Abstract
BACKGROUND:
Biomaterial cytotoxicity on dental stem cells plays a critical role in managing the regeneration of dental tissue.
AIM:
The aim of the present study was to evaluate the effect of Nano-hydroxy apatite (NHA), Mineral trioxide aggregate (MTA), and Calcium-enriched mixture (CEM) on the proliferation, and viability of human dental pulp stem cells (hDPSCs) isolated from third molar teeth.
METHODS:
Cultured DPSCs were characterized and the tested biomaterials were shaped into cylinders then inserted directly on the DPSCs. Proliferation and viability percentage of DPSCs were evaluated at 1, 3, 5, 7, 9, 11, and 14 days of culture.
RESULTS:
The biomaterials supplemented DPSCs showed a significant initial decrease in cell count and viability percentage at day one. Then, a rise in cell counts and viabilities was noticed after that. There was a decrease in cell counts, and viabilities in the NHA supplemented cells in comparison to other tested biomaterials.
CONCLUSIONS:
All tested biomaterials maintain the proliferation of DPSCs for different durations. NHA showed less proliferative and more cytotoxic effect than other tested materials.
Keywords: Cytotoxicity, Nano-hydroxy apatite (NHA), Mineral trioxide aggregate (MTA), calcium-enriched mixture (CEM), dental pulp stem cells (DPSCs)
Introduction
Successful dental regeneration process involves the proliferation of potent stem cells residing in the dental soft tissues followed by their differentiation into target secretory cells required for lost tissue replacement [1]. This proliferation is influenced intensely by the direct contact with dental materials. So, the biocompatibility of these materials is mandatory to maintain the viability of these cells during the regeneration process avoiding cell death with subsequent unfavourable immune and inflammatory reactions [2]. Several studies had described the Mineral trioxide aggregate (MTA) calcium silicate cement as biocompatible regenerative material [3, 4], using different cell cultures in direct and indirect contact. However, others reported some drawbacks including the unfavourable cytotoxic reactions regarding MTA metallic constituents [4].
Calcium enriched mixture (CEM) cement is a mixture of various calcium components that was introduced after MTA as an endodontic biomaterial and suggested to have similar biological behaviour on different cell types [5]. Additionally, Nano-hydroxyl apatite (NHA) biomaterial had been widely investigated for their regenerative and endodontic applications [5, 6]. It had distinct biological properties that were attributed to their biomimetic and nanoscale topography [7]. NHA was shown to have a positive proliferative effect on various mesenchymal cell types including stem cells [6], however other researches were doubtful about NHA cytotoxic effect [7].
The biologic effect of these mentioned cement was examined individually [8] however; limited studies were performed to evaluate their comparative biological effect especially on dental stem cells. Due to the clinical and regenerative importance of these materials in various endodontic applications, the present study aimed to evaluate and compare the cellular biocompatibility of these three endodontic types of cement (NHA, MTA and CEM) regarding evaluating their effect on the proliferation and the viability of DPSCs.
Materials and Methods
Cell culture
Seven sound human third molar teeth, indicated for extraction for orthodontic purposes from healthy adults aged 19-25 years, were extracted and collected under sterile conditions; informed consent was obtained from the patients before extraction from an outpatient clinic in the National Research Centre, Cairo, Egypt. Dental pulp cells were digested in 2 mg/ml collagenase solution (Collagenase NB4, SERVA, Germany). Then cultured with growth medium (GM) including; DMEM (4.5 g/L Glucose with L-Glutamine, Lonza Bioproducts, USA) supplemented with 2% antibiotic (Penicillin 10.000 U/ml Streptomycin 10.000 µg/ml; Lonza Bioproducts, USA), 10% Fetal bovine serum (FBS, SeraLab, UK) and 1 % antifungal (Amphotericin B, 250 µg/ml, Lonza Bioproducts, USA).
The isolated cells were incubated at 37ºC and 5% CO2 humidified atmosphere in the CO2 incubator. Then, cells were expanded into subsequent generations using 0.25% trypsin and 0.02% EDTA (Lonza Bioproducts, Belgium).
Colony formation efficiency testing
To measure the ability of single cell of human dental pulp stem cells (hDPSCs) for colony formation, 100 cells/ ml were gained from the first passage and cultured in a 3.5 cm culture dishes with culture GM. After 10 days of culturing, dental pulp cells in the dishes were fixed in 4% paraformaldehyde and colonies were visualised by 0.1 % toluidine blue staining (Lonza Bioproducts, Belgium, USA). Aggregates of more than or equal 50 cells were considered as a colony.
