Abstract
Highly efficient antioxidants based on superoxide dismutase (SOD) -loaded porous polymersomes are developed for treating neuropathic pain. The SOD-loaded porous polymersomes are highly permeable to superoxide radical, while retaining the antioxidant enzyme within their aqueous interiors. Administration of the antioxidant porous polymersomes following a painful nerve root compression is substantially more effective in preventing the onset of pain in rats than comparable or higher doses of free SOD alone.
Keywords: superoxide dismutase, antioxidant, porous polymersomes, neuropathic pain
ToC figure
Chronic pain is a highly prevalent and debilitating disorder affecting up to two-thirds of the general population in their lifetime and presenting staggering socioeconomic costs.[1] Neuropathic pain, or pain resulting from direct injury or secondary damage to neural tissue, affects approximately 10% of the U.S population and occurs in approximately 60–80% of persons with neural injuries including spinal cord injury and peripheral nerve trauma.[2] Despite its prevalence, neuropathic pain is largely resistant to treatment.[3] In many cases pain can persist for several years or a lifetime after an initial injury,[4] that often worsen into chronic disability.[5] Current treatments for neuropathic pain include surgical intervention and pharmacological approaches.[6] However, surgical intervention is usually an invasive procedure. Although many pharmacologic approaches, such as opioid analgesics[7] and non-opioid analgesics,[8] have been extensively pursued to treat neuropathic pain, none has emerged with any significant clinical success. In most cases, pharmacologic approaches are accompanied by marginal efficacy and undesired side effects including addiction and increased pharmacological tolerance. Accordingly, there is a tremendous need to develop new approaches for better treating neuropathic injury and its associated pain.
The spinal nerve root is a common source of neuropathic pain and can be injured directly from its compression by disc herniation, spondylosis or other spinal traumas.[9] Several animal models of nerve root compression have shown that even a transient, 15-minute compression applied to the nerve root is sufficient to induce sustained pain.[10, 11] In addition to pain, axonal damage and myelin degeneration are evident in the nerve root by 7 days after compression.[11] Such damage is hypothesized to relate to and cause neuronal dysfunction and contribute to the maintenance of pain by impaired axonal transport in the nerve root and disrupting the afferent signals that communicate to synapses in the spinal cord dorsal horn.[11]
Recent work has indicated that reactive oxygen species (ROS) play a critical role in initiating and maintaining neuropathic pain.[12] Neural tissue is particularly susceptible to ROS damage due to a high lipid content, a high rate of oxidative metabolic activity, intense production of reactive oxygen metabolites and the non-replicating nature of neurons.[13] Following neural injury, both the elevation and accumulation of ROS, such as superoxide, have been shown to induce secondary oxidative damage, including neuronal degeneration at the site of injury.[14] In addition, excessive ROS accumulation in the spinal cord has a critical role in the development of neuropathic pain through the activation of NMDA receptors on neurons[15, 16] and increased neuronal hyperexcitability in the spinal dorsal horn.[17] Despite the known role of excessive ROS in neuropathic pain induction, treatments to effectively reduce oxidative damage and attenuate pain are still lacking.
Antioxidants are molecules that can safely interact with ROS and terminate the deleterious cascades. There are multiple types of endogenous and exogenous antioxidants, such as phytochemicals, vitamins, and enzymes.[18] Among these, superoxide dismutase (SOD), which converts the potentially damaging superoxide radical (O2−) to hydrogen peroxide (H2O2), is one of the body’s primary internal antioxidant defenses, and plays a critical role in reducing the oxidative stress implicated in many life-threatening diseases.[19] Under normal healthy conditions, there are sufficient levels of SOD and other antioxidants in the spinal cord to maintain low levels of ROS.[20] However, after neural trauma, the levels of antioxidants are not sufficient to remove the excess ROS that are produced, resulting in a loss of cellular redox balance, and subsequent development of oxidative stress in the neurons and glia of the central nervous system.[21] SOD activity has also been found to decrease over time in injured neural tissue both centrally and peripherally.[21, 22] Thus, scavenging ROS by exogenous antioxidants has been investigated as a potential treatment for neuropathic pain.[15, 23, 24] For example, several different antioxidants (e.g., phenyl N-tert-Butyl-α-phenylnitrone, 5,5-dimethyl-pyrroline-N-oxide, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl, and vitamin E) have been explored for treating neuropathic pain.[25] However, the use of high-doses of these antioxidants is controversial due to significant dose-related side effects and relatively modest improvements in pain treatment.
