Abstract
Background
Aminoglycosides (AGs) and glycopeptides are antibiotics essential for treating life-threatening respiratory infections in patients with cystic fibrosis (CF). The goal of this study was to examine the effects of cumulative intravenous AG (amikacin and/or tobramycin) and/or glycopeptide (vancomycin) dosing on hearing status.
Methods
Hearing thresholds were measured from 0.25 to 16.0 kHz, in 81 participants with CF. Participants were categorized into two groups: normal hearing in both ears (≤25 dB HL for all frequency bands) or hearing loss (>25 dB HL for any frequency band in either ear). Participants were also characterized by their cumulative intravenous (IV)-AG (with or without vancomycin) exposure by comparing the total number of lifetime cumulative IV doses to a method that additionally accounts for the total number of doses per day (weighted method) per course of treatment.
Results
Participants in the hearing loss group were significantly older than those in the normal-hearing group. After adjusting for gender and age at the time of hearing test, participants in the two highest-quartile exposure groups were more likely to have permanent sensorineural hearing loss than those in the two lowest-quartile exposure groups.
Conclusions
Cumulative IV antibiotic dosing has a significant negative effect on hearing sensitivity in patients with CF, when controlling for age and gender effects. A trend for increasing odds of hearing loss was associated with increasing cumulative IV-antibiotic dosing.
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that can lead to life-threatening infections and reduced quality of life (1). Aminoglycoside (AG) (e.g., tobramycin) and glycopeptide (e.g., vancomycin) antibiotics are commonly prescribed to hospitalized patients to manage life-threatening lung infections caused by Pseudomonas aeruginosa (PA), methicillin-resistant Staphylococcus aureus (MRSA) and other organisms. These medications can also degrade auditory function in the inner ear – cochleotoxicity – most often resulting in permanent high-frequency sensorineural hearing loss (SNHL) (1–4).
It has been well-established in both clinical and preclinical studies that AG antibiotics induce cochleotoxicity (5–7). However, the incidence of hearing loss from intravenous (IV)-AG exposure in patients with CF remains unclear, likely contributing to the lack of ototoxic monitoring in many CF clinics (8). The prevalence of hearing loss from AG treatment in adult patients with CF ranges from 0–47% (2, 9–14) compared to 11–18% in age-matched groups of adults without a history of CF or AG exposure (15, 16). The high variability among studies of patients with CF is likely due to variations in hearing tests, age, type of AG, treatment duration, plasma drug levels, drug interactions, renal status, diabetes, gender, mitochondrial mutations, infection/inflammatory status and concomitant CF-related illnesses that might place a patient at higher risk of AG-induced SNHL (6, 7, 9, 15–17). Importantly, the co-administration of other drugs may increase the risk of AG-induced ototoxicity, including azithromycin (a macrolide), vancomycin, furosemide (a loop diuretic) (7, 18, 19). Al-Malky and colleagues (17) found no evidence of SNHL in pediatric patients (5–16 years old) with CF who had little or no dosing (≤ 5 courses) with IV-AGs (17). However, age-matched patients with CF that received > 5 courses of AG treatments had a greater risk of SNHL in the extended high frequency range (8.0–16.0 kHz) compared to patients with no exposure, or low exposure to AGs. All groups had similar hearing abilities in the 0.25–4.0 kHz region. Several significant risk factors were also associated with the prevalence of SNHL in patients with CF, including age, greater overall IV-AG dosing, and low pulmonary function scores (17). These data demonstrate the need to quantify the cumulative effect of IV-AG exposure, as well as accounting for frequency of dosing (weighted doses per day) on auditory function. This will allow a better understanding of how cumulative dosing can lead to cochleotoxicity in patients with CF.
A retrospective study by Scheenstra and colleagues (20) investigated the incidence of SNHL in 13 CF patients who received a baseline pretreatment hearing test and a follow-up hearing test after at least one course of IV-AG treatment. They did not find SNHL (up to 12.5 kHz) in CF patients who had up to 8 courses of IV-tobramycin treatment (10mg/kg, twice daily dosing for three weeks). These data contrast with those of Mulheran et al. (2) who reported a 17% (12/70) incidence of cochleotoxicity in their cohort using conventional audiometry (0.25–8 kHz), and significant threshold elevations in the extended high frequencies (9–16 kHz). Neither study addressed the potential synergistic effects of other treatments (e.g., glycopeptides) on hearing in their study groups.
It is a complicated endeavor to adequately estimate a patient’s lifelong exposure to IV-antibiotics, including AGs, although attempts have been made to identify the risk of ototoxicity in previous investigations (2, 3, 20, 21). Intravenous dosing of CF patients with AGs is traditionally based on body mass (e.g., 10mg/kg per day) using a single-dose administration per day in many different clinics (10, 22). However, the dosing frequency and treatment duration varies between patients and institutions, which can impact the onset of ototoxicity. Preclinical studies show that more-than-once daily dosing with AGs leads to higher rates of nephrotoxicity and cochleotoxicity compared to once-daily dosing (23–25). A confounding factor for many studies is the co-administration of glycopeptide antibiotics, such as vancomycin, that may synergistically increase the risk of nephrotoxicity and cochleotoxicity (19, 26).
The goal of this investigation was to determine the association between cumulative IV-AG (with or without [±] vancomycin) dosing and the prevalence of hearing loss in patients with CF.
MATERIALS AND METHODS
1. Participants
All study materials and test protocols were approved by the Oregon Health & Science University (OHSU) Institutional Review Board. Informed written consent was obtained at the participant’s clinic visit or at the beginning of the first scheduled test session. Parental consent was obtained for participants between 15 to 17 years of age with CF. All participants within this age group provided their assent to study test procedures and protocol by co-signing the consent form. Patients aged 15 years and older were recruited from the pediatric and adult CF centers at OHSU during routine clinic visits. Eighty-one participants, 34 females and 47 males with CF (mean age= 26 yr., SD ±10; range 15–63 years old) and a history of IV-AG with or without vancomycin treatments were enrolled in the study. The number of participants were skewed towards the lower age limit, and the upper age range was limited by the lifespan of patients with CF (27). Participants were excluded if they had abnormal 226-Hz tympanometric admittance (<0.03 mmho or >1.7 mmho) to rule out middle ear disorders, although there were no middle ear pressure or ear canal volume restrictions. Participants were also excluded if they had audiometric air-bone-gaps >10 dB HL from 0.25–4.0 kHz, indicative of conductive pathology (e.g., otitis media, eardrum perforation).