Osteogenic Differentiation
hDPSCs were seeded onto six-well plates; cultured to 70% confluence; and incubated in osteoinduction medium (DM) composed of previously prepared GM with 0.01 µmol/l dexamethasone and 1.8 mmol/l inorganic phosphate for 2 weeks (all purchased from Sigma, Aldrich, USA). Then, after 7 days (early stage of osteoblast differentiation), the samples were stained for alkaline phosphatase (ALP) activity using ALP kit according to the manufacturer’s instructions (Sigma–Aldrich, United States). Also, after 14 days (late stage of osteogenic differentiation), the samples were stained with 2% Alizarin red stain (Loba Chemie, India) [9] to reveal calcium accumulation in vitro.
Adipogenic Differentiation
hDPSCs cultures were supplemented with 0.5 μmol/L isobutylmethylxanthine, 0.5 μmol/L hydrocortisone, 60 μmol/L indomethacin and 10 μg ml insulin (Sigma-Aldrich, United States), The resultant differentiation was assessed at 21 days through the use of oil red O staining [9].
Measuring stem cell genes expression in the isolated DPSCs
The relative expression of stem cell genes; Bmi1 and Stat3 were evaluated and quantified by Two-Step PCR reaction using Power SYBR Green Master Mix and by real-time PCR System (StepOne™ Instrument, Applied Biosystems, USA).
Total Ribonucleic acid (RNA) was extracted from the isolated DPCs from the third and tenth passages using miRNeasy MiniKit (QIAGEN, Netherlands) according to manufacturer instructions. RNA was reversely transcribed into complementary DNA by reverse transcriptase (MultiScribe™ Reverse Transcriptase, Invitrogen) according to the manufacturer’s instructions. Quantitive real-time PCR (qPCR) was performed using Power SYBR Green Master Mix and the real-time PCR System according to the manufacturers’ instructions. All primers used in the present study were selected and designed according to Ahmed et al. [10] (Table 1) then synthesised by Invitrogen. PCR amplification experiment was triplicated, and then the PCR products were analysed.
Table 1.
Primers used in the present research
| Gene | 5’ DNA sequence 3’ | Product size (base pairs) |
|---|---|---|
| Bmi1 (Human) | Forward | 179 |
| ATATTTACGGTGCCCAGCAG | ||
| Reverse | ||
| GAAGTGGCCCATTCCTTCTC | ||
| Stat 3 (Human) | Forward | 191 |
| ATATTTACGGTGCCCAGCAG | ||
| Reverse | ||
| GAAGTGGCCCATTCCTTCTC | ||
| Beta Actin (Human) | Forward | 234 |
| ATATTTACGGTGCCCAGCAG | ||
| Reverse | ||
| GAAGTGGCCCATTCCTTCTC |
Amplification efficiency of different genes in all tested samples was determined relative to Beta-actin as an internal control. The fold change in differential gene expression relative to the control was calculated by 2- Δ Δ Ct livak method [11].
Samples preparation and cell seeding
All biomaterials were mixed according to manufacturer instructions and shaped into 2 mm diameter X 6 mm thickness cylinders. Then, the biomaterial cylinders were incubated in a CO2 incubator at 37°C and humidified atmosphere100% for 24 hours to ensure complete setting and then sterilised using ultra violet light.
Cultured cells from the third and fourth generations were seeded into 6-wells culture plates at a density of 15x103 cells per 3 ml GM in each well and incubated for 24 h.
To evaluate the effect of the tested biomaterials direct contact on the proliferation of hDPSCs, these cells were grouped into three groups according to biomaterial and media supplementation. Group I: DPSCs were cultured with GM and subdivided into three subgroups (A, B and C) according to the biomaterial (NHA, MTA and CEM respectively) supplementation, Group II: DPSCs were cultured with the only DM to evaluate the effect of differentiation on the proliferation. Group III: DPSCs were cultured with only GM (control group).
For each group and subgroup three 6-well plates were used and one biomaterial cylinder was inserted into each well of the 6-well plates (1cylinder /well). Media changed every three days for each plate without removing the biomaterial cylinders from the wells to keep them in contact with the DPSCs.
DPSCs in all groups and subgroups were then cultured and monitored by an inverted light microscope at the different time intervals: 1, 3, 5, 7, 9, 11 and 14 days. At these intervals, the trypan blue exclusion assay was used to calculate the viable mean cell counts as an indicator of the effect of the tested biomaterials on the DPSCs proliferation. Also, cell viability percentage calculation was done as an assessment of the tested materials cytotoxicity [11, 12]. All experiments were done in triplicates to assure reproducibility, and the resulted data were recorded.
