Abstract
BACKGROUND
Although exposure to stressful life events in adolescence has been associated with poor health as measured by number of physicians’ visits and symptom scores, little is known regarding stress in adolescence and either concurrent or subsequent asthma.
OBJECTIVE
The objective of this study was to explore whether life events in adolescence are associated with either concurrent or new active asthma.
METHODS
The Tucson Children’s Respiratory Study, a prospective population-based birth cohort, surveyed participants at 10 ages between 6 and 29 years regarding respiratory health. Asthma was defined as a physician-diagnosis of asthma with symptoms during the previous year. At age 16, participants (n = 318) were queried regarding stressful life events using the 67-item Life Events Questionnaire for Adolescents (LEQA). LEQA scores were examined in relation to both concurrent and new active asthma. Estimates were obtained with logistic regression and mixed models.
RESULTS
There was no relation between asthma prevalence at age 16 and LEQA scores in the overall sample, although males with high LEQA scores had higher prevalence of asthma compared with males with low scores (relative risk [RR]: 3.03; 95% confidence interval [CI]: 1.37, 6.69; P = .006). Among adolescents with no asthma through age 16, risk of new asthma was greater for those with high LEQA scores (adjRR: 4.07; 95% CI: 1.33, 12.43; P = .014), after adjustment for potential confounders including smoking. Emotional support from family and friends slightly diminished the relation of stress to new asthma.
CONCLUSIONS
Stressful life events during adolescence are associated with subsequent new asthma. Additional biological and psychological measures of stress would complement these findings.
Keywords: Adolescence, Asthma, Stress
Factors that determine new onset of asthma during adult life have not been extensively studied. Both environmental factors such as occupational exposures,1,2 smoking, and particulate exposure3 and host factors such as depression4 have been associated with risk of incident asthma in adults. There is also increasing evidence that childhood factors, including infections, obesity, and allergic phenotypes,5 may impact risk for adult asthma, suggesting that new onset asthma may have its roots well before symptoms develop.
The role of stress as a potential risk factor for adult-onset asthma has been minimally studied. Longer term chronic stressors such as job insecurity6 have been associated with a higher risk of self-reported asthma incidence among those with higher compared with lower levels of stressors.7,8 In particular, the Copenhagen City Heart Study showed perceived stress to be strongly associated with asthma incidence and hospitalizations in a cohort of adults free of atopic disorders at baseline.7 In contrast, other studies found no effect of daily stress on incidence of self-reported asthma.9 However, prospective studies on the association between stress before adulthood and adult-onset asthma do not, to our knowledge, exist.
In childhood, stress has been identified as a risk affecting onset, progression, and severity of asthma. Adolescents reporting 2 to 3 stressful events are 1.5 times as likely to have asthma as those with 0 to 1 event.10 Among asthmatic children, high levels of chronic stress increase the risk for an asthma attack within 2 weeks of a time-limited negative life event.11 Extreme stressors, such as community violence, are also associated with increased asthma risk.12 This is of particular interest given high levels of stress reported by teenagers13 and increasing trends in adolescent asthma prevalence worldwide.14 However, little is known regarding the effect of stress in adolescence on the development of asthma.
This study examines the role of stressful life events in adolescence (age 16) on concurrent asthma and newly diagnosed asthma from ages 18 to 29 within the population-based Tucson Children’s Respiratory Study (TCRS) cohort. We hypothesize that having additional stressful life events is associated with new active asthma diagnoses. We further examine the differential effects of these stressors on adolescent girls and boys as well as the potential mediating effects of emotional support. Some of the results of these studies have been previously published in the form of an abstract.15
METHODS
Study population
Participants were enrolled as newborns in the TCRS, a prospective, longitudinal study of the risk factors for acute and chronic respiratory illness in childhood and adult life, described previously.16,17 A total of 1246 healthy newborns and their families were enrolled at birth between 1980 and 1984. All participants were English speaking. Shortly after the child’s birth, parents completed a questionnaire reporting a history of physician-diagnosed asthma, ethnicity, and other demographics. They subsequently completed up to 7 questionnaires on their child’s respiratory health approximately every 2 years until the child was 16 years old. Active asthma was defined as physician diagnosis of asthma plus active symptoms in the previous year at any survey.
Asthma diagnosed before age 18 was determined from questionnaires completed at ages 6, 8, 11, 13, and 16. If active physician-diagnosed asthma was reported on any questionnaire, the participant was considered to have asthma. If the participant had 3 or more completed questionnaires without a report of active asthma, then they were considered nonasthmatic. Participants with less than 3 completed questionnaires were excluded (n = 8, Figure 1). Starting at age 18, participants completed questionnaires themselves, answering questions on asthma diagnoses and symptoms. Newly diagnosed asthma was defined as physician-diagnosed active asthma with symptoms reported on questionnaires in adult life (ages 18–29) among subjects who did not report asthma by age 16 (“new active asthma”). Parental smoking, education level, ethnicity, and self-reported smoking were determined by questionnaire; body mass index (BMI) was determined from nurse-measured height and weight.
