. Also, Kane D et al. [14] found that 38% had peripheral enthesopathy; 12% had plantar fasciitis; 6% had Achilles tendonitis; 37 (29%) had dactylitis of digits; 2% had tenosynovitis of the wrist; 10% had inflammatory spine pain. D’Agostino MA et al. [15] reported that the commonest sites of enthesopathy involvement in PsA are Achilles tendon, plantar fascia, patellar tendon and greater trochanter.
Moreover, De Filippis et al. [16] found that entheseal abnormalities, not detected at clinical examination, were present ultrasonography in six of 24 (25%) psoriasis patients. Naredo E et al. [12] reported that US enthesopathy was present in 11.6% of entheses in the psoriasis group and 5.3% of entheses in the control group (P < 0.0005). Ozcakar et al. [17] reported a significantly higher mean thickness of the Achilles tendon measured by the US in 30 psoriatic patients than in 20 healthy controls. De Simone et al [18] reported that ultrasonographic enthesopathic abnormalities were found in 11 of 15 psoriatic arthritis and involvement of the Achilles tendon found in 35 of 59 psoriatic patients, whereas clinical evaluation showed Achilles tendinitis in 18 (30.5%) of the 59 psoriatic patients and Achilles sonographic abnormalities in 35 of 59 psoriatic patients (59.2%). Astorri D et al. [19] reported that power Doppler ultrasound allows detecting structural and inflammatory abnormalities of enthesis in early PsA patients. But in the present study, we did not find power Doppler signal entheseal in PsA patients in present study.
In the present study, PASDAS was a direct significant correlation with Leeds Enteritis Index score (r = 0.452, p < 0.05) in active PsA patients. Moreover, PASDAS was a direct highly significant correlation with the thickness of plantar fascia and the thickness of Achilles tendon in active PsA patients, (r = 0. 823, p < 0.001 and r = 0. 796, p < 0. 001 respectively).
Similar findings were reported by Girolomoni G and Gisondi P [20] who found that monitoring of psoriasis patients has revealed that a higher baseline score for enthesitis may be associated with a more severe psoriasis outcome and nine patients with more severe clinical psoriasis outcome had a significantly higher baseline the Glasgow Ultrasound Enthesitis Scoring System (GUESS) score (9.29 vs. 4.25; P < 0.001) and a significantly higher baseline PASI score (14.5 vs. 3.8; P < 0.002) compared with the patients with a good clinical psoriasis outcome. But, no significant differences between the two groups were found for disease duration, age, waist circumference, BMI, or comorbid diabetes. Furthermore, Aydin SZ et al. [21] reported that psoriasis patients (with or without arthritis) were more likely to express higher inflammation-related enthesopathy scores and the psoriatic arthritis patients had higher ultrasound enthesopathy scores than psoriasis patients. Moreover, Gisondi et al. [22] reported that enthesitis score, as measured by the GUESS, correlates with BMI among patients with psoriasis alone.
In disagreement with our findings, Gisondi et al. [22] and Gutierrez et al. [23], did not find an association between most power Dopper ultrasound abnormalities of lower limbs and psoriasis severity, psoriasis duration. Interestingly, the presence of enthesopathy was directly correlated with age, body mass index, and waist circumference. However, Gisondi et al. [22] found a significantly higher mean GUESS score in psoriatic patients as compared with controls. By contrast, Gutierrez et al. [23] found significantly more US signs of enthesopathy and higher GUESS in the lower limbs of psoriatic patients as compared with healthy controls. Interestingly, the presence of enthesopathy was directly correlated with age, body mass index, and waist circumference and not with the duration and severity of psoriasis. However, Ash ZR et al. [24] showed that no link between the severity of psoriasis and enthesitis was evident. Our possible explanation of differences of correlation between enthesitis scores and severity of psoriasis from other authors may be due to different methods of severity assessment of enthesitis and psoriasis enthesitis in psoriatic arthritis.
Although the mechanism of enthesitis in PsA remains unclear, the roles of environmental, mechanical stress, immunologic and genetic factors have been studied. Gisondi et al. [22] reported the underlying causes of the enthesitis might be due to increased mechanical stress on the entheses of the lower limbs due to heavier weight, although obesity is also associated with systemic inflammation and high levels of proinflammatory cytokines. De Simone et al. [18] showed that the most common disorder was degenerative lesions of tendinitis with or without micro-calcification in psoriatic arthritis. Also, a hypothesis in PsA is that enthesitis arises at sites of high shear and compression forces, with the additive interaction between mechanical stress, microtrauma, and tissue repair mechanisms and bacterial molecules variably leading to inflammation [25].