2. Equipment
All testing was completed in a double-walled sound attenuated booth approved for clinical audiometric evaluations. A Grason Stadler Instrument (GSI) Tympstar V calibrated to ANSI standards (S3.39-R2007) (28) was used for assessments of middle-ear status that included 226-Hz tympanometry. A GSI-61 audiometer calibrated to ANSI standards (S3.6-1996) (29, 30) was used to measure pure-tone air conduction (AC) thresholds using standard transducers provided with the audiometer: ER3A insert earphones for conventional frequencies (0.25–8.0 kHz) and Sennheiser HDA 200 circumaural headphones for extended high frequencies (9.0–16.0 kHz). Clinical audiometric testing was done in dB hearing level (HL) based on the calibration of audiometers and transducers to meet current standards of clinical care (30). ER3A insert earphones were used because they are the preferred transducers for diagnostic audiometric testing, since they provide better interaural attenuation than supra-aural headphones (31, 32). The ER3A insert earphone was coupled to the ear with standard foam eartips unless a small eartip was required depending on the size of the participant’s ear canal. Sennheiser HDA 200 circumaural headphones were used for extended high-frequency testing because they provide excellent passive sound attenuation, a secure seal over the ear and good test-retest (33). Bone conduction (BC) thresholds were measured using a Model #B-71 bone oscillator.
3. Procedures
Testing was completed in a sound-attenuated booth in a single visit (~one hour) in either the Otolaryngology clinic or the Oregon Hearing Research Center at OHSU. The following clinical tests of auditory function were conducted using standard clinical equipment described above following otoscopy to ensure that the ear canal was clear and free from drainage and that the tympanic membrane was visible: 1) tympanometry with a 226-Hz probe tone; 2) pure-tone air conduction audiometry at conventional frequencies 0.25, 0.50, 1.0, 2.0, 3.0, 4.0, 6.0 and 8.0 kHz; 3) pure-tone air conduction audiometry at extended high-frequencies (HF): 9.0, 10.0, 11.2, 12.5, 14.0 and 16.0 kHz; and 4) pure-tone bone conduction audiometry from 0.25 to 4.0 kHz.
Tympanometry using a 226-Hz probe tone was conducted prior to behavioral hearing assessment. This test was used to obtain information about the transfer of sound energy through the middle ear, called acoustic admittance. Results of this test specify how well the eardrum and middle-ear bones (i.e., ossicles) function compared to normative standards. A normal tympanogram with acoustic admittance between (<0.03 mmho or >1.7 mmho) would indicate that the outer ear (i.e., ear-canal) was relatively clear and the middle-ear functioned normally to a low-pitch tone. An abnormal result would indicate pathology of the outer ear or middle ear (e.g., fluid behind the eardrum). Ears with abnormal admittance were excluded from data analyses.
Pure-tone hearing thresholds were then measured for conventional audiometric frequencies and extended HFs. All testing was completed by a licensed clinical audiologist using the Hughson-Westlake threshold searching technique (34). The tones were presented for 1–2 seconds, with varying intervals between tone presentations. The test level of each succeeding presentation was determined by the preceding response. After each failure to respond to a tone, the level was increased in 5-dB steps until a response occurred. After this response, tone intensity was decreased 10 dB, and another ascending series begun. Thresholds were defined as the lowest decibel hearing level (dB HL) at which responses occurred in at least one-half of a series of ascending trials. The minimum number of responses needed to determine thresholds of hearing was two responses out of three presentations at a single level. A threshold of >25 dB HL at one or more audiometric frequencies was considered to be outside the normal hearing range. These criteria are standard of care and meet the current National Guidelines for Classification of Hearing Loss (35, 36).
Participants were categorized into two groups: those with normal hearing and those with SNHL. Participants with hearing thresholds ≤ 25 dB HL at all frequencies for both ears were in the normal hearing group. Participants with a threshold of > 25 dB HL at any frequency tested in either ear were in the SNHL group. Our standard of clinical care required testing participants at 250 and 500 Hz, although these lower frequency bands may be affected by internal factors such as physiologic noise in participants with CF. All participants with a >25 dB HL threshold at 250 or 500 Hz also had >25 dB HL thresholds at higher frequencies. No participants had >25 dB HL thresholds at only 250 or 500 Hz, therefore categorization of these hearing groups were not affected by lower frequency thresholds. If both ears had SNHL, the poorer ear (i.e. higher pure tone average (PTA) threshold from 4.0–12.5 kHz) was chosen for analyses. For participants who had symmetrical average hearing thresholds, the left ear was chosen for analysis. Seven of the left ears chosen had unilateral conductive pathology, based on the presence of an air-bone gap exceeding 10 dB, and were excluded from the dataset. In these cases, the participant’s right ear was used for analysis if the hearing loss was sensorineural in nature.
4. Intravenous antibiotic dosing
All intravenous AG (tobramycin and/or amikacin) and vancomycin days of treatment were recorded in each participant’s personal electronic health record (EPIC) from 2007 until the date of study participation were reviewed and recorded. Paper-based medical records prior to 2007 were also examined for IV–AG and vancomycin dosing data. Vancomycin was included in the review because it can potentiate the cochleotoxicity of AGs (7, 19, 37–40), and is typically given to patients colonized with methicillin-resistant Staphylococcus aureus (MRSA) as clinical standard of care (41). All participants treated with aminoglycosides received clinical doses of either tobramycin and/or amikacin, which appear to have equivalent ototoxic potential, as reported previously (42–44). Individual doses of each aminoglycoside [amikacin (20–25 mg/kg/per day) and tobramycin (10 mg/kg/per day)] and glycopeptide [vancomycin (15–20mg/kg/per day)] were summed over each participant’s lifetime (total dosage). The cumulative lifetime dosing and its association with SNHL was determined by summing all doses and categorizing participants into quartiles of cumulative dosing from the lowest (Q1) to highest quartile (Q4) dosing groups.