Statistical Analysis
Statistical analysis was done using SPSS version (20) software program. The resulted viability percentages data were evaluated by an ordinary parametric One-way analysis of variance (ANOVA) followed by Duncan’s Multiple Range test. While the cell count data were analysed statistically by a group of non-parametric tests including; Kruskal-Wallis, Freidman’s tests of related samples, followed by Mann-Whitney and Wilcoxon singed-rank tests. Results were considered statistically significant at (P ≤ 0.05).
Results
The results showed that the isolated human dental pulp cells were including stem cell populations that had a positive self-renewal capability, colony forming efficiency, Multilineage differentiation (Isolated DPSCs exhibited positive staining for ALP activity (early osteogenic stage) after 7 days and calcium accumulation (late osteogenic stage) after 14 days by using alizarin red stain (Figure 1) and expressed oil red O-positive lipid clusters after 21 days of adipogenic induction) (Figure 2).
Figure 1.
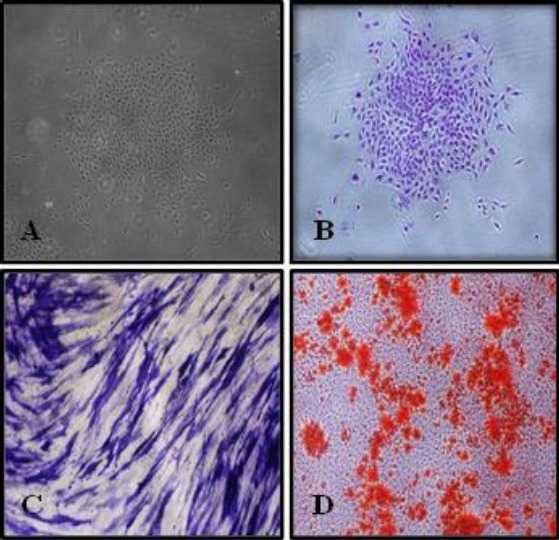
Phase contrast micrograph showing (A) a colony formed by dental pulp stem cells before staining by toluidine blue and (B) after staining by toluidine blue (X4), (C) positive reaction of dental pulp stem cells (DPSCs) for alkaline phosphatase kit after 7 days of osteogenic induction (X10), (D) Extracellular calcium accumulation (orange–red nodules) by differentiated DPSCs after 14 days of osteogenic induction (X4)
Figure 2.
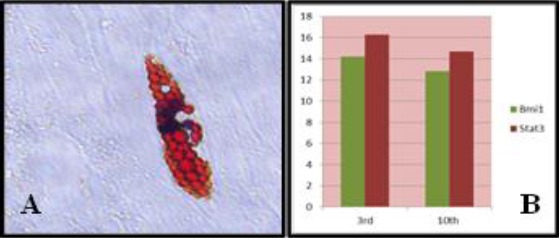
(A) Phase contrast micrograph showing fat droplets formed by dental pulp stem cells after 21 days of adipogenic induction (X40). (B) A chart showing the relative expression (fold increases) of Stat3 and Bmi1 mRNA in Human pulp cells up till the 10th generation
Moreover, DPSCs expressed stem cell genes (Stat3 and Bmi1) normalised to Human Beta-actin was high up till the10th generation, indicating the stemness of the isolated pulp cells and their ability to self-renew (Figure 2).
Regarding the morphologic observation for the cultured cells, it was shown that; DPSCs proliferated away from the NHA cylinders and showed noticeable signs of necrosis/ apoptosis (rounded cells) and cell-free zones surrounding the NHA cylinders in almost all culture plates especially after day five. On the other hand, DPSCs cultured with MTA, CEM and DM proliferated similarly. DPSCs proliferated towards the MTA and CEM cylinders with normal appearance and limited signs of necrosis/apoptosis or cell-free zones in the culture especially at and before day nine (Figure 3).
Figure 3.
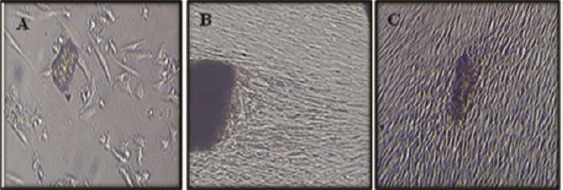
Phase contrast micrograph showing (A) DPSCs proliferated away from the NHA cylinders and showed noticeable signs of necrosis/ apoptosis (rounded cells) and cell-free zones surrounding the NHA cylinders, and (B&C) DPSCs proliferated towards the MTA and CEM cylinders with normal appearance and limited signs of necrosis/apoptosis or cell-free zones in the culture
According to the proliferation and viability percentage assays results (Figure 4 and Table 2), there was an initial statistically significant decrease in all biomaterials supplemented DPSCs counts and viabilities at day one in comparison to the GII and GIII. At the same time, there were no significant differences between the tested biomaterials.