Mease P [26] suggested that pathogenetic studies of PsA emphasize several areas: 1) immunologic factors including T cells and the Interleukin-23/17 pathway, 2) abnormal bone remodelling that involves bone erosion and bone proliferation; a unique pattern is seen in PsA, 3) genetics and 4) environmental factors. Moreover, mechanism of enthesitis in PsA may be explained by Coates LC et al. [27] who described that MRI and to a lesser extent the US allow visualization of localization of inflammatory change at the juxta-articular entheses suggests this as the primary site of inflammation.
In conclusion, musculoskeletal US is an accurate and low-cost method for assessment of enthesopathy with significant correlation to disease activities in psoriatic arthritis patients. A possible explanation of limitation of the present study is the low size of our population. Further studies would be interesting to validate our data in the large size of the population.
Acknowledgments
We would like to thank all the patients that participated in this study. Also, we acknowledge the assistance of our colleagues during this study.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. https://doi.org/10.1002/art.1780341003 PMid:1930310. [DOI] [PubMed] [Google Scholar]
- 2.Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, Burgos- Vargas R, Collantes-Estevez E, Davis J, Dijkmans B, et al. The development of Assessment of Spondylo Arthritis international Society classification criteria for axial spondyloarthritis (part I):classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68(6):770–6. doi: 10.1136/ard.2009.108217. https://doi.org/10.1136/ard.2009.108217 PMid:19297345. [DOI] [PubMed] [Google Scholar]
- 3.Dailey J. Differential diagnosis and treatment of heel pain. Clinics in Podiatry Medicine and Surgery. 1991;8(1):153–166. PMid:2015526. [PubMed] [Google Scholar]
- 4.Galluzzo E, Lischi DM, Taglione E, Lombardini F, Pasero G, Perri G, Riente L. Sonographic analysis of the ankle in patients with psoriatic arthritis. Scand J Rheumatol. 2000;29:52–5. doi: 10.1080/030097400750001806. https://doi.org/10.1080/030097400750001806 PMid:10722258. [DOI] [PubMed] [Google Scholar]
- 5.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis:assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59(5):686–91. doi: 10.1002/art.23568. https://doi.org/10.1002/art.23568 PMid:18438903. [DOI] [PubMed] [Google Scholar]
- 6.Kamel M, Eid H, Mansour R. Ultrasound detection of knee patellar enthesitis:a comparison with magnetic resonance imaging. Ann Rheum Dis. 2004;63:213–4. doi: 10.1136/ard.2003.010314. https://doi.org/10.1136/ard.2003.010314 PMid:14722216 PMCid:PMC1754891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis-Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project) Ann Rheum Dis. 2013;72:986–91. doi: 10.1136/annrheumdis-2012-201341. https://doi.org/10.1136/annrheumdis-2012-201341 PMid:22798567. [DOI] [PubMed] [Google Scholar]
- 8.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis:development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. doi: 10.1002/art.21972. https://doi.org/10.1002/art.21972 PMid:16871531. [DOI] [PubMed] [Google Scholar]
- 9.Ware JE. Measuring patients’views:the optimum outcome measure. BMJ. 1993;306:1429–30. doi: 10.1136/bmj.306.6890.1429. https://doi.org/10.1136/bmj.306.6890.1429 PMid:8518638PMCid:PMC1677908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acquacalda E, Albert C, Montaudie H, Fontas E, Danre A, Roux CH, Breuil V, Lacour JP, Passeron T, Ziegler LE. Ultrasound study of entheses in psoriasis patients with or without musculoskeletal symptoms:A prospective study. Joint Bone Spine. 2015 Jul;82(4):267–71. doi: 10.1016/j.jbspin.2015.01.016. https://doi.org/10.1016/j.jbspin.2015.01.016 PMid:25881759. [DOI] [PubMed] [Google Scholar]
- 11.Kavanaugh A, Cassell S. The assessment of disease activity and outcomes in psoriatic arthritis. Clin Exp Rheumatol. 2005;23(Suppl. 39):S142–S147. PMid:16273798. [PubMed] [Google Scholar]
- 12.Naredo E, Möller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, Mayordomo L, Moragues C, Uson J, de Agustín JJ, Martínez A, Rejón E, Rodriguez A, Daudén E. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis:a prospective case-control study. Rheumatology (Oxford) 2011;50(10):1838–48. doi: 10.1093/rheumatology/ker078. https://doi.org/10.1093/rheumatology/ker078 PMid:21700682. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, Yu C, Pei Z, Wang G, Shi B, Zhang F, Zhang Y, Zhang F. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25(12):1409–14. doi: 10.1111/j.1468-3083.2011.03985.x. https://doi.org/10.1111/j.1468-3083.2011.03985.x PMid:21349114. [DOI] [PubMed] [Google Scholar]