The cumulative lifetime dosing was then weighted by the frequency of dosing (# of dose administrations/day) for each participant in the secondary analysis “weighted” method. The frequency of IV dosing ranged from 6 to 24-hour intervals across treatments, and previous reports have shown that multiple dosing per day may be more toxic than single day dosing cochleotoxicity (23–25). The rationale for developing a weighting scheme was based on previous reports that utilize a dose-response relationship for evaluating the effects of an independent variable on a dependent variable. One example is evaluating the health consequences of smoking. Lung cancer incidence in smokers is roughly proportional to dose rate (cigarettes per day), but increases rapidly with duration of smoking. The assumption that the incidence rate is proportional to cumulative lifetime dose (the product of dose rate and duration) has been confirmed as incorrect for many years (45). Numerous studies have reported a strong dose-response relationship between cigarette consumption and severe diseases by using a “packs per day” method (i.e., higher risk for health exacerbations in patients who smoked more packs per day). Similar methods have been used in cancer-related research to analyze the effect of cisplatin treatments administered once per week versus every two or three weeks (46). Furthermore, murine studies show that more-than-once daily dosing with AGs leads to higher rates of nephrotoxicity and cochleotoxicity, and that once-daily dosing has the potential to reduce cochleotoxicity (23–25). In this study, there was a range of dosing frequencies from q6 (4 doses per day) to q24 (once daily). The weighted analyses were conducted as a secondary measure to cumulative dosing, so that the frequency of dosing per day across treatments was accounted for similar to studies in other disciplines reporting differences among these two methods (i.e., smoking research).
In this study’s weighting scheme, the most frequent (every 6-hours, or “q6”) dosing schedule was assigned the highest weight (1.0), and all other frequencies were weighted proportionally, i.e., the 8-hour dosing frequency (q8) was weighted lower at 0.75, q12 at 0.5, q24 at 0.25, q36 and q48 were given the lowest weights, at 0.1667 and 0.125 respectively. These weighted doses were then summed for each participant over their lifetime (when documented in available electronic and paper medical records) to obtain the cumulative weighted IV dose for AGs and glycopeptides. This weighting scheme has not previously been employed to investigate AG and glycopeptide cochleotoxicity.
Statistical analysis
Statistical analyses using Stata V.13 were designed and completed by a qualified biostatistician. Logistic regression modeling was used to obtain the odds of SNHL by quartile of cumulative IV dosing after adjusting for age at the time of each participant’s hearing test and gender. The models were also conducted with and without age at hearing test to assess confounding by age. In this cohort study, the odds ratio is expressed as the ratio of the number of cases (e.g., CF participants with SNHL) to the number of non-cases (e.g., CF participants with normal hearing). A significant trend of increasing odds of SNHL was also determined with increasing quartiles of cumulative dosing. A kappa statistic was computed to note the level of agreement between cumulative IV dosing and weighted quartiles of cumulative IV dosing from our secondary analysis.
The odds of SNHL were examined after excluding participants who only received vancomycin treatments. Further comparisons were made to determine if cumulative doses of AGs showed a synergistic association when counting days of vancomycin treatment, rather than cumulative dosing and, if the degree of hearing loss is related to age and/or IV dosing.
RESULTS
1. General findings
There were 81 participants (n=81 ears) with behavioral audiometry (hearing test) and IV-AG with or without glycopeptide exposure. Thirty-six participants (44%) had normal hearing in both ears (≤ 25dB HL for 0.25–16.0 kHz) and the remaining 45 participants (56%) had SNHL (>25dB HL at one or more audiometric frequencies between 0.25–16.0 kHz) in at least one ear (re: Table 1). In examining the poorer ear of each participant with SNHL, two ears had SNHLs between 0.25–8.0 kHz, 28 ears had SNHL between 9.0–16.0 kHz and 15 ears had SNHLs in both frequency ranges. There was no effect of gender (p=0.96) on SNHL. Participants with SNHL were significantly older at the time of hearing test with median age of 26 years compared to the median age of 21 years for their normal hearing counterparts (re: Table 1). The overall rates of SNHL in this cohort are larger than expected, with generally greater degrees of hearing loss in the high-frequency range (>8 kHz), when compared to age-matched normative data in controls without CF or a history of AG treatments (47). However, recent reports of age-related hearing loss in young adults have indicated there is a great degree of variability in the general population for frequencies between 12.5 and 16 kHz using the Sennheiser HDA 200 headphones (47, 48) (same headphones used in current study). The current study used the cut-off range for normal hearing at 25 dB HL, because this is similar to the highest mean hearing range between 8.0 to 16.0 kHz reported in normative studies for adults between 20–29 years of age (47).
Table 1.
Characteristics of CF patients recruited at OHSU.
Median age and range, % of males to females is displayed for 36 participants who had normal hearing in both ears and 45 participants who had SNHL in at least one ear (Total N = 81 ears). SNHL ears had more cumulative exposure to IV-antibiotics reflected for both cumulative IV antibiotic dosing (14/20 ears) and weighted (13/20 ears) methods compared to the normal hearing ears. The number of SNHL and normal hearing ears were comparable for the lowest quartile (Q1) for both methods.
| Characteristics | Total | NH | SNHL |
|---|---|---|---|
| Median Age years (range) | 23 (15 – 63) | 21 (15 – 35) | 26 (15 – 63) |
| N Male (%) | 47 (58.0) | 21 (58.3) | 26 (57.8) |
| N Female (%) | 34 (42.0) | 15 (41.7) | 19 (42.2) |
| Cumulative IV antibiotic doses | Total Ears (%) | NH ears (%) | SNHL ears (%) |
| Q1 (2 – 15) | 22 (27.2) | 10 (27.8) | 12 (26.7) |
| Q2 (16 – 52) | 20 (24.7) | 13 (36.1) | 7 (15.6) |
| Q3 (57 – 146) | 19 (23.5) | 7 (19.4) | 12 (26.7) |
| Q4 (152 – 647) | 20 (24.7) | 6 (16.7) | 14 (31.1) |
The lowest quartile of cumulative life-time dosing (Q1) ranged from 2 to 15 doses, the second quartile (Q2) ranged from 16 to 52 doses, the third quartile (Q3) ranged from 57 to 146 doses and the fourth and highest quartile (Q4) ranged from 152 to 647 doses (Table 1). The lowest dosing quartile had 12 of 22 ears with SNHL (15% of total N) and 10 of 22 ears with normal hearing (12% of total N). For the highest dosing quartile, 14 of 20 ears had SNHL (17% of total N) and 6 of 20 ears (1% of total N) had normal hearing (Table 1).