FIGURE 1.
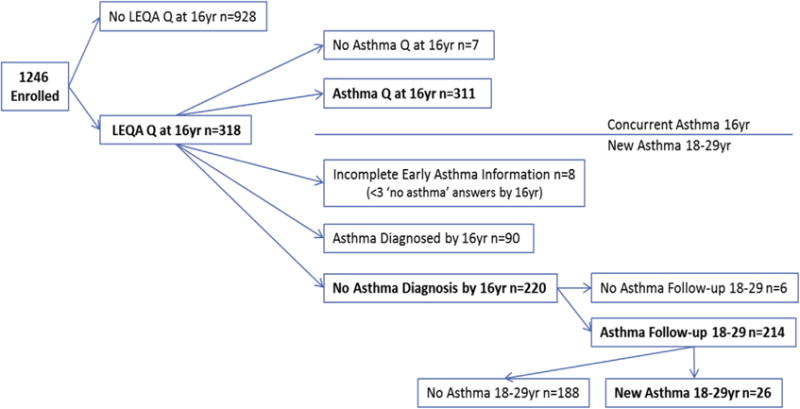
Study flow chart.
Informed consent was obtained from the parents for their children, or from the enrollees themselves as appropriate and the Institutional Review Board of the University of Arizona approved and monitored the study.
Exposures — stressful life events and emotional support
At age 16, participants who had an in-depth evaluation after July 1998 (n = 530) were eligible to complete the Life Events Questionnaire for Adolescents (LEQA)18 and the Emotional Support Scale.19 The LEQA consists of 67 items regarding potentially stressful events occurring in the past year. The number of events reported was summed to create a total life events score, and 10 subscale scores were created pertaining to particular domains (family, friends, physical, accomplishments, failures, medical, legal, safety, finance, and conflict) based on independent scoring of the 67 items by the study authors.
One-year test-retest correlations for the original LEQA version among adolescents have been moderately high and statistically significant. For negative, ambiguous, and total life events categories, correlations ranged from 0.53 to 0.64.20 Permission was obtained from the publisher (Wiley) for the use of the instrument.
The Emotional Support Scale asks whether the respondent feels comfortable speaking with parents, siblings, and friends on a range of issues. The scale is designed for ages 11–16 and questions show high internal consistency. Answers from questions were scored from 1 (no support) to 10 (high support) and then summed to create 3 indices measuring support from parents, siblings, and friends. These indices were also added to create a total support score (range: 0–150).
Statistical analysis
For demographic and exposure variables, differences in medians or proportions between adolescents with and without both concurrent (age 16) and new (ages 18–29) asthma were evaluated using χ2 or Student’s t-tests, as appropriate. Associations of stressful life events with demographic and exposure variables and the asthma outcomes were modeled with generalized estimating equations (GEE), using an independent correlation structure for concurrent asthma models and an exchangeable correlation structure for longitudinal models, as well as a log link function and a binomial distributional family with robust standard errors (STATA v.13). GEE allows for subjects to have missing data at any time point, accommodates correlated data by including an appropriate within-subject covariance structure, and can adjust for covariates. Exchangeable correlation structures were used assuming intrasubject correlation to be the same for all outcomes and their ability to handle unevenly spaced follow-up data.21 Results were reported by using robust standard error estimates.
Longitudinal associations of stressful events with asthma were adjusted for gender, ethnicity, self-reported smoking, maternal asthma, BMI, and finally for emotional support. Smoking was the only time-varying covariate assessed. The number of stressful life events was used both as a continuous measure, and categorized into high and low life events divided at the median. Similarly, support indices were used considered both as a continuous variable, and categorized into high and low life support divided at the median. Domain scores were treated as binary, 0 representing no events in that domain and 1 representing one or more events in that domain. The modifying effect of gender on the association was explored in models using a multiplicative term for the life event score (high vs low) with gender. Spearman correlations were examined among the emotional support subscales as well as associations between each subscale and the LEQA, while controlling for the other subscales. Kruskal-Wallis one-way analysis of variance was used to compare support by sex. Both full and sex-specific models were then examined adjusting further for total emotional support.
To examine the effect of neighborhood socioeconomic status (SES) on concurrent and new active asthma, principal component analyses (PCA) were conducted for SES characteristics22,23 after standardization, with the value of the first principal component included as an index of census-tract level SES at age 16 in regression models. PCA variables included were female (%), median age, minority race (%), Hispanic (%), family with female head of house (%), ≤ 12 years of schooling (%), do not speak English at home (%), household crowding, per capita income ($), unemployed ≥ 15 weeks (%), foreign born (%), and population density (people/square mile). The first principal component of at age 16 represented 66% of overall variance and was interpreted as a low SES index.