Despite the tremendous potential of using antioxidant scavengers to treat chronic pain, the analgesic effects are often transient and require repeated dosing.[15, 23] With enzyme-based antioxidant treatment, this is a consequence of poor pharmacokinetics and the rapid loss of enzyme activity in vivo. For example, the efficacy of treatment in the form of native SOD is largely limited because of a short circulation half-life in vivo (t1/2 of SOD=4–8min) and inadequate delivery to the site of injury.[20] Moreover, rapid inactivation of native SOD and its low affinity to tissue membranes in vivo has also prevented its clinical use.[26] While several methods to improve SOD retention in circulation have been performed, such as lecithinized SOD, their efficacy is still transient if administered later after injury.[26] Thus, the use of enzyme antioxidants is underscored by the need to develop a better delivery platform that protects against degradation, but maintains enzymatic activity.
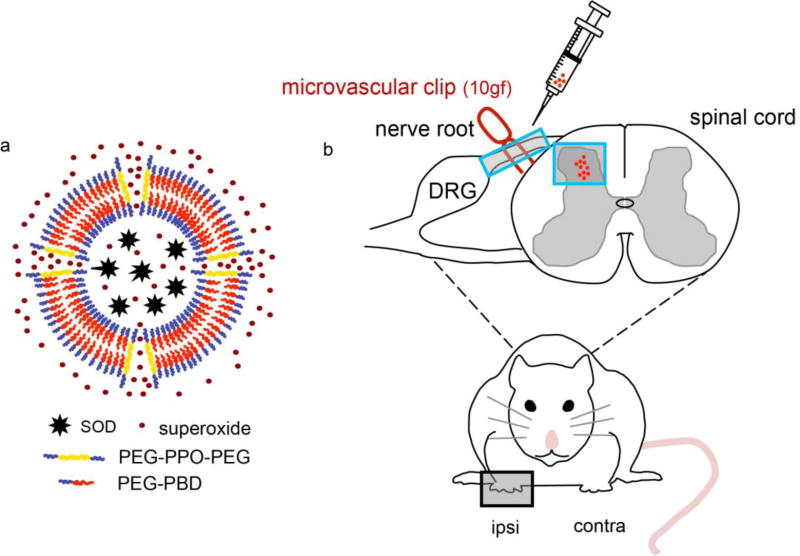
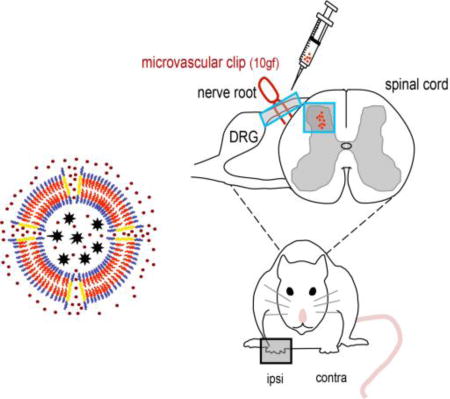
To date, several approaches have been developed to improve antioxidant enzyme therapy. One strategy involves the coupling of polyethylene glycol (PEG) to the enzymes or the encapsulation enzymes within nanoparticles, such as liposomes or poly(lactic-co-glycolic) acid (PLGA). These approaches typically increase enzyme bioavailability and provide a protection against degradation or proteolysis.[27] In other approaches, chemical modifications, genetic manipulations, and conjugation of antioxidant enzymes to antibodies have also been developed in order to provide more effective delivery.[28] While these approaches have shown promise for improving antioxidant enzyme therapy, several significant obstacles to clinical applications remain, including reduced enzyme activity, immunogenicity, toxicity, instability in vivo, and cost. Therefore, we sought to develop a platform that can overcome these barriers and allow for the efficient delivery of therapeutic antioxidant enzymes into injured spinal cord. To improve antioxidant enzyme delivery, we recently discovered that porous polymersomes could achieve this purpose.[29] Compared with existing carriers for enzyme delivery, the antioxidant enzyme-loaded porous polymersomes possess several key beneficial properties including: 1) the enzymes are unmodified (i.e. no conjugation and immobilization) and thus retain their original structure without altering functionality and potential diffusion limitation, 2) the polymersomes possess a highly porous membrane, providing encapsulated enzymes with efficient access to surrounding ROS, 3) the protective polymer shell protects the encapsulated enzymes from proteolysis, 4) a poly(ethylene glycol) (i.e. a PEG brush) surface, offers a stealth character and extended in vivo circulation, 5) the outer surface remains unobstructed, allowing for the highly efficient attachment of specific targeting agents. Therefore, porous polymersomes provide a unique platform for antioxidant enzyme delivery. In this study, we prepared SOD-loaded porous polymersomes and investigated whether they could form a highly efficient antioxidant formulation and prevent neuropathic pain after nerve root compression in a rat model (Figure 1).