Figure 4.
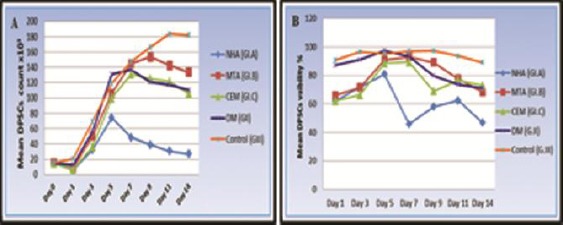
(A) Line chart showing the mean DPSCs count at all time intervals for (G-I) subgroups, (G-II) and (G-III). (B) Line chart showing change in the mean DPSCs viability percentage for (G-I) subgroups, (G-II) and (G III) at all-time intervals
Table 2.
The mean and standard deviation values for the DPSCs count and viability percentage, comparing all groups at each time interval
| Group Time | NHA (GI.A) | MTA (GI.B) | CEM (GI.C) | DM (GII) | Control (GIII) | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell count ×103 | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Day 0 | 15.00 | 0.00 | 15.00 | 0.00 | 15.00 | 0.00 | 15.00 | 0.00 | 15.00 | 0.00 | - |
| Day 1 | 6.22c | 1.20 | 8.56c | 1.33 | 6.88c | 1.36 | 12.89b | 1.54 | 20.44a | 6.98 | 0.001** |
| Day 3 | 32.22c | 7.81 | 50.22abc | 22.25 | 36.22bc | 13.84 | 55.67ab | 26.90 | 69.11a | 23.13 | 0.003** |
| Day 5 | 74.11b | 20.34 | 105.78ab | 36.17 | 99.78ab | 41.53 | 130.89a | 62.24 | 119.22ab | 52.05 | 0.050* |
| Day 7 | 48.33b | 21.16 | 144.89a | 64.35 | 131.11a | 49.73 | 137.22a | 53.54 | 146.89a | 46.09 | 0.001** |
| Day 9 | 39.11b | 16.94 | 153.67a | 49.71 | 125.11a | 42.26 | 120.78a | 54.68 | 166.11a | 48.16 | 0.001** |
| Day 11 | 30.56c | 18.84 | 142.67b | 44.89 | 121.44b | 35.69 | 117.11b | 49.02 | 183.78a | 40.31 | 0.001** |
| Day 14 | 6.67c | 15.50 | 133.44b | 45.97 | 105.56b | 39.33 | 110.33b | 49.68 | 181.78a | 46.83 | 0.001** |
| Viability% | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Day 1 | 62.07b | 13.26 | 65.89b | 13.89 | 61.98b | 12.25 | 87.21a | 10.04 | 90.81a | 9.04 | 0.001** |
| Day 3 | 71.67b | 14.21 | 71.54b | 10.99 | 66.39b | 13.12 | 91.14a | 10.07 | 96.47a | 6.14 | 0.001** |
| Day 5 | 80.79b | 13.29 | 90.89ab | 14.29 | 88.51ab | 12.71 | 97.33a | 4.01 | 95.21a | 7.12 | 0.026* |
| Day 7 | 45.77b | 18.44 | 93.02a | 12.18 | 89.34a | 14.29 | 93.40a | 7.89 | 96.96a | 5.37 | 0.001** |
| Day 9 | 57.78d | 13.74 | 89.12ab | 13.18 | 68.96cd | 12.97 | 79.81bc | 13.09 | 97.23a | 6.30 | 0.001** |
| Day 11 | 62.42c | 20.25 | 77.64b | 13.94 | 76.56bc | 12.98 | 73.76bc | 11.57 | 93.68a | 11.56 | 0.001** |
| Day 14 | 46.68c | 18.09 | 68.27b | 15.04 | 72.83b | 15.32 | 71.11b | 13.58 | 89.21a | 13.56 | 0.001** |
According to kruskal-Walls and Mann-Witney U test, within the same count row means with different superscripts (letters) are significantly different at (P ≤ 0.05),
: is significant at the 0.05 level of significance (P ≤ 0.05),
: is highly significant at the 0.001 level of significance (P ≤ 0.001). According to Duncan’s multiple range test (DMRT), within the same viability row means with different superscripts (letters) are significantly different at (P ≤ 0.05),
: is significant at the 0.05 level of significance (P ≤ 0.05),
: is highly significant at the 0.001 level of significance (P ≤ 0.001).