RESULTS
Stressful life events
Three-hundred and eighteen participants completed the LEQA at age 16. Individuals in the cohort who provided life events data were similar to those without life events data except that they were more likely to have parents who completed high school (Table I). Virtually all participants (99%) reported one or more stressful events during the past year (median life events score: 9, range 0–33, Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org). Fifty-one percent of males reported a score of more than 9 (median of 9) compared with 65.4% of females (median of 10) (P = .009, Table E1, available in this article’s Online Repository at www.jaciinpractice.org). A life events score above the median was positively associated with smoking (P < .001) and wheezing in the past year (P = .039, Table E1) but unrelated to parental smoking, education, age, or history of asthma.
TABLE I.
Characteristics of participants with life event data at age 16 years compared with those without life event data at age 16
| LEQA completed | LEQA not completed | P* | |||
|---|---|---|---|---|---|
| n | Percent | n | Percent | ||
| Participant characteristics | |||||
| Sex | |||||
| Male | 318 | 50.0 | 928 | 48.9 | .740 |
| Race/ethnicity† | |||||
| Non-Hispanic white | 318 | 59.4 | 928 | 58.7 | .825 |
| Skin prick test 6 y‡ | |||||
| ≥1 positive | 292 | 38.4 | 470 | 37.8 | .894 |
| Wheeze 6 y | |||||
| Active | 313 | 28.4 | 703 | 25.3 | .298 |
| MD asthma 6 y | |||||
| Active | 311 | 10.0 | 704 | 9.2 | .712 |
| Smoking 16 y | |||||
| Active | 301 | 12.0 | 209 | 11.5 | .869 |
| Parental characteristics at enrollment | |||||
| Smoking | |||||
| Active | 314 | 34.1 | 913 | 37.9 | .226 |
| Education | |||||
| ≤12 y | 313 | 34.5 | 916 | 42.7 | .011 |
| Age | |||||
| ≤28 y | 318 | 65.1 | 921 | 65.8 | .820 |
| MD asthma | |||||
| Yes | 294 | 20.8 | 795 | 23.5 | .333 |
LEQA, Life Events Questionnaire for Adolescents; MD, medical doctor.
P values (2-sided) based on Pearson χ2 statistic.
Participant race/ethnicity determined from parental information provided at enrollment.
At least one positive skin prick test at age 6 y based on 3-mm cutoff.
Participant characteristics and asthma
At age 16, 311 of those who completed the LEQA also provided data on asthma, with 18.3% (57/311) reporting active asthma. Adolescents with asthma were more likely to have a positive skin prick test (P = .002), to have wheezed in the past year (P < .001), and to be overweight at age 16 (P = .018, Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). At age 16, asthmatics did not differ from nonasthmatics in race, gender, or parental history of asthma (Table E2). A total of 11.2% of participants reported current smoking at age 16, but asthma was not associated with current smoking.
Stressful life events and asthma at age 16
There was no significant difference in asthma prevalence at age 16 among subjects with high versus low life event scores divided at the median (relative risk [RR]: 1.46; 95% confidence interval [CI]: 0.88, 2.41; P = .144, n = 311) (Figure 2). The RR did not change measurably after adjusting for gender, ethnicity, smoking and BMI at 16, and maternal asthma (adjRR: 1.32; 95% CI: 0.78, 2.22; P = .300, n = 290). However, the association between life events and concurrent asthma varied by gender, being statistically significant among males (RR: 3.03; 95% CI: 1.37, 6.69; P = .006, n = 157) but not among females (RR: 0.72; 95% CI: 0.37, 1.41; P = .339) with a significant interaction between life events and sex (P-interaction: 0.007, n = 311, Figure 2; adjusted model P-interaction: 0.014, n = 290). When life event scores were calculated for males and females separately and divided at the median, there was still no association between life events and asthma among females aged 16 (RR: 0.94; 95% CI: 0.48, 1.84; P = .849, n = 154), whereas the association among males remained significant (RR: 3.03; 95% CI: 1.37, 6.69; P = .006, n = 157).
FIGURE 2.
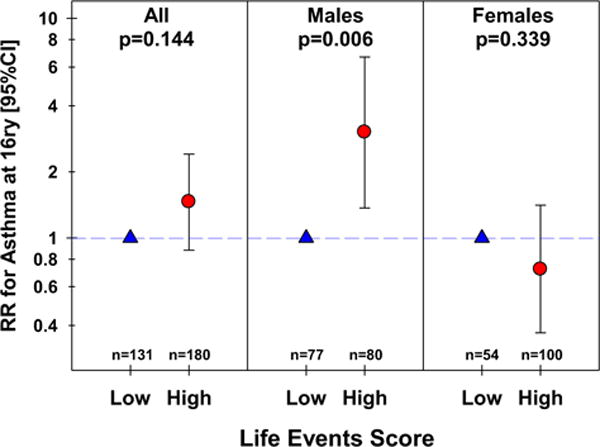
Relative risk for asthma at age 16, by the life events score, overall and stratified by gender. CI, Confidence interval; RR, relative risk.