Figure 1.
a) Schematic diagram of SOD-loaded porous polymersomes. Porous polymersomes were formed through the co-assembly of the diblock copolymer PEG-PBD and the triblock copolymer PEG-PPO-PEG. Antioxidant enzyme SOD was loaded into the aqueous lumen of the porous polymersomes. The SOD-loaded porous polymersomes have a high membrane permeability to the small superoxide radical, while retaining SOD within their aqueous interiors. b) Nerve root compression injury was performed using a 10gf microvascular clip applied to the C7 dorsal nerve root. SOD-loaded porous polymersomes were injected directly onto the nerve root ipsilateral to injury, immediately after compression was removed.
Porous polymersomes were prepared by using the diblock copolymer poly(ethylene glycol)-polybutadiene (PEG-PBD) and the triblock copolymer poly(ethylene glycol)-block-poly(propylene oxide)-block-poly(ethylene glycol) (PEG-PPO-PEG). It has been found that polymersomes made from 75mol% PEG-PBD/25mol% PEG-PPO-PEG exhibit improved membrane permeability to molecules less than 5 kDa, while molecules ≥10 kDa are retained within their aqueous interiors with no significant leakage.[29] In contrast, polymersomes made from pure PEG-PBD do not show any significant membrane permeability to small molecules. To prepare antioxidant porous polymersomes, Cu,Zn-SOD (Mr 32500 Da) was encapsulated into the aqueous interior of porous polymersomes made from 75mol% PEG-PBD/25mol% PEG-PPO-PEG using the film hydration method, followed by sonication, freeze-thaw and extrusion (100 nm filter membrane).[30] Control nonporous polymersomes were prepared using 100mol% PEG-PBD. Nonentrapped SOD molecules were removed via size exclusion chromatography packed with Sepharose 4B-CL. Dynamic light scattering (DLS) revealed that the SOD-encapsulated porous polymersomes had a mean diameter of 110 ± 5 nm (Figure 2a). The morphologies of porous vesicles were further confirmed by Cryo-TEM (Figure S1, Supporting Information). No significant difference in size was observed between SOD-encapsulate porous polymersomes and empty porous polymersomes (diameter 108 nm). To evaluate the structural stability of SOD-loaded porous polymersomes, the hydrodynamic diameter of the polymersomes was measured by DLS for 7 days following suspension in PBS buffer (0.1 mM phosphate, pH 7.4). It was found that antioxidant porous polymersomes did not exhibit any significant change in hydrodynamic diameter over this time frame (Figure S2, Supporting Information).
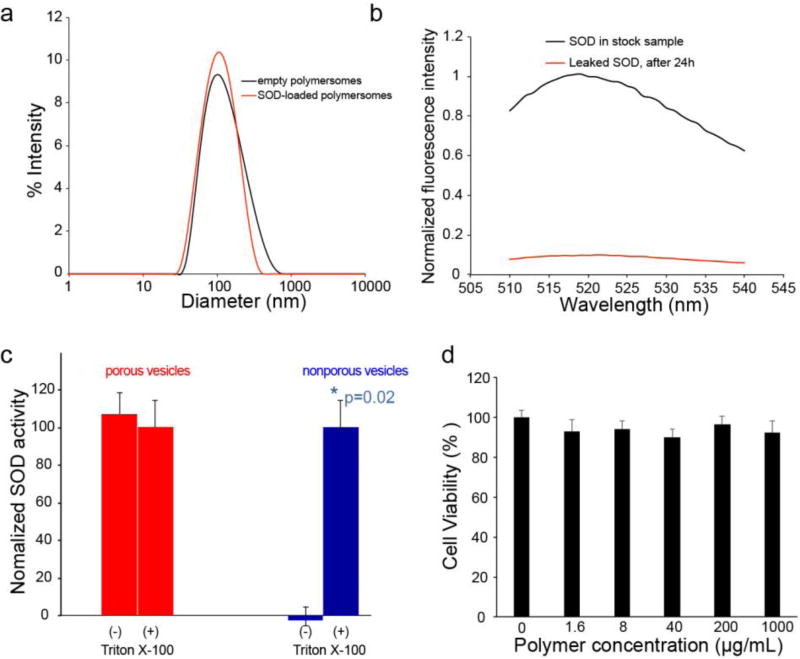
Figure 2.
a) Intensity-weighted size distribution of SOD-loaded porous polymersomes and empty polymersomes as measured by dynamic light scattering (DLS). b) Evaluation of SOD retention within porous polymersomes. Following 24 hours of incubation in PBS buffer (0.1 M, pH 7.4), the polymersomes were centrifuged on a Microcon filtering device with a 100 KDa MWCO membrane. The liquid that flowed through the filter was measured for fluorescence (red line). The fluorescence of unfiltered sample in the presence of Triton X-100 was also recorded (black line). The fluorescence intensity is normalized relative to the intensity of unfiltered sample at 518 nm. c) SOD activity within porous and nonporous polymersomes. SOD activity was tested before (−) and after (+) the addition of Triton-X-100. d) Cell viability in neuronal cultures was unchanged from control (0 µg/mL) following incubation with any polymersome concentration.