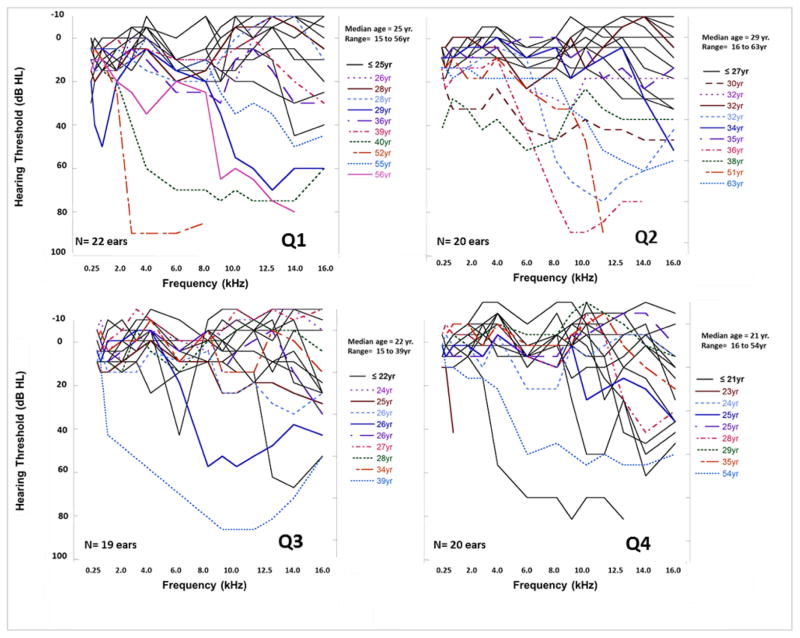
Figure 1 illustrates audiometric profiles for ears with SNHL and normal hearing for each cumulative dosing quartile. The number of ears with SNHL is similar to the number of ears with normal hearing for Q1, as previously described. There were fewer ears with SNHL in Q2, but in the higher antibiotic dosing quartiles (Q3 and Q4) the number of ears with SNHL was much higher (12 ears and 14 ears, respectively) than the number with normal hearing (7 ears and 6 ears, respectively). Median cumulative life-time doses for tobramycin q6 was 47 over the course of 11.75 days, for q8 this was 48 over 16 days, q12 this was 6.5 over 3.25 days, q24 this was 31 over 31 days, for q36 this was 3 over 4.5 days, and for q48 this was 4 over 8 days. Median cumulative life-time doses for vancomycin q6 was 39 over 9.75 days, for q8 this was 72 over 24 days, for q12 this was 7 over 3.5 days, and for q24 this was 36 over 36 days. Amikacin was the least common antibiotic in the dataset and median life-time cumulative doses for q8 was 2 over 0.7 days and, for q24 this was 35 over 35 days (Supplemental Table 1).
Figure 1.
Audiometric profiles ears in the lowest dosing group (Q1) up to the highest dosing group (Q4). Age (median and range) is also displayed for individual ears within each dosing quartile.
Individual hearing thresholds across the conventional (0.25 to 8.0 kHz) and extended high frequency (9.0 to 16.0 kHz) ranges for participants in the lowest dosing group (Q1) up to the highest dosing group (Q4). The ear with the greatest degree of hearing loss for each participant is displayed. Participants who were older than the median age for each quartile are displayed in colored lines.
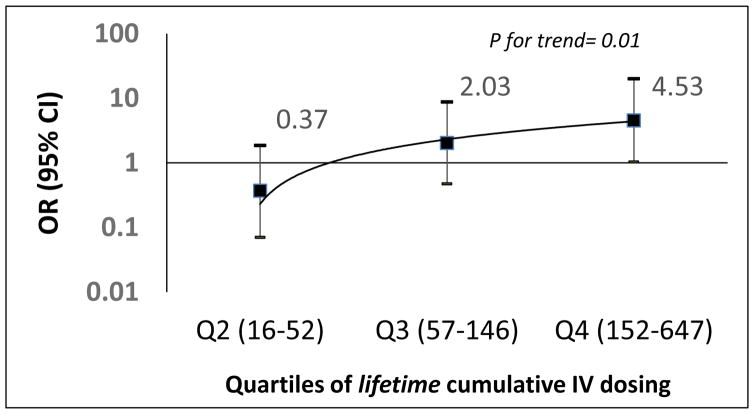
Table 2 shows the odds ratios for the cumulative dosing for participants who received IV- AGs (+/− vancomycin) treatments (N=81 ears). The median cumulative doses in the normal hearing group was 26.5, which was much lower than the median cumulative doses in the SNHL group of 69, although not statistically significant (p=0.08). Those in Q3 had 2.03 times higher odds of SNHL (95% CI: 0.47, 8.72), and those in the Q4 had 4.53 times higher odds of SNHL (95% CI: 1.03, 20.02) after adjusting for age at hearing test and gender. When we compared low dosing (Q1 + Q2 ears) and high dosing (Q3 + Q4 ears), the higher dosing group had 4.79 times higher odds of SNHL (p= 0.005). There was also a significant trend of increased odds of SNHL with increased dosing quartiles (p for trend = 0.01) (Figure 2). Age at the time of hearing test was significantly associated with SNHL after adjusting for medication dosage (p< 0.05) and gender. For each one-year increase in age at the time of hearing test, odds of developing SNHL was 1.16 (95% CI: 1.06 to 1.26, p= 0.001). Similar findings were evident for the same analyses excluding three participants who only received vancomycin treatments (N= 78 ears), however the association was not strengthened by pooling the low versus high dosing groups. (See bottom panel of Table 2). Excluding age at the time of the hearing test from the models attenuated the association (>10% change in odds ratios; data not shown), hence age at hearing test was included in all the models.
Table 2.
Cumulative IV antibiotic dosing and association with hearing loss (adjusted for age at hearing test and gender)
Odds ratios were calculated to estimate the probability of SNHL with low (Q1) to high (Q4) dosing groups. When Q1 was used as the referent groups, there was a significantly higher odds ratio that participants with the highest (Q4) IV antibiotic exposure would develop SNHL. This effect was observed with and without the inclusion of participants who only received vancomycin (bottom table). These effects remained statistically significant when the two lowest exposure groups (Q1 & Q2) were compared to the two highest exposure groups (Q3 & Q4).
| IV Antibiotic doses (N=81) | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Q1 (2 – 15) | 1.00 | 0.01 |
| Q2 (16 – 52) | 0.37 (0.07, 1.85) | |
| Q3 (57 – 146) | 2.03 (0.47, 8.72) | |
| Q4 (152 – 647) | 4.53 (1.03, 20.02) | |
| Low Dosing (Q1 & Q2) | 1.00 | 0.005 |
| High Dosing (Q3 & Q4) | 4.79 (1.62, 14.14) | |
| IV Antibiotic doses (excluding subjects who only received Vancomycin) (N=78) | ||
| Q1 (2 – 15) | 1.00 | 0.01 |
| Q2 (16 – 37) | 0.63 (0.14, 2.91) | |
| Q3 (38 – 80) | 1.01 (0.24, 4.31) | |
| Q4 (88 – 266) | 6.89 (1.41, 33.76) | |
| Low Dosing (Q1 & Q2) | 1.00 | 0.03 |
| High Dosing (Q3 & Q4) | 3.19 (1.15, 8.90) |
Figure 2. Odds ratios for association of cumulative IV dose for AG (± vancomycin) exposure.