Stressful life events and new active asthma ages 18–29
Of the 318 participants who completed the LEQA at age 16, 90 had previously diagnosed active asthma and 8 had fewer than 3 completed questionnaires between 6 and 16 years, leaving 220 with no diagnosis of active asthma by age 16 years (Figure 1). Among these 220, 6 did not have follow-up asthma information between 18 and 29 years of age, leaving 214 who were eligible for analyses of newly diagnosed asthma (Figure 1). Participants with new active asthma after age 16 (12.6%) had parents who were less likely to have a high school education and more likely to smoke compared with those without a new diagnosis (data not shown).
The proportion of adolescents who developed new active asthma between ages 18 and 29 are shown in Figure 3 in relation to life events. Subjects with high life event scores at age 16 had a higher prevalence of new active asthma at survey time points 26 and 29 years of age, compared with those with scores below the median (Figure 3). In longitudinal models, a high life events score was associated with increased risk of new active asthma up to age 29 (RR: 2.62; 95% CI: 1.05, 6.50; P = 038, n = 214). These relationships persisted after adjusting for gender, ethnicity, smoking at 16, maternal asthma, and BMI at age 16 (adjRR: 2.78; 95% CI: 1.01, 7.64; P = .048, n = 199). The association also persisted when limited to nonsmokers at 16 who remained nonsmokers between ages 18 and 29 (adjRR: 4.41; 95% CI: 1.38, 14.11; P = .012, n = 160) and was borderline significant after adjusting for smoking as a time-varying covariate from 18 to 29 instead of at age 16 (adjRR: 2.39; 95% CI: 0.92, 6.18; P = .072, n = 209). Further adjustment for parent education, parent smoking, or younger parental age at enrollment did not change the association (adjRR: 2.88; 95% CI: 1.10, 7.53; P = .031, n = 198). As an alternative SES measure, inclusion of a principal component that reflects a low neighborhood SES did not alter the magnitude of the observed association between life events and risk of new active asthma (adjRR: 2.69; 95% CI: 0.93, 7.78; P = .068, n = 148).
FIGURE 3.
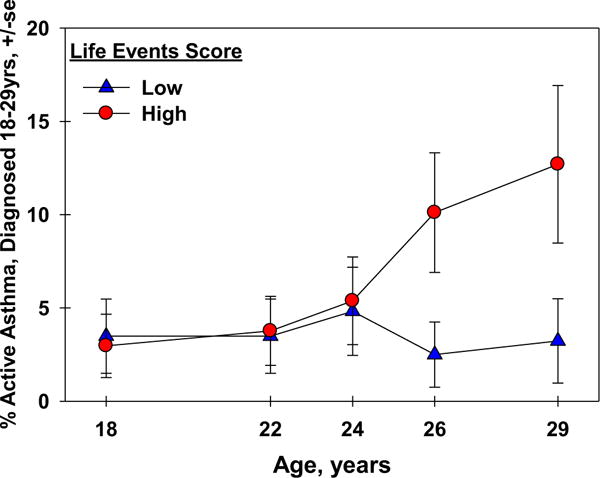
Percent new active asthma ages 18–29 by the life events score.
The relation between LEQA and asthma varied by sex. Males in the high life events group were 3 times more likely than those in the low life events group to develop active asthma between ages 18 and 29 (RR: 3.26; 95% CI: 0.88, 12.15; P = .078, n = 100). No significant association was observed among females (RR: 2.19; 95% CI: 0.55, 8.60; P = .261, n = 114), and there was no significant interaction with sex for life events as determinants of new active asthma between ages 18 and 29 (P = .582, n = 214).
Stressful life event domains and asthma
When the type of life events was considered, concurrent asthma (age 16) was significantly associated with events related to family, legal issues, and safety (for the group as a whole, Table II). When stratified by gender, the domains most strongly associated with concurrent asthma (vs no asthma) among males were family (RR: 3.10; 95% CI: 1.13, 8.46; P = .028, n = 157), legal (RR: 2.92; 95% CI: 1.51, 5.65; P = .002, n = 156), safety (RR: 3.25; 95% CI 1.75, 6.03; P < .001, n = 157), and physical (RR: 2.06; 95% CI: 1.08, 3.95; P = .028, n = 157). No statistically significant associations between life event domains and concurrent asthma were observed among females (data not shown).
TABLE II.
Unadjusted relative risks (95% CI) for concurrent and new asthma by life events domain considered as a binary variable
| Exposure domain | Concurrent asthma n = 311 |
New asthma 18–29 y n = 214 |
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| Family | 2.23 | 1.10, 4.51 | .026 | 1.65 | 0.54, 5.03 | .375 |
| Friends | 1.75 | 0.90, 3.41 | .097 | 3.65 | 0.97, 13.7 | .055 |
| Physical | 1.46 | 0.89, 2.42 | .137 | 1.06 | 0.40, 2.85 | .903 |
| Accomplishments | 1.58 | 0.67, 3.73 | .297 | 0.57 | 0.21, 1.56 | .272 |
| Failures | 1.17 | 0.72, 1.90 | .535 | 1.64 | 0.66, 4.05 | .286 |
| Medical | 0.97 | 0.59, 1.62 | .918 | 0.95 | 0.39, 2.30 | .911 |
| Legal | 1.60 | 1.00, 2.55 | .050 | 3.48 | 1.58, 7.67 | .002 |
| Safety | 2.25 | 1.42, 3.58 | .001 | 2.82 | 1.21, 6.58 | .017 |
| Finance | 1.28 | 0.80, 2.07 | .309 | 1.01 | 0.41, 2.49 | .984 |
| Conflict | 0.72 | 0.44, 1.17 | .188 | 2.25 | 0.69, 7.36 | .180 |
CI, Confidence interval; RR, relative risk.