- 14.Kane D, Stafford L, Bresnihan B, FitzGerald O. Prospective, clinical and radiological study of early psoriatic arthritis:an early synovitis clinic experience. Rheumatology (Oxford) 2003;42(12):1460–8. doi: 10.1093/rheumatology/keg384. https://doi.org/10.1093/rheumatology/keg384 PMid:14523223. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler:a cross-sectional study. Arthritis Rheum. 2003;48:523–33. doi: 10.1002/art.10812. https://doi.org/10.1002/art.10812 PMid:12571863. [DOI] [PubMed] [Google Scholar]
- 16.De Filippis LG, Caliri A, Lo Gullo R, Bartolone S, Miceli G, Cannavo SP, Borgia F, Basile G, Aloisi G, Zimbaro G, Scribano E, Bagnato GF. Ultrasonography in the early diagnosis of psoriasis-associated enthesopathy. Int J Tissue React. 2005;27:159–62. PMid:16440579. [PubMed] [Google Scholar]
- 17.Ozcakar L, Cetin A, Inanici F, Kaymak B, Gurer CK, Kolemen F. Ultrasonographical evaluation of the Achilles’tendon in psoriasis patients. Int J Dermatol. 2005;44:930–2. doi: 10.1111/j.1365-4632.2004.02235.x. https://doi.org/10.1111/j.1365-4632.2004.02235.x PMid:16336526. [DOI] [PubMed] [Google Scholar]
- 18.De Simone C, Guerriero C, Giampetruzzi AR, Costantini M, Di Gregorio F, Amerio P. Achilles tendinitis in psoriasis:clinical and sonographic findings. J Am Acad Dermatol. 2003;49:217–22. doi: 10.1067/s0190-9622(03)00904-6. https://doi.org/10.1067/S0190-9622(03)00904-6. [DOI] [PubMed] [Google Scholar]
- 19.Perrotta FM, Astorri D, Zappia M, Reginelli A, Brunese L, Lubrano E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int. 2016;36(11):1579–1583. doi: 10.1007/s00296-016-3562-8. https://doi.org/10.1007/s00296-016-3562-8 PMid:27600991. [DOI] [PubMed] [Google Scholar]
- 20.Girolomoni G, Gisondi P. Psoriasis and systemic inflammation:underdiagnosed enthesopathy. J Eur Acad Dermatol Venereol. 2009;23(Suppl 1):3–8. doi: 10.1111/j.1468-3083.2009.03361.x. https://doi.org/10.1111/j.1468-3083.2009.03361.x PMid:19686379. [DOI] [PubMed] [Google Scholar]
- 21.Aydin SZ1, Ash ZR, Tinazzi I, Castillo-Gallego C, Kwok C, Wilson C, Goodfield M, Gisondi P, Tan AL, Marzo-Ortega H, Emery P, Wakefield RJ, McGonagle DG. The link between enthesitis and arthritis in psoriatic arthritis:a switch to a vascular phenotype at insertions may play a role in arthritis development. Ann Rheum Dis. 2013;72(6):992–5. doi: 10.1136/annrheumdis-2012-201617. https://doi.org/10.1136/annrheumdis-2012-201617 PMid:22863575. [DOI] [PubMed] [Google Scholar]
- 22.Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, Girolomoni G. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy:a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30. doi: 10.1136/ard.2007.075101. https://doi.org/10.1136/ard.2007.075101 PMid:17720726. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez M, Filippucci E, De Angelis R, Salaffi F, Filosa G, Ruta S, Bertolazzi C, Grassi W. Subclinical entheseal involvement in patients with psoriasis:an ultrasound study. Semin Arthritis Rheum. 2011;40(5):407–12. doi: 10.1016/j.semarthrit.2010.05.009. https://doi.org/10.1016/j.semarthrit.2010.05.009 PMid:20688358. [DOI] [PubMed] [Google Scholar]
- 24.Ash ZR, Tinazzi I, Gallego CC, Kwok C, Wilson C, Goodfield M, Gisondi P, Tan AL, Marzo-Ortega H, Emery P, Wakefield RJ, McGonagle DG, Aydin SZ. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71(4):553–6. doi: 10.1136/annrheumdis-2011-200478. https://doi.org/10.1136/annrheumdis-2011-200478 PMid:22156725. [DOI] [PubMed] [Google Scholar]
- 25.McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy. Additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol. 2001;28:2155–9. PMid:11669149. [PubMed] [Google Scholar]
- 26.Mease P. Update on treatment of psoriatic arthritis. Bull NYU Hosp Jt Dis. 2012;70(3):167–71. PMid:23259623. [PubMed] [Google Scholar]
- 27.Coates LC, Anderson RR, Fitzgerald O, Gottlieb AB, Kelly SG, Lubrano E, McGonagle DG, Olivieri I, Ritchlin CT, Tan AL, De Vlam K, Helliwell PS. Clues to the Pathogenesis of Psoriasis and Psoriatic Arthritis from Imaging:A Literature Review. The Journal of Rheumatology. 2008;35:7. [PubMed] [Google Scholar]