Then, this decrease discontinued and instead a significant rise was noted starting from day three in all groups. This rise in cell counts and viabilities reached its peak at day five for the NHA and at day seven for CEM. But, the rise in proliferation reached its peak at day seven as well as viability reached its peak at day 5 for DM supplemented cells. Whereas, the MTA supplemented cells reached its peak in the cell counts and viabilities at day nine and seven respectively.
The MTA supplemented cells showed non-significantly slight more counts and viabilities than CEM at almost all the intervals. Whereas, there was a general decrease in the mean count and viabilities in the NHA supplemented cells in comparison to other tested biomaterials that was statistically significant after day five.
Discussion
Dental materials bioactivity can be assessed either in vitro or in vivo. In vitro cell cultures provide a convenient, controllable, repeatable and cost-effective tool for the preliminary evaluation of biological response for the test material. On the other hand, in vivo testing of dental materials may be influenced by the skill of the dentist, technical properties of the material and uncontrollable patient factors. Accordingly, the results obtained from in vitro assays might be indicative of the effects observed in vivo, and so it could be a suitable alternative [12].
In the present study, the stemness of the isolated cell populations was ensured by the expression of mesenchymal stem cell-specific gene markers; Bmi1 and Stat3 in the third and tenth generations cells using quantitive RT-PCR reaction. Furthermore, the stemness of the isolated population was confirmed by the positive colony forming efficiency and self-renewal capability of the isolated and cultured cells. Another finding to confirm the presence of stem cell population was the differentiation capability of isolated DPCs. These cells were successfully differentiated into odontoblast-like cells by odontogenic-induction medium, and this differentiation was confirmed by the positive mineralisation capability. Moreover, these cells successfully adipogenic differentiated and that was confirmed by the oil red O stain [13, 14, 10].
The culture of DPSCs in direct contact with standard cylinders of tested biomaterials combined the evaluation of the effect of the biomaterial itself and extracted components on hydration. In our opinion, it is better than using the insert-well system [15, 16] or the material elutes [17, 18] models that are limited in that it can only measure the effects of the extracted out components of the material ignoring the effect of non-extracted components and surface structure. Additionally, the effect of DM on DPSCs was compared to other tested biomaterials to evaluate the effect of cell differentiation on proliferation and viability.
In the present study, both a qualitative in-vitro assessment was done through optical microscopic observation of the DPSCs cultures in all experimental groups, and a quantitive assessment through using Trypan blue exclusion assay. This assay is quick, easily performed, and distinctively differentiates nonviable from viable cells based on the analysis of the integrity of the cell membrane [19, 20].
It was noticed in the present study that the control DPSCs had almost the greatest proliferation rate and mean viability percentage (> 80%) than other biomaterials supplemented cells. This could be explained as a false positive cytotoxic result due to the relative superficial roughness of the biomaterials that may inhibit the initial cellular attachment with subsequent reduction in actual numbers of seeded cells than those cells cultured on smoother tissue culture plastic [3]. Additionally, the leached out components from biomaterials such as calcium ions, together with their initial rising PH might cause some cell lysis and affect the cell growth [5, 21] which could explain the similar significant decrease in the tested biomaterials treated DPSCs counts and viabilities than the control and DM at day one. This was in agreement with other studies that showed an initial decrease in osteosarcoma [21] and osteoblast-like cells [22] viabilities after one day culture with MTA-sealers, MTA and CEM respectively.
On the other hands, the subsequent rise in cell counts and viabilities might be due to the formation of a hydroxyapatite layer (bio-mineralization) on the hydrated biomaterial surface that is more likely to cause alterations in cellular enzymatic activity than to change cell permeability thus, increasing the tested material biocompatibility [5]. Furthermore, it had been shown that this layer further stabilise the biomaterial structure, preventing the overdose of component leaching in the medium [22]. This controlled continuous release of calcium and phosphate ions from all biomaterials in addition to silica ions leached from calcium silicate biomaterials (MTA and CEM) together with the resultant adjusted alkaline PH, had been reported to promote cell adhesion, growth and proliferation [23, 24]. These results were in agreement with previous studies which stated that MTA direct contact [3], CEM and MTA extracts [17] showed a similar time-dependent increase in human DPSCs proliferation. Also, these results came in partial agreement with Liu et al., [24] and with other studies which demonstrated that NHA promoted the proliferation and viability of osteoblasts [7]. On the contrary, Mozayeni et al., [25] disagreed with our results which might be due to the difference in culture conditions such as different target cells (fibroblasts) used, application of tested materials elutes instead of direct contact which simulates more the clinical conditions.