Two key domains (legal and safety) were significantly associated with new active asthma between ages 18 and 29 (Table II). Among males, only legal (RR: 4.55; 95% CI: 1.43, 14.5; P = .010, n = 99) events were associated with higher prevalence of new active asthma. Among females, safety (RR: 6.00; 95% CI: 2.03, 17.71; P = .001, n = 114) and failure events (RR: 3.91; 95% CI: 1.03, 14.92; P = .046, n = 114) were positively associated with prevalence of new active asthma.
Role of emotional support
All 3 types of emotional support at age 16 (friend, sibling, parent) were positively correlated with each other (Spearman’s correlation coefficient 0.31–0.45, P < .001). Parental support was significantly related to a lower life events score independently of sibling and friend support (RR: 0.75; 95% CI: 0.60, 0.94; P = .013, n = 261). There was no relation of parental or sibling support with sex, although females reported significantly greater friend support than males (Kruskal-Wallis χ2; P < .001).
When the full models were further adjusted for total emotional support, the relationship between a high life events score and asthma did not markedly change, either for concurrent asthma at age 16 (not significant, data not shown) or for new active asthma between ages 18 and 29 (Table III). Among males, the relationship between a high life events score and concurrent asthma persisted after the addition of total emotional support to the model (adjRR: 2.89; 95% CI: 1.31, 6.37; P = .009, n = 142). No interaction was observed between life events and total support when assessed as modifiers of concurrent asthma (P = .539).
TABLE III.
Association between life events and asthma adjusted for emotional support
| Exposure | Concurrent asthma | New asthma 18–29 y | ||||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | P value | n | RR | 95% CI | P value | n | |
| Fully adjusted model* | 1.36 | 0.81, 2.29 | .249 | 294 | 2.78 | 1.01, 7.64 | .048 | 199 |
| Including total support* (high/low) | 1.40 | 0.84, 2.33 | .200 | 294 | 2.67 | 0.87, 8.20 | .086 | 199 |
CI, Confidence interval; RR, relative risk.
Adjusted for gender, ethnicity, smoking at 16 y, maternal asthma, body mass index at 16 y.
DISCUSSION
Among 16-year-old adolescents, stressful life events were strongly associated with higher prevalence of new active asthma between ages 18 and 29, but not with concurrent asthma. These relationships persisted after adjusting for the potential confounders of sex, ethnicity, maternal asthma, BMI, and current smoking. Although subjects with higher LEQA scores at age 16 were more likely to smoke and to start smoking, the relationship between life events and new active asthma remained after adjusting in our full model for smoking throughout the study period. Although emotional support diminished the relation of stressful events and asthma slightly, high life events remained a significant risk factor for new active asthma between ages 18 and 29.
In younger children, the effects of stress on asthma morbidity24 are well documented with both acute and chronic stressors in children increasing risk for asthma exacerbations. A prospective cohort following asthmatic children over 18 months showed that among participants with chronic stress, additional stressful events increased the risk of an asthma attack within 2 weeks of the event.11 In another study among a subgroup of children with moderate to severe asthma (n = 32), acute and chronic stress interacted to predict asthma symptoms.25 Data also support the possible contribution of psychosocial factors to asthma onset and persistence into childhood, and to the development of an atopic immune profile in young children predisposed to atopic asthma.26,27
However, the relation of stress to either concurrent or new asthma is understudied among adolescents of high school age, among whom 18.9% have been told by a doctor or nurse that they had asthma.28 Only one study has assessed the association between adolescent stress and concurrent asthma. Turyk et al10 found that self-reported life events were significantly related to both concurrent asthma and asthma morbidity among adolescents. However, because of its cross-sectional nature, the study could not assess whether asthma was the cause or the consequence of the stressful events. Our study among slightly older adolescents did not find an association between stressful life events and concurrent asthma.
In contrast, stress has been associated with asthma onset. In a large Finnish study, university students reported stressful life events, such as death of a family member, retrospectively for the year before the respiratory survey was conducted. Parental and personal conflicts during the preceding year were associated with an increased risk of asthma.29 Others have shown that high total exposure to stressful life events, as indicated by a cumulative severity score, predicted the onset of asthma among adults (hazard ratio: 1.96; 95% CI: 1.22, 3.13).8 However, to our knowledge, ours is the first study to prospectively examine stressors in adolescence and the subsequent development of adult asthma. Our study finds a strong relation between stressful events and the subsequent development of asthma symptoms severe enough to merit a new diagnosis of asthma.