Although diffusion of small molecules, i.e. superoxide anion, across the porous polymersome bilayer is desirable, the leakage of encapsulated SOD from the porous polymersome would be detrimental to the utility of this probe. To confirm that SOD was too large to pass through the porous membrane of the porous polymersomes, FITC-labeled SOD was encapsulated within the aqueous interior of the porous polymersomes. Following a 24 h incubation in 10 mM PBS buffer, the samples were centrifuged on a centrifugal filtering device (Amicon Ultra-4, 100K MWCO, Millipore Corp.) and the fluorescence of the flow-through was measured. It has been found that no fluorescence was detected in the flow-through, suggesting that the SOD is retained within the porous polymersome (Figure 2b). For comparison, transfer of free SOD was observed on a similar centrifugal filtering device (Figure S3, Supporting Information).
To assess the enzymatic activity of SOD-loaded porous polymersomes, the activity of SOD was measured by ferricytochrome c assay.[31] As shown in Figure 2c, SOD displayed the catalytic activity in porous polymersomes, suggesting that superoxide radical is capable of diffusing into the aqueous interior of polymersomes through the permeable porous membrane. To confirm this finding, SOD-loaded porous polymersomes were also treated with Triton X-100. The activity of the released SOD following Triton X-100 induced polymersome dissolution was measured. It has been found the SOD activity following the complete release of the enzyme from polymersomes was similar to it in SOD-loaded porous polymersomes that had not been treated with Triton X-100. In contrast, when SOD was encapsulated in nonporous polymersomes made from 100mol% PEG-PBD, SOD activity of trapped enzyme was negligible and was only detected after dissolving of the polymersomes with Triton X-100 treatment. These findings indicate that SOD-encapsulated porous polymersomes does not affect dismutase activity compared to non-porous polymersomes, providing a permeable membrane that allows free superoxide radicals to pass into the aqueous interior and interact with the encapsulated antioxidant enzyme SOD.
Prior to administering antioxidant polymersomes in vivo, the cytotoxicity of the porous polymersome was tested with dorsal root ganglia (DRG) neurons. Incubation of porous polymersomes did not reduce cell viability at any concentration compared to untreated cultures (Figure 2d). Similarly, polymersomes did not induce cell lysis compared to untreated cultures, and less than 1% cell lysis was observed for both the treated and untreated groups (Figure S4 in the Supporting Information). Cell lysis in treated cultures was also not different amongst all treatment concentrations.
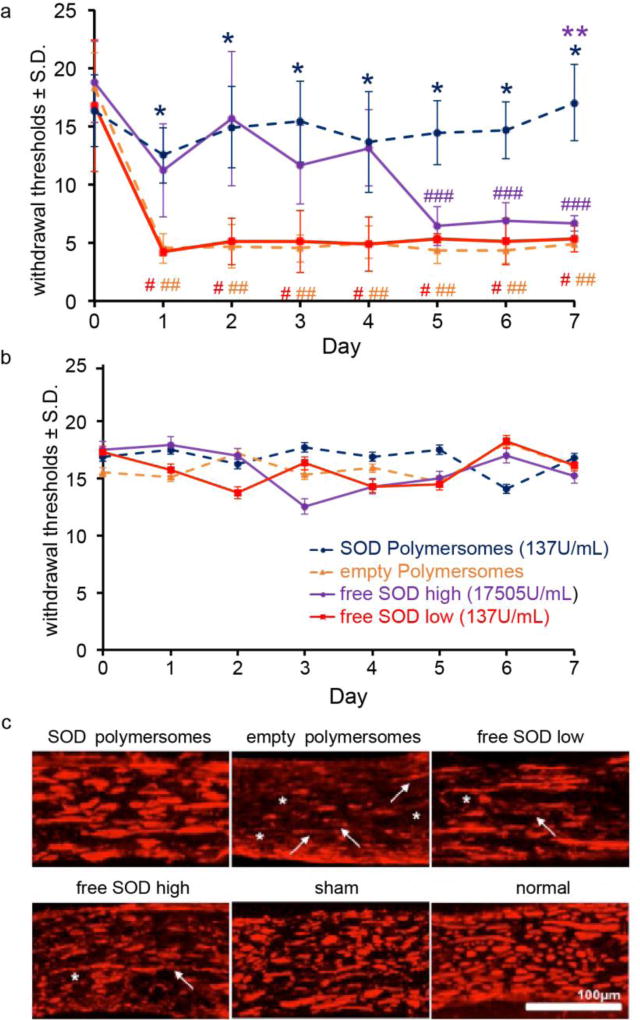
To test the efficacy of SOD-loaded porous polymersomes in treating pain, male Holtzman rats underwent a unilateral painful C7 root compression.[32] Immediately after compression, either 100 µl (137 U/mL) of SOD-loaded porous polymersomes in saline, empty polymersomes (no SOD encapsulation), or free SOD at either a comparable dose (137 U/mL) or higher dose (17505 U/mL) of free SOD in saline was administered directly to the nerve root. As shown in Figure 3a, administration of antioxidant polymersomes prevents the development of pain that is typically induced by a nerve root compression. The withdrawal thresholds in the ipsilateral injured forepaw are significantly higher following treatment with SOD-loaded porous polymersomes than with empty polymersomes, on days 1 through 7 post-treatment (p<0.01), indicating less pain. In addition, thresholds following treatment with SOD-loaded porous polymersomes are significantly higher than the same dose of free SOD, on days 1 through 7 (p<0.01). Both the empty polymersomes and low dose of free SOD are significantly lower than their baseline (p<0.0002), indicating a painful response. The higher dose of free SOD only transiently prevents pain, lasting until day 5; at days 6 and 7 thresholds are significantly below baseline (p<0.0012). At day 7, thresholds following treatment with free high SOD are significantly lower than thresholds for rats treated with SOD-loaded porous polymersomes (p<0.0001). There are no differences in contralateral forepaw withdrawal thresholds between any of the groups, on days 1 through 7 post-treatment (Figure 3b).