It was also observed that the mean cell viability percentage of the DM supplemented cells were high (>70%) and that of MTA and CEM supplemented cells were kept above 60% for all intervals. According to Bin et al, [23] the percentage of cell viability was considered optimal when the average value obtained was 50% or higher. Therefore, both MTA and CEM materials can be categorized as non-cytotoxic materials, this finding come in agreement with other studies that tested the cytotoxicity of these materials [16, 27].
On the other hand, there was a general decrease in the mean count and viabilities in the NHA supplemented cells than other tested biomaterials and that was significant after day five (below 50%). This might be due to the role of calcium silicate and other calcium compounds present in MTA and CEM that persistently control the rate of soluble silicon and calcium ions released in the medium thus, promoting similar more cell survival and biocompatibility [25] than NHA.
In addition, the differences in the biomaterials surface area and topography including particle-size and shape, had a key role on their cytotoxic behavior [7, 28]. Jaberiansari et al., [17] found that a nano sized MTA formulation exerted more toxic effects on DPSCs when compared with three commercially available MTA formulations and CEM cement. Similarly, the decreased particle size in NHA (nano range) might lead increased surface reactivity and hence, decreased biocompatibility in comparison to MTA and CEM that are in micron range [29, 30].
It was noticed that our proliferation and viability assays results were almost in accordance with each other and with our morphologic observations, which agreed with Ghoddusi et al., [30] who stated that, MTA and CEM do not induce cytotoxicity on L929 when observed by optical microscopy.
At long time intervals (day eleven and fourteen), the significant decrease in count and viability of the DM, CEM and MTA supplemented DPSCs in comparison to control cells which showed high cell densities (confluency) in most culture wells. This might be attributed to the odontogenic differentiation potential of the tested materials [5] rather than to be due to material cytotoxicity. Also, Semeghini et al., [31] observed a decrease in the proliferation rate of undifferentiated pulp cells (OD-21) supplemented with odontogenic-induction medium, especially after seven and ten days of culture. Therefore, it was unacceptable to evaluate the cell proliferation for longer than fourteen days.
In conclusion, all tested biomaterials had an initial inhibitory effect on DPSCs proliferation and viability; they were biocompatible to DPSCs except the NHA that showed questionable inhibitory effects at long culture periods. Therefore, MTA and CEM can be used safely for pulp capping and regenerative purposes while, the NHA need further cytotoxicity investigations for regenerative endodontic applications.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Sloan AJ, Waddington RJ. Dental pulp stem cells:What, where, how? Int J Paediatr Dent. 2009;19(1):61–70. doi: 10.1111/j.1365-263X.2008.00964.x. https://doi.org/10.1111/j.1365-263X.2008.00964.x PMid:19120509. [DOI] [PubMed] [Google Scholar]
- 2.Gomes Cornelio AL, Salles LP, Campos Da Paz M, Cirelli JA, Guerreiro-Tanomaru JM, Tanomaru Filho M. Cytotoxicity of Portland cement with different radiopacifying agents:A cell death study. J Endod. 2011;37(2):203–10. doi: 10.1016/j.joen.2010.11.017. https://doi.org/10.1016/j.joen.2010.11.017 PMid:21238803. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Jeon M, Shin DM, Lee JH, Song JS. [Effects of mineral trioxide aggregate on the proliferation and differentiation of human pulp cells from primary and permanent teeth] Korean Acad Pediatr Dent. 2013;40:185–193. https://doi.org/10.5933/JKAPD.2013.40.3.185. [Google Scholar]
- 4.Minamikawa H, Yamada M, Deyama Y, Suzuki K, Kaga M, Yawaka Y, et al. Effect of N-acetylcysteine on rat dental pulp cells cultured on mineral trioxide aggregate. J Endod [Internet] 2011;37(5):637–41. doi: 10.1016/j.joen.2011.02.012. https://doi.org/10.1016/j.joen.2011.02.012 PMid:21496663. [DOI] [PubMed] [Google Scholar]