(Q1 = Referent; horizontal line at 1)
Odds ratios for association of cumulative IV antibiotic dosing. There was a significant trend of increased odds of SNHL with increased dosing quartiles (p for trend = 0.01) when Q1 was used as the referent group.
In the secondary weighted analysis, for the same participants and treatments, those in Q3 had 2.34 times higher odds of SNHL (95% CI: 0.54, 10.10), and those in the highest quartile (Q4) had 3.71 times higher odds of SNHL (95% CI: 0.86, 15.97) compared to the lowest dosing quartile (Q1), after adjusting for age at the time of hearing test and gender. There was a significant trend of increasing odds of SNHL with increasing quartiles of cumulative dosing (p for trend = 0.05). Those in the higher two weighted dosing quartiles were 2.83 times (95% CI: 0.98, 8.16) more likely to have SNHL compared to the lower dosing quartiles (Q1 and Q3) (p=0.06) compared to 4.79 times more likely with cumulative IV dosing. These associations were strengthened when excluding ears that only received vancomycin. Those in the higher two weighted dosing quartiles were 3.60 times (95% CI: 1.25, 1.34) more likely to have SNHL compared to the lower dosing quartiles (p=0.02).
A moderate level of agreement was evident with a kappa statistic of 0.61 (95% CI: 0.48, 0.73) between cumulative dosing quartiles and weighted quartiles of cumulative antibiotic dosing (tobramycin, amikacin and vancomycin). When vancomycin was excluded, the level of agreement strengthened slightly but still remained moderate with a kappa statistic of 0.69 (95% CI: 0.57, 0.84).
Exploratory analysis for different scenarios of AGs with or without vancomycin did not reveal any association between cumulative doses of AGs (+/− vancomycin) and SNHL (p= 0.76).
Discussion
Our data show that increased cumulative lifetime IV-AG dosing was associated with greater odds for developing sensorineural hearing loss. This suggests that ongoing clinical monitoring of cumulative lifetime IV-AG dosing in patients with cystic fibrosis is needed. Furthermore, a dose-relationship model to estimate the dose “threshold “when a patient is at higher risk for hearing loss may be useful for patient care. Tracking the cumulative dose may also be useful to the clinical care team (e.g., pulmonologists, pharmacologists, nurses) to identify when a patient is at higher risk of hearing loss, and refer patients for immediate audiological monitoring.
The data also provide strong evidence for routine hearing evaluations with extended high-frequency testing (9.0 to 16.0 kHz) on every CF patient who has or will receive IV-AG treatments in their lifetime. We found a prevalence of 56% (45 of 81 ears) for SNHL in CF ears with a history of IV-AG (+/− vancomycin) treatments. The prevalence of SNHL in this study is slightly higher than reported in previous studies by others that ranged between 0–47% (2, 9, 11–14, 20, 21). The majority of prior studies tested pediatric patients 0 to18 years of age, and their methodological approach for classifying hearing loss was limited to a standard hearing test range of 0.25–8.0 kHz. However, our study tested participants 15 years old or over in both conventional and extended higher frequencies through to 16 kHz (displayed in Figure 1). The current study is in line with previous work that used a similar age group, and hearing test methods for identifying ototoxicity. For example, Fausti et al. (1992) detected SNHL in 51% of ototoxic drug-exposed ears in conventional and extended high-frequency hearing thresholds (up to 20 kHz) (14). This group further reported that 74% of these ears with SNHL had hearing loss in the frequency range ≥9.0 kHz (14). A retrospective study by Tarshish (12), reported on the risk factors and prevalence of SNHL in a large cohort of CF patients (mean age= 18 yr., SD = 10 yr.) who had received audiometric evaluations between 2007 and 2010. They found that 21% (37 of 178) had SNHL in at least one ear. However, of these ears 94.1% had unilateral SNHL, and 77.8% had bilateral SNHL at their highest test frequencies (6.0–8.0 kHz) (12). When we age matched our study (re: mean age of 26 yr., SD ±10) to Tarshish’s cohort, patients with CF between 20 to 49 years old, we found a prevalence for SNHL of ~42% within the conventional frequency range, all those had SNHL in the 6.0–8.0 kHz range, reflecting the normal progression of ototoxic damage. Tarshish and colleagues would have likely found an even higher prevalence of SNHL in their groups if audiometric data for frequencies >8.0 kHz were evaluated.
Another important finding of this investigation, was the significant age effect between the normal hearing (mean age= 22 yr., SD ±6) and SNHL (mean age= 30 yr., SD ±12) groups. There was also a non-significant trend for an increasing degree of hearing loss related to increasing exposure to IV-antibiotics and older age. This is in line with other studies that reported age effects in patients receiving IV-AG treatments. Gatell and colleagues (42) reported on both ototoxicity and nephrotoxicity rates of a heterogeneous group of CF and non-CF patients treated with aminoglycosides for sepsis, urinary or biliary tract infection. Patients who developed ototoxicity were found to be significantly older and had higher serum aminoglycoside trough levels compared to younger participants, and that the risk for developing drug-induced SNHL increased from 3 to 26% as age increased from 14 to 90 years old (42).
Despite the association observed between increased cumulative dosing and hearing loss, it was clear that participants in all four quartiles had great variability in their hearing thresholds, particularly in the extended frequency ranges (Figure 1), notably those in the lowest exposure quartile (Q1). Although many ears in Q1 had normal hearing for all frequencies, 9/22 ears had some degree of SNHL. These tended to be the older CF patients between 29 to 56 years of age indicating that age was associated with hearing loss, even in the lowest exposure group. Once we controlled for age, greater cumulative dosing significantly increased the risk for developing hearing loss. The diversity of hearing thresholds in each quartile suggests that using cumulative dosing exclusively to determine who should be screened for hearing loss is inadequate, but, may be used to identify participants at higher risk for hearing loss. Other factors likely also contribute to the variability of the presence of hearing loss in each quartile, including (but not limited to) renal function and inflammation. These findings and factors suggest that it is imperative to routinely monitor hearing in any patient receiving IV-AG treatments.