Figure 3.
a) SOD porous polymersomes prevent pain developmentwith significantly higher withdrawal thresholds in the ipsilateral forepaw compared to both the empty polymersomes or free SOD low (*p<0.0001) treatments. Empty polymersome and free SOD treatment do not prevent pain with thresholds significantly lower than baseline on all days (#,## p<0.002). Pain develops after free SOD treatment after day 5 from baseline (### p<0.012) with thresholds significantly lower from SOD porous polymersomes on day 7 (**p<0.01). b) Withdrawal thresholds in the contralateral forepaw were not significantly different between groups on any day tested. c) NF200 labeling in the ipsilateral nerve roots after SOD porous polymersome treatment was similar to both sham and normal roots, yet treatment with empty polymersomes displayed the greatest evidence of axonal thinning (asterisk) and discontinuous labeling (arrows), with lesser axonal damage observed in both free SOD groups.
Treatment with antioxidant polymersomes partially prevents the axonal swelling and damage that typically arises after a painful nerve root compression (Figure 3c, and Table S1 in Supporting Information). Even 7 days after nerve root compression, axons in the injured nerve root treated with SOD-loaded porous polymersomes display similar axonal pathology to both normal and sham nerve roots with 4 out of 6 rats receiving a score of – or −/+ (Table S1). In contrast, for treatment with empty porous polymersomes, roots display the most extensive axonal pathology including axonal thinning, periodic varicosities and discontinuous NF200 labeling. Roots treated with empty polymersomes are scored as having the most extensive damage, with all but 1 rat receiving the highest score (++) (Table S1). Treatment with either concentration of the free SOD antioxidants does not seem to prevent the full extent of damage in the injured nerve root, including disrupted axonal labeling and altered morphology.
This is the first study to show that the encapsulation of SOD in a porous polymersome formulation preserves SOD enzymatic activity (Figures 2c&3a) and are non-cytotoxic to neuronal and glial cells (Figure 2d). Moreover, administration of these SOD-loaded porous polymersomes, immediately after painful nerve root compression, prevents the onset of pain as well as axonal damage more effectively than a comparable or higher dose of free SOD (Figure 3 and Supplementary Table 1).
Compared with phospholipid liposomes, polymersomes (i.e. polymeric vesicles) made from high-molecular-weight copolymers exhibit enhanced stability and a long blood circulation time.[33] As a result, polymersomes maintain their structural integrity in vivo for longer periods of time and are expected to provide encapsulated antioxidant enzymes with better protection against inactivation. However, the impermeability of the thick polymersome membrane can be problematic for the efficient removal of ROS. In order for antioxidant enzyme-polymersomes to be used as an efficient antioxidant, they should allow ROS, e.g. O2−, to pass into the aqueous interior and interact with encapsulated antioxidant enzymes. To tackle this problem, we prepared porous polymersomes as a nanocarrier for antioxidant enzymes. The porous polymersomes can be easily synthesized using a facile method by simply incorporating PEG-PPO-PEG.[29] Compared with other methods for producing porous polymersome that require free radical cross-linking[34] or acid hydrolysis[35], which could have an affect on the activity of encapsulated enzyme and therefore likely reduce the efficacy of therapy, the current method applied in this work was performed using more mild and gentle conditions. As shown in Figure 2c, these SOD-loaded porous polymersomes can act as highly efficient antioxidants.