- 5.Utneja S, Nawal RR, Talwar S, Verma M. Current perspectives of bio-ceramic technology in endodontics:calcium enriched mixture cement - review of its composition, properties and applications. Restor Dent Endod. 2015;40(1):1. doi: 10.5395/rde.2015.40.1.1. https://doi.org/10.5395/rde.2015.40.1.1 PMid:25671207 PMCid:PMC4320271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseinzade M, Soflou RK, Valian A, Nojehdehian H. Physicochemical properties of MTA, CEM, hydroxyapatite and nano hydroxyapatite-chitosan dental cements. Biomed Res. 2016;27(2):442–8. [Google Scholar]
- 7.Xu Z, Liu C, Wei J, Sun J. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J Appl Toxicol [Internet] 2012;32(6):429–35. doi: 10.1002/jat.1745. https://doi.org/10.1002/jat.1745 PMid:22162110. [DOI] [PubMed] [Google Scholar]
- 8.Vitti RP, Prati C, Sinhoreti MAC, Zanchi CH, Souza E, Silva MG, Ogliari FA, et al. Chemical-physical properties of experimental root canal sealers based on butyl ethylene glycol disalicylate and MTA. Dent Mater. 2013;29(12):1287–94. doi: 10.1016/j.dental.2013.10.002. https://doi.org/10.1016/j.dental.2013.10.002 PMid:24183503. [DOI] [PubMed] [Google Scholar]
- 9.Abdelfattah MI, Hassib NF. The efficacy of vitamin C in the formation of periodontal ligament stem cell sheet. Egypt J Histol. 2015;38(4):837–43. https://doi.org/10.1097/01.EHX.0000475425.12581.33. [Google Scholar]
- 10.Ahmed NE-MB, Aboul-Ezz EHA, Zakhary SY, El Badry TH, Ramzy MI. Isolation of Dental Pulp Stem Cells and their In Vitro Differentiation into Odontoblast-like Cells. Maced J Med Sci [Internet] 2011;4(3):253–60. https://doi.org/10.3889/MJMS.1857-5773.2011.0142. [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. https://doi.org/10.1006/meth.2001.1262 PMid:11846609. [DOI] [PubMed] [Google Scholar]
- 12.Samyuktha V, Ravikumar P, Nagesh B, Ranganathan K, Jayaprakash T, Sayesh V. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J Conserv Dent. 2014;17(5):467. doi: 10.4103/0972-0707.139844. https://doi.org/10.4103/0972-0707.139844 PMid:25298650PMCid:PMC4174709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronthos S, Mankani M, Brahim J, Robey PG SS. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. https://doi.org/10.1073/pnas.240309797 PMid:11087820 PMCid:PMC17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelfattah MI, Nasry SA, Mostafa AA. Characterization and cytotoxicity analysis of a ciprofloxacin loaded Chitosan/Bioglass scaffold on cultured human periodontal ligament stem cells:A preliminary report. Open Access Maced J Med Sci. 2016;4(3):461–7. doi: 10.3889/oamjms.2016.052. https://doi.org/10.3889/oamjms.2016.052 PMid:27703576 PMCid:PMC5042636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider R, Rex Holland G, Chiego D, Hu JCC, Nor JE, Botero TM. White Mineral Trioxide Aggregate Induces Migration and Proliferation of Stem Cells from the Apical Papilla. J Endod. 2014;40(7):931–6. doi: 10.1016/j.joen.2013.11.021. https://doi.org/10.1016/j.joen.2013.11.021 PMid:24935538 PMCid:PMC4426880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margunato S1, Tasl? PN2, Ayd?n S2, Karap?nar Kazandag M1 SF. In Vitro Evaluation of ProRoot MTA, Biodentine, and MM-MTA on Human Alveolar Bone Marrow Stem Cells in Terms of Biocompatibility and Mineralization. J Endod. 2015;41(10):1646–52. doi: 10.1016/j.joen.2015.05.012. https://doi.org/10.1016/j.joen.2015.05.012 PMid:26410417. [DOI] [PubMed] [Google Scholar]
- 17.Jaberiansari Z, Naderi S FST. Cytotoxic Effects of Various Mineral Trioxide Aggregate Formulations, Calcium-Enriched Mixture and a New Cement on Human Pulp Stem Cells. Iran Endod J. 2014;9(4):271–276. PMid:25386208 PMCid:PMC4224765. [PMC free article] [PubMed] [Google Scholar]