To date, there are few studies that have addressed the association of age, degree of hearing loss and dosage of IV-AG (+/− vancomycin) with cochleotoxicity in a large cohort of patients (1, 4, 9, 17). The present study is unique in that the above factors were investigated using a method that quantifies cumulative IV-antibiotic dose exposure and a separate method that takes into account the daily dosing schedule during these treatments (weighted). A significant effect of cumulative IV-AG dosing (+/− vancomycin) on SNHL in CF participants was discovered using both methods, after accounting for age at the time of test and gender. Specifically, the cumulative IV dosage calculation showed participants with the highest quartile of dosage were 4.79 times more likely to develop SNHL than the lowest quartile. With the weighted dosage scheme, this was 3.71 times more likely. Both methods showed dose-dependent ototoxicity effects which were stronger for the cumulative-dose method.
The rationale for developing a weighting scheme was based on previous reports that utilize a dose-response relationship for evaluating the effects of an independent variable on a dependent variable. Studies in other fields have accounted for both the cumulative and frequency effects when investigating health outcomes of a specific independent variable (re: accounting for packs per day when researching smoking effects on health). This is relevant to the current study because a CF patient who receives an IV-AG in separate doses per day may have more difficulty clearing the drug through their system compared to a person who received the same total dose in a single daily administration. The most frequent dosing regimen was q6 (four doses per day), which received the highest weight compared to a less frequent dosing regimen such as a q24 (one dose per day). In contrast, cumulative IV doses are calculated as the total lifetime number of doses that a participant received whereby a more frequent dosing regimen such as a q6 is counted the same as a less frequent dosing regimen like a q24. This has the potential to inflate the effect of the less frequent dosing regimen leading to a larger effect size and a potentially stronger association. Thus, weighted associations are thought to be more conservative and reflect frequency of dosing. This could be an explanation of why, in this study, we see stronger associations with the cumulative IV dosing quartiles compared to the weighted dosing quartiles. This might also indicate that the weighted method was a better representation of the ototoxic effects because daily dose administration was considered.
Our data differs from that reported by Scheenstra et al. (20) who did not find significant SNHL in 13 participants following IV-tobramycin treatment. Although our cumulative IV-dosing method is comparable to that by Scheenstra et al., there are several methodological differences that may account for this discrepancy. First, treatments were administered at 10 mg/kg, twice-daily for up to 21 days for each course in the Scheenstra study, whereas this study enrolled participants treated with antibiotics once- to four-times daily (~14 days) for each course of treatment over their lifetime. The present investigation also had a much larger cohort (N=81) and larger range of cumulative dose exposure over a lifetime was determined in our cohort compared to Scheenstra (N=19). This difference alone may indicate that patients in the present study were likely more ill given the substantially higher cumulative dose exposure over their lifetime. In addition, participants who are more likely to be ill from respiratory infections may have greater levels of systemic inflammation that can further predispose them to aminoglycoside-induced ototoxicity, as suggested recently (49, 50). Finally, our participants began receiving AG dosing at younger ages than in Scheenstra et al. (20), further supporting the suggestion that our participants were more likely to be ill than in the Scheenstra cohort, potentially predisposing them to cochleotoxicity.
The results of this investigation and others (3, 14, 17) provide direct evidence of AG-induced damage to the auditory system. It is recommended that clinics monitor patients for early changes in hearing function, particularly at higher frequencies, pre- and post IV-AG treatments. This allows for identification of hearing damage before it reaches frequencies important for speech discrimination (e.g., 0.5–8.0 kHz). This information will allow both the patient and the physician to discuss possible modifications to the treatment regimen, particularly if an alternative approach is or becomes available, or at a minimum provides a basis for recommendation for rehabilitation.
One limitation of the present investigation is the estimate of cumulative (lifelong) IV-AG and/or glycopeptide drug exposure for each patient. Although a rigorous review of each patient’s electronic and paper medical records were undertaken, some IV-AG treatments may have been missed, or not documented at the time of treatment. It is difficult to account for this degree of error; therefore, separating participants into quartiles of dosing was adopted to overcome this risk, as previously described. The higher dosing groups were still significantly more likely to have SNHL. We also did not account for nebulized treatments, such as inhaled AG azithromycin, which is common in CF treatment; however, their total dose per inhalation is a fraction of that given intravenously. It is unknown if there is a synergistic effect between inhaled and IV-AG treatment on SNHL. Assessment of serum drug peak and trough levels, and consequently trough levels during treatments may provide more insight.
The results of this study may have been confounded with other drugs co-administered during or between IV- AG or glycopeptide treatments throughout each patient’s lifetime. This was taken into consideration but difficult to account for statistically due to the small cohort size in this study, and the reduced incidence of the types of drug treatments being co-administered across subjects.
Another limitation of this investigation is that a single hearing test was conducted to estimate hearing status, unlike Scheenstra et al. (20) who had a baseline and post-treatment hearing test. The purpose of this study was to estimate the prevalence of SNHL in a cohort of CF patients, rather than estimate the incidence of developing SNHL post-treatments. A repeated measures design would provide a better estimate of changes in post-treatment hearing status from baseline following one or more intravenous antibiotic drug treatments. Accounting for these limitations may improve the estimates reported here; however, the current findings have strong statistical effects that would likely remain significant if both limitations were adequately addressed in a subsequent study.
Previous studies, as well as the current study, show that further research is necessary to clarify both the incidence and prevalence of AG-induced ototoxicity in patients with CF. It will also be important to further consider the effects of age, methodological differences, treatment regimen, pulmonary and renal function, and the co-administration of other medications with AGs and their pharmacokinetics.
Conclusion
There was a significant effect of cumulative IV-AG dosing (± vancomycin) on SNHL in CF participants, after accounting for age and gender. Older CF patients tended to have more medication exposure and a greater degree of hearing loss compared to younger CF patients. Therefore, in addition to a regular cochleotoxicity monitoring program for all CF patients taking IV-AGs, it is recommended that patients at greater risk for SNHL (e.g., older age, and higher total IV-AG courses) be more closely monitored for cochleotoxicity as a part of their treatment plan. Preventing or ameliorating the effects of permanent SNHL is crucial for patients with CF who already have a significantly compromised quality of life due to the disease.
Supplementary Material
Highlights.
Aminoglycosides and glycopeptides are antibiotics essential for treating life-threatening respiratory infections in patients with cystic fibrosis (CF).