Incubation of the SOD porous polymersomes with DRG neuronal cultures did not significantly alter cell viability (Figure 2d) as measured by the MTS assay or induce cell lysis at any concentration tested (Figure S4). For cytotoxicity assays, the MTS assay measures cell viability through the reductase activity in the mitochondria, and therefore is a surrogate measurement for mitochondrial health.[36] In this experiment, several different incubation concentrations were tested, all of which, showed no mitochondrial compromise. Similarly, incubation with SOD-loaded porous polymersomes did not show any evidence of lysis as measured by the LDH assay, a measurement utilizing membrane disruption as a surrogate marker for cell death.[37] These findings are consistent with other cytotoxicity assays using similarly sized polymersomes.[36]
Additionally, administering the SOD-loaded porous polymersomes in-vivo immediately after a painful nerve root injury is more effective in preventing pain development than the same dose of free SOD (Figure 3a). Moreover, treatment with the higher dose of free SOD only transiently alleviated pain. This finding is consistent with outcomes of antioxidant treatment of other neuropathic injuries, specifically treatment with a higher dose of free antioxidant did not fully abolish pain after spinal nerve ligation.[15] Similar studies, which used superoxide dismutase or its mimetic M40403, have also shown a transient attenuation of pain.[24, 38] In this study, the SOD-loaded porous polymersomes were administered locally after nerve root compression. We hypothesize that polymersomes were internalized by neuronal and glial cells and then degraded in their lysosomes. For any polymersomes that were not retained at the injury site, they would enter the blood circulation and ultimately be cleared by the liver.[30] Another study has shown that the delivery method may affect the efficacy of antioxidant treatments to reduce pain after neural injury.[39] Local administration of the ROS antioxidant Tempol intrathecally produced anti-nociceptive effects following chronic constriction injury, yet systemic, intraperotineal injection of Tempol did not reduce behavioral sensitivity or mitigate the effects of oxidative damage.[39] Therefore, future studies are needed to investigate if systemic administration of the SOD porous polymersomes can sufficiently prevent the development of pain.
In addition to alleviating pain, antioxidant treatments have been shown to rescue neuronal health following neuronal injuries.[40] In this study, administration of the SOD-loaded porous polymersomes partially prevented the axonal damage and disorganization (Figure 3c) that usually occurs after painful nerve root compression.[11] Treatment with the empty polymersomes exhibited the most disruption in NF200 labeling and axonal thinning, while treatment with both free SOD regiments displayed axonal damage to a lesser extent, suggesting that free SOD antioxidant treatment administration may delay the onset of pain, but alone may not sufficiently reduce ROS to rescue neuronal health after injury. The prevention of axonal damage with SOD-loaded porous polymersomes also suggests that these polymersomes may have greater antioxidant activity than free antioxidant alone, especially since it is known that free antioxidants are rapidly degraded in-vivo.[41] In fact, SOD enzymatic activity has been shown to be neuroprotective following SCI,[42, 43] with administration of SOD enzyme rescuing motor neuron health in the injured spinal cord.[42] In this study the SOD-loaded porous polymersomes were administered immediately after injury, although oxidative stress following neural injury is thought to occur within hours after injury,[38, 44] it is unknown if administration of these polymersomes later after injury would be as effective, when pain and axonal damage are established. However, these studies demonstrate that SOD-encapsulated porous particles can be used as a novel potential pain treatment, that is more effective than treatment with free antioxidant alone.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health NCI R01CA175480 (ZC), the Cervical Spine Research Society, Catherine Sharpe Foundation, and the PENN ITMAT-CT3N Pilot Project.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Sonia Kartha, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA.