The mechanisms underlying the association between stress in adolescence and the development of asthma are unknown. Possible mechanisms include stress related alterations in immune function through release of hypothalamic-pituitary-adrenal (HPA) and Sympathomedullary Pathway hormones that bind to and alter functions of immunologically active cells. For example, Chen et al30 demonstrated in adolescents that the experience of stress partially mediates the association of SES with inflammatory immune markers implicated in asthma (ie, IL-5 and IFN-γ). Although relations are complex, stress is associated with the magnitude of inflammatory responses including higher eosinophil counts, greater allergen associated lymphocyte proliferative responses, and heightened production of inflammatory cytokines (IL-5, IL-13, IL-4, IFN-γ).31 Throughout adolescent development, many shifts are observed in neuroendocrine function,32 with a dramatic change in reactivity exhibited by the HPA axis in response to stressors.33 Alternatively, stress-induced behavioral changes, such as increased smoking and poor sleep quality, may increase exacerbation and possibly development of asthma.
Female gender has been found to be a risk factor for the development of asthma in young adulthood.34 Females in our population reported more life events at age 16 than males, consistent with other reports that girls are more likely to report cumulative stress.35,36 However, the association between stressful life events and both concurrent and new active asthma was evident only for males. What accounts for this difference by sex is unknown. In our population, females reported a higher level of social support, consistent with reports that female adolescents are more likely to seek social support,37,38 perhaps mediating the role of stressors. It is unclear whether this gender difference in response to stress translates into differential susceptibility for prevalence or development of disease, with conflicting evidence to date.39–41 Marked differences by sex emerge in adolescence in physiological response systems that include corticolimbic circuitries, the HPA axis, and the autonomic nervous system.42 Clearly, both social and physiologic factors may contribute to the difference in asthma risk by sex.
Two key life event domains (legal and safety) were associated with new active asthma between ages 18 and 29. One review documented that childhood asthma is strongly affected by the psychological functioning of the parent, interactions between parent and the child, and the child’s functioning.43 Others have shown that safety or a lack thereof is a key risk factor for asthma. In a 3-year longitudinal study, children living in areas with high crime rates were at increased risk for asthma44 as were children exposed to greater community violence.12
We evaluated the role of emotional support in the stressasthma pathway and found that support from someone emotionally close to the adolescent (parent, sibling, and/or friends) reduced the association only modestly. Social support mechanisms have been shown to provide buffering effects to both stress40 and asthma onset. For example, parental social support is inversely linked to asthma prevalence among young children and to cortisol responses to stress among youth.45,46 However, the type of social support seems to be important in conferring resilience to stressors47 and further work is needed to explore this dimension.
Loss to follow-up is one limitation of prospective studies. However, of the 220 subjects with life events data at age 16 who did not have prior asthma, 97% provided questionnaire and asthma data at least once between 18 and 29. We were able to adjust for several confounding factors, but associations observed in the study may be attributable to residual confounding. For example, environmental triggers more common in homes of subjects with high stress levels may differentially elicit stress re-sponses.48 A self-administered questionnaire was used to assess life events that may have introduced recall bias. On the other hand, answering written questions may have allowed participants to more freely report stressful events than if they had to voice them to the nurse.49 A second limitation of the questionnaire is that participants did not rate the event as positive or negative, although our subscales distinguished between achievements and more negative events. LEQA respondents were significantly more likely to have a high-school education than nonrespondents. Finally, life events were only assessed at a single time point, so the potential confounding effects of earlier or later life stressors is unknown. Others have shown that life events predict daily stress50 and that chronic stress may persist into adulthood,51 so it is plausible that the stressors identified by the LEQA were chronic in nature.
Despite these limitations, the prospective nature of our study and the long-term follow-up provide rich data for investigating the relationship between stressful life events and asthma. By virtue of prospective follow-up, recall bias on the life events and potential confounders such as smoking is reduced. Further, the cohort was population based and included minimal loss to follow-up and long-term asthma outcomes. Finally, study participants differed minimally from the broader TCRS cohort.
In conclusion, major stressful events are associated with future asthma in a general population sample and may represent an additional risk factor associated with susceptibility for the development of adult-onset asthma. Although additional research is needed into the immunologic and/or neuroendocrine mechanisms underlying the observed associations, it remains to be seen whether interventions directed at reducing adolescent stress will reduce incidence of asthma in adulthood.
Supplementary Material
What is already known about this topic?
Adolescents exposed to stressful life events have been shown to be at increased risk for poor health as well as concurrent asthma.
What does this article add to our knowledge?
This study provides prospective evidence that objectively defined life events in adolescence were associated with new active asthma diagnosed between ages 18 and 29.
How does this study impact current management guidelines?
Major stressful events in adolescence are associated with future asthma in a general population sample and may represent an additional risk factor associated with susceptibility for the development of adult-onset asthma.