CF patients with sensorineural hearing loss tend to be older than patients with normal hearing
Cumulative IV antibiotic dosing has a significant negative effect on hearing sensitivity in patients with CF, when controlling for age and gender effects.
Acknowledgments
This work is supported by the following: National Institutes of Health CTSA grant (UL1TR000128) to the Oregon Clinical and Translational Research Institute (PSS) and NIH-NIDCD Grant Awards: R01 DC004555, R01 DC012588, and R01 DC10202
Preliminary data from this study was presented at the 2014 and 2015 Association for Research in Otolaryngology (ARO) Midwinter meetings
Footnotes
Disclosures: The opinions presented here are the private views of the author and do not represent the official views of the Department of Veterans Affairs, National Institutes of Health or the United States Government
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martins LM, Camargos PA, Becker HM, Becker CG, Guimaraes RE. Hearing loss in cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2010;74(5):469–73. doi: 10.1016/j.ijporl.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Mulheran M, Degg C, Burr S, Morgan DW, Stableforth DE. Occurrence and risk of cochleotoxicity in cystic fibrosis patients receiving repeated high-dose aminoglycoside therapy. Antimicrob Agents Chemother. 2001;45(9):2502–9. doi: 10.1128/AAC.45.9.2502-2509.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng AG, Johnston PR, Luz J, Uluer A, Fligor B, Licameli GR, et al. Sensorineural hearing loss in patients with cystic fibrosis. Otolaryngol Head Neck Surg. 2009;141(1):86–90. doi: 10.1016/j.otohns.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Prayle A, Smyth AR. Aminoglycoside use in cystic fibrosis: therapeutic strategies and toxicity. Curr Opin Pulm Med. 2010;16(6):604. doi: 10.1097/MCP.0b013e32833eebfd. [DOI] [PubMed] [Google Scholar]
- 5.Moore RDSC, Lietman PS. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis. 1984;149:23–30. doi: 10.1093/infdis/149.1.23. [DOI] [PubMed] [Google Scholar]
- 6.Md Daud MK, Mohamadl H, Haron A, Rahman NA. Ototoxicity screening of patients treated with streptomycin using distortion product otoacoustic emissions. B-ENT. 2014;10(1):53. [PubMed] [Google Scholar]
- 7.Hirose K, Li SZ, Ohlemiller KK, Ransohoff RM. Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide. Journal of the Association for Research in Otolaryngology : JARO. 2014;15(4):555–70. doi: 10.1007/s10162-014-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummett RE, Fox KE. Aminoglycoside-induced hearing loss in humans. Antimicrob Agents Chemother. 1989;33(6):797–800. doi: 10.1128/aac.33.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore RD, Smith CR, Lietman PS. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis. 1984;149(1):23–30. doi: 10.1093/infdis/149.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Riethmueller J, Ballmann M, Schroeter TW, Franke P, von Butler R, Claass A, et al. Tobramycin once- vs thrice-daily for elective intravenous antipseudomonal therapy in pediatric cystic fibrosis patients. Infection. 2009;37(5):424–31. doi: 10.1007/s15010-009-8117-4. [DOI] [PubMed] [Google Scholar]
- 11.Pilcher OB, deOliveira TM, et al. The prevalence of neurosensorineural hearing loss among cystic fibrosis patients from Hospital de Clinicals de Porto Alegre. Int J Pediatr Otorhinolaryngol. 2003:939–41. doi: 10.1016/s0165-5876(03)00135-6. [DOI] [PubMed] [Google Scholar]
- 12.Tarshish Y, Huang L, Jackson FI, Edwards J, Fligor B, Wilkins A, et al. Risk Factors for Hearing Loss in Patients with Cystic Fibrosis. J Am Acad Audiol. 2016;27(1):6–12. doi: 10.3766/jaaa.14104. [DOI] [PubMed] [Google Scholar]
- 13.Geyer LB, Menna Barreto SS, Weigert LL, Teixeira AR. High frequency hearing thresholds and product distortion otoacoustic emissions in cystic fibrosis patients. Braz J Otorhinolaryngol. 2015;81(6):589–97. doi: 10.1016/j.bjorl.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, McDonald WJ. High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. J Infect Dis. 1992;165(6):1026–32. doi: 10.1093/infdis/165.6.1026. [DOI] [PubMed] [Google Scholar]
- 15.Yoon PJ, Price M, Gallagher K, Fleisher BE, Messner AH. The need for long-term audiologic follow-up of neonatal intensive care unit (NICU) graduates. Int J Pediatr Otorhinolaryngol. 2003;67(4):353–7. doi: 10.1016/s0165-5876(02)00400-7. [DOI] [PubMed] [Google Scholar]
- 16.Gurtler N, Schmuziger N, Kim Y, Mhatre AN, Jungi M, Lalwani AK. Audiologic testing and molecular analysis of 12S rRNA in patients receiving aminoglycosides. Laryngoscope. 2005;115(4):640–4. doi: 10.1097/01.mlg.0000161355.28073.f5. [DOI] [PubMed] [Google Scholar]
- 17.Al-Malky G, Suri R, Dawson SJ, Sirimanna T, Kemp D. Aminoglycoside antibiotics cochleotoxicity in paediatric cystic fibrosis (CF) patients: A study using extended high-frequency audiometry and distortion product otoacoustic emissions. Int J Audiol. 2011;50(2):112–22. doi: 10.3109/14992027.2010.524253. [DOI] [PubMed] [Google Scholar]
- 18.Mick P, Westerberg BD. Sensorineural hearing loss as a probable serious adverse drug reaction associated with low-dose oral azithromycin. J Otolaryngol. 2007;36(5):257–63. doi: 10.2310/7070.2007.0047. [DOI] [PubMed] [Google Scholar]
- 19.Bailie GR, Neal D. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Exp. 1988;3(5):376–86. doi: 10.1007/BF03259891. [DOI] [PubMed] [Google Scholar]
- 20.Scheenstra RJ, Heijerman HG, Zuur CL, Touw DJ, Rijntjes E. No hearing loss after repeated courses of tobramycin in cystic fibrosis patients. Acta Otolaryngol. 2010;130(2):253–8. doi: 10.3109/00016480903015150. [DOI] [PubMed] [Google Scholar]