- 18.Hengameh A, Reyhaneh D, Nima MM, Hamed H. Effects of two bioactive materials on survival and osteoblastic differentiation of human mesenchymal stem cells. J Conserv Dent. 2014;17(4):349–353. doi: 10.4103/0972-0707.136509. https://doi.org/10.4103/0972-0707.136509 PMid:25125848 PMCid:PMC4127694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Er K, Polat ZA, Ozan F, Tasdemir T, Sezer U, Siso SH. Cytotoxicity analysis of strontium ranelate on cultured human periodontal ligament fibroblasts:a preliminary report. J Formos Med Assoc. 2008;107(8):609–15. doi: 10.1016/S0929-6646(08)60178-3. https://doi.org/10.1016/S0929-6646(08)60178-3. [DOI] [PubMed] [Google Scholar]
- 20.Gomes-Cornelio AL, Rodrigues EM, Salles LP, Mestieri LB, Faria G, Guerreiro-Tanomaru JMT-FM. Bioactivity of MTA Plus, Biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int Endod J. 2017;50AD(1):39–47. doi: 10.1111/iej.12589. [DOI] [PubMed] [Google Scholar]
- 21.Saeed Asgary a Laleh Alim Marvasti b and AK. Indications and Case Series of Intentional Replantation of Teeth. Iran Endod J. 2014;9(1):71–78. PMid:24396380. [PMC free article] [PubMed] [Google Scholar]
- 22.Salles LP, Gomes-Cornelio AL, Guimaraes FC, Herrera BS, Bao SN, Rossa-Junior C, et al. Mineral trioxide aggregate-based endodontic sealer stimulates hydroxyapatite nucleation in human osteoblast-like cell culture. J Endod. 2012;38(7):971–6. doi: 10.1016/j.joen.2012.02.018. https://doi.org/10.1016/j.joen.2012.02.018 PMid:22703663. [DOI] [PubMed] [Google Scholar]
- 23.Bin CV, Valera MC, Camargo SEA, Rabelo SB, Silva GO, Balducci I, et al. Cytotoxicity and Genotoxicity of Root Canal Sealers Based on Mineral Trioxide Aggregate. J Endod. 2012;38(4):495–500. doi: 10.1016/j.joen.2011.11.003. https://doi.org/10.1016/j.joen.2011.11.003 PMid:22414836. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Zhao M, Lu J, Ma J, Wei J, Wei S. Cell responses to two kinds of nanohydroxyapatite with different sizes and crystallinities. Int J Nanomedicine. 2012;7:1239–50. doi: 10.2147/IJN.S28098. https://doi.org/10.2147/IJN.S28098 PMid:22419871 PMCid:PMC3299575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozayeni MA, Milani AS, Marvasti LA, Asgary S. Cytotoxicity of calcium enriched mixture cement compared with mineral trioxide aggregate and intermediate. Aust Endo J. 2012;38:70–5. doi: 10.1111/j.1747-4477.2010.00269.x. https://doi.org/10.1111/j.1747-4477.2010.00269.x PMid:22827819. [DOI] [PubMed] [Google Scholar]
- 26.Saberi EA, Karkehabadi HMN. Cytotoxicity of Various Endodontic Materials on Stem Cells of Human Apical Papilla. Iran Endod J. 2016;11(1):17–22. doi: 10.7508/iej.2016.01.004. PMid:26843872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Ng S, Heng BC, Guo J, Ma L, Tan TTY, et al. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch Toxicol. 2013;87(6):1037–52. doi: 10.1007/s00204-012-0827-1. https://doi.org/10.1007/s00204-012-0827-1 PMid:22415765. [DOI] [PubMed] [Google Scholar]
- 28.Xiaochen Liu, Minzhi Zhao, Jingxiong Lu, Jian Ma, Jie Wei, SW Cell responses to two kinds of nanohydroxyapatite with different sizes and crystallinities. Int J Nanomedicine. 2012;7:1239–1250. doi: 10.2147/IJN.S28098. https://doi.org/10.2147/IJN.S28098 PMid:22419871 PMCid:PMC3299575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soheilipour E, Kheirieh S, Madani M, Baghban AA, Asgary S. Particle size of a new endodontic cement compared to Root MTA and calcium hydroxide. Iran Endod J. 2009;4(3):112–116. PMid:24003332 PMCid:PMC3758862. [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoddusi J, Afshari JT, Donyavi Z, Brook A, Disfani R, Esmaeelzadeh M. Cytotoxic effect of a new endodontic cement and mineral trioxide aggregate on L929 line culture. Iran Endod J. 2008;3(2):17–23. PMid:24171015 PMCid:PMC3808562. [PMC free article] [PubMed] [Google Scholar]
- 31.Semeghini MS, Fernandes RR, Chimello DT, Oliveira FS B-PK. In vitro evaluation of the odontogenic potential of mouse undifferentiated pulp cells. Braz Dent J. 2012;23(4):328–36. doi: 10.1590/s0103-64402012000400004. https://doi.org/10.1590/S0103-64402012000400004 PMid:23207845. [DOI] [PubMed] [Google Scholar]