Acknowledgments
The authors gratefully acknowledge the contributions of Dr. Lynn Taussig who started the Tucson Children’s Respiratory Study in 1980. They thank Bruce Saul and Justin Frere for data management, Nathan Lothrop and Paloma Beamer for mapping files, Stefano Guerra for helpful comments, and the study nurses, Marilyn Lindell, Lydia de la Ossa, and Nicole Pargas, for data collection and participant follow-up.
This work was supported by grants HL-056177 and HL-095021 from the National Heart Lung and Blood Institute.
E. Oren received support from a University of Arizona Health Sciences Career Development Award. F. D. Martinez has received research support from National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (HL056177, HL098112, HL091889, HL130045, HL132523), NIH/National Institute of Environmental Health Sciences (NIEHS) (ES006694), NIH/National Institute of Allergy and Infectious Diseases (NIAID) (AI126614), and Johnson & Johnson (UA009253-0001); and has received consultancy fees from Copval. A. L. Wright has received research support from the NIH.
Abbreviations used
- BMI
Body mass index
- CI
Confidence interval
- GEE
Generalized estimating equations
- HPA
Hypothalamic-pituitary-adrenal
- LEQA
Life Events Questionnaire for Adolescents
- PCA
Principal component analyses
- RR
Relative risk
- SES
Socioeconomic status
- TCRS
Tucson Children’s Respiratory Study
Footnotes
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology. 2009;14:814–21. doi: 10.1111/j.1440-1843.2009.01562.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoy RF, Burgess JA, Benke G, Matheson M, Morrison S, Gurrin L, et al. Occupational exposures and the development of new-onset asthma: a population-based cohort study from the ages of 13 to 44 years. J Occup Environ Med. 2013;55:235–9. doi: 10.1097/JOM.0b013e31827edefb. [DOI] [PubMed] [Google Scholar]
- 3.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med. 2014;190:914–21. doi: 10.1164/rccm.201403-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner WM, Schreiner PJ, Sood A, Jacobs DR., Jr Depression and risk of incident asthma in adults. The CARDIA study. Am J Respir Crit Care Med. 2014;189:1044–51. doi: 10.1164/rccm.201307-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svanes C. What has the ECRHS told us about the childhood risks of asthma, allergy and lung function? Clin Respir J. 2008;2(Suppl 1):34–44. doi: 10.1111/j.1752-699X.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- 6.Loerbroks A, Bosch JA, Douwes J, Angerer P, Li J. Job insecurity is associated with adult asthma in Germany during Europe’s recent economic crisis: a prospective cohort study. J Epidemiol Community Health. 2014;68:1196–9. doi: 10.1136/jech-2014-204274. [DOI] [PubMed] [Google Scholar]
- 7.Rod NH, Kristensen TS, Lange P, Prescott E, Diderichsen F. Perceived stress and risk of adult-onset asthma and other atopic disorders: a longitudinal cohort study. Allergy. 2012;67:1408–14. doi: 10.1111/j.1398-9995.2012.02882.x.. [DOI] [PubMed] [Google Scholar]
- 8.Lietzen R, Virtanen P, Kivimaki M, Sillanmaki L, Vahtera J, Koskenvuo M. Stressful life events and the onset of asthma. Eur Respir J. 2011;37:1360–5. doi: 10.1183/09031936.00164609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med. 2003;97:273–80. doi: 10.1053/rmed.2003.1419. [DOI] [PubMed] [Google Scholar]
- 10.Turyk ME, Hernandez E, Wright RJ, Freels S, Slezak J, Contraras A, et al. Stressful life events and asthma in adolescents. Pediatr Allergy Immunol. 2008;19:255–63. doi: 10.1111/j.1399-3038.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, et al. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–7. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- 12.Sternthal MJ, Jun HJ, Earls F, Wright RJ. Community violence and urban childhood asthma: a multilevel analysis. Eur Respir J. 2010;36:1400–9. doi: 10.1183/09031936.00003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychological Association. Stress in America: Are Teens Adopting Adults’ Stress Habits? Available from: http://www.apa.org/news/press/releases/stress/2013/stress-report.pdf. Accessed October 22, 2016.