- 21.Mulheran M, Hyman-Taylor P, Tan KH, Lewis S, Stableforth D, Knox A, et al. Absence of cochleotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times-daily tobramycin in cystic fibrosis patients. Antimicrob Agents Chemother. 2006;50(7):2293–9. doi: 10.1128/AAC.00995-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Meter DJ, Corriveau M, Ahern JW, Lahiri T. A survey of once-daily dosage tobramycin therapy in patients with cystic fibrosis. Pediatr Pulmonol. 2009;44(4):325–9. doi: 10.1002/ppul.20985. [DOI] [PubMed] [Google Scholar]
- 23.Nordstrom L, Lerner SA. Single daily dose therapy with aminoglycosides. J Hosp Infect. 1991;18(Suppl A):117–29. doi: 10.1016/0195-6701(91)90013-x. [DOI] [PubMed] [Google Scholar]
- 24.Tran Ba Huy P, Deffrennes D. Aminoglycoside ototoxicity: influence of dosage regimen on drug uptake and correlation between membrane binding and some clinical features. Acta Otolaryngol. 1988;105(5–6):511–5. doi: 10.3109/00016488809119511. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert DN. Once-daily aminoglycoside therapy. Antimicrob Agents Chemother. 1991;35(3):399–405. doi: 10.1128/aac.35.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43(7):1549–55. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foundation CF. Cystic Fibrosis Foundation Patient Registry Annual Data Report 2013 to the Center Directors. Bethesda, Maryland: 2014. [Google Scholar]
- 28.(ANSI) ANSI; Author, editor. Specification for instruments to measure aural acoustic impedance and admittance (aural acoustic immittance) (S3.39-1987(R2007) New York: 2007. [Google Scholar]
- 29.(ANSI) ANSI; Author, editor. Specifications for Audiometers (ANSI 3.6-1996) New York: 1996. [Google Scholar]
- 30.Institute ANSI. Specification for audiometers (ANSI S3.6-2010) New York: Author; 2010. [Google Scholar]
- 31.Cox RM, MM Reference equivalent threshold levels for pure tone and 13 octare noise bands: insert earphone and TDH 49 earphone. J Acoust Soc Am. 79(2):443–6. doi: 10.1121/1.393531. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen T. Reference thresholds for EARTONE 3A insert earphones. Scand Audiol. 1991;20(3):205–7. doi: 10.3109/01050399109074955. [DOI] [PubMed] [Google Scholar]
- 33.Frank T. High-frequency hearing thresholds in young adults using a commercially available audiometer. Ear Hear. 1990;11(6):450–4. doi: 10.1097/00003446-199012000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Carhart R, Jerger J. Preferred Method For Clinical Determination Of Pure-Tone Thresholds. Journal of Speech and Hearing Disorders. 1959;24:330–45. [Google Scholar]
- 35.Association ASLH. Scope of practice in audiology [Scope of practice] 2004 [Available from: www.asha.org/policy.
- 36.Audiology AAo. Guidelines and Standards Adult Diagnostics. 2016 [Available from: http://www.audiology.org/publications/guidelines-and-standards.
- 37.Sinxadi P, Blockman M. Drug-induced ototoxicity.(Clinical pharmacology)(Clinical report) CME: Your SA Journal of CPD. 2009;27(8):372. [Google Scholar]
- 38.Bates DE, Beaumont SJ, Baylis BW. Ototoxicity induced by gentamicin and furosemide. Ann Pharmacother. 2002;36(3):446–51. doi: 10.1345/aph.1A216. [DOI] [PubMed] [Google Scholar]
- 39.Bruniera FR, Saviolli LRM, Bacci MR, Feder D, da Luz Goncalves Pedreira M, Sorgini Peterlini MA, et al. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19(4):694–700. [PubMed] [Google Scholar]
- 40.Chiodo AA, Alberti PW. Experimental, clinical and preventive aspects of ototoxicity. Eur Arch Otorhinolaryngol. 1994;251(7):375–92. doi: 10.1007/BF00181963. [DOI] [PubMed] [Google Scholar]
- 41.Pleasants RA, Michalets EL, Williams DM, Samuelson WM, Rehm JR, Knowles MR. Pharmacokinetics of vancomycin in adult cystic fibrosis patients. Antimicrob Agents Chemother. 1996;40(1):186–90. doi: 10.1128/aac.40.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatell JM, San Miguel JG, Zamora L, Araujo V, Bonet M, Bohe M, et al. Comparison of the nephrotoxicity and auditory toxicity of tobramycin and amikacin. Antimicrob Agents Chemother. 1983;23(6):897–901. doi: 10.1128/aac.23.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matz GJ, Lerner SA. Prospective studies of aminoglycoside ototoxicity in adults. In: Lerner SA, Matz GJ, Hawkins JE, editors. Aminoglycoside ototoxicity. Boston: Little, Brown and Company; 1981. pp. 327–36. [Google Scholar]
- 44.Brummett RE, Fox KE, Bendrick TW, Himes DL. Ototoxicity of tobramycin, gentamicin, amikacin and sisomicin in the guinea pig. J Antimicrob Chemother. 1978;4(Suppl A):73–83. doi: 10.1093/jac/4.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 45.Lubin JHCN, Wichmann HE, Schaffrath-Rosario A, Alavanja MC. Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiology. 2007;18:639–48. doi: 10.1097/EDE.0b013e31812717fe. [DOI] [PubMed] [Google Scholar]
- 46.Rademaker-Lakhai JM, Crul M, Zuur L, Baas P, Beijnen JH, Simis YJ, et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol. 2006;24(6):918–24. doi: 10.1200/JCO.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez Valiente A, Trinidad A, Garcia Berrocal JR, Gorriz C, Ramirez Camacho R. Extended high-frequency (9–20 kHz) audiometry reference thresholds in 645 healthy subjects. Int J Audiol. 2014;53(8):531–45. doi: 10.3109/14992027.2014.893375. [DOI] [PubMed] [Google Scholar]
- 48.Frank T. High-frequency (8 to 16 kHz) reference thresholds and intrasubject threshold variability relative to ototoxicity criteria using a Sennheiser HDA 200 earphone. Ear Hear. 2001;22(2):161–8. doi: 10.1097/00003446-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Koo JW, Quintanilla-Dieck L, Jiang M, Liu J, Urdang ZD, Allensworth JJ, et al. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci Transl Med. 2015;7(298):298ra118. doi: 10.1126/scitranslmed.aac5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross CPLS, Urdang ZD, Srikanth P, Garinis AC, Steyger PS. Effect of sepsis and systemic inflammatory response syndrome on neonatal hearing screening outcomes following gentamicin exposure. Int J Pediatr Otorhinolaryngol. 2015;79(11):1915–9. doi: 10.1016/j.ijporl.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.