Dr. Lesan Yan, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA
Christine L. Weisshaar, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA
Meagan E. Ita, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA
Dr. Vladimir V. Shuvaev, Department of Systems Pharmacology and Translational Therapeutics, Center for Translational Targeted Therapeutics and Nanomedicine of the Institute for Translational Medicine and Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA
Prof. Vladimir R. Muzykantov, Department of Systems Pharmacology and Translational Therapeutics, Center for Translational Targeted Therapeutics and Nanomedicine of the Institute for Translational Medicine and Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA
Prof. Andrew Tsourkas, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA
Prof. Beth A. Winkelstein, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA
Prof. Zhiliang Cheng, Department of Bioengineering, University of Pennsylvania, 210 South 33rd Street, 240 Skirkanich Hall, Philadelphia, PA 19104, USA
References
- 1.Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, Cote P, Haldeman S, Ammendolia C, Carragee E, Hurwitz E, Nordin M, Peloso P. Bone, P. Joint Decade - Task Force on Neck, D. Its Associated, Spine (Phila Pa 1976) 2008;33:S39. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]; Waters TR. J Electromyogr Kinesiol. 2004;14:7. doi: 10.1016/j.jelekin.2003.09.004. [DOI] [PubMed] [Google Scholar]; Cote P, Cassidy JD, Carroll LJ, Kristman V. Pain. 2004;112:267. doi: 10.1016/j.pain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Yawn BP, Wollan PC, Weingarten TN, Watson JC, Hooten WM, Melton LJ., 3rd Pain Med. 2009;10:586. doi: 10.1111/j.1526-4637.2009.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rutkowski MD, Winkelstein BA, Hickey WF, Pahl JL, DeLeo JA. Spine (Phila Pa 1976) 2002;27:1604. doi: 10.1097/00007632-200208010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moalem G, Tracey DJ. Brain Res Rev. 2006;51:240. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]; Harden N, Cohen M. J Pain Symptom Manage. 2003;25:S12. doi: 10.1016/s0885-3924(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 4.Scholz J, Woolf CJ. Nat Neurosci. 2007;10:1361. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan K, Litchy WJ, O'Fallon WM, Kurland LT. Brain. 1994;117(Pt 2):325. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]
- 6.Colangelo AM, Bianco MR, Vitagliano L, Cavaliere C, Cirillo G, De Gioia L, Diana D, Colombo D, Redaelli C, Zaccaro L, Morelli G, Papa M, Sarmientos P, Alberghina L, Martegani E. J Neurosci. 2008;28:2698. doi: 10.1523/JNEUROSCI.5201-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNicol ED, Midbari A, Eisenberg E. The Cochrane database of systematic reviews. 2013;8:Cd006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore RA, Chi CC, Wiffen PJ, Derry S, Rice AS. The Cochrane database of systematic reviews. 2015;10:Cd010902. doi: 10.1002/14651858.CD010902.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbed KM, Coumans JV. Neurosurgery. 2007;60:S28. doi: 10.1227/01.NEU.0000249223.51871.C2. [DOI] [PubMed] [Google Scholar]; Hagen EM, Rekand T. Pain Ther. 2015;4:51. doi: 10.1007/s40122-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JR, Syre PP, Oake SA, Nicholson KJ, Weisshaar CL, Cruz K, Bucki R, Baumann BC, Janmey PA, Winkelstein BA. PLoS One. 2013;8:e80006. doi: 10.1371/journal.pone.0080006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson KJ, Zhang S, Gilliland TM, Winkelstein BA. J Neurosurg Spine. 2014;20:751. doi: 10.3171/2014.2.SPINE13672. [DOI] [PubMed] [Google Scholar]
- 12.Hassler SN, Johnson KM, Hulsebosch CE. Journal of neurochemistry. 2014;131:413. doi: 10.1111/jnc.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yowtak J, Wang J, Kim HY, Lu Y, Chung K, Chung JM. Pain. 2013;154:2469. doi: 10.1016/j.pain.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gwak YS, Hassler SE, Hulsebosch CE. Pain. 2013;154:1699. doi: 10.1016/j.pain.2013.05.018. [DOI] [PubMed] [Google Scholar]; Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14851. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans PH. Br Med Bull. 1993;49:577. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- 14.Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Spinal Cord. 2012;50:264. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]; Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Brain Res Rev. 2009;60:202. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kallenborn-Gerhardt W, Hohmann SW, Syhr KMJ, Schroder K, Sisignano M, Weigert A, Lorenz JE, Lu RR, Brune B, Brandes RP, Geisslinger G, Schmidtko A. Pain. 2014;155:2161. doi: 10.1016/j.pain.2014.08.013. [DOI] [PubMed] [Google Scholar]; Salvemini D, Little JW, Doyle T, Neumann WL. Free Radical Bio Med. 2011;51:951. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Kim HK, Chung JM, Chung K. Pain. 2007;131:262. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye L, Xiao L, Bai X, Yang SY, Li Y, Chen Y, Cui Y, Chen Y. Neurosci Lett. 2016;634:79. doi: 10.1016/j.neulet.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Kim HY, Lee I, Chun SW, Kim HK. Neural Plast. 2015;2015:293423. doi: 10.1155/2015/293423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman K. Clin Interv Aging. 2007;2:219. [PMC free article] [PubMed] [Google Scholar]