- 14.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 15.Oren E, Gerald LB, Stern DA, Wright AL, Martinez FD. Self-reported stressful life events during adolescence and asthma. Proceedings from the American Thoracic Society 2015 International Conference; May 15–20, 2015; Denver, CO. Abstract 97. [Google Scholar]
- 16.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–46. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 17.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–31. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 18.Masten AS, Neeman J, Andenas S. Life events and adjustment in adolescents: the significance of event independence, desirability, and chronicity. J Res Adolesc. 1994;4:71–97. [Google Scholar]
- 19.Wills TA, Vaccaro D, McNamara G. The role of life events, family support, and competence in adolescent substance use: a test of vulnerability and protective factors. Am J Community Psychol. 1992;20:349–74. doi: 10.1007/BF00937914. [DOI] [PubMed] [Google Scholar]
- 20.Garmezy N, Tellegen A. Studies of stress-resistant children: methods, variables, and preliminary findings. In: Morrison F, Lord C, Keating D, editors. Advances in Applied Developmental Psychology. New York: Academic Press; 1984. pp. 231–87. [Google Scholar]
- 21.Shults J, Sun W, Tu X, Kim H, Amsterdam J, Hilbe JM, et al. A comparison of several approaches for choosing between working correlation structures in generalized estimating equation analysis of longitudinal binary data. Stat Med. 2009;28:2338–55. doi: 10.1002/sim.3622. [DOI] [PubMed] [Google Scholar]
- 22.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83:1041–62. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beamer PI, Lothrop N, Lu Z, Ascher R, Ernst K, Stern DA, et al. Spatial clusters of child lower respiratory illnesses associated with community-level risk factors. Pediatr Pulmonol. 2016;51:633–42. doi: 10.1002/ppul.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 25.Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosom Med. 2009;71:378–84. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108:E69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- 27.Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health. 2004;94:625–32. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Self-reported asthma among high school students—United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54:765–7. [PubMed] [Google Scholar]
- 29.Kilpelainen M, Koskenvuo M, Helenius H, Terho EO. Stressful life events promote the manifestation of asthma and atopic diseases. Clin Exp Allergy. 2002;32:256–63. doi: 10.1046/j.1365-2222.2002.01282.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65:984–92. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 31.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ojeda SR, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. New York: Elsevier; 2002. pp. 589–659. [Google Scholar]
- 33.McCormick CM. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiol Behav. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Abramson M, Kutin JJ, Raven J, Lanigan A, Czarny D, Walters EH. Risk factors for asthma among young adults in Melbourne, Australia. Respirology. 1996;1:291–7. doi: 10.1111/j.1440-1843.1996.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 35.Berton MW, Stabb SD. Exposure to violence and post-traumatic stress disorder in urban adolescents. Adolescence. 1996;31:489–98. [PubMed] [Google Scholar]
- 36.Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999;77:1061–72. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- 37.Frydenberg E, Lewis R. Boys play sport and girls turn to others: age, gender and ethnicity as determinants of coping. J Adolesc. 1993;16:253–66. doi: 10.1006/jado.1993.1024. [DOI] [PubMed] [Google Scholar]
- 38.Seiffge-Krenke I. Causal links between stressful events, coping style, and adolescent symptomatology. J Adolesc. 2000;23:675–91. doi: 10.1006/jado.2000.0352. [DOI] [PubMed] [Google Scholar]
- 39.Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: sex differences in the role of cortisol reactivity to interpersonal stress. J Clin Child Adolesc Psychol. 2009;38:513–24. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escobar M, Alarcon R, Blanca MJ, Fernandez-Baena FJ, Rosel JF, Trianes MV. Daily stressors in school-age children: a multilevel approach. Sch Psychol Q. 2013;28:227–38. doi: 10.1037/spq0000020. [DOI] [PubMed] [Google Scholar]
- 41.Tout K, de Haan M, Campbell EK, Gunnar MR. Social behavior correlates of cortisol activity in child care: gender differences and time-of-day effects. Child Dev. 1998;69:1247–62. [PubMed] [Google Scholar]
- 42.Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37:1135–57. doi: 10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaugars AS, Klinnert MD, Bender BG. Family influences on pediatric asthma. J Pediatr Psychol. 2004;29:475–91. doi: 10.1093/jpepsy/jsh051. [DOI] [PubMed] [Google Scholar]
- 44.Shankardass K, Jerrett M, Milam J, Richardson J, Berhane K, McConnell R. Social environment and asthma: associations with crime and No Child Left Behind programmes. J Epidemiol Community Health. 2011;65:859–65. doi: 10.1136/jech.2009.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berz JB, Carter AS, Wagmiller RL, Horwitz SM, Murdock KK, Briggs-Gowan M. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2- to 3-year olds. J Pediatr Psychol. 2007;32:154–66. doi: 10.1093/jpepsy/jsj123. [DOI] [PubMed] [Google Scholar]
- 46.Kelsay K, Leung DY, Mrazek DA, Klinnert MD. Prospectively assessed early life experiences in relation to cortisol reactivity in adolescents at risk for asthma. Dev Psychobiol. 2013;55:133–44. doi: 10.1002/dev.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyman SM, Gold SN, Cott MA. Forms of social support that moderate PTSD in childhood sexual abuse survivors. J Fam Violence. 2003;18:295–300. [Google Scholar]
- 48.O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121:1133–1139.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Duggal S, Malkoff-Schwartz S, Birmaher B, Anderson BP, Matty MK, Houck PR, et al. Assessment of life stress in adolescents: self-report versus interview methods. J Am Acad Child Adolesc Psychiatry. 2000;39:445–52. doi: 10.1097/00004583-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Wagner BM, Compas BE, Howell DC. Daily and major life events: a test of an integrative model of psychosocial stress. Am J Community Psychol. 1988;16:189–205. doi: 10.1007/BF00912522. [DOI] [PubMed] [Google Scholar]
- 51.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