- 19.Pham-Huy LA, He H, Pham-Huy C. Int J Biomed Sci. 2008;4:89. [PMC free article] [PubMed] [Google Scholar]
- 20.Kabu S, Gao Y, Kwon BK, Labhasetwar V. J Control Release. 2015;219:141. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CY, Chen JK, Wu YT, Tsai MJ, Shyue SK, Yang CS, Tzeng SF. J Biomed Sci. 2011;18:13. doi: 10.1186/1423-0127-18-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur G, Bedi O, Sharma N, Singh S, Deshmukh R, Kumar P. J Basic Clin Physiol Pharmacol. 2016;27:9. doi: 10.1515/jbcpp-2015-0026. [DOI] [PubMed] [Google Scholar]
- 23.Xie YG, Mu HJ, Li Z, Ma JH, Wang YL. Exp Ther Med. 2014;8:1137. doi: 10.3892/etm.2014.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvernini D. J Pharmacol Exp Ther. 2004;309:869. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 25.Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Pain. 2011;152:844. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takenaga M, Ohta Y, Tokura Y, Hamaguchi A, Nakamura M, Okano H, Igarashi R. Journal of Controlled Release. 2006;110:283. doi: 10.1016/j.jconrel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. Am J Physiol. 1989;256:H589. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]; Luisa Corvo M, Jorge JC, van't Hof R, Cruz ME, Crommelin DJ, Storm G. Biochim Biophys Acta. 2002;1564:227. doi: 10.1016/s0005-2736(02)00457-1. [DOI] [PubMed] [Google Scholar]; Reddy MK, Wu L, Kou W, Ghorpade A, Labhasetwar V. Appl Biochem Biotechnol. 2008;151:565. doi: 10.1007/s12010-008-8232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita T, Nishikawa M, Tamaki C, Takakura Y, Hashida M, Sezaki H. J Pharmacol Exp Ther. 1992;263:971. [PubMed] [Google Scholar]
- 29.Yan L, Higbee E, Tsourkas A, Cheng Z. J Mater Chem B Mater Biol Med. 2015;3:9277. doi: 10.1039/C5TB02067K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng ZL, Thorek DLJ, Tsourkas A. Advanced Functional Materials. 2009;19:3753. doi: 10.1002/adfm.200901253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuvaev VV, Han J, Tliba S, Arguiri E, Christofidou-Solomidou M, Ramirez SH, Dykstra H, Persidsky Y, Atochin DN, Huang PL, Muzykantov VR. PLoS One. 2013;8:e77002. doi: 10.1371/journal.pone.0077002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorek DL, Weisshaar CL, Czupryna JC, Winkelstein BA, Tsourkas A. Mol Imaging. 2011;10:206. [PubMed] [Google Scholar]
- 33.Discher DE, Eisenberg A. Science. 2002;297:967. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Z, Tsourkas A. Langmuir. 2008;24:8169. doi: 10.1021/la801027q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Z, Thorek DL, Tsourkas A. Adv Funct Mater. 2009;19:3753. doi: 10.1002/adfm.200901253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhu BM, Ali SF, Murdock RC, Hussain SM, Srivatsan M. Nanotoxicology. 2010;4:150. doi: 10.3109/17435390903337693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan FK, Moriwaki K, De Rosa MJ. Methods Mol Biol. 2013;979:65. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bains M, Hall ED. Bba-Mol Basis Dis. 2012;1822:675. [Google Scholar]
- 39.Zhao B, Pan Y, Wang Z, Tan Y, Song X. Cell Mol Neurobiol. 2016;36:893. doi: 10.1007/s10571-015-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marklund N, Clausen F, Lewen A, Hovda DA, Olsson Y, Hillered L. Acta Neurochir (Wien) 2001;143:73. doi: 10.1007/s007010170141. [DOI] [PubMed] [Google Scholar]
- 41.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. Br J Pharmacol. 2003;140:445. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yune TY, Lee JY, Jiang MH, Kim DW, Choi SY, Oh TH. Free Radic Biol Med. 2008;45:1190. doi: 10.1016/j.freeradbiomed.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Sugawara T, Lewen A, Gasche Y, Yu FS, Chan PH. Faseb J. 2002;16:1997. doi: 10.1096/fj.02-0251fje. [DOI] [PubMed] [Google Scholar]
- 44.von Leden RE, Yauger YJ, Khayrullina G, Byrnes KR. J Neurotrauma. 2017;34:755. doi: 10.1089/neu.2016.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.